Abstract
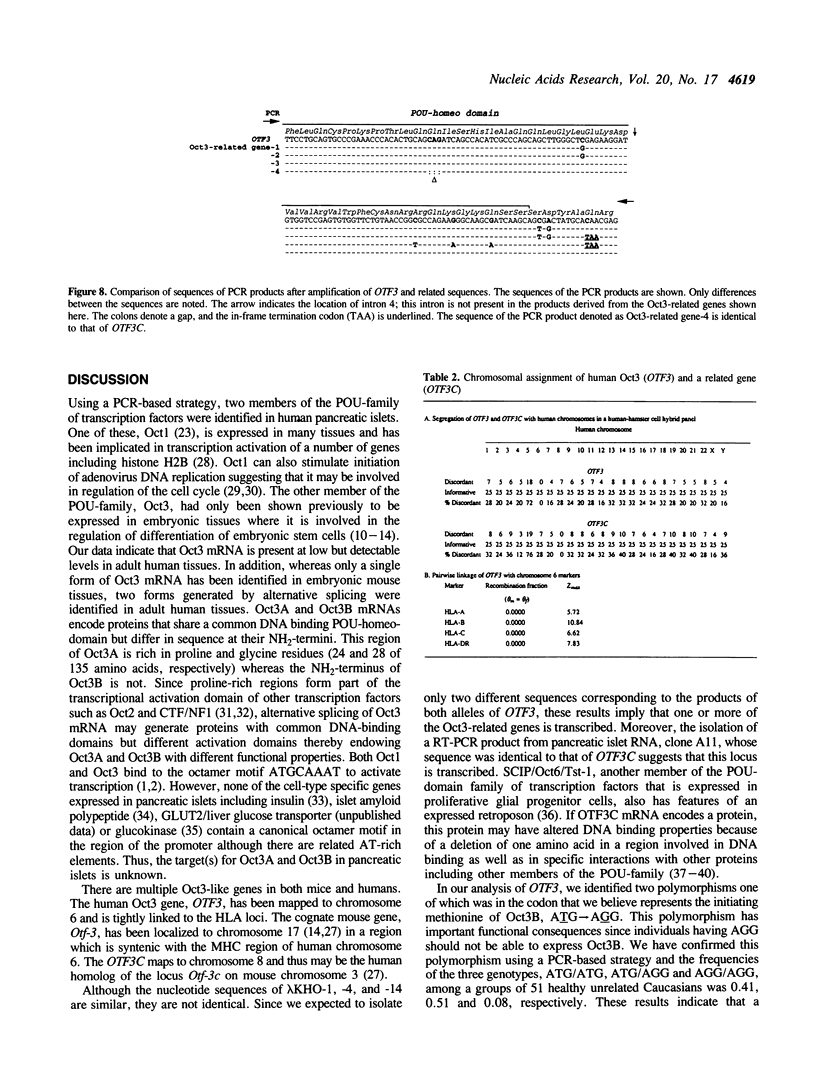
Transcription factors containing the POU-domain have been shown to be important regulators of tissue-specific gene expression in the pituitary and lymphoid cells. Using a polymerase chain reaction (PCR)-based strategy, we have searched for similar factors that may be expressed in adult human pancreatic islets. This approach resulted in the amplification of sequences encoding the octamer binding proteins Oct1 and Oct3 (also called Oct4). The isolation of cDNAs encoding Oct3 revealed the expression of two isoforms of this transcription factor termed Oct3A and Oct3B that are generated by alternative splicing. Human Oct3A and Oct3B are composed of 360 and 265 amino acids, respectively, of which the 225 amino acids at the COOH-termini are identical. The sequence of human Oct3A shows 87% amino acid identity with mouse Oct3. Reverse-transcriptase PCR showed low levels of expression of both Oct3A and Oct3B mRNA in all adult human tissues examined. We also isolated and characterized the human Oct3 gene (OTF3) and a related gene, OTF3C. The human Oct3 gene, localized to human chromosome 6 in the region of the MHC complex, spans about 7 kb and consists of five exons. The Oct3-related gene, OTF3C, is a retroposon and has been localized to human chromosome 8. Southern blotting and PCR amplification of human DNA indicated the presence of other OTF3-related genes as has been previously noted in the mouse. Two polymorphisms which can be typed using PCR were identified in OTF3 which will facilitate genetic studies of this gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Pictet R. L., Rutter W. J., Cordell B., Tischer E., Goodman H. M. Sequence of the human insulin gene. Nature. 1980 Mar 6;284(5751):26–32. doi: 10.1038/284026a0. [DOI] [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Clerc R. G., Corcoran L. M., LeBowitz J. H., Baltimore D., Sharp P. A. The B-cell-specific Oct-2 protein contains POU box- and homeo box-type domains. Genes Dev. 1988 Dec;2(12A):1570–1581. doi: 10.1101/gad.2.12a.1570. [DOI] [PubMed] [Google Scholar]
- Dausset J., Cann H., Cohen D., Lathrop M., Lalouel J. M., White R. Centre d'etude du polymorphisme humain (CEPH): collaborative genetic mapping of the human genome. Genomics. 1990 Mar;6(3):575–577. doi: 10.1016/0888-7543(90)90491-c. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster T., Balmaceda C. G., Roeder R. G. The cell type-specific octamer transcription factor OTF-2 has two domains required for the activation of transcription. EMBO J. 1990 May;9(5):1635–1643. doi: 10.1002/j.1460-2075.1990.tb08283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Albert V. R., Chen R. P., Crenshaw 3d E. B., Elsholtz H. P., He X., Kapiloff M. S., Mangalam H. J., Swanson L. W., Treacy M. N. A family of POU-domain and Pit-1 tissue-specific transcription factors in pituitary and neuroendocrine development. Annu Rev Physiol. 1990;52:773–791. doi: 10.1146/annurev.ph.52.030190.004013. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Flynn S. E., Voss J. W., Albert V. R., Kapiloff M. S., Wilson L., Rosenfeld M. G. The POU-specific domain of Pit-1 is essential for sequence-specific, high affinity DNA binding and DNA-dependent Pit-1-Pit-1 interactions. Cell. 1990 Jun 15;61(6):1021–1033. doi: 10.1016/0092-8674(90)90067-o. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A reappraisal of non-consensus mRNA splice sites. Nucleic Acids Res. 1991 Jul 25;19(14):3795–3798. doi: 10.1093/nar/19.14.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet. 1985 May;37(3):482–498. [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Magnuson M. A., Shelton K. D. An alternate promoter in the glucokinase gene is active in the pancreatic beta cell. J Biol Chem. 1989 Sep 25;264(27):15936–15942. [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Müller M. M., Ruppert S., Schaffner W., Matthias P. A cloned octamer transcription factor stimulates transcription from lymphoid-specific promoters in non-B cells. Nature. 1988 Dec 8;336(6199):544–551. doi: 10.1038/336544a0. [DOI] [PubMed] [Google Scholar]
- Nishi M., Sanke T., Seino S., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I., Steiner D. F. Human islet amyloid polypeptide gene: complete nucleotide sequence, chromosomal localization, and evolutionary history. Mol Endocrinol. 1989 Nov;3(11):1775–1781. doi: 10.1210/mend-3-11-1775. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Okazawa H., Okamoto K., Ishino F., Ishino-Kaneko T., Takeda S., Toyoda Y., Muramatsu M., Hamada H. The oct3 gene, a gene for an embryonic transcription factor, is controlled by a retinoic acid repressible enhancer. EMBO J. 1991 Oct;10(10):2997–3005. doi: 10.1002/j.1460-2075.1991.tb07850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. B., Segil N., Heintz N. Differential phosphorylation of the transcription factor Oct1 during the cell cycle. Science. 1991 Aug 30;253(5023):1022–1026. doi: 10.1126/science.1887216. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G. POU-domain transcription factors: pou-er-ful developmental regulators. Genes Dev. 1991 Jun;5(6):897–907. doi: 10.1101/gad.5.6.897. [DOI] [PubMed] [Google Scholar]
- Rosner M. H., Vigano M. A., Ozato K., Timmons P. M., Poirier F., Rigby P. W., Staudt L. M. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990 Jun 21;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Ruvkun G., Finney M. Regulation of transcription and cell identity by POU domain proteins. Cell. 1991 Feb 8;64(3):475–478. doi: 10.1016/0092-8674(91)90227-p. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Cromlish J. A., Gerster T., Kawakami K., Balmaceda C. G., Currie R. A., Roeder R. G. A human lymphoid-specific transcription factor that activates immunoglobulin genes is a homoeobox protein. Nature. 1988 Dec 8;336(6199):551–557. doi: 10.1038/336551a0. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Dressler G. R., Balling R., Rohdewohld H., Gruss P. Oct-4: a germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990 Jul;9(7):2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Hatzopoulos A. K., Balling R., Suzuki N., Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989 Sep;8(9):2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. M., Voss J. W., Ingraham H. A., Holloway J. M., Broide R. S., Rosenfeld M. G., Swanson L. W. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990 May;4(5):695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- Siracusa L. D., Rosner M. H., Vigano M. A., Gilbert D. J., Staudt L. M., Copeland N. G., Jenkins N. A. Chromosomal location of the octamer transcription factors, Otf-1, Otf-2, and Otf-3, defines multiple Otf-3-related sequences dispersed in the mouse genome. Genomics. 1991 Jun;10(2):313–326. doi: 10.1016/0888-7543(91)90314-5. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Roeder R. G. Interaction of a common factor with conserved promoter and enhancer sequences in histone H2B, immunoglobulin, and U2 small nuclear RNA (snRNA) genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6382–6386. doi: 10.1073/pnas.83.17.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Tanaka M., Herr W. The Oct-1 homoeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989 Oct 19;341(6243):624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- Vanin E. F. Processed pseudogenes: characteristics and evolution. Annu Rev Genet. 1985;19:253–272. doi: 10.1146/annurev.ge.19.120185.001345. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., Kal A. J., Van der Vliet P. C. The DNA binding domain (POU domain) of transcription factor oct-1 suffices for stimulation of DNA replication. EMBO J. 1990 Jun;9(6):1883–1888. doi: 10.1002/j.1460-2075.1990.tb08314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrijzer C. P., van Oosterhout J. A., van der Vliet P. C. The Oct-1 POU domain mediates interactions between Oct-1 and other POU proteins. Mol Cell Biol. 1992 Feb;12(2):542–551. doi: 10.1128/mcb.12.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J. W., Wilson L., Rosenfeld M. G. POU-domain proteins Pit-1 and Oct-1 interact to form a heteromeric complex and can cooperate to induce expression of the prolactin promoter. Genes Dev. 1991 Jul;5(7):1309–1320. doi: 10.1101/gad.5.7.1309. [DOI] [PubMed] [Google Scholar]