Abstract
Abstinent methamphetamine (Meth) dependent individuals demonstrate poorer performance on tests sensitive to attention/information processing speed, learning and memory, and working memory when compared to non-Meth dependent individuals. The poorer performance on these tests may contribute to the morbidity associated with Meth-dependence. In light of this, we sought to determine the effects of acute, low-dose Meth administration on attention, working memory, and verbal learning and memory in 19 non-treatment seeking, Meth-dependent individuals. Participants were predominantly male (89%), Caucasian (63%), and cigarette smokers (63%). Following a four day, drug-free washout period, participants were given a single-blind intravenous infusion of saline, followed the next day by 30 mg of Meth. A battery of neurocognitive tasks was administered before and after each infusion, and performance on measures of accuracy and reaction time were compared between conditions. While acute Meth exposure did not affect test performance for the entire sample, participants who demonstrated relatively poor performance on these tests at baseline, identified using a median split on each test, showed significant improvement on measures of attention/information processing speed and working memory when administered Meth. Improved performance was seen on the following measures of working memory: choice reaction time task (p≤0.04), a 1-back task (p≤0.01), and a 2-back task (p≤0.04). In addition, those participants demonstrating high neurocognitive performance at baseline experienced similar or decreased performance following Meth exposure. These findings suggest that acute administration of Meth may temporarily improve Meth-associated neurocognitive performance in those individuals experiencing lower cognitive performance at baseline. As a result, stimulants may serve as a successful treatment for improving cognitive functioning in those Meth-dependent individuals experiencing neurocognitive impairment.
Keywords: Attention, Cognition, Methamphetamine, Processing, Working memory
1. Background/Introduction
More people worldwide use amphetamine-type stimulants than any other illicit drug besides cannabis (UNODC, 2010). According to the most recent (2008) National Survey on Drug Use and Health (NSDUH), 314,000 residents of the US aged 12 or older used Meth in the prior month. Moreover, the number of recent new users of Meth was 95,000. While these numbers reflect a decrease from previous years of the survey, a significant segment of the population continues to experiment with this dangerous drug (SAMHSA, 2008). Meth use is associated with neurocognitive impairment (for review see: Kalechstein and Newton 2007; Quinton and Yamamoto 2006), including poor performance on measures of attention/information processing speed, learning and memory, and frontal lobe functioning. Also, a recently published manuscript reported that more than 40% of previously Meth-dependent individuals still experienced neurocognitive impairments after prolonged abstinence from Meth (Cherner et al., 2010). These neurocognitive abnormalities have been linked to deficits in presynaptic dopamine (DA) neuronal markers (Johanson et al., 2006; Volkow et al., 2001; Wang et al., 2004).
Given this association, it is reasonable to hypothesize that administration of dopaminergic agents such as Meth might ameliorate Meth-associated neurocognitive impairments. For example, a recently published study showed that, in those participants who experienced baseline working memory deficits, modafinil administration ameliorated them (Kalechstein et al., 2010). Furthermore, in a study of cocaine dependent individuals, it was reported that cocaine exposure improved cocaine users’ neurocognitive performance (Woicik, et al., 2009; Johnson et al., 2005). Along similar lines, the administration of d-amphetamine has been proven effective to reliably improve aspects of cognitive function (Silber et al., 2006). Using the same reasoning, we assessed whether Meth administration improves neurocognitive functioning, specifically in those domains most affected in Meth-dependence: attention/information processing speed, learning and memory, and frontal lobe functioning.
2. Methods
2.1. Subjects
All subjects were non-treatment-seeking and met DSM-IV-TR criteria for current Meth dependence. They were 18 to 45 years old, smoked or injected Meth at least twice per week in 4 out of the 6 weeks prior to study entry, and provided a positive urine toxicology for Meth prior to admission. Furthermore, participants were in good health and had normal laboratory assessments and physical examinations. Potential participants were excluded if they were diagnosed with another Axis I psychiatric disorder, were currently dependent on any other drugs (including alcohol) aside from nicotine, and/or had a history of seizure disorder, head trauma, or concomitant use of any psychotropic medication. The Institutional Review Board of the University of California Los Angeles (UCLA) approved this study and all subjects gave informed consent after being made aware of the possible risks of participation. Subjects were recruited through advertisements in the community, and were paid for their participation.
Nineteen participants, 17 men and 2 women, completed the study. The average age of the participants was 35.58±8.24 (mean±S.D.). Twelve participants identified themselves as White or Caucasian, 5 as Hispanic or Latino, 1 as Asian, and 1 as African-American or Black. Participants in this study averaged 13.63±2.14 years of education. Twelve of the 19 participants were cigarette smokers. With respect to Meth usage patterns, on average, participants used Meth for 8.50± 6.03 years, had used 16.78±8.26 days out of the last 30 prior to study entry, reported using 3.25±2.52 g of Meth per week, and had average Beck Depression Inventory-II scores of 6.65±6.93.
2.2. Study design
This study was conducted as part of a medication trial conducted in the UCLA General Clinical Research Center (CRC) (De La Garza et al., 2008). Following admission to the CRC, participants completed baseline assessments, including the Addiction Severity Index (ASI)-Lite CF Version (McLellan et al., 1992), and the BDI-II (Beck et al., 1996). On the fourth day of the inpatient stay, participants received a single-blinded intravenous infusion of saline (placebo) and on the fifth day, subjects received a single-blinded infusion of Meth (30 mg, IV). One hour prior to drug (saline or Meth) administration, participants completed a baseline battery of neurocognitive tasks (described later). One hour following drug administration, participants completed the same battery of tasks that was administered at baseline, as well as the Hopkins Verbal Learning Task-Revised (HVLT-R) (Shapiro et al., 1999).
2.3. Drugs
A NIDA contractor (RTI International, Research Triangle Park, NC) provided sterile Meth solution for human use and a saline solution of equal volume and appearance was used as the control. An IND was obtained from the FDA for the use of Meth in this study. Meth or saline was administered over 2 min using an intravenous pump.
2.4. Neurocognitive Tasks
2.4.1. Simple reaction time task (SRT)
The SRT involves pseudo-random presentation of a series of letters (from the set A, a, G, g, T, t, H, h), one at a time, at the center of a computer screen. Participants were instructed to press a red button on the response box with their dominant forefinger as quickly as possible following presentation of the letter. Letters were black on a white background, subtended approximately 1.9°×1.6°. Each letter was presented for 500 ms, with a subsequent letter presented 2500 ms later. A total of 32 trials were presented. The dependent variable was the difference in reaction time (msec) between the second and first administrations of the task (SRT2−SRT1).
2.4.2. Choice reaction time task (CRT)
The CRT involves presentation of the same set of letters seen during the SRT. In this task, however, participants are instructed to press a red button on the response box with their dominant forefinger upon presentation of G, g, H, or h. Upon presentation of A, a, T, or t, participants were instructed to press a blue button on the response box. Letters were black on a white background, subtended approximately 1.9×1.6°. Each letter was presented for 500 ms, with a subsequent letter presented 2500 ms later. A total of 32 trials were presented. The dependent variables were reaction time (msec) and response accuracy, indexed as the ratio of actual accurate responses to total possible responses.
2.4.3. N-back task (working memory task)
The working memory task was a variation of an N-back that has been used previously (Smith et al., 1996). Participants were presented with a series of letters from the same set as seen on the SRT and CRT. In the 1-back condition, participants were to signal a ‘yes’ response (pressing a blue button with their dominant forefinger) if the presented letter matched the letter presented immediately beforehand. If the two letters did not match, a ‘no’ response (pressing a red button with their dominant forefinger) was required. In the 2-back condition, a ‘yes’ response was required if the presented letter matched the letter two trials previous. Otherwise, a ‘no’ response was required. Case of the letter was not relevant to matching verbal identity. Letters were black on a white background, subtended approximately 1.9°×1.6°. Each letter was presented for 500 ms, with a subsequent letter presented 2500 ms later. After completing at least 20 trials of practice, participants completed a total of 32 trials for each condition. The dependent variables were reaction time (msec) and response accuracy, indexed as the ratio of actual accurate responses to total possible accurate responses.
2.4.4. Hopkins Verbal Learning Test-Revised (HVLT-R, verbal learning and memory task) (Brandt and Benedict, 2005)
The HVLT was administered with the above battery of neurocognitive tasks following Meth or saline administration. Participants were read a list of 12 words, and asked to recall as many as they could. This procedure was repeated two times (for a total of 3 learning trials). Following a 20–25 min delay, participants were asked to recall the words without the aid of cues (Delayed Recall). After delayed recall, participants were then read a list of 24 words, and had to identify the 12 words from the original list (Recognition). The dependent variables of interest for the HVLT-R were total words recalled during the three learning trials and number of words remembered on the delayed recall subtest. A different version of the test was administered pre- and post-infusion to eliminate the possibility of practice effects.
2.4.5. Order of test administration
The battery of neurocognitive tests were administered in the following order: The HVLT-R learning recall trials, SRT, CRT, the N-back tests, delayed recall of the HVLT-R, followed by re-administration of the SRT. Difference score between the two SRT administrations was used as a measure of psychomotor fatigue. The reaction time tests were programmed on a laptop computer using SuperLab (SuperLab 1997). All responses for computerized tasks were given using a RB-730 response box (Cedrus, Phoenix AZ). A standardized set of instructions was given to the participants both written and orally prior to administration of each task, and participants were always reminded to respond as quickly and accurately as possible.
2.5. Statistical analysis
Data were calculated and analyzed using SPSS 11.0. Descriptive statistics were compiled for demographic variables and analyzed using appropriate parametric or non-parametric tests. Reaction time cutoffs of shorter than 100 ms and longer than 1500 ms for each computerized task were established to eliminate the possibility of anticipating the appearance of the stimuli, as well as the possibility of a delayed response intruding on the presentation of the subsequent stimulus. Main effects were determined using paired sample t-tests, comparing performance following administration of saline versus performance following Meth. A repeated measures ANOVA was used to verify the lack of practice effects and the absence of significant differences in performance across three time points (baseline, following saline, and pre-Meth administration), coinciding with days 1, 4, and 5 respectively. Significance for all analyses was set at p<0.05 (Keppel 1982), and effect size was indexed as η2 (eta squared).
The sample was divided into high versus low performers based on performance at baseline using median split (fast and slow for reaction time; high and low for accuracy and fatigue). The purpose of this approach was to determine if those participants demonstrating greater levels of impairment at baseline would be more likely to respond to Meth administration.
3. Results
3.1. Preliminary analyses
Neither demographic (e.g. age, ethnicity and education) nor drug use history variables were correlated with performance on any of the neurocognitive assessments administered at baseline. Because of this, covariates were not included in the analyses.
3.2. Effect of acute Meth administration on neurocognition for the entire sample
Accuracy of responses on the working memory tests increased in the Meth condition relative to placebo, but did not reach statistical significance. Mean percent correct (±SD) increased from 90.88±12.39 to 93.42±11.26 on the CRT (t16 = −1.76, p =0.10, η2 =0.16), from 81.07±20.18 to 91.24±7.71 on the 1-back (t17 = −2.05, p =0.06, η2=0.19), and from 73.72±13.84 to 81.26±16.84 on the 2-back (t16 = −1.86, p=0.08, η2=0.18).
On the HVLT-R, participants recalled 23.44±0.98 words (out of a possible 36) following saline, and 23.05±1.18 words following Meth (t17 =0.40, p =0.69, η2 =0.09). On the delayed recall trial, participants recalled 86.72±4.59% and 88.28±5.21% of the previously recalled words following saline and Meth, respectively (t16 = −0.56, p=0.59, η2=0.02). During the recognition trial, participants correctly recognized 10.94±0.37 (out of 12) words following saline, and 11.06±0.38 words following Meth (t16 = −0.40, p =0.69, η2 =0.01).
3.3. Effects of Meth on neurocognition of high and low performers
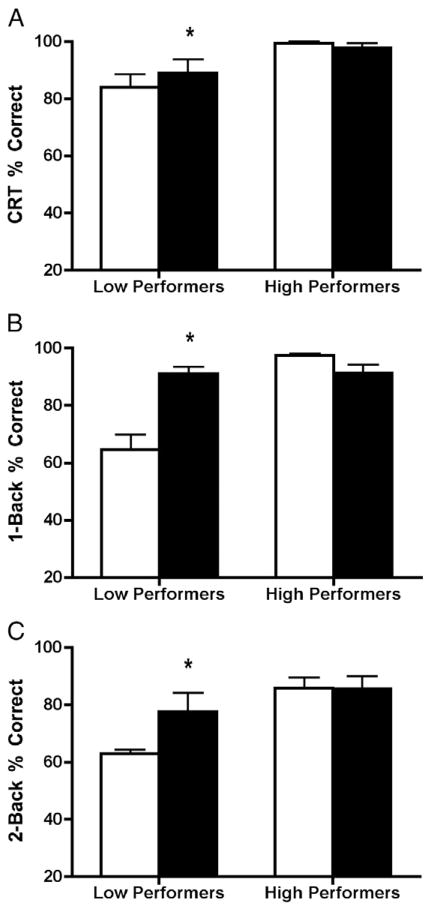
Using baseline data, the sample was divided into high versus low performance using median split. Demographic information for high versus low performers can be found in Tables 1a, 1b, 1c, and 1d. For the CRT (Fig. 1A), mean percent correct for low performers increased following Meth administration (t8 = −2.58, p ≤0.04, η2 =0.45), though mean percent correct for high performers did not change following Meth administration (t7 =0.97, p =0.38). For the 1-back (Fig. 1B), mean percent correct for low performers increased following Meth administration (t8 = −4.87, p ≤0.01, η2 =0.75), while mean percent correct for high performers resulted in a non-significant decrease following Meth (t7 =2.18, p =0.07). For the 2-back (Fig. 1C), mean percent correct for low performers increased following Meth administration (t8 = −2.53, p≤0.04, η2=0.45), while mean percent correct for high performers did not change (t7 =0.07, p =0.95).
Table 1a.
Demographic and drug use data for high versus low performers.
| CRT
|
1-back
|
2-back
|
||||
|---|---|---|---|---|---|---|
| High performers | Low performers | High performers | Low performers | High performers | Low performers | |
| Performance average | 98.77±1.87* | 84.00±13.82 | 97.53±1.86* | 64.61±15.88 | 85.79±10.26* | 63.00±4.03 |
| Gender | 9M/0F | 7M/2F | 9M/0F | 7M/2F | 7M/1F | 8M/1F |
| Age | 40.78±5.63* | 31.11±7.91 | 36.89±6.57 | 33.56±9.89 | 38.13±5.49 | 31.11±8.34 |
| Education | 14.56±2.70 | 12.89±1.05 | 14.22±2.44 | 13.33±1.73 | 14.88±2.17 | 13.00±1.73 |
| Years of Meth use | 6.33±4.72 | 10.94±6.83 | 10.00±5.67 | 7.28±6.67 | 8.00±6.65 | 9.83±6.01 |
| Recent Meth use | 18.50±8.51 | 15.33±8.73 | 12.44±7.53* | 21.00±7.25 | 13.71±10.13 | 17.56±6.73 |
| Grams used per week | 3.53±3.26 | 3.01±1.84 | 2.44±1.93 | 3.13±1.76 | 2.50±2.43 | 3.17±1.33 |
p≤0.05 for high versus low performers.
Table 1b.
Demographic and drug use data for fast versus slow responders.
| CRT
|
1-back
|
2-back
|
||||
|---|---|---|---|---|---|---|
| Fast responders | Slow responders | Fast responders | Slow responders | Fast responders | Slow responders | |
| Response average | 819.75±45.74* | 929.78±49.52 | 779.85±58.06* | 954.11±135.24 | 802.11±36.79* | 910.78±73.72 |
| Gender | 9M/1F | 8M/1F | 10M/0F | 7M/2F | 9M/1F | 8M/1F |
| Age | 37.80±9.30 | 33.11±6.51 | 35.80±8.10 | 35.33±8.86 | 33.70±8.95 | 37.67 7.30 |
| Education | 13.60±2.46 | 13.67±1.87 | 13.70±1.83 | 13.56±2.55 | 14.20±1.81 | 13.00 2.40 |
| Years of Meth use | 5.85±3.38* | 11.44±7.09 | 7.30±4.30 | 9.83±7.56 | 7.55±6.83 | 9.56 5.17 |
| Recent Meth use | 19.67±8.23 | 13.89±7.66 | 18.56±7.67 | 15.00±8.90 | 17.89±7.42 | 15.67 9.34 |
| Grams used per week | 3.86±2.96 | 2.73±2.11 | 2.24±1.54 | 4.14±2.95 | 2.56±1.74 | 4.28 3.27 |
p≤0.05 for fast and slow responders.
Table 1c.
Demographic and drug use data for those demonstrating high versus low fatigue.
| SRT difference
|
||
|---|---|---|
| High fatigue | Low fatigue | |
| Response average | 39.83±52.14* | −42.00±23.96 |
| Gender | 8M/1F | 9M/1F |
| Age | 33.89±8.27 | 37.10±8.33 |
| Education | 15.22±2.05* | 12.20±0.79 |
| Years of Meth use | 7.17±6.99 | 9.70±5.08 |
| Recent Meth use | 15.38±7.73 | 17.90±8.91 |
| Grams used per week | 2.60±1.87 | 4.00±3.07 |
p≤0.05 for those demonstrating high and low fatigue.
Table 1d.
Individual breakdown of high versus low performers and fast versus slow responders.
| Subject # | CRT % correcta | CRT reaction timeb | 1-back % correcta | 1-back reaction timeb | 2-back % correcta | 2-back reaction timeb | SRT (difference)c |
|---|---|---|---|---|---|---|---|
| 1 | Low | Slow | High | Fast | High | Fast | High |
| 2 | ^ | Slow | High | Fast | Low | Slow | Low |
| 3 | High | Fast | Low | Fast | ^ | ^ | Low |
| 4 | Low | Fast | Low | Slow | Low | Fast | High |
| 5 | Low | Fast | Low | Slow | Low | Slow | Low |
| 6 | High | Slow | Low | Slow | High | Fast | High |
| 7 | High | Slow | High | Fast | Low | Fast | High |
| 8 | High | Fast | High | Slow | High | Slow | High |
| 9 | High | Fast | High | Fast | High | Fast | High |
| 10 | High | Fast | Low | Fast | High | Fast | High |
| 11 | High | Fast | ^ | Slow | ^ | Slow | Low |
| 12 | Low | Slow | Low | Fast | Low | Fast | High |
| 13 | Low | Slow | Low | Slow | Low | Fast | Low |
| 14 | Low | Slow | High | Slow | Low | Fast | High |
| 15 | High | Fast | High | Fast | High | Slow | Low |
| 16 | Low | Slow | Low | Slow | High | Slow | Low |
| 17 | High | Fast | High | Fast | Low | Slow | Low |
| 18 | Low | Fast | Low | Fast | Low | Slow | Low |
| 19 | Low | Slow | High | Slow | High | Slow | Low |
Missed data point.
– High versus low performers.
– Fast versus slow responders.
– High versus low fatigue.
Fig. 1.
Analyses comparing high and low performers following saline (open bars) and methamphetamine (filled bars) administration for (A) CRT, (B) 1-back, and (C) 2-back. *=Significantly different from post-saline; p <0.05.
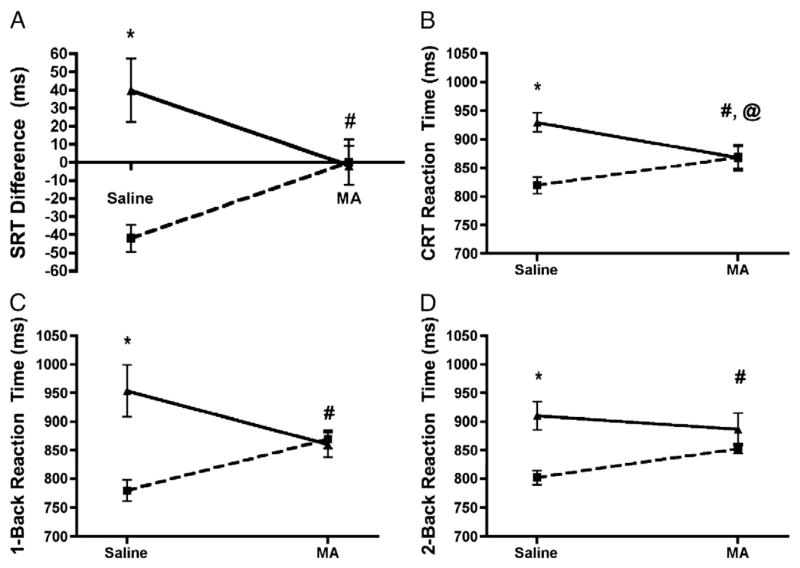
Reaction time analyses using median splits showed significant effects of Meth for all tasks (Fig. 2). For SRT (Fig. 2A), change in reaction time from the beginning of the battery to the end of the battery was calculated as a difference score (SRT2−SRT1). A higher score was considered to be indicative of psychomotor fatigue. Individuals with higher fatigue following saline showed less fatigue following Meth administration (t9 = −2.37, p ≤0.05, η2=0.38). Individuals with lower fatigue following saline showed no change following Meth administration (t8 =1.95, p =0.087, η2 =0.32).
Fig. 2.
Analyses comparing fast responders (square) and slow responders (triangle) following saline and methamphetamine administration for (A) SRT Difference Score, (B) CRT, (C) 1-back, and (D) 2-back. SRT Difference Score is a measure of psychomotor fatigue, with two groups: low fatigue (square) and high fatigue (triangle). #=Fast responding group was significantly slower following methamphetamine; less fatigued group showed increased fatigue following MA. @=Slow responding group was significantly faster following methamphetamine. *, #, @=p<0.05.
For CRT (Fig. 2B), individuals with faster reaction time following saline displayed slower reaction time after Meth (t9 = −2.81, p ≤0.03, η2=0.47), and individuals slower performance following saline displayed faster reaction time after Meth (t8 =3.44, p ≤0.01, η2 =0.60).
For the 1-back (Fig. 2C), individuals with faster reaction time following saline displayed slower reaction time after Meth (t9 = −3.89, p≤0.01, η2=0.63). Individuals with slower reaction time on the 1-back following saline displayed faster reaction time following Meth, however this improvement was not significant (p=0.15). For the 2-back (Fig. 2D), individuals with faster reaction time following saline displayed slower reaction time following Meth (t8 = −3.78, p ≤0.01, η2 =0.64).
For the HVLT-R, neither total recall on learning trials nor delayed recall showed significant effects of Meth administration in either the high or low performing groups (total recall, p =0.79 for low performers, p=0.24 for high performers; delayed recall, p=0.39 for low performers, p=0.74 for high performers).
4. Discussion
The data in the current report reveal that acute administration of Meth improved attention/information processing speed and working memory in Meth-dependent individuals who performed poorly at baseline. Moreover, for those who performed relatively well at baseline, acute Meth exposure did not affect performance on measures of working memory, but was associated with slower speed of information processing. Episodic memory was not affected by acute Meth administration.
The current findings may explain, at least in part, why three of every five Meth users relapse during abstinence (Rawson et al., 2006). Specifically, Meth may partially improve the neurocognitive performance seen in some abstinent Meth users. This argument is intriguing as it is consistent with the self-medication hypothesis of substance abuse (for review see: Khantzian 1997). It remains unknown whether poorer neurocognitive performance precedes the development of Meth dependence, or whether it is a result of Meth use. Either way, poorer neurocognitive abilities could contribute to the maintenance of Meth dependence.
Several other points emerge from the current data set. First, there is a great deal of variability in neurocognitive functioning among otherwise similar Meth-dependent individuals, at least with regard to basic demographics and other drug use history. This supports our rationale for performing the median split, i.e., to reduce the possibility that ceiling effects suppressed a potential medication effect. Second, low-dose stimulants may be effective treatments for neurocognitive dysfunction in Meth dependence, though stimulants with lower abuse potential than Meth would be preferable, and the potential benefits would need to be weighed against risks, such as relapse-induced stimulant exposure. Finally, screening for cognitive impairments should be part of a standard battery prior to initiation of treatment.
A specific example of the variability among participant responding was evident on the CRT, a relatively simple task, where participants need to only make one determination, whether the letter presented is of the set A, a, T, t, or G, g, H, h, prior to responding. The post-saline results showed that six individuals answered correctly 100% of the time, but that five answered correctly less than 85% of the time. Some individuals performed remarkably poorly, given that this is a relatively easy task. The data are even more noteworthy for the more complex working memory tasks. The 1-back task asks participants to recall only the letter that immediately preceded the letter currently on the screen, assign an answer of ‘match’ or ‘no match’, and press the appropriate button. After the saline infusion, eight participants answered correctly less than 83% of the time, while eight participants answered correctly more than 96% of the time. Performance on the 2-back task, the most difficult probe of working memory evaluated, showed a broad range of accuracy (ranging from 56% to 100%). Again, and not surprisingly, there were individuals who performed poorly, and individuals who performed well on this task at baseline. These data provide strong evidence for the theory that not all Meth users will exhibit the same set of symptoms, and may benefit from individually-tailored treatments (De La Garza and Newton, 2006). In addition, there was a subset of Meth-dependent individuals that performed at nearly 100% on the tests administered. While this does introduce the possibility of “ceiling effects”, a more important finding is that there are individuals that can become dependent on Meth, but show no cognitive dysfunction as a result.
Another interesting finding was that acute Meth administration improved performance on working memory tasks, but not on the episodic verbal learning and memory task. These two distinct cognitive domains are associated with different neuroanatomical structures and pathways; working memory has been linked to the dorsolateral pre-frontal cortex, and episodic memory to temporal lobe structures such as the hippocampus and parahippocampal gyrus (for review see: Baddeley 1986; D’Esposito et al., 1995; Schacter and Tulving 1994). One potential explanation for this discrepancy may be that the dose of Meth administered (30 mg, IV) was not large enough to activate the hippocampus, which might be rendered less sensitive to the effects of Meth due to prolonged exposure.
The overall poor performance (by both high and low performers) reported on the HVLT-R in this study is consistent with the observation that hippocampal volume is reduced in some Meth users (Thompson et al., 2004). In that study (which had comparable demographic and drug use profile to our study with the exception of the male/female ratio) reduction in hippocampal volume were correlated with impaired recall on a word learning task. One possible explanation for that finding was that, as a result of Meth exposure a subset of users experienced irreversible damage to their left hippocampus. Because we did not conduct structural imaging, it was not possible to correlate performance on the HVLT-R with changes in brain structure for this sample. Nonetheless, given the relatively poor performance of these participants on the HVLT-R, it may be they experienced a level of Meth-associated neurotoxicity in areas of the brain that modulate memory such that they would not be responsive to a pharmaceutical intervention. Indeed, in a separate study from our research group, methamphetamine addicts’ performance on a measure of verbal learning and memory did not improve following the administration of modafinil (Kalechstein et al., 2010).
In a separate study, it was reported that Meth dependence was associated with deficient strategic (i.e. executive) control of verbal encoding and retrieval relative to matched controls (Woods et al., 2005). The present sample differed from the sample recruited by Woods et al. insofar as it was not ethically feasible to recruit matched controls for this study. Moreover, the sample in the current study showed a relatively greater level of memory impairment relative to the sample recruited by Woods et al. and, as a result, may not have been responsive to the acute Meth administration. Additionally, it is noteworthy that acute Meth administration was associated with significantly improved response accuracy in those individuals who demonstrated relatively poor performance at baseline. Therefore, it may be that performance on the HVLT-R is only modestly affected by executive/frontal functioning. The results of Woods et al. support this conclusion given that, in their sample, performance on measures of executive/frontal function accounted for a small portion of the variation in performance on memory tests.
An alternative hypothesis implicates retrieval of episodic memory, rather than encoding. Retrieval is believed to be a function of the anterior pre-frontal cortex (McDermott et al., 2000; Schacter and Tulving 1994). This is an attractive explanation, as the individuals in the current study performed poorly on the immediate recall trials of the HVLT-R, while performance on the recognition trial was not impaired. Participants recalled 23 words (out of a possible 36) following saline and Meth. In both instances, participants recalled approximately 64% of the words. However, during the recognition trial, when individuals were read a list of 24 words, and had to select the 12 from the original list, participants performed markedly better, correctly recognizing approximately 11 (out of 12) words following both saline and Meth. During both conditions, false positive responses were less than 1, so it was not simply a matter of the individuals answering “yes” to every choice. These results point to a deficit in uncued retrieval, such as occurs on each learning trial, as when cues (the words themselves) are presented during the recognition trial, Meth-dependent individuals are able to correctly identify 92% of the words they had heard previously. To our knowledge, there are no published reports assessing effects of Meth on anterior pre-frontal cortex structure or function, and this is an area that warrants further investigation. However, there is literature showing that Meth abusers had lower glucose metabolism in the anterior cingulate and insula and higher glucose metabolism in the lateral orbitofrontal area, middle and posterior cingulate, amygdala, ventral striatum, and cerebellum (London et al., 2004).
It is important to concede some limitations with this study. First, the cognitive status of these individuals prior to onset of drug use was not determined, so it is unknown whether lower neurocognitive performance preceded the development of Meth addiction or resulted from it. Also, since there was not a control group included, we could only compare the responses of the Meth-dependent participants to one another and not to what could be considered “normal” responses. Another limitation is the rather small sample size of 19 participants and a study with a larger number of individuals would increase confidence in the results. In addition, Meth users often take much larger doses on a day-to-day basis (450 mg/use) than those doses administered in this study (30 mg). Also, it is possible that nicotine withdrawal may be driving some of the performance decrements that are then being improved by Meth administration in individuals with low baseline performance (since participants were abstinent from nicotine for 2 h prior to cognitive testing). However, after comparing smokers to non-smokers, there were no significant performance differences between those two groups, indicating that nicotine withdrawal was not a factor in performance. Finally, since peak subjective effects of Meth occur at approximately 30 min (Newton et al., 2005), obtaining neurocognitive measures closer to the peak may provide a more accurate representation of the effect of Meth administration on cognition. The current results suggest that not all Meth-dependent individuals display cognitive dysfunction (similar to Cherner et al., 2010), and that acute Meth administration can improve cognitive function in a sub-group of Meth-dependent individuals who perform poorly on tasks of attention and working memory. It follows that screening for, and subsequently treating cognitive impairment in abstinent Meth users who display poorer neurocognitive performance should be a priority, as it may help to improve treatment outcomes (Kalechstein et al., 2010).
An additional finding that became apparent in the preparation of Table 1d is that there were substantial intra-individual differences in baseline task performance, i.e., different participants comprised the low- and high-performing groups, depending on the test administered. In other words, there were not 2 consistent groups of high or low performers and fast or slow responders across all tasks administered. One explanation is that, for a subset of better performers, a ceiling effect is present. As illustrated in Table 1d, some participants performed well on some tasks, but not others. This makes sense as one would expect to observe level of impairment on a spectrum such that some individuals showed no impairment, some individuals showed variable performance on the measures, and others showed consistent levels of impairment. This reflects the range of impairment one might expect to see within a cohort of Meth addicts. Furthermore, it shows that Meth-related neurocognitive impairment is not a unitary, all-encompassing phenomenon, that impairment is not a dichotomous variable, and the variability observed is consistent with that seen in other neuropsychiatric conditions. Additionally, these results suggest a possible reason for continued use, particularly among individuals with low cognitive function. In fact, poorer neurocognitive functioning is associated with low treatment retention in a sample of cocaine-dependent individuals receiving cognitive–behavioral therapy (Aharonovich et al., 2006). This is of particular importance since cognitive–behavioral therapy is the only empirically validated option for treatment (Rawson et al., 2002). Adequate cognitive skills are necessary to benefit from this form of treatment. In addition to the basic cognitive function needed for therapy, it is also necessary in order to obtain and maintain a job or complete other common everyday tasks, which may be impacted by cognitive dysfunction (Heaton et al., 1994; Kalechstein et al., 2003).
Acknowledgments
The authors acknowledge grants from the National Institutes of Health DA 18185, DA 014593, DA 017754 and RR-00865.
Abbreviations
- Meth
Methamphetamine
- NSDUH
National Survey Drug Use and Health
- S.D
Standard Deviation
- BDI
Beck Depression Inventory
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–22. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford University Press; 1986. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RH. Hopkins Verbal Learning Test - Revised (HVLT-R) Lutz, FL: Psychological Assessment Resources, Inc; 2005. [Google Scholar]
- Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T, Vaida F, Atkinson JH, Grant I, Heaton RK. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug Alcohol Depend. 2010;106:154–63. doi: 10.1016/j.drugalcdep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–81. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- De La Garza R, II, Newton TF. Individual variability in addiction profiles: importance for medications development. Unpublished findings presented at the 68th Annual Meeting of the College on Problems of Drug Dependence; Scottsdale, AZ. 2006. [Google Scholar]
- De La Garza R, Shoptaw S, Newton TF. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Int J Neuropsychopharmacol. 2008;11:729–41. doi: 10.1017/S1461145708008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Velin RA, McCutchan JA, Gulevich SJ, Atkinson JH, Wallace MR, Godfrey HP, Kirson DA, Grant I. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group. HIV Neurobehavioral Research Center. Psychosomatic Medicine. 1994;56:8–17. doi: 10.1097/00006842-199401000-00001. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacol Berl. 2006;186:620. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Wallace C, Wells LT, Wang Y. Effects of isradipine on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition. Psychopharmacology (Berl) 2005;178(2–3):296–302. doi: 10.1007/s00213-004-1998-0. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, Newton TF. Modafinil Administration Improves Working Memory in Methamphetamine-Dependent Individuals Who Demonstrate Baseline Impairment. Am J Addict. 2010;9(4):340–4. doi: 10.1111/j.1521-0391.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF. Methamphetamine. In: Kalechstein AD, van Gorp WG, editors. Neuropsychology and Substance Use: State-of-the-Art and Future Directions. New York: Taylor & Francis; 2007. [Google Scholar]
- Kalechstein AD, Newton TF, van Gorp WG. Neurocognitive functioning is associated with employment status: a quantitative review. J Clin Exp Neuropsychol. 2003;25:1186–91. doi: 10.1076/jcen.25.8.1186.16723. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher’s handbook. Prentice Hall: Prentice Hall; 1982. [Google Scholar]
- Khantzian E. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–44. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61(1):73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger IHL. Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: an event related MRI study. J Cogn Neurosci. 2000;12:965–76. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth eddition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, II, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav. 2005;82:90–7. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8:E337–47. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Brethen P. Treatment of methamphetamine use disorders: an update. J Subst Abuse Treat. 2002;23:145–50. doi: 10.1016/s0740-5472(02)00256-8. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive–behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–74. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- SAMHSA. National Survey on Drug Use and Health. Rockville, MD: 2008. [Google Scholar]
- Schacter DL, Tulving E. What are the memory systems of 1994? In: Schacter DL, Tulving E, editors. Memory Systems 1994. Cambridge: MIT Press; 1994. pp. 1–38. [Google Scholar]
- Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13:348–58. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- Silber BY, Croft RJ, Papafotiou K, Stough C. The acute effects of d-amphetamine and methamphetamine on attention and psychomotor performance. Psychopharmacol Berl. 2006;187:154–69. doi: 10.1007/s00213-006-0410-7. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- SuperLab. SuperLab. Cedrus Corporation; Phoenix, AZ: 1997. [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. World Drug Report. United Nations Publication; 2010. Sales No. E.10.XI.13. [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–8. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34(5):1112–22. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Cherner M, Heaton RK, Grant I. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]