Abstract
A variety of preclinical models have been constructed to emphasize unique aspects of addiction-like behavior. These include Negative Reinforcement (“Pain Avoidance”), Positive Reinforcement (“Pleasure Seeking”), Incentive Salience (“Craving”), Stimulus Response Learning (“Habits”), and Inhibitory Control Dysfunction (“Impulsivity”). We used a survey to better understand why methamphetamine-dependent research volunteers (N = 73) continue to use methamphetamine, or relapse to methamphetamine use after a period of cessation of use. All participants met DSM-IV criteria for methamphetamine abuse or dependence, and did not meet criteria for other current Axis I psychiatric disorders or dependence on other drugs of abuse, other than nicotine. The questionnaire consisted of a series of face-valid questions regarding drug use, which in this case referred to methamphetamine use. Examples of questions include: “Do you use drugs mostly to make bad feelings like boredom, loneliness, or apathy go away?”, “Do you use drugs mostly because you want to get high?”, “Do you use drugs mostly because of cravings?”, “Do you find yourself getting ready to take drugs without thinking about it?”, and “Do you impulsively take drugs?”. The scale was anchored at 1 (not at all) and 7 (very much). For each question, the numbers of participants rating each question negatively (1 or 2), neither negatively or affirmatively (3–5), and affirmatively (6 or 7) were tabulated. The greatest number of respondents (56%) affirmed that they used drugs due to “pleasure seeking.” The next highest categories selected were “impulsivity” (27%) and “habits”(25%). Surprisingly, many participants reported that “pain avoidance” (30%) and “craving” (30%) were not important for their drug use. Results from this study support the contention that methamphetamine users (and probably other drug users as well) are more heterogeneous than is often appreciated, and imply that treatment development might be more successful if treatments targeted subtypes of patients, though a range of limitations to the approach used are acknowledged.
INTRODUCTION
Many theories of addiction have been proposed with the intention of identifying the mechanism(s) that best explains the behaviors observed in addicts.1 These theories frequently rely on preclinical data, primarily because they permit research into the underlying neural mechanisms in ways not possible using human subjects. The theories addressed here include:
Negative Reinforcement-NR (“Pain Avoidance”)
Positive Reinforcement-PR (“Pleasure Seeking”)
Incentive Salience-IS (“Craving”)
Stimulus Response Learning-SRL (“Habits”) and
Inhibitory Control Dysfunction-IIC (“Impulsivity”)
The names used for these models are descriptive and are not necessarily those originally proposed by the authors. The names in quotations are colloquial. This list is not exhaustive, but serves to outline broad areas of active research. Other theories have been suggested, but have not yet gained wide acceptance (eg. reward deficiency2).
Negative reinforcement provided one of the earliest theoretical explanations of addictive behavior.3 The basic premise is that drug use reduces withdrawal dysphoria. A more recent and sophisticated example of this model highlights the cumulative negative effects produced by repeated cycles of intoxication and withdrawal,4–6 and falls under the rubric of the opponent process theory of emotional regulation.7
Positive reinforcement, based on classical learning theory,8 is probably the most familiar preclinical model of addiction. Quite simply, this theory states that users will say they take drugs because they enjoy using them.9,10
The Incentive Salience model posits links between sensitization of particular brain systems and motivation, which is distilled into the concept of drug craving. In this theory, the attribution of incentive salience to drug-related stimuli is increased by exposure to abused drugs.11–13 Hence, according to Incentive Salience theory drug use is attributable to craving.
The stimulus response learning model identifies habit learning as the key to understanding addiction.14 In classical learning theory, stimuli and responses are associated with outcomes, and the outcome determines the likelihood that the response will follow the stimulus in the future. In stimulus response learning, the outcome is less important, and the stimulus itself elicits a habitual response. Conditioned reinforcement and impulsivity are key features of this theory.15 This model predicts that users will describe drug taking as habitual or compulsive.
The inhibitory control dysfunction model implicates impulsivity as the factor that underlies addiction.16–20 Impulsivity has been linked to appetitive approach systems21 as well as drug-related impairments in new learning and to perseveration.20,22 Thus, models involving inhibitory control deficits predict that users will attribute drug use to impulsivity or perseveration.
Preclinical research on these theories examines behavior, or neural activity underlying behavior. By contrast, clinical research often involves the surveying of participants in order to understand the motivation(s) that underlie their behavior. While it is true that clinical studies often lack the experimental control that is typically observed in preclinical studies, clinical studies are not solely reliant on inference in order to determine the motivations of the study participants.
Methamphetamine abuse can potentially be explained by the aforementioned preclinical models of addiction. Namely, methamphetamine produces typical stimulant effects in many users, including self-reported euphoria and withdrawal.23–25 Methamphetamine exposure also increases self-reported craving in some users,26 and craving for methamphetamine is associated with methamphetamine use in clinical trials.27 Methamphetamine users also exhibit deficits in response inhibition,28 suggesting that deficits in inhibitory control may play a role methamphetamine use as well. Although the role of stimulus response learning in methamphetamine use has not been investigated clinically, there is a large preclinical literature suggesting stimulus response learning plays a role.14,29,30
To investigate users’ perceptions of the reasons for their methamphetamine use, we constructed a survey in which participants’ self-perceived reasons for taking methamphetamine or for relapsing to methamphetamine use were assessed on a 7-point scale for each of the five domains mentioned above. This survey was intended to sample the opinions of methamphetamine dependent volunteers regarding the reasons for their drug use. We hypothesized that methamphetamine dependent volunteers would be comprised of subgroups that varied in terms of identifying the best explanation for their methamphetamine use.
METHODS
Participants
Participants included 73 non-treatment-seeking, methamphetamine-dependent men and women. Subjects were recruited from the community via radio and newspaper advertisements. All participants met DSM-IV criteria for methamphetamine-dependence, and did not meet criteria for other current Axis I psychiatric disorders or dependence on other drugs aside from nicotine. The study procedures and informed consent document were approved by the Institutional Review Board of the University of California Los Angeles. After the risks of the study had been fully explained, all subjects provided informed consent, and were paid for participation upon completion of the study. The subject demographics are detailed in Table 1.
TABLE 1.
Participant characteristics
| N = 73 | |
|---|---|
| Age (± SD) | 36.3 ± 9.2 |
| Number of women | 12 (16%) |
| Mean days of methamphetamine use in past 30 days | 13.6 ± 8.7 |
| Mean duration of methamphetamine use in years | 10.6 ± 8.2 |
| Number with positive methamphetamine urine test at interview | 38 (52%) |
| Number describing themselves as: | |
| Caucasian | 40 (55%) |
| Hispanic | 20 (27%) |
| African-American | 5 (6.8%) |
| Asian | 2 (2.7%) |
| Other or mixed | 6 (8.2%) |
| Addiction Severity Index subscales | n = 51 for ASI |
| Medical complications | .0587 (.1657) |
| Economic complications | .6287 (.3098) |
| Alcohol complications | .0801 (.1142) |
| Drug complications | .1349 (.0956) |
| Legal complications | .0562 (.1214) |
| Family complications | .1093 (.1404) |
| Psychiatric complications | .0871 (.1307) |
Procedure/Measures
Participants completed an initial battery of questionnaires including demographic, locator, and recruitment surveys. Subjects were administered a variety of assessments including the SCID (Structured Clinical Interview for DSM-IV-TR diagnoses)31 or MINI,32 Addiction Severity Index-Lite,33 Beck Depression Inventory,34 and a timeline follow-back to assess drug use in the prior 6 weeks. At the time of assessment, a toxicology screen was performed to assess recent substance use.
Participants then completed the main instrument, a questionnaire assessing self-perceived reasons for taking drugs or for relapsing. The questionnaire used a 7-point scale and included questions addressing each of the five domains described above. The scale ranged from 1 (not at all) to 7 (very much). The specific questions are shown in Table 2.
TABLE 2.
Theories of addiction
| Theory Question | Mean Score (SD) Range: 1 to 7 | Not at all (1 or 2) | Very Much (6 or 7) | X2(df) p < |
|---|---|---|---|---|
| Negative reinforcement (“Pain Avoidance”) | ||||
| Do you use drugs mostly to make bad feelings like boredom, loneliness, or apathy go away? | 3.75 (1.98) | 30.1% | 23% | — |
| Do drugs lessen withdrawal symptoms? | 3.71 (2.18) | 36% | 26% | — |
| Do you often use drugs even though you are feeling OK? | 5.18 (1.52) | 3% | 47% | .000 |
| Do you relapse mostly to make bad feelings like boredom, loneliness, or apathy go away? | 3.59 (2.15) | 36% | 26% | — |
| Positive reinforcement (“Pleasure Seeking”) | ||||
| Do you use drugs mostly because you want to get high? | 5.34 (1.63) | 7% | 56% | .000 |
| Do you enjoy many things besides drugs? | 5.22 (1.74) | 10% | 52% | .000 |
| Do you often use drugs without thinking about getting high? | 3.60 (2.07) | 41% | 22% | — |
| Do you relapse mostly because you want to get high? | 4.80 (2.03) | 19% | 44% | .03 |
| Incentive salience (“Craving”) | ||||
| Do you use drugs mostly because of cravings? | 3.72 (1.86) | 30% | 19% | — |
| Do things paired with drugs, like pipes or razors, make you crave? | 3.32 (2.24) | 47% | 26% | .016 |
| Do you use drugs even though you aren’t craving? | 4.19 (1.98) | 26% | 27% | — |
| Do you relapse mostly because of cravings? | 3.37 (1.86) | 40% | 15% | — |
| Do things paired with drugs, like pipes or razors, make you relapse? | 2.93 (2.01) | 51% | 15% | .005 |
| Stimulus-Response learning (“Habits”) | ||||
| Do you find yourself getting ready to take drugs without thinking about it? | 3.56 (2.18) | 41% | 25% | — |
| Does using drugs seem like a habit, like nail-biting or picking? | 3.96 (2.19) | 40% | 23% | — |
| Do you find yourself relapsing without thinking about it? | 3.65 (2.07) | 33% | 31% | — |
| Impaired inhibitory control (“Impulsivity”) | ||||
| Do you impulsively take drugs? | 3.90 (2.11) | 29% | 27% | — |
| Once you begin to get ready to use, do you find you can’t stop? | 4.13 (2.11) | 26% | 31% | — |
| Do you impulsively relapse? | 3.27 (2.18) | 47% | 22% | — |
Data Analysis
The mean and standard deviation for each question in the questionnaire were calculated. The internal consistency of the questionnaire was assessed using Cronbach’s alpha. This calculation was performed including all questions shown in Table 2.
Participants’ responses on each question were then analyzed using chi-square statistics. Responses were recoded into three categories (1–2, not at all; 3–5, somewhat; 6–7, very much) to simplify data analysis. The frequency at which participants selected each category (eg, not at all) across all questions was calculated and this frequency was used as the expected frequency for each question. The frequency at which participants selected each category for each question was then used as the observed frequency. A chi-square test was used to determine whether each category was selected at frequencies that were greater or lesser than expected for each question. Significance was set at p < 0.05. Pearson correlations were used to relate depression severity, indexed by the Beck Depression Inventory, with responses to the question “Do you use drugs mostly to make bad feelings … go away.”
Next, we conducted a correlation and factor analysis. To limit the number of variables, we included only the first question in each category as being representative (see Table 2). Varimax rotation was used.
RESULTS
Table 1 shows demographics and drug use data for participants completing this study. Participants reported using methamphetamine 13.6 ± 8.7 days out of the past 30 days. As typical for studies of methamphetamine-dependent participants, the most were male (84%) and Caucasian (55%). None of the participants met criteria for abuse or dependence on cocaine. Not surprisingly, participants rated economic problems and drug problems highly on the ASI. Further analysis was not conducted because ASI scores were available for only a subset of participants (n = 51).
The test items were internally consistent (Cronbach’s α = 898, based on the responses from 69 individuals with complete data for all questions shown in Table 2. Table 2 also shows the lowest (1 or 2: Not at all) and highest (6 or 7: Very much) ratings by participants for each question on the questionnaire. Most respondents (56%) reported that they used drugs due to positive reinforcement, and a similar proportion (44%) identified that they relapsed for the same reason. However, 56% of respondents reporting that they used drugs due to positive reinforcement (comprising 52% of the total sample) also responded very much to the question “do you enjoy many things besides drugs?” This suggests that reinforcers other than methamphetamine remained effective for most participants. The next highest categories rated highly were impaired inhibitory control (27%) and negative reinforcement (23%), and similar numbers reported that they relapsed for these reasons as well. Among those identifying negative reinforcement as an important motivation, only 7 of 17 (41%) responded that they frequently used drugs due to withdrawal symptoms. Somewhat smaller numbers responded that stimulus response learning (25%) or incentive salience (19%) were very important for ongoing drug use or for relapse.
Of the 56% of participants who rated positive reinforcement as a highly important motivation for using methamphetamine, only 8 of these (11% of the total) also rated negative reinforcement highly, so these two categories were mostly non-overlapping. Fourteen of the 16 subjects who rated stimulus-response learning highly also rated positive reinforcement as a motivation for use. Similarly, 12 of 20 subjects who rated impaired inhibitory control highly also rated positive reinforcement highly. By contrast, only 8 of 14 participants who rated incentive salience highly also rated positive reinforcement highly whereas 5 of 15 rating incentive salience highly also rated negative reinforcement highly.
Examination of response patterns suggests participants were consistent in their answers. Most (14 of 18) individuals who rated stimulus-response learning as very important motivations for using methamphetamine (“Do you find yourself getting ready to take drugs without thinking about it”) also reported that methamphetamine use seems “like a habit, like nail-biting or picking.” Twenty participants rated impaired inhibitory control as a very important motivation for using drugs. Of these, 14 rated highly “Once you begin to get ready to use, do you find you can’t stop?” Sixteen of these 20 also rated positive reinforcement highly as a motivation for using drugs. The smallest number of participants, 14 (19.2%), rated incentive salience highly as a reason for using drugs. Of these, 8 also rated positive reinforcement highly, whereas 5 rated negative reinforcement highly as an explanation for their behavior. By contrast, an even larger number (35 or 47%) reported that objects paired with drug use, such as pipes or razors did not produce craving.
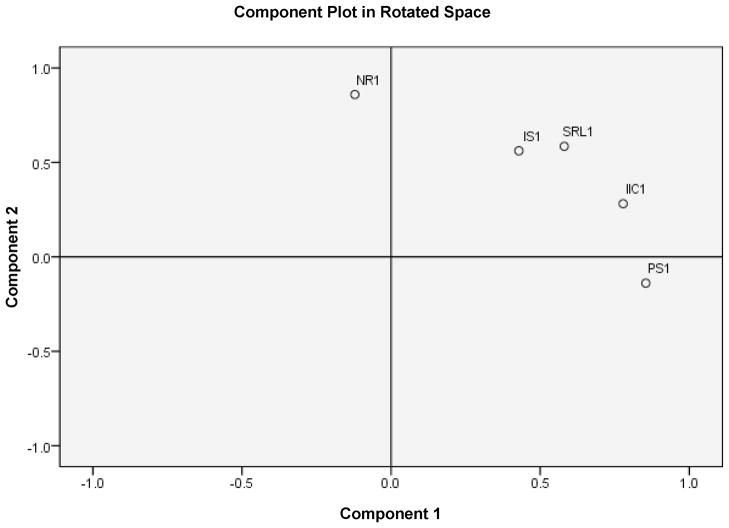
The correlation matrix for results from the first question from each category is shown in Table 3. The factor analysis resulted in the emergence of two factors, accounting for 67.3% of the variance (Figure 1). One factor corresponded basically to negative reinforcement and the other factor corresponded basically to positive reinforcement. Responses on the remaining questions correlated with each of these to varying degrees. Very similar results were obtained using an unrotated matrix (not shown). When the factor analysis was run with the negative reinforcement question excluded, only one component could be extracted, and it accounted for 55% of the variance (not shown).
TABLE 3.
Correlations between responses to the first question in each category (n = 71)
| NR 1 | PS 1 | IS 1 | SRL 1 | IIC 1 | |
|---|---|---|---|---|---|
| NR 1 | — | ||||
| PS 1 | −.03 | — | |||
| IS 1 | .21 | .21 | — | ||
| SRL 1 | .31* | .33* | .47† | — | |
| IIC1 | .19 | .49† | .35* | .51† | — |
p < .01.
p < .001.
FIGURE 1.
In this figure, the questions are coded according to the name of the theory. Thus NR1 is the first question from the negative reinforcement theory, IS1 refers to incentive salience, SLR1 refers to stimulus-response learning, IIC1 refers to impaired inhibitory control, and PS1 refers to positive reinforcement.
Next we examined the relationship between responses to key questions and mood. To do this, we examined the correlations between ratings on the first question in each category and the BDI score. Significant correlations were observed for most questions. Negative reinforcement (r = .307, p < .01), incentive salience (r = .397, p < .001), stimulus-response learning (r = .384, p < .001), and impaired inhibitory control (r = .318, p < .01) all correlated with scores on the BDI. Only ratings on the positive reinforcement scale did not correlate with scores on the BDI (r = .100, ns).
We also explored relationships between responses to key questions, demographic and drug use data, and addiction severity. Responses to none of the key questions correlated with age, days used in the past month, or years of use. Responses on negative reinforcement correlated significantly with economic complications (r = −.32, p < .05), alcohol complications (r = −.30, p < .05), and drug complications (r = .30, p < .05). No other correlations were significant.
DISCUSSION
The greatest number of participants rated positive reinforcement or “pleasure seeking” as a very important motivation for their drug use, with a smaller but generally non-overlapping group reporting “pain avoidance” or negative reinforcement as a very important motivation for drug use. Many of those identifying positive reinforcement as important also rated stimulus-response learning, incentive salience, or inhibitory control dysfunction as important, suggesting that the obvious motivation of pleasure seeking frequently co-exists with other, perhaps equally relevant, reasons for using drugs. This overlap has been emphasized previously in theoretical papers on addiction,11,35 but, to our knowledge, has not been tested in clinical models of addiction. Surprisingly, however, participants rating positive reinforcement as very important to their drug use also reported that they continued to enjoy other activities as well. Ratings on this scale did not correlate with depressive symptoms, either. This is at odds with the idea that chronic drug exposure produces dysfunction in brain reward systems rendering users less able to enjoy other activities,36 though we could not directly test that hypothesis in this study.
Among those rating negative reinforcement highly, less than half also reported that drugs lessen withdrawal symptoms. This was unexpected, as this aspect of negative reinforcement seems critical for theories of addiction based on opponent-process concepts.4 According to negative reinforcement theory, drug use is motivated by withdrawal symptoms that are reversed by drug use early in the addictive process, but later they are only partially and transiently reversed by drug use. A minority of our participants rated negative reinforcement or withdrawal as important to their ongoing drug use. This was unexpected, as methamphetamine has a long elimination half-life,37 produces marked changes in brain dopamine,38 and has been reported to produce withdrawal symptoms.24,25
The stimulus-response learning model was rated highly by a substantial subgroup of participants. This group described their drug use as habitual and thoughtless. This is consistent with theories of aberrant learning,39 which purports that drug-seeking behavior progresses from an “action-outcome” stage, in which reinforcing effects control behavior, to a habit stage, in which stimulus-response associations take over control. Addiction is conceived of, in part, as habitual responding that is relatively insensitive to the value of the reward. One fifth (20%) of our participants identified drug taking as having prominent habitual characteristics.
Impairments in inhibitory control, or more broadly, impulsivity,16,17 is another conceptualization of addiction with a strong basis in neuroscience research.20,22 About a quarter of our participants reported that impaired inhibitory control plays a role in ongoing drug use and relapse. Impulsivity is a multi-dimensional construct, including such concepts as perseveration, acting in the absence of forethought, and undue risk-taking.40 Further work is needed to characterize the aspects of impulsivity that participants believe are most important.
Incentive salience or “craving” was rated highly as a motivation for drug use and relapse by the smallest number of participants, a finding at odds with common conceptualizations of addiction.41–43 Strikingly, 36% believed that craving contributed not at all to their drug use, and a majority (55%) did not believe that objects associated with drug use produced cravings. Explanations for this contradictory finding could include the suggestion that participants have a response bias against reporting craving, that they are unaware of cravings, or that they themselves define craving idiosyncratically and do not attribute relevant feeling to the word “craving.” Clinical experience suggests that some subjects, for example, identify craving with specific somatic experiences, and would use the words “desire” or “want” to express what we referred to as craving. If this were so, participants might have rated this item differently if we had used the word “want” rather than “crave.” On the other hand, the modest magnitude of cue-induced increases in craving seen in experimental studies has generally not been appreciated. For example, in a large study in this area,44 about one-third of 150 cocaine-dependent volunteers did not report any increase in craving following exposure to cocaine cues. In another study,45 among those reporting increases in craving the magnitude of increase averaged 3.4 points out of 10.45 This frequency and magnitude of craving may not be sufficient to account for a great deal of drug use. This is consistent with a recent qualitative study that found that most methamphetamine users did not perceive craving as insurmountable.46 By contrast, in clinical trials craving has been shown to significantly predict subsequent drug use,27,47,48 suggesting that there may well be an important role for craving as an important motivation for drug use. This issue clearly requires further investigation.
The results from the correlation and factor analysis suggest that positive and negative reinforcement reflect different constructs, with the other constructs being related somewhat to each. This suggests that concepts associated with the other theories of addiction are needed for a comprehensive understanding of participants’ perceived motivations for using methamphetamine.
Depressive symptoms, indexed by the BDI, were positively associated with ratings for the first question each category, excepting positive reinforcement, which did not correlate with the BDI. This suggests that depressive symptoms may contribute to negative reinforcing and incentive effects of drugs, and may play a role in impulsive drug use. This also suggests that people reporting they use drugs for their pleasurable effects do so irrespective of self-reported depressive symptoms.
Results from this study support the contention that methamphetamine users may be more heterogeneous than is often appreciated, and imply that treatment development might be more successful if aimed at particular subtypes of patients.42,49 A formidable challenge is identifying valid subgroups of users. Further research is needed to define coherent subgroups of users that could guide the development of individualized treatments for methamphetamine dependence. In addition, it is unclear whether subgroups, if they could be identified reliably, would be stable over time. Nevertheless, results from this study indicate that users identify a range of motivations for using drugs, suggesting that further research in this area would likely contribute to advances in treatment development research.
In the absence of external validation, a key limitation of this survey approach is that we sampled participants’ opinions regarding reasons for using methamphetamine and did not employ questionnaires with established reliability and validity. Although the instrument had substantial internal consistency as evidenced by the high Cronbach alpha level, this report must serve as preliminary evidence of the usefulness of this novel instrument. The potential pitfalls of self-report instruments are well known.50 A further limitation of these findings includes the under-representation of women and minority racial and ethnic groups among methamphetamine users in West Los Angeles, the area where the survey was conducted. Nevertheless, this initial attempt to characterize responses of self-identified subgroups of methamphetamine dependent volunteers may inform treatment development research by facilitating the identification of more homogenous subgroups of patients. This may facilitate the identification of treatments with differing degrees of efficacy for different subgroups of patients.42,49,51
References
- 1.West R. Theories of addiction. Addiction. 2001;96:3–13. doi: 10.1046/j.1360-0443.2001.96131.x. [DOI] [PubMed] [Google Scholar]
- 2.Blum K, Braverman ER, Holder JM, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl):i–iv. 1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 3.Wikler A. Recent progress in research on the neurophysiological basis of morphine addiction. Am J Psychiatry. 1948;105:329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- 6.Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine adminstration on brain reward function in rats. Psychopharmacology (Berl) 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- 7.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 8.Spence KW. Behavior theory and conditioning. New Haven: Yale University Press; 1956. [Google Scholar]
- 9.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 10.Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. 1985;3:445–460. [PubMed] [Google Scholar]
- 11.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 12.Berridge KC, Robinson TE. The mind of an addicted brain: Neural sensitization of wanting versus liking. Current Directions in Psychological Science. 1995;4:71–76. [Google Scholar]
- 13.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 14.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 15.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- 17.de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 18.Miller L. Predicting relapse and recovery in alcoholism and addiction: neuropsychology, personality, and cognitive style. J Subst Abuse Treat. 1991;8:277–291. doi: 10.1016/0740-5472(91)90051-b. [DOI] [PubMed] [Google Scholar]
- 19.Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- 20.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 21.Gray JA. Perspectives on anxiety and impulsivity: A commentary. Journal of Research in Personality. 1987;21:493–509. [Google Scholar]
- 22.Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 23.Newton TF, Roache JD, De La Garza R, 2nd, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- 24.McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 25.Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- 26.De La Garza R, Shoptaw S, Newton TFD. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Int J Neuropsychopharmacol. 2008;11:729–741. doi: 10.1017/S1461145708008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63:269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- 28.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 30.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 33.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 35.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 36.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 37.Newton TF, De La Garza R, 2nd, Fong T, et al. A comprehensive assessment of the safety of intravenous methamphetamine administration during treatment with selegiline. Pharmacol Biochem Behav. 2005;82:704–711. doi: 10.1016/j.pbb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Wilson JM, Kalasinsky KS, Levey AI, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 39.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 40.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 41.Addolorato G, Leggio L, Abenaveli L, Gasbarrini G. Neurobiochemical and clinical aspects of craving in alcohol addiction: a review. Addict Behav. 2005;30:1209–1224. doi: 10.1016/j.addbeh.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 43.Shiffman S, West R, Gilbert D. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res. 2004;6:599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- 44.Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- 45.Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 46.Bruehl AM, Lende DH, Schwartz M, Sterk CE, Elifson K. Craving and control: methamphetamine users’ narratives. J Psychoactive Drugs. 2006;(Suppl 3):385–392. doi: 10.1080/02791072.2006.10400602. [DOI] [PubMed] [Google Scholar]
- 47.Weiss RD, Griffin ML, Mazurick C, et al. The relationship between cocaine craving, psychosocial treatment, and subsequent cocaine use. Am J Psychiatry. 2003;160:1320–1325. doi: 10.1176/appi.ajp.160.7.1320. [DOI] [PubMed] [Google Scholar]
- 48.Kosten TR, Scanley BE, Tucker KA, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 49.De La Garza IR, Newton TF. Individual variability in addiction profiles: Importance for medications development. Annual Meeting of The College on the Problems of Drug Dependence; 2006; Scottsdale, AZ. [Google Scholar]
- 50.Williamson A. Using self-report measures in neurobehavioural toxicology: can they be trusted? Neurotoxicology. 2007;28:227–234. doi: 10.1016/j.neuro.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Monterosso J, Ehrman R, Napier KL, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]