Abstract
Background
Intravenous metoclopramide is effective as primary therapy for acute migraine but the optimal dose of this medication is not yet known.
Methods
This was a randomized, double-blind, dose finding study conducted on patients who presented to our emergency department (ED) meeting International Classification of Headache Disorders criteria for migraine without aura. We randomized patients to 10mg, 20mg, or 40mg of intravenous metoclopramide. We co-administered diphenhydramine to all patients to prevent extra-pyramidal side effects. The primary outcome was improvement in pain on an 11 point Numerical Rating Scale (NRS) at one hour. Secondary outcomes included sustained pain freedom at 48 hours and adverse effects.
Results
In this study, 356 patients were randomized. Baseline demographics and headache features were comparable among the groups. At one hour, those who received 10mg improved by a mean of 4.7 NRS points (95%CI: 4.2, 5.2); those who received 20mg improved by 4.9 (95%CI: 4.4, 5.4), and those who received 40mg improved by 5.3(95%CI: 4.8, 5.9). Rates of 48 hour sustained pain freedom in the 10, 20, and 40mg groups were: 16% (95%CI:10,24%), 20% (95%CI:14,28%), and 21% (95%CI:15,29%), respectively. The most commonly occurring adverse event was drowsiness, which impaired function in 17% (95%CI: 13,21%) of the overall study population. Akathisia developed in 33 patients. Both drowsiness and akathisia were evenly distributed across the 3 arms of the study. One month later, no patient had developed tardive dyskinesia.
Conclusions
20mg or 40mg of metoclopramide are no better for acute migraine than 10mg of metoclopramide.
The majority of the two million headache patients who present to US emergency departments (ED) annually are suffering an acute migraine [1, 2]. The goal of ED therapy is to deliver rapid relief of pain, with a minimum of unpleasant side effects, and without recurrence of headache after ED discharge [3]. The ideal medication would have few contra-indications and would not exacerbate the underlying migraine disorder. Intravenous metoclopramide, when compared to sumatriptan, non-steroidals, opioids, and other anti-emetics, has demonstrated many of the characteristics described above [4–10]. Additionally, metoclopramide is inexpensive, widely available, and well known to emergency physicians because of its use for the treatment of nausea and gastroparesis. The standard dose of metoclopramide for treatment of migraine is 10mg. Limited clinical data suggest that 20mg of intravenous metoclopramide is more efficacious than 10mg [11–13]. One study reported excellent anti-migraine efficacy with doses of metoclopramide up to 60mg, administered over 45 minutes[14]. However, the hypothesis that more metoclopramide is more efficacious has not yet been subject to rigorous experimental design. The purpose of this study was to compare the efficacy and safety of three different doses of intravenous metoclopramide--10mg versus 20mg versus 40mg--for the treatment of acute migraine. We tested three primary hypotheses, adjusting our risk of type 1 error appropriately:
40mg of metoclopramide would improve the headache more than 10mg of metoclopramide, as measured by the change in an 11 point Numerical Rating Scale for pain between baseline and one hour
20mg of metoclopramide would improve the headache more than 10mg of metoclopramide, as measured by the change in an 11 point Numerical Rating Scale for pain between baseline and one hour
40mg of metoclopramide would improve the headache more than 20mg of metoclopramide, as measured by the change in an 11 point Numerical Rating Scale for pain between baseline and one hour
Methods
Study Overview
This was a randomized, double-blind, three-armed clinical trial comparing three doses of parenteral metoclopramide for treatment of acute migraine in an emergency department. There was no placebo arm. This trial was registered at http://www.clinicaltrials.gov and approved by the Montefiore Medical Center IRB.
Rapid administration of metoclopramide at times causes akathisia, an extra-pyramidal side-effect characterized by restlessness and agitation [15, 16], which can be treated with anti-cholinergics such as diphenhydramine. Among ED migraineurs, subjective restlessness has been reported in 20% of patients who received 20mg of metoclopramide without diphenhydramine and in 10% who received diphenhydramine prophylactically.[17] To prevent this side effect, 25mg of diphenhydramine was prophylactically co-administered to all subjects. We believed prophylactic co-administration of diphenhydramine would eliminate a dose-related incidence of restlessness, which otherwise may have resulted in a dose-related disparity in administration of diphenhydramine by the clinical team. Since diphenhydramine may have independent anti-migraine activity[18], administering diphenhydramine to all subjects maintained the internal validity of this study.
Setting
We performed this study in the emergency department of Montefiore Medical Center, an urban emergency department that receives over 100,000 adult visits annually. The emergency department is staffed around the clock by salaried, trained, bilingual (English and Spanish) technician-level research associates who execute research studies under the supervision of the principal investigators.
Selection of Participants
Adult patients younger than 70 years who had an acute exacerbation of a migraine without aura as defined by the International Classification of Headache Disorders-2 [19] were eligible for participation. If the acute headache met all migraine criteria with the exception of prolonged duration (>72 hours) or insufficient duration (<4 hours) they were included in the study. We excluded patients if they had a secondary headache (an “organic” headache), if the patient was to receive a lumbar puncture in the ED, or for a maximum documented temperature greater than 100.3 degrees, a new objective neurologic abnormality, allergy or intolerance to a study medication, previous enrollment, or pregnancy. After randomization but prior to un-blinding, it was determined that some patients received off-protocol ketorolac at the same time as the investigational medication. We excluded these patients from all analyses.
Intervention
Arm 1) Metoclopramide 10mg + diphenhydramine 25mg, infused intravenously over 20 minutes
Arm 2) Metoclopramide 20mg + diphenhydramine 25mg, infused intravenously over 20 minutes
Arm 3) Metoclopramide 40mg + diphenhydramine 25mg, infused intravenously over 20 minutes
Randomization and Blinding
The research pharmacist generated a randomization list in blocks of six using computer generated random number tables available at http://www.randomization.com. This was done in a location removed from the emergency department and inaccessible to emergency department personnel. In an order determined by these computer-generated random number tables, the pharmacist inserted medication into identical vials and placed these vials into sequentially numbered identical research bags. These research bags were then used in order by the research team. Only the pharmacist knew the assignment. Every research bag had two vials, one containing metoclopramide and one containing 25 milligrams of diphenhydramine. The pharmacist added normal saline to the vials containing 10mg and 20mg of metoclopramide so that each vial contained 8mL of clear solution. The two vials from each research bag were placed in a 50cc bag of normal saline by a clinical nurse. The 50cc bag containing the medication was then administered as a slow intravenous drip.
Protocol
After obtaining informed consent, we performed a brief pain assessment and then administered the investigational medication as an intravenous drip between time zero and twenty minutes. Research associates returned every thirty minutes to ascertain the subject’s headache level. At one hour and two hours after medication administration, the research associates asked a more detailed series of ten questions regarding pain, functional limitations, and adverse events. If subjects required more pain medication at or after one hour had elapsed, they were administered additional medication at the discretion of the treating physician. We contacted all of our research subjects by telephone 48 hours after ED discharge to ascertain headache status, satisfaction with treatment, and presence of adverse events. We reviewed our medical center’s database on an ongoing basis to determine if any study patient had returned to any clinic, medical office or ED within one month of enrollment. If so, we performed a blinded review of the medical record to determine if an adverse events possibly related to the investigational medication had occurred.
Methods of measurement
As a primary measure of headache intensity, we utilized a standard, validated, and reproducible 11-point numerical rating scale (NRS)[20]. This scale asks patients to assign their pain a number between 0 and 10, with 0 representing no pain and ten representing the worst pain imaginable. Secondary measurement tools included a standard four-point pain intensity categorical scale, in which patients describe their pain as “severe”, “moderate”, “mild”, or “none” and a four-point functional disability scale, in which patients describe their headache-related disability as severe (“cannot get up from bed or stretcher”), moderate (“great deal of difficulty doing what I usually do and can only do very minor activities” ), mild (“little bit of difficulty doing what I usually do”), or none. All of these scales are recommended for use in migraine research by the International Headache Society[21]. One hour after medication administration, we asked all of our patients if they needed more medication for pain. Finally, we assessed our patients’ satisfaction with treatment by asking each of them, 48 hours after enrollment, whether they would want to receive the same medication the next time they visited the ED with an acute migraine. This latter question allowed each patient to weigh for himself the relative efficacy and tolerability of the medication.
We assessed adverse events one hour, two hours, and 48 hours after medication administration. Because akathisia is a known side effect of metoclopramide[22, 23], this reaction was assessed for specifically using the Short Akathisia Instrument during the one hour following medication administration[24]. On this scale, akathisia is diagnosed by an increase in subjective restlessness after medication administration with objective corroboration. We considered patients to have isolated subjective akathisia if they reported severe restlessness or anxiety at any time following medication administration without objective signs.
Data collection and processing
Data acquisition was performed by the research associates, supervised by the principal investigator. Research associates entered data directly in real time onto a standardized paper data collection instrument. A trained research secretary then transcribed the data into SPSS Data Entry V.4.0 (SPSS Inc., Chicago, Illinois). The principal investigator, who remained blinded during the process, double-checked all data for accuracy.
Outcome measures
The primary outcome for this study was a comparison of change in numerical rating scale score between baseline and one hour for every pairing of the three investigational arms. Although two hours is a more standard endpoint for outpatient migraine trials[21], we have found in previous work that many of our patients are eager to be discharged before the two hour endpoint has arrived.
Secondary outcomes include desire to receive the same medication at the next ED visit for migraine, sustained headache freedom (defined as achieving a headache-free state within two hours of medication administration and maintaining it for 48 hours without use of additional medication), sustained headache response (defined as achieving headache intensity of “mild” or “none” within two hours of medication administration and maintaining it for 48 hours), achieving a normal functional status by two hours, patient request for rescue medication, and dwell time in the ED.
Sample size calculation
This was a three-armed study with three pair-wise comparisons. Using the conservative Bonferroni correction for multiple comparisons, we set α=.017. A review of our recent acute migraine studies revealed a typical standard deviation of 2.8 NRS points. For the sample size calculation we assumed a normal distribution and a between group difference of 1.3 units on the numerical rating scale. 1.3 NRS units is a validated and reproducible minimum clinically significant difference in pain severity [25, 26]. With these parameters, we calculated the need for 100 subjects in each arm, for a total of 300 subjects. After adding to this a 10% rate for protocol violations, we planned to enroll 330 subjects (110 patients per arm).
Analysis
The primary outcome is mean change in NRS score within each group, bounded by unadjusted 95%CIs. Student’s t-test for independent sample was used to compare mean differences in pain scores between each of the three arms. We report all proportions with 95%CI and compared them using a chi-square test. We included patients who received rescue medication in all analyses.
Results
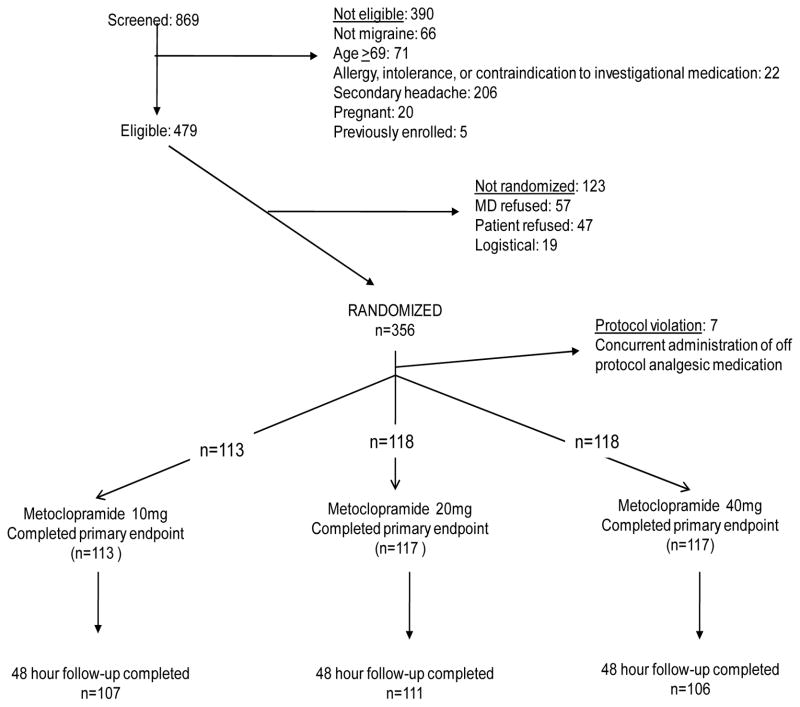
Enrollment commenced in May of 2008 and continued for 21 months. We screened 869 patients with non-traumatic headache for enrollment and ultimately randomized 356. Please see CONSORT flow diagram, Figure 1. We excluded from all analyses 13 patients who received the investigational medication because blinded chart review revealed they were ineligible due to fever or recent lumbar puncture. We excluded seven patients from the study post-randomization because blinded chart review revealed concurrent administration of off- protocol analgesic medication. Including these 20 patients in the primary analysis did not alter the primary or secondary outcomes.
Figure 1.
CONSORT flow diagram
Baseline demographics and headache features were comparable among the three groups (Table 1). Of note, more than 1/3 of patients took no medication before presenting to the ED.
Table 1.
Baseline Characteristics
| Variable | Metoclopramide 10mg (n=113) | Metoclopramide 20mg (n= 118 ) | Metoclopramide 40mg (n=118 ) |
|---|---|---|---|
| Age in years (mean, SD) | 39 (11) | 37 (10) | 38 (12) |
| Female | 83% (75, 89%) | 87% (80, 92%) | 82% (74, 88%) |
| Race* | |||
| Black/African American | 28% (21, 37%) | 28% (21, 37%) | 20% (14, 28%) |
| Latino/Hispanic | 70% (61, 78%) | 70% (61, 78%) | 76% (68, 83%) |
| Asian/Pacific Islander | 0% (0, 4%) | 1% (0, 5%) | 1% (0, 5%) |
| White | 18% (12, 26%) | 20% (14, 28%) | 19% (13, 27% |
| Headache duration in hours (median, IQR) | 48 (19, 72) | 68 (24, 96) | 24 (14, 72) |
| Aura symptoms | 27% (20, 36%) | 32% | 27% (20, 36%) |
| Median pain score (0–10) at baseline (IQR) | 8 (7, 10) | 9 (7, 10) | 9 (8, 10) |
| Took migraine/headache medication prior to ED presentation | 57% (48, 66%) | 68% (59, 76%) | 64% (55, 72%) |
| Median days of functional disability (IQR)** | 4 (2, 10) | 4 (1, 9) | 4 (2, 7) |
Reported as percent and 95%CI unless otherwise noted
Race: Some patients were multi-racial
“How many headaches have you had in the last 90 days that were so severe you were unable to do most of your usual daily activities?”
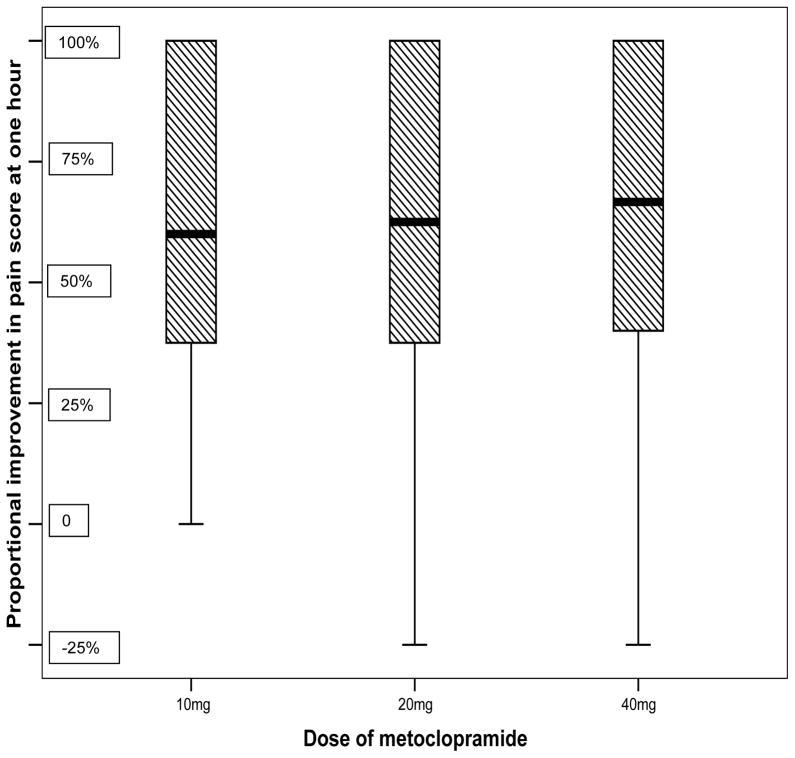
One hour after medication administration the 10mg metoclopramide group improved by 4.7 NRS units (unadjusted 95%CI: 4.2, 5.2), the 20mg metoclopramide group improved by 4.9 (unadjusted 95%CI: 4.4, 5.4), and the 40mg metoclopramide group improved by 5.3 (unadjusted 95%CI: 4.8, 5.9). Most patients improved by more than 50% and 75% improved by at least 1/3 (Figure 2). Pairwise comparison after adjustment of alpha did not reveal differences between any two groups that surpassed established minimum clinically significant differences for acute pain [25, 26]. An ANOVA analysis was not statistically significant (p=0.19). The 95%CI for the 0.2 NRS unit difference between the 10mg and 20mg group was −0.5, 0.9. The 95%CI for the 0.4 NRS unit difference between the 20mg and 40mg group was −0.3, 1.1. The 95%CI for the 0.6 NRS unit difference between the 10mg and 40mg group was −0.1, 1.3. We report secondary outcomes in Table 2.
Figure 2.
Box and whiskers plot demonstrating median (bold line), inter-quartile range (hatched box), and complete range of the percent improvement in pain by 1 hour (whiskers). 3/4 of patients improved by >33%. Most improved by >50%.
Table 2.
Secondary outcomes
| Metoclopramide 10mg | Metoclopramide 20mg | Metoclopramide 40mg | |
|---|---|---|---|
| Wish to receive this medication the next ER visit | 77/104 * 74% (65, 81%) |
83/108* 77% (68, 84%) |
80/104* 77% (68, 84%) |
| 2 hour headache relief | 93/113 82% (74, 88%) |
94/117* 80% (72, 86%) |
100/117* 86% (79, 91%) |
| 2 hour headache freedom | 49/113 43% (34, 52%) |
53/117* 45% (36, 54%) |
51/117* 44% (35, 53%) |
| 2 hour normal functional status | 70/112* 63% (54, 71%) |
68/117* 58% (49, 67%) |
80/115* 70% (61, 78%) |
| Sustained relief | 58/107* 54% (45, 63%) |
63/116* 54% (45, 63%) |
69/106* 65% (56, 73%) |
| Sustained pain free | 18/111* 16% (10, 24%) |
23/116* 20% (14, 28%) |
24/113* 21% (15, 29%) |
| Time to discharge, minutes (median [IQR]) | 160 (108, 275) | 154 (97, 226) | 158 (114, 254) |
| Requested rescue medication one hour later | 23/113* 20% (14, 28%) |
18/116* 16 % (10, 24%) |
21/114* 18% (12, 26%) |
Reported as percent and 95%CI unless otherwise noted
Some secondary outcome data is missing either because patients were lost-to-follow-up or because they refused/couldn’t answer the question
Akathisia developed in 33 patients (9% [95%CI: 6, 12%]) and was evenly distributed across the study arms. This included 18 with subjective restlessness and anxiety, and 15 with objective akathisia that caused them to elope from the emergency department or to be treated with additional diphenhydramine. Altogether, clinicians administered diphenydramine to 23 patients for subjective or objective akathisia: eight patients in both the 10mg and 20mg groups and seven patients in the 40mg group. One hour after medication administration, 69% (95%CI: 64, 74%) of the sample reported drowsiness, which was evenly distributed across the study arms. One-third of these, or 17% (95%CI: 13, 21%) of all patients reported that the drowsiness impaired function. No patient required admission to the hospital due to drowsiness. Dizziness after medication administration was reported by 29 patients (8%, 95%CI: 6, 11% ), including 14 in the 10mg arm, 10 in the 20mg arm, and 5 in the 40 mg arm. Weakness and myalgias were each reported by four separate patients. No more than two patients reported any other specific side effects. No patient was diagnosed with tardive dyskinesia within one month of medication administration. No patient developed a clinically relevant dysrhythmia. One patient randomized to the 20mg dose developed de novo status epilepticus 1.5 days after investigational medication administration and was ultimately diagnosed with encephalitis. It seems most plausible that this was related to an underlying inflammatory process in the brain and not the investigational medication.
Age had a small negative correlation with the primary outcome, which was not statistically significant after adjustment for multiple comparisons. Otherwise, the primary outcome was not associated with gender, baseline nausea, aura symptoms, duration of headache, use of medication at home, or use of prophylactic medications.
Limitations
This study has several limitations. We did not include a placebo arm. However, the efficacy of metoclopramide for acute migraine has been so well established that a placebo arm seemed unnecessary.[7]
We did not measure the efficacy of metoclopramide alone—in all arms of this study it was combined with diphenhydramine. We used diphenhydramine to minimize the risk of metoclopramide induced akathisia, which otherwise may have resulted in a dose-related disparity in administration of diphenhydramine by the clinical team. Since diphenhydramine may have independent anti-migraine activity[18], administering diphenhydramine to all research patients was necessary to maintain the internal validity of this study. Thus, we believe our conclusion is valid, though our estimates of the efficacy of metoclopramide may be inflated. We cannot rule-out an important dose-dependent effect of metoclopramide blunted by the co-administration of diphenhydramine.
We conducted this study in one urban ED, serving a large Latino population. Women were over-represented in this study compared to expected prevalence rates [36, 37]. However, we are not aware of gender or ethnicity differences that should limit the generalizability of this data to other populations.
We searched our medical center database to identify any cases of tardive dyskinesia that occurred beyond our 48 hour follow-up phone call. It is possible that a patient visited another hospital in our area and was not captured in our hospital’s database.
We did not determine serum levels of metoclopramide.
There are some differences in baseline variables but as none of these baseline variables are associated with the primary outcome we do not believe they confound our results.
Discussion
In this randomized, double-blind, three-arm dose-finding clinical trial, we found no evidence of increased efficacy of 40 mg or 20 mg of IV metoclopramide when compared head-to-head to the standard 10mg dose. We therefore recommend 10mg as the initial dose of intravenous metoclopramide for treatment of acute migraine in the ED. This study was not designed to determine whether or not diphenhydramine should be co-administered with metoclopramide.
We were surprised by the results, although it is in keeping with dose-finding studies of parenteral droperidol and prochlorperazine [27, 28], other anti-dopaminergics with demonstrated efficacy in acute migraine. The efficacy of droperidol peaked at a relatively low dose—2.75mg--in a randomized dose-finding study. In a quasi-experimental study, increasing the dose of prochlorperazine beyond 3.5mg, in combination with dihydroergotamine 1.0mg, did not result in increased efficacy.
We found relatively low rates of sustained headache freedom in this trial, similar to other ED-based trials [12, 29, 30]. The goal of sustained headache freedom remains elusive for many ED patients, though many patients are satisfied with only a modest 1/3 reduction in headache intensity[3].
Failure to achieve sustained headache freedom was mediated partly by incomplete response to the medication in the ED and partly by recurrence of headache after ED discharge. Headache after ED discharge is quite common—1/3 of migraineurs report moderate or severe headache in the 24 hours following ED discharge and ½ remain functionally disabled[31]. Emergency physicians and others who treat acute headaches are urged to provide their patients with appropriate treatment options for use following discharge. Oral naproxen and oral sumatriptan are comparably and modestly effective for the treatment of recurrent headache post-ED discharge [32]. Dexamethasone has a small benefit with a number needed to treat of nine to ten [33, 34].
Drowsiness was a common side effect in all three treatment arms in this study. Given that all patients received diphenhydramine in addition to the metoclopramide, this is not surprising. However, drowsiness or functional impairment is common in ED migraine patients, even when administered medications not expected to cause this symptom, such as subcutaneous sumatriptan. In fact, drowsiness or sedation has been as common in patients randomized to sumatriptan as in those who received high-dose metoclopramide + diphenhydramine [9] or prochlorperazine + diphenhydramine [35].
As far as we could determine, none of our patients were diagnosed with tardive dyskinesia within one month of study enrollment. This is consistent with the absence of any reports of development of tardive dyskinesia after isolated parenteral doses of metoclopramide. There were no occurrences of clinically relevant dysrhythmias in our study. Although there are isolated case reports of cardiac arrest after metoclopramide, a causal relationship has not been established.
Variability in response to the investigational medication is marked and easily seen in the box and whiskers plot (Figure 2). Baseline clinical features such as nausea or duration of headache do not predict response and therefore cannot be used to guide therapy.
This study did not assess the benefit of re-dosing in those who do not achieve a satisfactory response to the initial dose. Previous studies has suggested higher rates of sustained headache freedom and medication satisfaction among patients treated with sequential IV doses of 20mg of metoclopramide[9, 30]. This is a hypothesis left for future work.
In conclusion, we found no evidence that administration of parenteral metoclopramide in doses greater than 10mg were any more efficacious for treatment of acute migraine than the standard 10 mg dose. Therefore, we recommend 10mg of intravenous metoclopramide as the initial dose for the treatment of acute migraine.
Acknowledgments
Funding: Some of this research was conducted with Dr. Friedman’s K23 Career Develoment Award from the National Institute of Neurological Disorders and Stroke 1K23NS051409
Footnotes
Conflict of interest: None of authors report any conflict of interest
References
- 1.Friedman BW, et al. Applying the International Classification of Headache Disorders to the emergency department: an assessment of reproducibility and the frequency with which a unique diagnosis can be assigned to every acute headache presentation. Ann Emerg Med. 2007;49(4):409–19. 419, e1–9. doi: 10.1016/j.annemergmed.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Vinson DR. Treatment patterns of isolated benign headache in US emergency departments. Ann Emerg Med. 2002;39(3):215–22. doi: 10.1067/mem.2002.121400. [DOI] [PubMed] [Google Scholar]
- 3.Friedman BW, Bijur PE, Lipton RB. Standardizing emergency department-based migraine research: an analysis of commonly used clinical trial outcome measures. Acad Emerg Med. 17(1):72–9. doi: 10.1111/j.1553-2712.2009.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belgrade MJ, et al. Comparison of single-dose meperidine, butorphanol, and dihydroergotamine in the treatment of vascular headache. Neurology. 1989;39(4):590–2. doi: 10.1212/wnl.39.4.590. [DOI] [PubMed] [Google Scholar]
- 5.Cete Y, et al. A randomized prospective placebo-controlled study of intravenous magnesium sulphate vs. metoclopramide in the management of acute migraine attacks in the Emergency Department. Cephalalgia. 2005;25(3):199–204. doi: 10.1111/j.1468-2982.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- 6.Cicek M, et al. Prospective, randomised, double blind, controlled comparison of metoclopramide and pethidine in the emergency treatment of acute primary vascular and tension type headache episodes. Emerg Med J. 2004;21(3):323–6. doi: 10.1136/emj.2002.000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colman I, et al. Parenteral metoclopramide for acute migraine: meta-analysis of randomised controlled trials. Bmj. 2004;329(7479):1369–73. doi: 10.1136/bmj.38281.595718.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis GL, et al. The efficacy of metoclopramide in the treatment of migraine headache. Ann Emerg Med. 1993;22(2):191–5. doi: 10.1016/s0196-0644(05)80201-x. [DOI] [PubMed] [Google Scholar]
- 9.Friedman BW, et al. A trial of metoclopramide vs sumatriptan for the emergency department treatment of migraines. Neurology. 2005;64(3):463–8. doi: 10.1212/01.WNL.0000150904.28131.DD. [DOI] [PubMed] [Google Scholar]
- 10.Tek DS, et al. A prospective, double-blind study of metoclopramide hydrochloride for the control of migraine in the emergency department. Ann Emerg Med. 1990;19(10):1083–7. doi: 10.1016/s0196-0644(05)81508-2. [DOI] [PubMed] [Google Scholar]
- 11.Coppola M, Yealy DM, Leibold RA. Randomized, placebo-controlled evaluation of prochlorperazine versus metoclopramide for emergency department treatment of migraine headache. Ann Emerg Med. 1995;26(5):541–6. doi: 10.1016/s0196-0644(95)70001-3. [DOI] [PubMed] [Google Scholar]
- 12.Friedman BW, et al. A Randomized Controlled Trial of Prochlorperazine Versus Metoclopramide for Treatment of Acute Migraine. Ann Emerg Med. 2007 doi: 10.1016/j.annemergmed.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Jones J, et al. Randomized double-blind trial of intravenous prochlorperazine for the treatment of acute headache. Jama. 1989;261(8):1174–6. [PubMed] [Google Scholar]
- 14.Corbo J, et al. Randomized clinical trial of intravenous magnesium sulfate as an adjunctive medication for emergency department treatment of migraine headache. Ann Emerg Med. 2001;38(6):621–7. doi: 10.1067/mem.2001.119424. [DOI] [PubMed] [Google Scholar]
- 15.Seviour C, Harrison D, Abu-Laban R. Incidence of Akathisia from Intravenous Metoclopramide for Migraine Headache (abstract) Academic Emergency Medicine. 2000;7(5):536b. [Google Scholar]
- 16.Vinson DR. Diphenhydramine in the treatment of akathisia induced by prochlorperazine. J Emerg Med. 2004;26(3):265–70. doi: 10.1016/j.jemermed.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Friedman BW, et al. A randomized trial of diphenhydramine as prophylaxis against metoclopramide-induced akathisia in nauseated emergency department patients. Ann Emerg Med. 2009;53(3):379–85. doi: 10.1016/j.annemergmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Swidan SZ, Lake AE, 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep. 2005;9(1):65–70. doi: 10.1007/s11916-005-0077-5. [DOI] [PubMed] [Google Scholar]
- 19.Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia. 1988;8(Suppl 7):1–96. [PubMed] [Google Scholar]
- 20.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10(4):390–2. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 21.Tfelt-Hansen P, et al. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia. 2000;20(9):765–86. doi: 10.1046/j.1468-2982.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 22.Parlak I, et al. Rate of metoclopramide infusion affects the severity and incidence of akathisia. Emerg Med J. 2005;22(9):621–4. doi: 10.1136/emj.2004.014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parlak I, et al. Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: a randomized controlled trial. Acad Emerg Med. 2007;14(8):715–21. doi: 10.1197/j.aem.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Vinson DR. Development of a simplified instrument for the diagnosis and grading of akathisia in a cohort of patients receiving prochlorperazine. J Emerg Med. 2006;31(2):139–45. doi: 10.1016/j.jemermed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38(6):633–8. doi: 10.1067/mem.2001.118863. [DOI] [PubMed] [Google Scholar]
- 26.Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med. 1996;3(2):142–6. doi: 10.1111/j.1553-2712.1996.tb03402.x. [DOI] [PubMed] [Google Scholar]
- 27.Saadah HA. Abortive headache therapy in the office with intravenous dihydroergotamine plus prochlorperazine. Headache. 1992;32(3):143–6. doi: 10.1111/j.1526-4610.1992.hed3203143.x. [DOI] [PubMed] [Google Scholar]
- 28.Silberstein SD, et al. Acute migraine treatment with droperidol: A randomized, double-blind, placebo-controlled trial. Neurology. 2003;60(2):315–21. doi: 10.1212/01.wnl.0000042477.63516.b2. [DOI] [PubMed] [Google Scholar]
- 29.Akpunonu BE, et al. Subcutaneous sumatriptan for treatment of acute migraine in patients admitted to the emergency department: a multicenter study. Ann Emerg Med. 1995;25(4):464–9. doi: 10.1016/s0196-0644(95)70259-8. [DOI] [PubMed] [Google Scholar]
- 30.Friedman BW, et al. Randomized trial of IV dexamethasone for acute migraine in the emergency department. Neurology. 2007;69(22):2038–44. doi: 10.1212/01.WNL.0000281105.78936.1d. [DOI] [PubMed] [Google Scholar]
- 31.Friedman BW, et al. Recurrence of primary headache disorders after emergency department discharge: frequency and predictors of poor pain and functional outcomes. Ann Emerg Med. 2008;52(6):696–704. doi: 10.1016/j.annemergmed.2008.01.334. [DOI] [PubMed] [Google Scholar]
- 32.Friedman BW, et al. Treating Headache Recurrence After Emergency Department Discharge: A Randomized Controlled Trial of Naproxen Versus Sumatriptan. Ann Emerg Med. doi: 10.1016/j.annemergmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colman I, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ. 2008;336(7657):1359–61. doi: 10.1136/bmj.39566.806725.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med. 2008;15(12):1223–33. doi: 10.1111/j.1553-2712.2008.00283.x. [DOI] [PubMed] [Google Scholar]
- 35.Kostic MA, et al. A Prospective, Randomized Trial of Intravenous Prochlorperazine Versus Subcutaneous Sumatriptan in Acute Migraine Therapy in the Emergency Department. Ann Emerg Med. 2009 doi: 10.1016/j.annemergmed.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Lipton RB, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–9. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 37.Lipton RB, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58(6):885–94. doi: 10.1212/wnl.58.6.885. [DOI] [PubMed] [Google Scholar]