Abstract
Purified carbohydrates and fats are usually palatable to humans and other animals, and their consumption often induces weight gain and accumulation of fat. In this study, we examined consumption of complex carbohydrates (cornstarch and Polycose) and fats (soybean oil and margarine) in mice from two inbred strains, C57BL/6ByJ and 129P3/J. At lower concentrations of liquid nutrients tested using two-bottle tests, when the amounts consumed had negligible energy content, the C57BL/6ByJ mice had higher acceptance of Polycose and soybean oil. This was probably due to strain differences in chemosensory perception of Polycose and oil. At higher concentrations, the mice consumed a substantial part of their daily energy from the macronutrient sources, however, there were no or only small strain differences in nutrient consumption. These small differences were probably due to strain variation in body size. The two strains also did not differ in chow intake. Despite similar energy intakes, access to the nutrients resulted in greater body weight (BW) gain in the C57BL/6ByJ mice than in the 129P3/J mice. The diet-induced weight gain was examined in detail in groups of 2-month-old C57BL/6ByJ and 129P3/J mice given ether chow, or chow and margarine to eat. Access to margarine did not increase total energy consumption of either strain. It increased BW and adiposity of the C57BL/6ByJ mice, but only after they reached the age of ~3 months. There were no differences in BW and adiposity between control and margarine-exposed 129P3/J mice. The results suggest that diet-induced adiposity in the B6 mice depends on age and does not depend on hyperphagia.
Keywords: Carbohydrate, Fat, Polycose, Starch, Energy intake, Obesity, Mouse strains, Genotype
1. Introduction
Human obesity depends on a combination of genetic and environmental factors and is most frequently expressed when individuals are exposed to ample, energy-dense diets. There is evidence that diet selection is in part genetically mediated [1], which can contribute to the development of obesity. Mouse strains differ in nutrient selection and in predisposition to diet-induced adiposity, and thus can serve as a model to examine interactions between genetic and environmental contributions to obesity. In this study, we compared the nutrient appetite and nutrient-induced adiposity of mice from C57BL/6ByJ (B6) and 129P3/J (129) strains. Dietary carbohydrate was provided as either cornstarch or Polycose, and dietary fat was provided as margarine or soybean oil.
2. Materials and methods
2.1. Animals
Mice of the B6 (n = 83) and 129 (n = 85) strains were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in individual cages in a temperature-controlled room at 23°C on a 12-h light:12-h dark cycle. In Experiment 5, naïve mice were used. Mice used in Experiments 1–4 and 6 had been previously tested in two-bottle tests with several non-nutritive sweeteners or sugars; no strain differences in body weight (BW) changes during these tests were noted.
2.2. Food and nutrients
During all experiments, mice had ad libitum access to deionized water and pelleted chow Teklad Rodent Diet 8604 (Harlan Teklad, Madison, WI; 24.5% protein, 50.3% carbohydrate and 4.4% fat; 3.93 kcal/g gross energy).
Dietary carbohydrate was provided as either cornstarch (Sigma, St. Louis, MO; 4.0 kcal/g) or Polycose (Ross Laboratories, Columbus, OH; 3.8 kcal/g). Dietary fat was provided as margarine (Fleishmann’s Unsalted Spread, Nabisco, East Hanover, NJ; 6.4 kcal/g) or soybean oil (“BestYet Vegetable Oil,” Scrivner; 8.6 kcal/g).
Nutrient solutions and emulsions were prepared every 48 h. Polycose was dissolved in deionized water and provided as a solution. The cornstarch and soybean oil (except for 100% oil) were provided as emulsions and prepared using deionized water stabilized with 0.3% (weight/volume) xanthan gum (Sigma). Xanthan gum is tasteless to humans, it does not affect animal growth [2], and 0.3% xanthan gum has negligible caloric value. Neither strain distinguished 0.3% xanthan gum from water (Experiments 2, 4). Therefore, the use of xanthan gum was unlikely to have affected strain-dependent differences in nutrient-emulsion intake. The starch and oil emulsions were homogenized using a motor-driven propeller. No separation of the emulsions was noted during the tests.
2.3. Measurement of food and fluid intakes
Food and fluid intakes were measured daily in the middle of the light period. The BW of individual mice was measured before and after each test series.
Chow intake was measured by weighing the cage tops containing food pellets to the nearest 0.1 g. The bottoms of the cages were covered with corrugated cardboard sheets. When the cage tops were weighed, the cardboard sheets were changed, and any spilled food was collected and weighed. Food intake was corrected for spillage, although this did not exceed 0.1 g/day/mouse.
Solid margarine was offered to the mice in foil cups. Each day, a new cup with margarine was weighed to the nearest 0.1 g and placed into the cage; the next day, it was removed from the cage, weighed, and discarded. The cage floors were covered with cardboard sheets to detect spillage, although none was noted.
Cornstarch (0.03–6%), Polycose (0.003–30%), and soybean oil (1–100%, all weight/volume) were tested in increasing order of concentrations using two-bottle preference tests in which one bottle contained the nutrient and the other bottle contained either deionized water (Polycose solutions and 100% oil) or 0.3% xanthan gum (cornstarch and soybean oil emulsions). Each concentration was tested for 2 days. The positions of the tubes were switched every 24 h to control for side preferences. The construction of the drinking tubes and other experimental details have been previously described [3]. The drinking tubes were positioned to the right of the feeder with their tips 15-mm apart, and each extended 25 mm into the cage. Each tube had a stainless steel tip with a 3.175-mm diameter hole from which the mice could lick the fluids. Fluid volumes were measured to the nearest 0.2 ml.
2.4. Dissection of fat depots
Mice were sacrificed with carbon dioxide, weighed, and their length from the base of the lower incisors to the anus was measured to the nearest millimeter. Bilateral adipose tissue deposits were excised and weighed to the nearest 0.01 g. In Experiment 5, the gonadal (epididimal), retroperitoneal, mesenteric, inguinal, and subscapular depots were excised. In Experiment 6, only gonadal (epididimal) and retroperitoneal depots were excised.
2.5. Analysis of body fat composition
Mice were shaved. After dissecting fat depots, the gastrointestinal tract from the esophagus to the anus including the stomach was removed, weighed, and discarded. The carcass, together with the dissected adipose tissue, was autoclaved, homogenized and dried. A sample of the dry homogenate was mixed with petroleum ether, centrifuged, and the supernatant was collected; this was repeated three times. The homogenate and supernatant samples were left overnight to allow the ether to evaporate. Fat content of samples was calculated as average of (i) dry weight of the supernatant, and (ii) the difference between the sample weights before and after fat extraction, and expressed as a percentage of the wet BW.
2.6. Data analyses
For each mouse, we calculated average daily (24 h) food and fluid intakes. Preference scores in two-bottle tests were calculated for each mouse as the ratio of the average nutrient solution or emulsion intake to average total fluid intake, expressed in percent (%).
The B6 mice were heavier and larger than the 129 mice. Often, they also had higher intakes, more fat, and greater BW gain, which could be attributed, at least partially, to their bigger size. To account for these differences in body size, we used two approaches. First, we used BW or length as a covariate in ANOVA to assess significance of differences. Second, we calculated ratios: BW gain in percent relative to initial BW, intakes per 30 g of initial BW, and adiposity measures per 10 cm of body length (the approximate weight and length of an adult mouse). We used initial BW (measured before access to the nutrients) for both corrections because nutrient consumption was often accompanied by BW gain, and thus affected final BW. Although using ratios to account for differences in body size has disadvantages [4,5], its advantage is in adjusting mean values, as well as significance levels. Generally, the results of both corrections were similar, with a few exceptions (see Results).
To summarize, food and fluid intakes were analyzed (i) using initial BW as a covariate, and (ii) expressing intake per 30 g BW. The results of the two-bottle tests with starch, Polycose, and soybean oil were analyzed using two-way ANOVAs with strain as the between-group factor and concentration as the within-group factor. ANOVAs for energy intakes from Polycose or soybean oil were conducted using only higher concentrations (3–30% for Poly-cose and 3–100% for oil). The lower concentrations were excluded because these solutions had negligible caloric content compared with higher concentrations. Thus, including all concentrations in the ANOVA complex would result in strong correlation between means and variability, violating one of the ANOVA assumptions.
Three approaches were used to analyze BW gain in Experiments 2–4: (i) Initial and final BW were analyzed using two-way ANOVAs with strain as the between-group factor and period (before and after the tests) as the within-group factor; (ii) BW increase was calculated in grams (final BW – initial BW) and strain differences were analyzed using one-way ANOVA with initial BW as a covariate; (iii) BW increase was calculated in percent [(final – initial)/initial × 100] and the strains were compared using t tests.
Because BW depends on the amount of body fat, BW is not suitable for correcting adiposity. Instead, we used body length to account for differences in body size. Weight of fat depots was analyzed (i) using body length as a covariate in ANOVA, and (ii) by calculating adiposity index (weight of dissected fat depots per 10 cm of body length).
Differences between individual means were evaluated using Tukey Honestly Significant Difference post hoc tests or planned comparison tests. These tests used a two-tailed criterion for significance of P < .05. To assess the significance of preference or avoidance of the nutrients in two-bottle tests, the intake from one tube was compared to the intake from the second tube using paired t tests. This comparison was made for each nutrient concentration for each strain, so the total number of comparisons was 44. To avoid a potential inflation of significance levels due to multiple testing, we applied a Bonferroni correction, which set the critical level of statistical significance at P < .05/44 or .0011.
2.7. Experiments
2.7.1. Experiment 1: food intake
Six-month-old B6 (n = 11) and 129 (n = 12) male mice had free access to water and Teklad Rodent diet. Food intake was measured daily for 3 days. BW was measured on the last day of testing.
2.7.2. Experiment 2: starch and margarine intake
Four-month-old B6 (n = 12) and 129 (n = 12) male mice were offered six concentrations of cornstarch emulsions. After the last cornstarch emulsion, the mice received water in both drinking tubes for 2 days, and for the next 2 days, they received one tube containing 0.3% xanthan gum and another tube containing deionized water. Next, margarine intake was examined using the same mice over an 8-day period. On the first 4 days, margarine was given to mice, but no measurements of intake were made. On the next 4 days, margarine intake was measured daily. Chow intake was not measured in this experiment.
2.7.3. Experiment 3: Polycose intake
Four-month-old B6 (n = 12) and 129 (n = 12) male mice were offered 11 concentrations of Polycose solutions. Chow intake was not measured in this experiment.
2.7.4. Experiment 4: oil intake
Four-month-old B6 (n = 11) and 129 (n = 12) male mice were offered six concentrations of soybean oil. One to thirty percent oil emulsions was tested with 0.3% xanthan gum. Pure (100%) oil was tested with deionized water. After the last oil concentration was tested, the mice received water in both drinking tubes for 4 days, and for the next 2 days they were presented with one tube containing 0.3% xanthan gum and the other tube containing deionized water. Chow intake was not measured in this experiment.
2.7.5. Experiment 5: effect of margarine on BW gain and adiposity
In 2-month-old B6 (n = 19) and 129 (n = 20) male mice with ad libitum access to chow and water, BW and chow intake were measured daily for 4 days (Week 0). Based on these BW measurements, the mice from each strain were distributed into two groups with similar average BW and similar BW variation. For the next 10 weeks, one group (control) from each strain was maintained on water and chow, and the other group was given water, chow, and margarine. Chow and margarine intakes were measured daily on 3 days each week. BW was measured twice a week. Individual intake and BW measurements were averaged for each week and analyzed using ANOVA. For BW, all data before and during access to margarine (i.e., for Weeks 0–10) were analyzed together using ANOVA with strain, group and period factors, and with no covariates. The data for intakes were analyzed using BW before access to margarine (Week 0) as a covariate. We did separate analyses for (i) period before access to margarine (two-way ANOVA with strain and group factors), and (ii) period during access to margarine (three-way ANOVA with strain, group, and period factors).
At the end of the experiment, the mice were sacrificed, their gonadal (epididimal), retroperitoneal, mesenteric, inguinal, and subscapular adipose tissue deposits were dissected, and their fat content was measured. An adiposity index was calculated using total weight of all these depots. The dissected fat and carcasses were used to analyze body fat composition.
2.7.6. Experiment 6: adiposity in older male and female mice
B6 (nine males and nine females) and 129 (eight males and nine females) mice were maintained on Teklad Rodent diet from the time they were purchased at the age of 2 months until they were 7 to 9 months old, when they were sacrificed. Their retroperitoneal and gonadal adipose depots were excised. An adiposity index was calculated using total weight of these two depots. Although only retroperitoneal and gonadal depots were analyzed in this experiment, these measurements predict total adiposity, because weight of these depots highly correlates with percentage body fat (r = .922; Reed et al., unpublished results).
3. Results
In all groups, the B6 mice were heavier than the 129 mice. In all experiments where variable concentrations of solutions and emulsions were tested, the nutrient concentration significantly affected all indices measured (Table 1).
Table 1.
ANOVA results for two-bottle tests in Experiments 2, 3, and 4
| Nutrient | Effect of: | Solution or emulsion consumption
|
Energy consumption
|
|||||
|---|---|---|---|---|---|---|---|---|
| df |
F values
|
dfa
|
F values | |||||
| Intake per mouseb | Intake per 30 g BWc | Preferencec | Intake per mouseb | Intake per 30 g BWc | ||||
| Starch | Strain | 1,21 | 2.8 | 0.0 | 2.6 | 1,21 | 2.4 | 0.0 |
| Concentration | 5,110 | 21.1*** | 19.1*** | 55.1*** | 5,110 | 90.4*** | 76.8*** | |
| Strain × Concentration | 5,110 | 0.9 | 0.6 | 1.6 | 5,110 | 0.5 | 0.2 | |
| Polycose | Strain | 1,21 | 4.9 * | 0.0 | 17.5*** | 1,21 | 4.8 * | 0.8 |
| Concentration | 10,220 | 248.8*** | 222.4*** | 114.7*** | 4,88 | 910.2*** | 759.5*** | |
| Strain × Concentration | 10,220 | 2.5** | 0.6 | 10.7*** | 4,88 | 3.5 * | 2.6 * | |
| Oil | Strain | 1,20 | 5.8 * | 41.1*** | 20.5*** | 1,20 | 0.0 | 3.7 |
| Concentration | 4,84 | 39.5*** | 39.4*** | 63.9*** | 3,63 | 211.3*** | 250.7*** | |
| Strain × Concentration | 4,84 | 12.2*** | 10.0*** | 13.7*** | 3,63 | 4.3** | 8.1*** | |
ANOVAs for energy intakes were conducted using 0.03–6% starch, 3–30% Polycose, and 3–100% oil (see explanation in Materials and methods).
ANOVA with initial BW as a covariate.
ANOVA with no covariates.
P < .05.
P < .01.
P < .001.
3.1. Experiment 1: food intake
The B6 and 129 mice did not differ in intake of chow and energy derived from chow. The chow (Teklad diet pellets) intakes were respectively 3.2 ± 0.2 and 2.8 ± 0.05 g/mouse (ns, ANOVA with BW as a covariate), or 3.0 ± 0.1 and 2.9 ± 0.04 g/30 g BW (ns, t tests). The intakes of energy derived from chow were respectively 12.5 ± 0.6 and 11.2 ± 0.2 kcal/mouse, or 11.7 ± 0.4 and 11.3 ± 0.1 kcal/30 g BW (ns). The B6 mice were heavier than the 129 mice (32.1 ± 0.4 and 29.5 ± 0.5 g, respectively; P= .0013, t test).
3.2. Experiment 2: starch and margarine intake
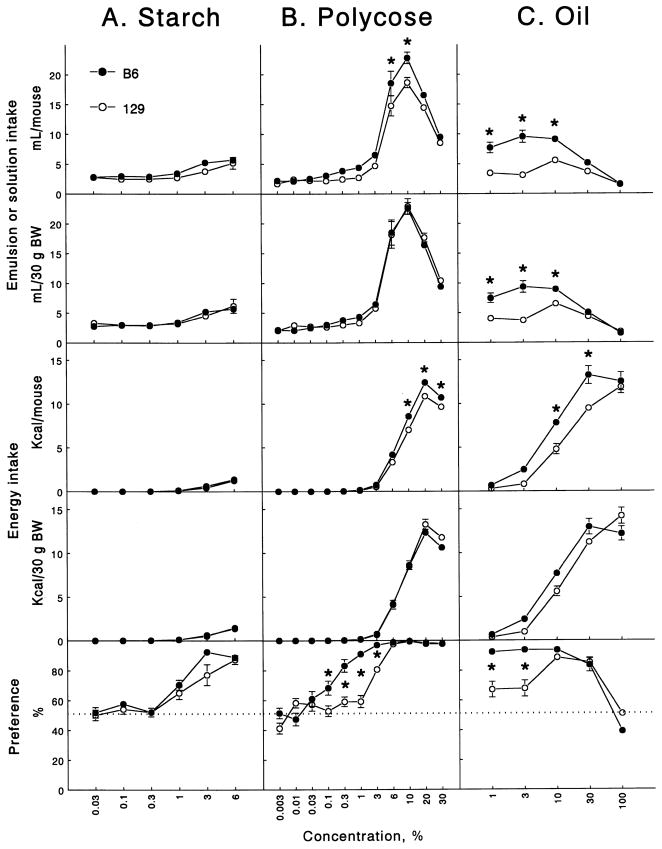
The B6 and 129 mice did not differ in consumption of starch, energy derived from starch, and starch preferences (Fig. 1A, Table 1). The B6 mice were indifferent to 0.03–0.3% starch and preferred 1–6% starch (i.e., consumed significantly more starch emulsion than 0.3% xanthan gum, P < .0011, paired t tests; Fig. 1A, bottom). The 129 mice were indifferent to 0.03–1% starch and preferred 3–6% starch. Thus, starch preference thresholds were lower in the B6 mice than in the 129 mice.
Fig. 1.
Average daily intakes of nutrient emulsions or solutions (the top two rows), energy derived from the nutrients (the third and fourth rows), and nutrient preferences (the bottom row) by B6 and 129 mice in two-bottle 48-h preference tests. (A) Cornstarch emulsions (Experiment 2); (B) Polycose solutions (Experiment 3); (C) Soybean oil emulsions (Experiment 4). Vertical bars represent S.E.M. * Significant difference between B6 and 129 mice; P < .05, post hoc tests, ANOVA with initial BW as a covariate (intakes per mouse), or with no covariates (intakes per BW and preferences).
In a separate 48-h test conducted 2 days after the end of testing cornstarch, the mice were presented with one tube containing 0.3% xanthan gum and the other tube containing water. The B6 mice drank daily 3.1 ± 0.4 ml xanthan gum and 1.7 ± 0.3 ml water (ns, paired t test). The 129 mice drank 2.1 ± 0.3 ml xanthan gum and 2.1 ± 0.3 ml water (ns). Preference scores for xanthan gum over water were 64 ± 7% in the B6 and 50 ± 7% in the 129 strain (ns, t test).
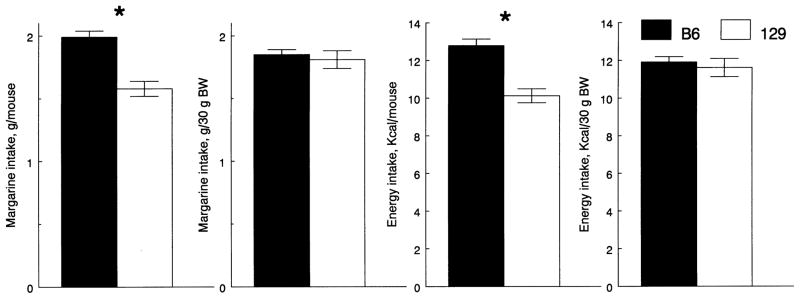
The B6 mice ate more margarine and energy derived from margarine per mouse compared with the 129 mice, but the two strains did not differ when intakes were expressed per BW (Fig. 2).
Fig. 2.
Average daily intakes of margarine and energy derived from margarine by B6 and 129 mice in Experiment 2. Vertical bars represent S.E.M. * Significant difference between B6 and 129 mice; P < .05, ANOVA with initial BW as a covariate (intakes per mouse), or t tests (intakes per BW).
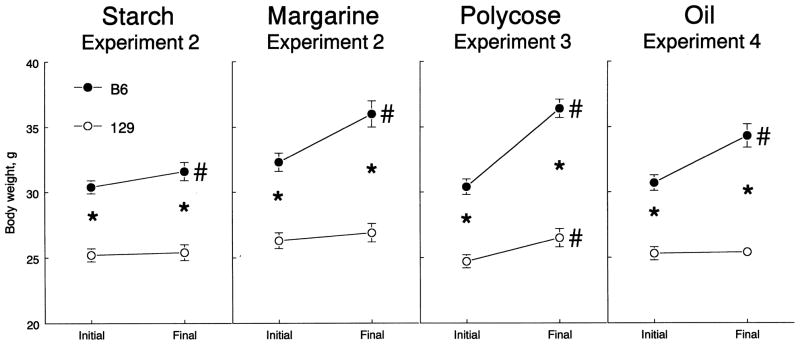
During access to starch and margarine, the BW of the B6 mice increased, whereas it did not change in the 129 strain (significant Strain × Period interaction; Table 2, tFig. 3). Although BW gain in percent (compared using tests) was greater in the B6 mice, the strains did not differ significantly when BW gain was expressed in grams and analyzed with initial BW as a covariate (Table 2).
Table 2.
BW changes of B6 and 129 mice during access to nutrients in Experiments 2, 3, and 4 (Mean ± S.E.M.)
| Nutrient | Test duration (days) | BW increasea
|
df |
F valuesb for effects of:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| grams (g)c
|
%d
|
||||||||
| B6 | 129 | B6 | 129 | Strain | Periode | Strain × Periode | |||
| Starch | 12 | 1.2 ± 0.3 | 0.2 ± 0.2 | 3.9 ± 1.0 | 0.8 ± 0.9 * | 1,22 | 53.0*** | 14.1** | 6.8 * |
| Margarine | 8 | 3.7 ± 0.4 | 0.6 ± 0.5 | 11.2 ± 1.1 | 2.3 ± 1.8*** | 1,22 | 49.2*** | 47.6*** | 25.4*** |
| Polycose | 22 | 6.0 ± 0.3 | 1.8 ± 0.3*** | 19.9 ± 0.8 | 7.4 ± 1.0*** | 1,22 | 80.4*** | 432.5*** | 124.0*** |
| Oil | 10 | 3.6 ± 0.4 | 0.03 ± 0.18*** | 11.6 ± 1.2 | 0.3 ± 0.7*** | 1,21 | 80.7*** | 73.0*** | 70.3*** |
Difference between BWs before and after testing a nutrient.
ANOVA with no covariates.
Effect of strain assessed using one-way ANOVA with initial BW as a covariate.
Relative to initial BW. Strain differences were assessed using t tests.
Effect of period assessed difference between BW before and after tests with the nutrients.
P < .05.
P < .01.
P < .001.
Fig. 3.
BW changes of B6 and 129 mice during access to nutrients in Experiments 2, 3, and 4. Vertical bars represent S.E.M. Initial and final BWs were measured before and after tests with a nutrient, respectively. Significant differences are indicated by * (B6 vs. 129 strains) or # (initial vs. final BW); P < .05; post hoc tests, ANOVA with no covariates (Table 2).
3.3. Experiment 3: Polycose intake
The B6 mice had higher absolute intakes of Polycose solutions and energy derived from Polycose, but the two strains did not differ when Polycose and energy intakes were expressed per BW (Fig. 1B; Table 1). The B6 mice preferred 0.3–30% Polycose to water, whereas the 129 mice preferred 3–30% Polycose to water. Thus, Polycose preference thresholds were lower in the B6 mice than in the 129 mice. The B6 mice had higher preference scores than did the 129 mice at 0.1–3% Polycose concentrations.
Compared with the 129 mice, the B6 mice had a greater BW gain during access to Polycose (significant Strain × Period interaction; Table 2, Fig. 3).
3.4. Experiment 4: oil intake
Compared with the 129 mice, the B6 mice drank more 1–10% oil emulsions expressed per mouse or per BW (Fig. 1C, Table 1). Compared with the 129 mice, they also consumed more calories from 10% and 30% oil, when energy intake was expressed per mouse. Energy intake expressed per BW was significantly affected by Strain × oncentration interaction, but in post hoc tests no significant strain differences for individual oil concentrations were found. The B6 mice preferred 1–30% oil; the 129 mice preferred 10% and 30% oil. Thus, oil preference thresholds were lower in the B6 mice than in the 129 mice. The B6 mice had significantly higher preference scores than did the 129 mice at 1% and 3% oil concentrations.
During access to soybean oil, the BW of the B6 mice increased, but it did not change significantly in the 129 strain (significant Strain × Period interaction; Table 2, Fig. 3).
In a separate 48-h test conducted 4 days after the end of testing the soybean oil, the mice were presented with one tube containing 0.3% xanthan gum and the other tube containing water. The B6 mice drank daily 2.4 ± 0.5 ml xanthan gum and 2.0 ± 0.4 ml water (ns). The 129 mice drank 1.9 ± 0.3 ml xanthan gum and 1.4 ± 0.2 ml water (ns). Preference scores for xanthan gum over water were 53 ± 8% in the B6 and 55 ± 5% in the 129 strain (ns). These results are similar to the results of testing xanthan gum in Experiment 2.
3.5. Experiment 5: effect of margarine on BW gain and adiposity
In Experiments 2–4, access to nutrients caused a greater weight gain in the B6 than in the 129 mice. But in these tests, we did not measure intake of chow, and thus, could not estimate total energy intake. Experiment 5 was designed to assess whether the greater weight gain in the B6 mice is accompanied by hyperphagia, and whether it is due to body fat accumulation.
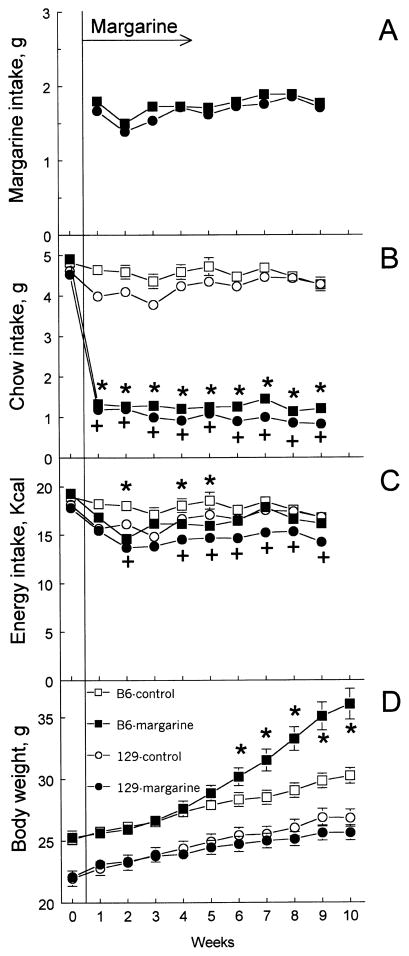
Before access to margarine (during the first 4 days of the experiment), there were no differences between the B6 and 129 strains in chow and energy intakes (Fig. 4B,C). When the mice were given margarine in addition to chow, they consumed more margarine (in grams) than they consumed chow (Fig. 4A,B). Although margarine consumption changed during the experiment [effect of period, F(8,144) = 23.6, P < .001], there were neither significant strain differences nor a Strain × Period interaction. Margarine-exposed mice had much lower chow intake compared with chow intakes during the initial period and by the control mice [effect of group, F(1,34) = 1303.7, P < .001]. Although there were some changes in chow intake during the period of the experiment [effects of period F(8,280) = 7.3, P < .001; Group × Period interaction F(8,280) = 6.2, P < .001; Strain × Group × Period interaction F(8,280) = 5.4, P < .001], they were minor relative to the difference between control and margarine-exposed groups. Intake of energy from chow (in the control group) or chow and margarine (in the margarine-exposed group) did not differ significantly between the B6 and 129 strains, but margarine-exposed mice from both strains consumed less energy than did the control mice [effect of group F(1,34) = 18.2, P < .001]. Although there were some changes in energy intake during the period of the experiment [effects of period F(8,280) = 12.6, P < .001; Strain × Period interaction F(8,280) = 2.5, P < .05; Group × Period interaction F(8,280) = 4.8, P < .001; Strain × Group × Period interaction F(8,280) = 3.2, P < .01], it remained more-or-less constant.
Fig. 4.
Average daily intakes of margarine (A), chow (B), and energy derived from all food sources (C), and BW (D) of B6 and 129 mice in Experiment 5. Vertical bars represent S.E.M. On Week 0, all mice had only chow to eat. On Weeks 1–10, margarine groups had margarine in addition to chow. Significant differences between control and margarine-exposed mice are indicated by * (B6 mice) or + (129 mice); P < .05; post hoc tests, ANOVA with BW before access to margarine (Week 0) as a covariate (A–C) or with no covariates (D).
Throughout the experiment, the B6 mice were heavier than the 129 mice [effect of strain F(1,35) = 50.3, P < .001], and all mice gained weight [effect of period F(10,350) = 257.9, P < .001; Fig. 4D]. BW differences between control and margarine-exposed mice were expressed differently depending on strain and period of experiment [interactions Strain × Period, F(10,350) = 34.1, P < .001; Group × Period, F(10,350) = 12.3, P < .001; Strain × Group × Period, F(10,350) = 29.1, P < .001]. The control and margarine-exposed 129 mice had similar BWs throughout the experiment. BWs of the control and margarine-exposed B6 mice did not differ during the first 5 weeks of access to margarine, but starting from the sixth week, the margarine-exposed B6 mice began gaining more weight than did the control B6 mice. By the end of the experiment (after 10 weeks of access to margarine), the margarine-exposed B6 mice were 20% heavier than the control B6 mice (Table 3).
Table 3.
BW, length, and adiposity of B6 and 129 mice maintained on chow (control) or chow and margarine (margarine) in Experiment 5 (Mean ± S.E.M.)
| Index | Units | B6
|
129
|
ANOVA, F(1,35), effect of:
|
||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 9) | Margarine (n = 10) | Control (n = 10) | Margarine (n = 10) | Strain | Group | Strain × Group | ||
| Lengtha,b | cm | 8.4 ± 0.1 | 8.7 ± 0.1 | 8.3 ± 0.2 | 8.3 ± 0.1 | 6.5 * | 1.6 | 2.1 |
| BWb | g | 30.2 ± 0.7 | 36.3 ± 1.3# | 26.9 ± 0.8 | 25.3 ± 0.5† | 65.1*** | 6.4 * | 18.4*** |
| Retroperitoneal fatc | g | 0.20 ± 0.03 | 0.57 ± 0.06# | 0.12 ± 0.02 | 0.15 ± 0.02† | 35.1*** | 27.8*** | 18.1*** |
| Gonadal fatc | g | 0.59 ± 0.06 | 2.05 ± 0.21# | 0.43 ± 0.05 | 0.74 ± 0.07† | 25.3*** | 48.6*** | 18.7*** |
| Mesenteric fatc | g | 0.43 ± 0.03 | 0.80 ± 0.07# | 0.41 ± 0.03 | 0.40 ± 0.03† | 13.4*** | 13.6*** | 14.9*** |
| Inguinal fatc | g | 0.40 ± 0.04 | 1.20 ± 0.15# | 0.35 ± 0.03 | 0.55 ± 0.04† | 12.7** | 33.4*** | 11.1** |
| Subscapular fatc | g | 0.28 ± 0.03 | 0.98 ± 0.12# | 0.19 ± 0.02 | 0.30 ± 0.04† | 24.0*** | 32.9*** | 16.5*** |
| Total fat dissectedc | g | 1.89 ± 0.16 | 5.60 ± 0.58# | 1.50 ± 0.13 | 2.14 ± 0.19† | 23.9*** | 39.1*** | 18.0*** |
| Adiposity indexb,d | g/10 cm | 2.24 ± 0.19 | 6.39 ± 0.65# | 1.81 ± 0.15 | 2.58 ± 0.21† | 33.0*** | 44.1*** | 20.8*** |
| Body fat contentb | % | 11.8 ± 1.2 | 26.1 ± 2.0# | 12.4 ± 1.3 | 16.1 ± 1.4† | 9.9** | 37.0*** | 13.0*** |
Post hoc tests were not conducted because there was no significant Strain × Group interaction.
ANOVA with no covariates.
ANOVA with body length as a covariate.
Total fat dissected/length × 10.
P < .05, ANOVA.
P < .01, ANOVA.
P < .001, ANOVA.
Significant difference between the control and margarine-exposed mice from the same strain, P < .05, post hoc tests.
Significant difference between the B6 and 129 mice from the same experimental group, P < .05, post hoc tests.
The B6 mice had longer bodies than did the 129 mice, but there were no significant differences in body length between the control and margarine-exposed mice (Table 3). BW, weight of fat depots, adiposity index, and body fat content had similar pattern of differences: The margarine-exposed B6 mice were heavier and fatter than the control B6 mice and both groups of the 129 mice. The last three groups did not differ significantly from each other (ns, post hoc tests), except for the B6 controls being heavier than the margarine exposed 129 mice (P < .01).
3.6. Experiment 6: adiposity in older male and female mice
In Experiment 5, the 2–4-month-old control B6 and 129 mice maintained on chow did not differ in adiposity. However, it is possible that obesity develops in the chow-maintained mice spontaneously in older age. The goal of Experiment 6 was to compare the spontaneous adiposity of older B6 and 129 mice, and to assess the role of gender.
Although there was a small variation in mouse ages, the groups did not differ significantly (Table 4). ANOVAs with age as a covariate (not shown) yielded similar results to ANOVA without using age as a covariate (Table 4). The B6 males had longer bodies than did the 129 males, but body length was similar in females from the two strains. Both males and females from the B6 strain were heavier than the 129 mice of the corresponding gender. Although overall, the B6 mice had more body fat than did the 129 mice (significant effects of strain on retroperitoneal and total fat, and adiposity index), planned comparison tests detected significant differences only between B6 and 129 males, but not females. However, the B6 females also tended to have more fat than did the 129 females. Males were longer, heavier, and fatter than females.
Table 4.
Age, BW, length, and adiposity of B6 and 129 mice in Experiment 6 (Mean ± S.E.M.)
| Index | Units | Males
|
Females
|
ANOVA: F values for effect of:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| B6 (n = 9) | 129 (n = 8) | B6 (n = 9) | 129 (n = 9) | df | Strain | Gender | Strain × Gender | ||
| Agea | months | 8.1 ± 0.3 | 8.2 ± 0.2 | 7.4 ± 0.3 | 8.2 ± 0.2 | 1,31 | 3.2 | 1.2 | 1.2 |
| Lengtha | cm | 9.3 ± 0.1 | 8.8 ± 0.1# | 8.4 ± 0.1 | 8.6 ± 0.1 | 1,31 | 5.7 * | 39.2*** | 16.2*** |
| BWa | g | 33.7 ± 0.6 | 27.8 ± 0.7# | 26.4 ± 0.5 | 22.8 ± 0.8# | 1,31 | 54.4*** | 89.2*** | 3.2 |
| Retroperitoneal fatb | g | 0.46 ± 0.05 | 0.23 ± 0.03# | 0.16 ± 0.02 | 0.08 ± 0.02 | 1,30 | 13.1** | 9.9** | 0.2 |
| Gonadal fatb | g | 1.21 ± 0.13 | 0.77 ± 0.07# | 0.50 ± 0.05 | 0.43 ± 0.09 | 1,30 | 3.0 | 4.4 * | 0.0 |
| Total fat dissectedb | g | 1.67 ± 0.18 | 1.00 ± 0.10# | 0.66 ± 0.06 | 0.51 ± 0.11 | 1,30 | 5.3 * | 5.8 * | 0.1 |
| Adiposity indexa,c | g/10 cm | 1.79 ± 0.19 | 1.13 ± 0.10# | 0.78 ± 0.07 | 0.59 ± 0.11 | 1,31 | 10.7** | 36.3*** | 3.2 |
ANOVA with no covariates.
ANOVA with body length as a covariate.
Total fat dissected/length × 10.
P < .05, ANOVA.
P < .01, ANOVA.
P < .001, ANOVA.
Significant difference between the B6 and 129 strains, P < .05, planned comparisons.
4. Discussion
Although consumption of diets with various macronutrient contents has been extensively studied in rodents, research on the chemosensory perception of fats and complex carbohydrates has involved only rats. To our knowledge, this is the first study of the acceptance of fats and complex carbohydrates by Mus musculus. We demonstrated that mice, like other rodents [6–10], avidly consume starch, Polycose, soybean oil, and margarine. We also have shown differences between the B6 and 129 mouse strains in acceptance of Polycose and soybean oil. In addition, these two stains differ in BW gain when they consume these nutrients.
The larger size and heavier weight of the B6 mice compared with the 129 mice likely contributed to strain differences in nutrient consumption. The B6 mice had higher absolute (expressed per mouse) intakes of Polycose, soybean oil, and margarine (only in Experiment 2, but not 5). However, these differences dissipated when intakes were calculated per BW, except for consumption of dilute oil emulsions. Preference scores do not depend on BW, and they were higher in the B6 than in the 129 mice for low concentrations of Polycose and soybean oil. Altogether, the strain differences in indexes of acceptance free from effects of body size were observed only for dilute Polycose (0.1–3%) and oil (1–10%), with almost no calories. More concentrated nutrients were more palatable (as indicated by higher preference scores) to mice and provided them with more energy, but no strain differences free from effects of BW were observed for these concentrations. This suggests that the strain differences in acceptance of Polycose and oil may be attributed to chemosensory perception of their flavor, rather than to appetite for their nutritive value. In addition, the acceptance of starch was similar in the B6 and 129 mice, suggesting that the strain differences in Polycose and oil consumption are specific rather than dependent on generalized mechanisms like neophobia or exploration of novel foods. Similar chow intakes by the B6 and 129 mice (Experiments 1 and 5) also suggest that the strains do not differ in general feeding motivation.
The higher acceptance of the dilute Polycose solutions by the B6 mice may be explained by higher sweet taste sensitivity of this strain [3,11–15]. Polycose contains approximately 9% glucose and maltose, sugars that have a sucrose-like taste to mice [16,17]. In addition, the two strains differed in acceptance of Polycose, but not starch, a non-sweet carbohydrate with similar nutritive value. However, studies using rats suggest that chemosensory perception of Polycose is complex and involves a mechanism distinct from those transducing tastes of sweeteners or starch [18–21]. Thus, the strain differences in Polycose consumption could also be due to variation in this Polycose-specific chemosensation.
The strain difference in acceptance of soybean oil may be due to either sensory [10,22,23] or postingestive [24,25] factors. Fat perception may involve the sensation of fatty acid taste [22,23] or flavors of fat impurities [10], and both might have mediated strain differences in oil acceptance. Postingestive factors, such as the ease and speed of oil digestion might also differ by strain. Several obesity-prone mouse and rat strains have elevated voluntary consumption of dietary fat compared with obesity-resistant animals [26–29]. Mutations of the leptin receptor gene [29] and possibly other genes that lead to obesity may have a pleiotropic effect on the acceptance of dietary fat. Thus, the greater acceptance of oil by the B6 mice might be related to their genetic predisposition to become obese.
Our previous studies have shown that B6 and 129 mice differ in consumption of sucrose and ethanol [3,11]. Both these compounds have caloric value for animals. Thus, higher intakes of sucrose and ethanol by the B6 mice could possibly be due to a greater appetite for calories in this strain compared with the 129 strain. In the previous studies, sucrose and ethanol consumption was much higher in the B6 strain compared with the 129 strain, regardless of whether it was expressed per mouse or per BW. On the contrary, in this study, the two strains did not differ in energy intake expressed per BW. This suggests that the strain differences in sucrose and ethanol intakes are not caused by the appetite for calories.
Although the B6 and 129 mice did not show large differences in consumption of energy from nutrients tested in Experiments 1–4, during access to these nutrients, the B6 mice gained more weight than did the 129 mice. Experiment 5 was designed to assess whether this higher BW gain by the B6 mice is explained by hyperphagia, and whether it is due to body fat accumulation. In this experiment, access to margarine did not cause hyperphagia (increased total energy intake) in either mouse strain. However, it resulted in greater BW gain and adiposity in the B6 mice compared to control B6 mice on chow. On the contrary, margarine-exposed and control 129 mice did not differ in BW gain and adiposity. In addition, in Experiment 6 with older mice fed chow, the B6 mice were fatter than the 129 mice despite similar food intakes (measured in Experiments 1 and 5). In previous studies using rats, access to concentrated sources of carbohydrates or fats promoted hyperphagia and stimulated weight gain [8,9,30–32]. However, under some conditions, access to high-fat diets stimulated body fat accumulation in the absence of hyperphagia [26,33]. This suggests that dietary fat may have genotype-dependent metabolic effects that induce obesity [34,35], which seems to be also the case with the B6 mice. This is consistent with results of other studies showing that the C57BL/6J mouse strain is predisposed to obesity [26,34,36–39], whereas the 129/Sv strain is lean [40].
In Experiment 5, the B6 mice started gaining more weight after 5 weeks of access to margarine, whereas in Experiment 2, the effect of margarine in the B6 mice was noticed after only 8 days of margarine consumption. Age could account for this difference between the two experiments. In Experiment 5, the mice were 2 months old when they first had access to margarine, and the B6 mice did not gain excess weight compared to controls until they became older than 3 months. In Experiment 2, the mice were 4.5 months old when they first had access to margarine. Although we did not have control (given only chow) groups in Experiment 2, the gain of 11% BW by the B6 mice in 1 week is much higher than the usual growth rate of mice on a regular diet and is most likely due to accumulation of body fat. These data suggest that predisposition of the B6 mice to diet-induced obesity develops only after the age of 3 months.
Further evidence for the role of age was obtained in Experiment 6, in which ~8-month-old B6 mice maintained on chow were fatter than the 129 mice. In contrast, ~ 4-month-old control B6 and 129 mice on chow had similar fatness in Experiment 5. This suggests that fat accumulation in B6 mice is facilitated by access to purified sources of energy, and it develops more slowly (or at a later stage) on the regular diet. Experiment 6 also demonstrated that males of both strains are fatter than females, and that the strain differences in adiposity are expressed more strongly in males than in females.
In summary, the B6 and 129 mice appear to differ in chemosensory perception of Polycose solutions and oil emulsions, but they have no or only minor differences in energy consumption. The addition of a carbohydrate or fat source to the standard diet resulted in large weight gain in the B6 mice compared with little or no gain in the 129 mice, despite similar energy intakes. These results confirm that B6 mice are susceptible to dietary obesity, whereas 129 mice are resistant. The data also suggest that the B6 mice develop a propensity to become fat only after they become 3 months old, and that this propensity increases with age. We conclude that the B6 and 129 strains provide a model to characterize genes involved in dietary obesity under conditions of free dietary choice.
Acknowledgments
This work was supported by the following funding sources: NIH-DC00882 (GKB), NIH-R03DC03509 (DRR), NIH-RO1DK36339 (MGT), NIH-R01DK44073 and NIH-R01DK48095 (RAP). We appreciate the advice of Dr. Israel Ramirez concerning the preparation and testing of the nutrient emulsions. We thank Jessica Santo and Maria Theodorides for technical assistance in conducting the experiments.
References
- 1.Reed DR, Bachmanov AA, Beauchamp GK, Tordoff MG, Price RA. Heritable variation in food preferences and their contribution to obesity. Behav Genet. 1997;27:373–87. doi: 10.1023/a:1025692031673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez I. Diet texture, moisture and starch type in dietary obesity. Physiol Behav. 1987;41:149–54. doi: 10.1016/0031-9384(87)90145-4. [DOI] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol: Clin Exp Res. 1996;20:201–6. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord. 1995;19:644–52. [PubMed] [Google Scholar]
- 5.Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc. 1993;156:379–92. [Google Scholar]
- 6.Ackroff K, Vigorito M, Sclafani A. Fat appetite in rats: the response of infant and adult rats to nutritive and non-nutritive oil emulsions. Appetite. 1990;15:171–88. doi: 10.1016/0195-6663(90)90018-4. [DOI] [PubMed] [Google Scholar]
- 7.Feigin MB, Sclafani A, Sunday SR. Species differences in polysac-charide and sugar taste preferences. Neurosci Biobehav Rev. 1987;11:231–40. doi: 10.1016/s0149-7634(87)80031-3. [DOI] [PubMed] [Google Scholar]
- 8.Sclafani A, Vigorito M, Pfeiffer CL. Starch-induced overeating and overweight in rats: influence of starch type and form. Physiol Behav. 1988;42:409–15. doi: 10.1016/0031-9384(88)90169-2. [DOI] [PubMed] [Google Scholar]
- 9.Sclafani A, Xenakis S. Sucrose and polysaccharide induced obesity in the rat. Physiol Behav. 1984;32:169–74. doi: 10.1016/0031-9384(84)90125-2. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez I. Chemoreception for fat: do rats sense triglycerides directly? Appetite. 1992;18:193–206. doi: 10.1016/0195-6663(92)90197-e. [DOI] [PubMed] [Google Scholar]
- 11.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996;26:563–73. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, Beauchamp GK. Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mamm Genome. 1997;8:545–8. doi: 10.1007/s003359900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capeless CG, Whitney G. The genetic basis of preference for sweet substances among inbred strains of mice: preference ratio phenotypes and the alleles of the Sac and dpa loci. Chem Senses. 1995;20:291–8. doi: 10.1093/chemse/20.3.291. [DOI] [PubMed] [Google Scholar]
- 14.Lush IE. The genetics of tasting in mice: VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res. 1989;53:95–9. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- 15.Lush IE, Hornigold N, King P, Stoye JP. The genetics of tasting in mice: VII. Glycine revisited, and the chromosomal location of Sac and Soa. Genet Res. 1995;66:167–74. doi: 10.1017/s0016672300034510. [DOI] [PubMed] [Google Scholar]
- 16.Ninomiya Y, Higashi T, Katsukawa H, Mizukoshi T, Funakoshi M. Qualitative discrimination of gustatory stimuli in three different strains of mice. Brain Res. 1984;322:83–92. doi: 10.1016/0006-8993(84)91183-1. [DOI] [PubMed] [Google Scholar]
- 17.Ninomiya Y, Mizukoshi T, Higashi T, Katsukawa H, Funakoshi M. Gustatory neural responses in three different strains of mice. Brain Res. 1984;302:305–14. doi: 10.1016/0006-8993(84)90244-0. [DOI] [PubMed] [Google Scholar]
- 18.Sclafani A. Carbohydrate taste, appetite and obesity: an overview. Neurosci Biobehav Rev. 1987;11:131–53. [PubMed] [Google Scholar]
- 19.Sclafani A. Starch and sugar tastes in rodents: an update. Brain Res Bull. 1991;27:383–6. doi: 10.1016/0361-9230(91)90129-8. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez I. Does starch taste like Polycose? Physiol Behav. 1991;50:389–92. doi: 10.1016/0031-9384(91)90083-z. [DOI] [PubMed] [Google Scholar]
- 21.Sako N, Shimura T, Komure M, Mochizuki R, Matsuo R, Yamamoto T. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiol Behav. 1994;56:741–5. doi: 10.1016/0031-9384(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 22.Gilbertson TA. Gustatory mechanisms for the detection of fat. Curr Opin Neurobiol. 1998;8:447–52. doi: 10.1016/s0959-4388(98)80030-5. [DOI] [PubMed] [Google Scholar]
- 23.Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–10. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- 24.Elizalde G, Sclafani A. Fat appetite in rats: flavor preferences conditioned by nutritive and non-nutritive oil emulsions. Appetite. 1990;15:189–97. doi: 10.1016/0195-6663(90)90019-5. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez I, Tordoff MG, Friedman MI. Satiety from fat? Adverse effects of intestinal infusion of sodium oleate. Am J Physiol. 1997;273:R1779–85. doi: 10.1152/ajpregu.1997.273.5.R1779. [DOI] [PubMed] [Google Scholar]
- 26.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–32. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 27.Smith BK, Andrews PK, West DB. Macronutrient diet selection in thirteen mouse strains. Am J Physiol: Regul, Integr Comp Physiol. 2000;278:R797–805. doi: 10.1152/ajpregu.2000.278.4.R797. [DOI] [PubMed] [Google Scholar]
- 28.Smith BK, West DB, York DA. Carbohydrate vs. fat preference: evidence for differing patterns of macronutrient selection in two inbred mouse strains. Obes Res. 1995;3(Suppl 3):411s. doi: 10.1152/ajpregu.1997.272.1.R357. [DOI] [PubMed] [Google Scholar]
- 29.Castonguay TW, Burdick SL, Guzman MA, Collier GH, Stern JS. Self-selection and the obese Zucker rat: the effect of dietary fat dilution. Physiol Behav. 1984;33:119–26. doi: 10.1016/0031-9384(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 30.Sclafani A. Carbohydrate-induced hyperphagia and obesity in the rat: effects of saccharide type, form, and taste. Neurosci Biobehav Rev. 1987;11:155–62. doi: 10.1016/s0149-7634(87)80020-9. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez I, Friedman MI. Dietary hyperphagia in rats: role of fat, carbohydrate, and energy content. Physiol Behav. 1990;47:1157–63. doi: 10.1016/0031-9384(90)90367-d. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez I, Tordoff MG, Friedman MI. Dietary obesity and hyperphagia: what causes them? Physiol Behav. 1989;45:163–8. doi: 10.1016/0031-9384(89)90180-7. [DOI] [PubMed] [Google Scholar]
- 33.West DB, York B. Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr. 1998;67(Suppl 3):505S–12S. doi: 10.1093/ajcn/67.3.505S. [DOI] [PubMed] [Google Scholar]
- 34.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Serive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–51. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 35.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague–Dawley rats. Am J Physiol. 1997;273:R725–30. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 36.York B, Lei K, West DB. Sensitivity to dietary obesity linked to a locus on chromosome 15 in a CAST/Ei × C57BL/6J F2 intercross. Mamm Genome. 1996;7:677–81. doi: 10.1007/s003359900204. [DOI] [PubMed] [Google Scholar]
- 37.Mehrabian M, Wen PZ, Fisler J, Davis RC, Lusis AJ. Genetic loci controlling body fat, lipoprotein metabolism, and insulin levels in a multifactorial mouse model. J Clin Invest. 1998;101:2485–96. doi: 10.1172/JCI1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surwit RS, Wang S, Petro AE, Sanchis D, Raimbault S, Ricquier D, Collins S. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc Natl Acad Sci USA. 1998;95:4061–5. doi: 10.1073/pnas.95.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsunoda N, Ikemoto S, Takahashi M, Maruyama K, Watanabe H, Goto N, Ezaki O. High-monounsaturated fat diet-induced obesity and diabetes in C57BL/6J mice. Metabolism. 1998;47:724–30. doi: 10.1016/s0026-0495(98)90037-3. [DOI] [PubMed] [Google Scholar]
- 40.Taylor BA, Phillips SJ. Detection of obesity QTLs on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics. 1996;34:389–98. doi: 10.1006/geno.1996.0302. [DOI] [PubMed] [Google Scholar]