Abstract
A detailed analysis of the 5' end of the rudimentary gene of Drosophila melanogaster is presented. Rudimentary transcripts are heterogeneous at their 5' ends indicating that transcription is initiated at multiple sites within a region of approximately 50 bp. These transcription initiation sites are within a region that is preferentially susceptible to nuclease cleavage in isolated nuclei. Additional nuclease hypersensitive regions were found within the first exon and the first intron. Within these internal nuclease hypersensitive regions are the insertion sites for previously identified P element transposons which disrupt rudimentary expression. One of these P element insertions, located in the first intron, is removed from the rudimentary transcript with the splicing of this intron. Another P element insertion, within the first exon, is removed from the rudimentary transcript by novel first intron splicing involving a cryptic splice donor site, located 5' to the insertion, and either the normal acceptor site or a cryptic splice acceptor site within the second exon.
Full text
PDF





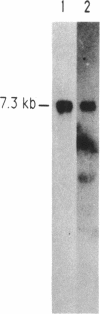
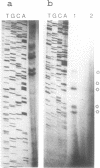
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronow B., Lattier D., Silbiger R., Dusing M., Hutton J., Jones G., Stock J., McNeish J., Potter S., Witte D. Evidence for a complex regulatory array in the first intron of the human adenosine deaminase gene. Genes Dev. 1989 Sep;3(9):1384–1400. doi: 10.1101/gad.3.9.1384. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronmiller C., Schedl P., Cline T. W. Molecular characterization of daughterless, a Drosophila sex determination gene with multiple roles in development. Genes Dev. 1988 Dec;2(12A):1666–1676. doi: 10.1101/gad.2.12a.1666. [DOI] [PubMed] [Google Scholar]
- DeLotto R., Schedl P. Internal promoter elements of transfer RNA genes are preferentially exposed in chromatin. J Mol Biol. 1984 Nov 15;179(4):607–628. doi: 10.1016/0022-2836(84)90158-x. [DOI] [PubMed] [Google Scholar]
- Doerig R. E., Suter B., Gray M., Kubli E. Identification of an amber nonsense mutation in the rosy516 gene by germline transformation of an amber suppressor tRNA gene. EMBO J. 1988 Aug;7(8):2579–2584. doi: 10.1002/j.1460-2075.1988.tb03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988 Dec 25;263(36):19259–19262. [PubMed] [Google Scholar]
- Farnham P. J., Kollmar R. Characterization of the 5' end of the growth-regulated Syrian hamster CAD gene. Cell Growth Differ. 1990 Apr;1(4):179–189. [PubMed] [Google Scholar]
- Freund J. N., Jarry B. P. The rudimentary gene of Drosophila melanogaster encodes four enzymic functions. J Mol Biol. 1987 Jan 5;193(1):1–13. doi: 10.1016/0022-2836(87)90621-8. [DOI] [PubMed] [Google Scholar]
- Freund J. N., Zerges W., Schedl P., Jarry B. P., Vergis W. Molecular organization of the rudimentary gene of Drosophila melanogaster. J Mol Biol. 1986 May 5;189(1):25–36. doi: 10.1016/0022-2836(86)90378-5. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Corces V. G. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1987 Nov;1(9):996–1004. doi: 10.1101/gad.1.9.996. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Garrard W. T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Han S., Udvardy A., Schedl P. Transcriptionally active chromatin is sensitive to Neurospora crassa and S1 nucleases. J Mol Biol. 1984 Nov 5;179(3):469–496. doi: 10.1016/0022-2836(84)90076-7. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Jarry B. P. Genetical and cytological location of the structural parts coding for the first three steps of pyrimidine biosynthesis in Drosophila melanogaster. Mol Gen Genet. 1979 May 4;172(2):199–202. doi: 10.1007/BF00268283. [DOI] [PubMed] [Google Scholar]
- Jarry B., Falk D. Functional diversity within the rudimentary locus of Drosophila melanogaster. Mol Gen Genet. 1974;135(2):113–122. doi: 10.1007/BF00264779. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of Drosophila pre-mRNAs. Nucleic Acids Res. 1985 Jul 11;13(13):4971–4981. doi: 10.1093/nar/13.13.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley M. R., Kidd S., Berg R. L., Young M. W. Restriction of P-element insertions at the Notch locus of Drosophila melanogaster. Mol Cell Biol. 1987 Apr;7(4):1545–1548. doi: 10.1128/mcb.7.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen A., Weintraub H. An altered DNA conformation detected by S1 nuclease occurs at specific regions in active chick globin chromatin. Cell. 1982 Jun;29(2):609–622. doi: 10.1016/0092-8674(82)90177-5. [DOI] [PubMed] [Google Scholar]
- Louis C., Schedl P., Samal B., Worcel A. Chromatin structure of the 5S RNA genes of D. melanogaster. Cell. 1980 Nov;22(2 Pt 2):387–392. doi: 10.1016/0092-8674(80)90349-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby S. The biochemical genetics of rudimentary mutants of Drosophila melanogaster. I. Aspartate carbamoyltransferase levels in complementing and non-complementing strains. Hereditas. 1973;73(1):11–16. [PubMed] [Google Scholar]
- Rawls J. M., Freund J. N., Jarry B. P., Louis C., Segraves W. A., Schedl P. Organization of transcription units around the Drosophila melanogaster rudimentary locus and temporal pattern of expression. Mol Gen Genet. 1986 Mar;202(3):493–499. doi: 10.1007/BF00333283. [DOI] [PubMed] [Google Scholar]
- Rawls J. M., Fristrom J. W. A complex genetic locus that controls of the first three steps of pyrimidine biosynthesis in Drosophila. Nature. 1975 Jun 26;255(5511):738–740. doi: 10.1038/255738a0. [DOI] [PubMed] [Google Scholar]
- Roiha H., Rubin G. M., O'Hare K. P element insertions and rearrangements at the singed locus of Drosophila melanogaster. Genetics. 1988 May;119(1):75–83. doi: 10.1093/genetics/119.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K., Cline T. W., Schedl P. Functional changes associated with structural alterations induced by mobilization of a P element inserted in the Sex-lethal gene of Drosophila. Genetics. 1987 Oct;117(2):221–231. doi: 10.1093/genetics/117.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E., Evans T., Welsh J., Efstratiadis A. Conformation of promoter DNA: fine mapping of S1-hypersensitive sites. Cell. 1983 Dec;35(3 Pt 2):837–848. doi: 10.1016/0092-8674(83)90116-2. [DOI] [PubMed] [Google Scholar]
- Searles L. L., Greenleaf A. L., Kemp W. E., Voelker R. A. Sites of P element insertion and structures of P element deletions in the 5' region of Drosophila melanogaster RpII215. Mol Cell Biol. 1986 Oct;6(10):3312–3319. doi: 10.1128/mcb.6.10.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves W. A., Louis C., Tsubota S., Schedl P., Rawls J. M., Jarry B. P. The rudimentary locus of Drosophila melanogaster. J Mol Biol. 1984 May 5;175(1):1–17. doi: 10.1016/0022-2836(84)90441-8. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota S., Ashburner M., Schedl P. P-element-induced control mutations at the r gene of Drosophila melanogaster. Mol Cell Biol. 1985 Oct;5(10):2567–2574. doi: 10.1128/mcb.5.10.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota S., Schedl P. Hybrid dysgenesis-induced revertants of insertions at the 5' end of the rudimentary gene in Drosophila melanogaster: transposon-induced control mutations. Genetics. 1986 Sep;114(1):165–182. doi: 10.1093/genetics/114.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardy A., Schedl P. Chromatin organization of the 87A7 heat shock locus of Drosophila melanogaster. J Mol Biol. 1984 Feb 5;172(4):385–403. doi: 10.1016/s0022-2836(84)80013-3. [DOI] [PubMed] [Google Scholar]
- Voelker R. A., Graves J., Gibson W., Eisenberg M. Mobile element insertions causing mutations in the Drosophila suppressor of sable locus occur in DNase I hypersensitive subregions of 5'-transcribed nontranslated sequences. Genetics. 1990 Dec;126(4):1071–1082. doi: 10.1093/genetics/126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]