Abstract
Tolerance to the analgesic effects of opioids has been demonstrated in laboratory animals after repeated drug administration, yet this effect has been studied less frequently under controlled laboratory conditions in humans. This within-subject, double-blind, placebo-controlled study was designed to determine if tolerance developed to the analgesic, subjective, and physiological effects of the commonly prescribed opioid oxycodone when it was administered daily for 5 days. The effects of oxycodone’s (0, 5, and 20 mg/70 kg, p.o.) were compared, using a within-session cumulative dosing procedure, on the 1st and 5th days of the ‘daily’ dosing phase to assess for tolerance; active oxycodone was administered on the 2nd-4th days of the daily dosing phase. Changes in the effects of oxycodone were also compared when the medication was only administered on the 1st and 5th day of a 5-day ‘intermittent’ dosing phase; placebo medication was administered on the 2nd–4th days of the intermittent dosing phase. A 9-day ‘washout’ period occurred between phases when no medication was administered. Healthy volunteers (N=10) with no history of drug dependence or current drug use participated in this outpatient study. Analgesia was assessed using the Cold-Pressor Test (CPT), pain and drug effects were measured using a variety of questionnaires, and pupil diameter was monitored as an index of physiological effects. When administered daily, no differences were observed in oxycodone-induced analgesia between the 1st and 5th days, but tolerance did develop to some of the positive subjective effects of oxycodone. In contrast, oxycodone-induced analgesia and participant ratings of some positive subjective drug effects were greater on the 5th day compared to the 1st day of the intermittent dosing phase. No differences in the miotic effects of oxycodone between the 1st and 5th days of either dosing phase were detected. Though obtained under limited experimental conditions, these findings suggest that tolerance may not develop to the analgesic effects of therapeutic doses of oxycodone under short-term daily dosing conditions, even though some of its subjective effects may decrease. These data also suggest that intermittent administration may enhance the analgesic effects of oxycodone, while also increasing some of the drug’s positive subjective effects related to abuse liability.
Keywords: Analgesia, Tolerance, Opioid, Oxycodone, Abuse Liability, Subjective Effects, human
INTRODUCTION
Tolerance to the behavioral effects of mu-opioid agonists after repeated administration is an extensively documented, well-established phenomenon in a wide range of species (Bodner, 2007). Of clinical relevance is the tolerance observed to the analgesic effects of opioid agonists in mice, rats, and non-human primates using an array of nociceptive stimuli. The magnitude of tolerance to the analgesic effects of opioids has been shown to be dependent upon the pharmacological efficacy of the opioid administered, its duration of action, dosing schedule (i.e., continuous infusion, daily interdose interval), route of administration, and nociceptive assay used to determine drug effect (Ferguson et al., 1969; Mucha et al., 1996; Fairbanks and Wilcox, 1997; Pawar et al., 2007; Sirohi et al., 2008; Kumar et al., 2008). Despite its clinical implications, relatively few controlled laboratory studies in humans have sought to determine the extent to which tolerance develops to the analgesic effects of opioids. Increasing doses required to maintain analgesia in patient populations suggests tolerance development, but disease progression may be the ultimate reason for increasing dosage needs, rather than tolerance (Jage, 2005).
Though tolerance to the analgesic effects of opioids has not been explored extensively in humans, several studies have investigated the relationship between long-term opioid administration and hyperalgesia, increased sensitivity to painful stimuli. Hyperalgesia has been reported to occur among patients requiring long-term opioid agonist maintenance therapy for opioid dependence (i.e., methadone administration; Compton et al., 2000, 2001) and chronic pain (King et al., 2005). Opioid-induced hyperalgesia has also been demonstrated in humans under well-controlled laboratory conditions with continuous infusions of the ultra-short-acting mu-opioid agonist, remifentanil (Lugenbuhl, et al., 2003; Troster et al., 2006; Singler et al., 2007). Given these reports, it is difficult to decipher whether the increasing dose requirement of analgesics needed to suppress pain is 1) due to direct tolerance to the analgesic effects of opioids, 2) a result of increased pain due to disease progression, as observed among cancer patients, or 3) driven by opioid-induced hyperalgesia. Therefore, this study sought to determine under well-controlled conditions, and in the absence of disease progression, the extent to which direct tolerance to opioid-induced analgesia occurs as a result of repeated, daily administration of oxycodone, a popularly prescribed analgesic.
In laboratory animals, opioid-induced tolerance develops to many opioid-elicited behavioral effects. It is therefore conceivable that if repeated exposure to prescription opioids alters their analgesic effects in humans, other behavioral effects including those that are predictive of abuse potential would be affected similarly. With repeated dosing, tolerance develops to the discriminative stimulus effects of opioids in laboratory animals (Colpaert et al., 1976; Young et al., 1991a,b; Galici et al., 2005), which are thought to be related to subjective drugs effects in humans (Schuster and Johanson, 1988). In an effort to clarify the nature by which repeated opioid exposure alters its subjective effects, specifically those that are predictive of a drug’s abuse potential, the current study assessed how daily oxycodone administration altered participant ratings of subjective drug effects in addition to its analgesic effects.
In summary, defining the effects of daily administration of opioids is a clinically relevant issue from both an abuse liability and a pain perspective. The objective of the present human laboratory study was to assess whether tolerance develops to the analgesic, subjective, and physiological effects of the frequently prescribed analgesic, oxycodone, when administered daily and when administered intermittently to healthy men and women.
METHODS
Participants
Normal, healthy volunteers aged 21–55 years were recruited through local newspaper advertisements. Those who met inclusion/exclusion criteria after an initial telephone screen were invited to the laboratory for further screening. Prior to enrollment, participants gave written informed consent, received a psychiatric and medical evaluation, and provided detailed drug use and medical histories. Participants were accepted into the study if they were healthy, as determined by a physical examination (including electrocardiogram, and urine and blood chemistries). They had to have taken opioids at least twice previously for medical purposes with no serious side effects, and to have had no history of recreational opioid use. Those who reported chronic pain, used over-the-counter analgesics more than four times a month, consumed more than 500 mg of caffeine daily, or met Diagnostic and Statistical Manual (of Mental Disorders), fourth edition, revised criteria for substance abuse or dependence, or had current Axis I psychopathology were excluded from the study. Women were excluded if they were pregnant, nursing, dysmenorrheic, or experienced moderate to severe premenstrual symptoms. Participants were admitted into the study only after written informed consent to participate was given and eligibility criteria were verified. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute and were in accord with the Declaration of Helsinki.
Design and Procedures
The study consisted of two 5-day phases (Monday-Friday) during which the frequency of oxycodone administration varied according to the phase (daily versus intermittent dosing; Table 1), and required 14-outpatient visits over the course of 4 weeks at the New York State Psychiatric Institute. On the 1st and 5th days of each phase, participants received an oral solution of 0, 5, and 15 mg/70 kg of oxycodone (cumulative doses of 0, 5, and 20 mg/70 kg, p.o.) at 45 minute intervals. On the intermediate days (2nd, 3rd, and 4th days), participants received capsules of either oxycodone (daily dosing; 15 mg BID, p.o.), or placebo (intermittent dosing; 0 mg BID, p.o.); the morning dose was administered in the laboratory, and the evening dose was dispensed to the participant in bottles with child-safety caps and ingested at home after 5 PM. Participants were instructed to abstain from taking any other medications or alcohol, and not to drive after taking the capsule. Oxycodone was not administered during a 10-day ‘washout’ period between phases (a total of 9 no-dose days). Two laboratory visits were scheduled during this period and during the week following the completion of the second phase to maintain contact with participants throughout the study. Daily and intermittent dosing phases were counterbalanced across participants so that half of the participants (n = 5) completed the daily dosing phase first and half of the participants (n = 5) completed the intermittent dosing phase first. Before study onset, participants completed 1–2 training sessions during which they were familiarized with the CPT, computerized tasks, and study procedures. Study drugs were not administered during these sessions.
Table 1.
Representative Study Schedule
| MON | TUES | WED | THURS | FRI | |
|---|---|---|---|---|---|
| DOSING PHASE | Intermittent | Intermittent | Intermittent | Intermittent | Intermittent |
| PHASE DAY | 1 | 2 | 3 | 4 | 5 |
| OXY DOSE | 0, 5, 20 mg/70 kg | 0 mg BID | 0 mg BID | 0 mg BID | 0, 5, 20 mg/70 kg |
| DOSING PHASE | Daily | Daily | Daily | Daily | Daily |
| PHASE DAY | 1 | 2 | 3 | 4 | 5 |
| OXY DOSE | 0, 5, 20 mg/70 kg | 15 mg BID | 15 mg BID | 15 mg BID | 0, 5, 20 mg/70 kg |
Laboratory Visits and Sessions
Participants were instructed to arrive at the laboratory at 09.00h, Monday-Friday of both phases. Breath alcohol levels were assessed, possible use of illicit drugs was determined by a urine drug toxicology screen, and urine pregnancy tests were completed for female participants. On cumulative dosing days, a standardized breakfast was provided prior to the session, and physiological monitoring (blood pressure, heart rate, oxygen saturation, measurement of pupil diameter) began prior to drug administration, and continued throughout the session (Table 2). Baseline pain responsivity and subjective effects were assessed before and at specified time points after dosing. Time production assessment, and cognitive and psychomotor performance measures were also completed throughout the sessions. Pain responsivity, subjective and physiological effects, time production assessment, and cognitive and psychomotor performance measures were also completed before and after capsule administration on the intermediate days (2nd, 3rd, and 4th days of each phase), to investigate the development of tolerance during these days. These data did not reveal consistent trends of altered analgesic, behavioral, or physiological effects of oxycodone or placebo as a function of dosing condition and will not be discussed further.
Table 2.
Session Schedule
| TIME (min) | EVENT |
|---|---|
| −90 | Urine drug toxicology, breathalyzer, light breakfast |
| −60 | Physiological monitoring begins (oxygen saturation, blood pressure) |
| −55 | Baseline assessments (Subjective effects, tasks, pupils, CPT) |
| 0 | Dose administration #1 (Placebo) |
| 15 | Subjective effects, pupils |
| 30 | Subjective effects, tasks, pupils, CPT |
| 45 | Dose administration #2 (5 mg oxycodone) |
| 60 | Subjective effects, pupils |
| 75 | Subjective effects, tasks, pupils, CPT |
| 90 | Dose administration #3 (15 mg oxycodone [cumulative dose of 20 mg]) |
| 105 | Subjective effects, pupils |
| 120 | Subjective effects, tasks, pupils, CPT |
| 150 | Subjective effects, tasks, pupils, CPT |
No oxycodone-induced effects on performance were observed during the cumulative dosing sessions, and this will not be discussed further. Oxycodone-induced effects on time production will be reported in a separate publication. At the end of the session, participants were free to leave the laboratory once sobriety was determined using field sobriety and balancing tasks (Evans et al., 1990). In the case of persistent intoxication, participants were driven home by a car service; this occurred on 7 out of the 70 (10 participants X 7 active medication days) sessions.
Analgesic Effects
The cold pressor test (CPT) was used to assess analgesic responses. The cold pressor apparatus consisted of two water coolers, fitted with metal frameworks. One cooler was filled with warm water (37oC) and the other was filled with cold water (4oC). An aquarium pump constantly circulated the water in each cooler. The coolers were equipped with a wire cradle upon which the participant was instructed to rest his/her hand during the test. Participants were instructed to remove all jewelry and to spread the fingers of the hand during the test.
The CPT began with an immersion of the hand into the warm-water bath for three minutes. During this time, blood pressure and heart rate were measured. Immediately after removal of the hand from the warm water, skin temperature of the thumbpad was recorded and the experimenter read a standardized script to the participant describing the procedures of the test. Immediately after measurement of skin temperature following warm water immersion, participants immersed the hand into the cold-water bath. Participants were instructed to report the first painful sensation after immersion. They were asked to tolerate the stimulus as long as possible, but were permitted to withdraw their hand from the cold water if the stimulus was too uncomfortable. Maximum immersion time was three minutes, although participants were not informed of this limit. Latency to first feel pain and latency to withdraw the hand from the water were recorded by the experimenter. During the test, blood pressure and heart rate were measured using the arm that was not immersed in the water bath. Upon withdrawal, skin temperature of the thumbpad was recorded and participants completed the short form of the McGill Pain Questionnaire (see below), which included questions about the sensations experienced during the cold-water immersion. The CPT was completed before and 30 minutes after each dose administration, and 60 minutes after the final dose. During the intermediate days of each phase, the CPT was completed before and 30 and 60 minutes after the morning dose was administered.
Subjective Effects
McGill Pain Questionnaire (MPQ)
A 15-item shortened form of the McGill Pain Questionnaire (Melzack, 1987) was used to assess the sensory and affective dimensions of the pain experience immediately following the CPT. Participants described their experience of pain by choosing among a series of possible answers (None [score=1], Mild [score=2], Moderate [score=3], or Severe [score=4]). They were asked to describe the pain as “Throbbing,” “Shooting,” “Stabbing,” “Sharp,” “Cramping,” “Gnawing,” “Hot-Burning,” “Aching,” “Heavy,” “Tender,” “Splitting,” “Tired-Exhausting,” “Sickening,” “Fearful,” and “Punishing-Cruel.” Scores were added across all 15 items to generate a sum score, which ranged between 15 and 60. This questionnaire was completed immediately after participants withdrew their hand from the cold water.
Drug Effects Questionnaire (DEQ)
The DEQ consisted of 6 questions presented one at a time after oxycodone administration. The participants were asked to rate the positive and negative subjective effects produced by the drug and drug quality by selecting a phrase that most accurately reflected their feelings at that moment. The following questions were included: “How strong a drug effect do you feel?” “Do you feel any good effects from the drug?” “Do you feel any bad effects from the drug?” “Rate the degree to which you would be willing to take today’s drug again.” The participant was asked to indicate on a scale of 0–4, 0 corresponding to “No effects at all” and 4 corresponding to “Very strong (good, bad, etc.) effects.” Participants also were asked whether they liked the drug and were instructed to choose from among several possible answers with -4 corresponding to “Dislike very much” to 4 corresponding to “Like very much.” And finally, they were asked whether the drug was most like placebo, a sedative, or a stimulant.
Visual Analog Scales (VAS)
To further determine the mood and physical subjective effects produced by the drug, participants completed visual analog scales consisting of 18 items describing various mood and physiological states (“I feel…” “Able to concentrate,” “Alert,” “Anxious,” “Bad effect,” “Calm,” “Confused,” “Depressed,” “Focused,” “Good effect,” “High,” “Hungry,” “Irritable,” “Sedated,” “Self-confident,” “Social,” “Stimulated,” “Talkative,” and “Tired”). Participants were instructed to indicate on a 100-mm line, anchored on the left with “Not at all” and on the right with “Extremely,” how they were feeling at that moment. Items were presented to participants before and approximately 15 and 30 minutes after each oxycodone dose, and 60 minutes after the final dose of the session.
Physiological Effects
Pupil diameter was measured before drug administration, 15 and 30 minutes after each dose, and 60 minutes after the final oxycodone dose of the session. A pulse oximeter was used to monitor respiratory function. A soft sensor was placed on a finger, which continuously monitored oxygen saturation (%SpO2.) If %SpO2 decreased below 93%, participants were prompted verbally by staff to take a deep breath. Blood pressure was measured every 15 minutes by a blood pressure cuff attached to the participant’s right arm. Blood pressure and %SpO2 were evaluated for safety only and not recorded for data analysis purposes.
Drugs
Oxycodone HCl [Oxyfast® Immediate-Release Oral Concentrate Solution (20 mg/ml), Purdue Pharma] was prepared at doses of 0, 5 and 15 mg per 70 kg. On the 1st and 5th days of each phase, the solution was mixed in orange-flavored Gatorade with 1 ml peppermint oil floated on top to mask the taste of the drug. A total volume of 200 ml was administered at each dosing, and was consumed within 5 min. We have used a similar procedure in other protocols to successfully mask the flavor of the beverage (Comer et al., 2010). On the 2nd, 3rd, and 4th days of each phase, oxycodone capsules (placebo or 15 mg) were administered in size 00 opaque capsules with lactose filler prepared by the New York State Psychiatric Institute Research Pharmacy. The oxycodone doses tested were based upon the recommended dose range of orally administered oxycodone for treating pain at the time the study was designed (5–30 mg every 6 h; Physicians’ Desk Reference, 2001).
Data Analysis
Repeated-measures analyses of variance (ANOVA) with planned comparisons were conducted to determine if daily or intermittent opioid exposure produced tolerance to the analgesic, subjective, and miotic effects of oxycodone, as assessed on the 1st and 5th days of each phase. For each endpoint, a total of 12 pairwise comparisons were performed. For each session, the effects of oxycodone (cumulative doses of 5 and 20 mg/70 kg) were compared to placebo (1 comparison X 4 sessions). Within each phase, the effects of oxycodone (cumulative doses of 5 and 20 mg/70 kg) were compared between the 1st and 2nd sessions (1 comparison X 2 phases); placebo data were also compared between these two sessions (1 comparison X 2 phases). The effects of oxycodone (cumulative doses of 5 and 20 mg/70 kg) were compared between phases by assessing differences between data on the first session of the two phases, and differences between the second sessions of both phases (1 comparison X 2 sessions); placebo effects were also compared between phases for both sessions (1 comparison X 2 sessions). Dependent variables included latency to report pain during the CPT, latency to withdraw the hand from the cold water, participant ratings of pain as assessed by the MPQ, participant ratings of subjective drug effects as assessed by the DEQ and VAS, and pupil diameter. For dependent measures with multiple observations under each dose condition (i.e., subjective effects), peak values were used for analysis. To assess miotic effects, trough values of pupil diameter were used for analysis. Results were considered statistically significant when p values were equal to or less than 0.05 using Huynh-Feldt corrections.
RESULTS
Participants
Fifteen volunteers enrolled in the study and 10 participants completed it (7 men; 3 women; 8 White; 2 Hispanic). Of the 5 volunteers who discontinued study participation, 2 dropped out due to medication side effects (excessive sedation, nausea, vomiting, intense headache), 1 was discontinued due to elevated blood pressure during the CPT, and 2 were unable to be contacted. The average age of the completers was 31 years (range: 23–47). Of the completers, eight participants reported previous marijuana use with maximum lifetime use ranging from 3 times ever smoked to once per week; last marijuana use for these participants ranged from 6 weeks to 25 years prior to screening. Two participants reported past cocaine use, with use ranging from twice ever used to once per week; last cocaine use ranged from 4 to 25 years prior to the study screening process. One participant reported past recreational use of Ecstasy and Ritalin®: both drugs were used once, with Ecstasy used 8 years prior to study screening and Ritalin® used 2 months before study screening. Two participants reported daily tobacco use (8–10 cigarettes per day). Participants did not have a history of drug or alcohol dependence and had not used drugs (with the exception of cigarettes) for at least one month prior to screening. Participants did not test positive for illicit drug use during the course of the study.
Analgesic Effects
CPT: Latency to report pain and withdraw hand from water
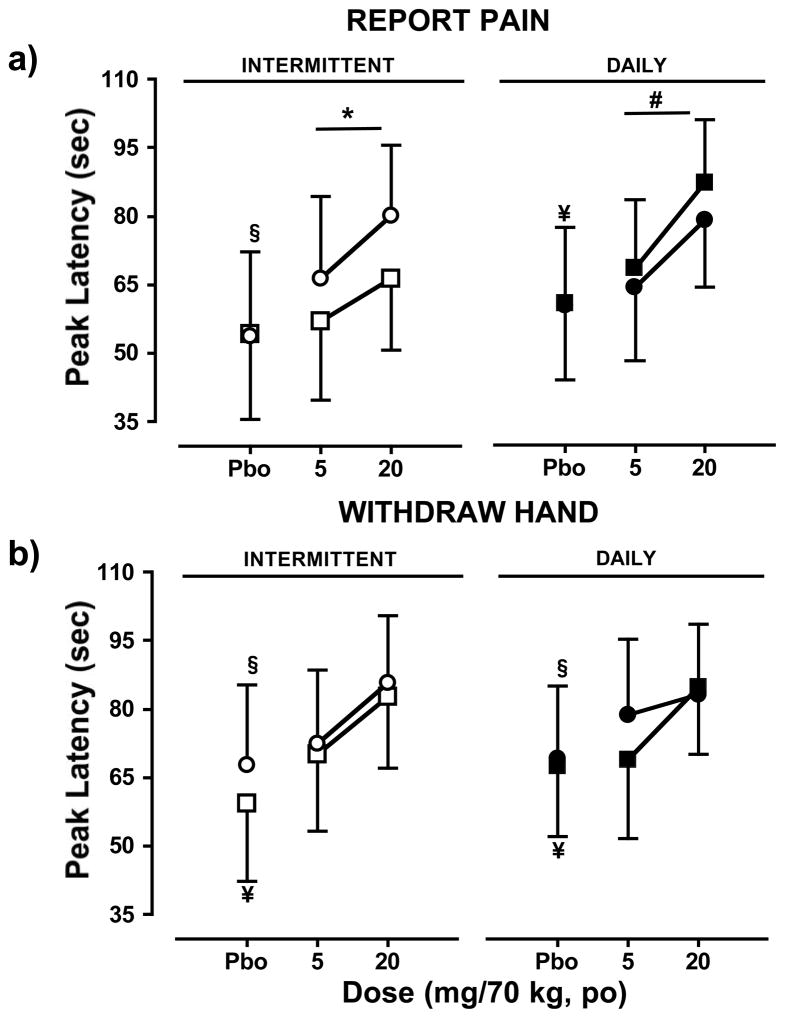
Figure 1 shows the latency to first report pain (top panels), and the latency to withdraw the hand from the cold water (bottom panels) during the first and second sessions for each phase (intermittent dosing phase, left panels; daily dosing phase, right panels). During the intermittent phase, oxycodone increased the latency to report pain relative to placebo during the second sessions (p ≤ 0.01), but not the first. Latency to report pain was significantly greater after oxycodone administration during the second session of the intermittent phase relative to the first (p ≤ 0.05). Relative to placebo, oxycodone increased the latency to withdraw the hand from the water during both the first (p ≤ 0.01) and second (p ≤ 0.05) sessions of the intermittent phase; oxycodone effects for this measure of analgesia did not differ between the two sessions. Placebo effects did not differ between the two sessions for either latency to report pain or withdraw the hand from the water.
Figure 1.
Peak values (mean ± SEM) of latency to (a) report pain during the CPT and (b) withdraw the hand from the cold water (mean ± SEM ) during the first session (intermittent phase, □; daily phase, ▮) and second session (intermittent phase, ○; and daily phase, ●) as a function of cumulative oxycodone dose. Significant differences between placebo and oxycodone (cumulative doses of 5 and 20 mg/70kg) during the first session are indicated by ¥ (p ≤ 0.05), and second session by § (p≤ 0.05). Within each phase, significant differences between sessions in placebo or oxycodone effects are indicated by * (p ≤ 0.05). Between phases, significant differences between corresponding sessions for placebo or oxycodone effects are indicated by # (p ≤ 0.05).
For the daily dosing phase, oxycodone increased the latency to report pain relative to placebo during the first session (p ≤ 0.01) but not the second; however, oxycodone increased the latency to withdraw the hand from the water during both sessions relative to placebo (p ≤ 0.05). Oxycodone and placebo effects did not differ between the two sessions for these measures.
A comparison of oxycodone and effects between phases revealed that oxycodone-induced increases in latency to report pain were higher during the first session of the daily dosing phase relative to the first session of the intermittent dosing phase (p ≤ 0.01). No other between-phase differences were observed.
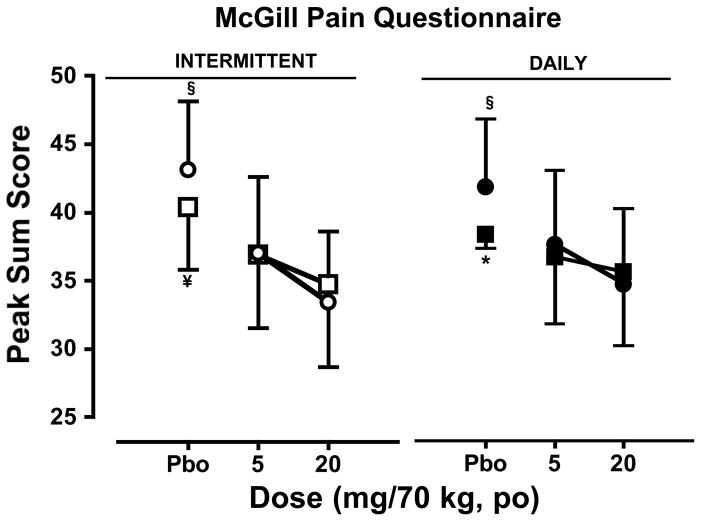
Subjective Pain Response: McGill Pain Questionnaire
The effects of oxycodone on subjective pain ratings as measured by the MPQ are shown on Figure 2. During the intermittent dosing phase (left panel), oxycodone decreased subjective ratings of pain compared to placebo during the first and second sessions (p ≤ 0.01); oxycodone and placebo effects on these ratings did not differ between the two sessions.
Figure 2.
Peak ratings (mean ± SEM) of subjective pain response as measured by the MPQ during the first session and second session as a function of cumulative oxycodone dose. See Figure 1 legend for details.
For the daily dosing phase (right panel), oxycodone decreased subjective ratings of pain compared to placebo during the second session (p ≤ 0.05), but not the first. Though the effects of oxycodone did not differ between the two sessions, the subjective pain ratings were significantly higher after placebo administration on the second session relative to the first (p ≤ 0.05)
A comparison of oxycodone and placebo effects between phases revealed no differences in oxycodone or placebo effects on subjective pain rating for either session.
Subjective Drug Effects
Drug Effects Questionnaire
Ratings of subjective drug effect as a function of oxycodone dose and session for each phase as measured by the DEQ are depicted in Table 3. During the intermittent dosing phase, oxycodone increased subjective effect ratings of ‘Strong’ and ‘Like’ for both sessions relative to placebo (p ≤ 0.01). Ratings for these subjective effects were higher during the second session relative to the first after oxycodone administration (p ≤ 0.05); no differences in ratings between the two sessions after placebo administration emerged. Oxycodone also increased the subjective effect ratings for ‘Take Again’ for the second session relative to placebo (p ≤ 0.01), but not the first. These ratings did not differ between sessions after oxycodone was administered; however, subjective effect ratings after placebo was administered were significantly lower during the second session relative to the first (p ≤ 0.05).
Table 3.
Peak participant ratings (mean ± SEM) of subjective drug effects as measured by the DEQ during the intermittent and daily dosing phases during the first and second session as a function of cumulative oxycodone dose. Participants rated their subjective drug liking by indicating on a scale from -4, corresponding to “Dislike very much,” to 4, corresponding to “Like very much.” Participants rated subjective drug strength and desire to take the dose again on a scale of 0–4, 0 corresponding to “No effects at all” and 4 corresponding to “Very strong effects.”
| Dose (mg/70 kg) | INTERMITTENT | DAILY | |||
|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 1 | Session 2 | ||
| LIKE | 0 | 0.1 (0.1)* | 0.5 (0.3)* | 0.6 (0.3)* | 0.1 (0.1)* |
| 5 | 0.8 (0.4)# | 1.4 (0.4)† | 1.1 (0.2) | 0.6 (0.2) | |
| 20 | 1.3 (0.4) | 2.1 (0.5) | 1.6 (0.4) | 1.3 (0.4) | |
| STRONG | 0 | 0.8 (0.4)* | 0.8 (0.3)* | 0.7 (0.2)* | 0.8 (0.3)* |
| 5 | 1.6 (0.8)#,† | 2.2 (0.3)† | 2.1 (0.3)# | 0.9 (0.3) | |
| 20 | 3.1 (0.2) | 3.3 (0.2) | 3.3 (0.2) | 2.8 (0.3) | |
| TAKE AGAIN | 0 | 1.9 (0.5)# | 1.3 (0.4)* | 1.7 (0.4) | 1.4 (0.3) |
| 5 | 1.9 (0.5) | 2.2 (0.5)† | 1.7 (0.4)# | 1.4 (0.3) | |
| 20 | 1.8 (0.5) | 2.1 (0.5) | 2.0 (0.4) | 1.3 (0.4) | |
Significant differences between placebo and oxycodone (cumulative doses of 5 and 20 mg/70kg) within a session are indicated by * (p ≤ 0.05). Within each phase, significant differences between sessions in placebo or oxycodone effects are indicated by # (p ≤ 0.05). Between phases, significant differences between corresponding sessions for placebo or oxycodone effects are indicated by † (p ≤ 0.05).
For the daily dosing phase, oxycodone increased the subjective effect ratings for ‘Strong’ and ‘Like’ for both sessions relative to placebo (p ≤ 0.01). Ratings for ‘Strong’ were higher during the second session relative to the first after oxycodone administration (p ≤ 0.001); no differences in ratings between the two sessions after placebo administration emerged for either Strong’ or ‘Like.’ No differences in ratings for ‘Take Again’ between placebo and oxycodone administration were detected for either session; however, these ratings were significantly lower during the second session relative to the first after oxycodone administration (p ≤ 0.05). No differences in ratings for the two sessions after placebo was administered were detected.
A comparison of subjective effect ratings for ‘Strong, ‘ Like,’ and ‘Take Again’ between phases demonstrated that subjective effect ratings were higher during the second session of the intermittent phase relative to the daily dosing phase after oxycodone administration (p ≤ 0.01), whereas subjective effects ratings for ‘Strong’ were higher during the first session of the daily dosing phase relative to the intermittent dosing phase after oxycdone administration (p ≤ 0.05). No differences in ratings were detected between phases for either session after placebo administration.
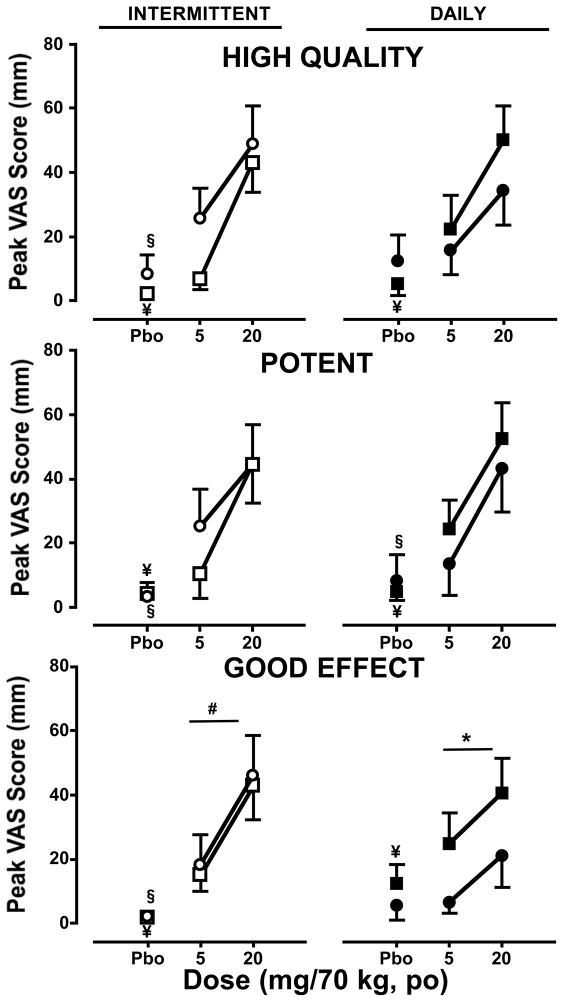
Visual Analog Scales
Subjective effect ratings as measured with the VAS are shown in Figure 3. During the intermittent dosing phase (left panels), oxycodone increased subjective effect ratings for ‘High Quality,’ ‘Potent,’ and ‘Good Effect’ for both sessions relative to placebo (p ≤ 0.01). Ratings for these subjective effects did not differ between the two sessions either for oxycodone or placebo.
Figure 3.
Peak participant ratings (mean ± SEM) of subjective drug effects as measured by the VAS during the intermittent (left panels) and daily (right panels) dosing phases during the first and second sessions as a function of cumulative oxycodone dose. See Figure 1 legend for details.
For both sessions of the daily dosing phase, oxycodone increased ratings of ‘High Quality,’ ‘Potent,’ and ‘Good Effect’ during the first session relative to placebo (p ≤ 0.05); however, during the second session oxycodone only increased the subjective ratings for ‘Potent’ relative to placebo (p ≤ 0.05). Ratings for ‘Good Effect’ after oxycodone administration were significantly lower for the second session relative to the first (p ≤ 0.05), however no differences in these ratings emerged between the two sessions after placebo administration.
Though no differences emerged in the effects of oxycodone on subjective ratings for ‘High Quality,’ ‘Potent, and Good Effect’ between phases for the first session, subjective ratings of ‘Good Effect’ after oxycodone administration were higher during the second session of the intermittent dosing phase relative to the daily dosing phase (p ≤ 0.05). No differences between phases for either session were detected after placebo administration.
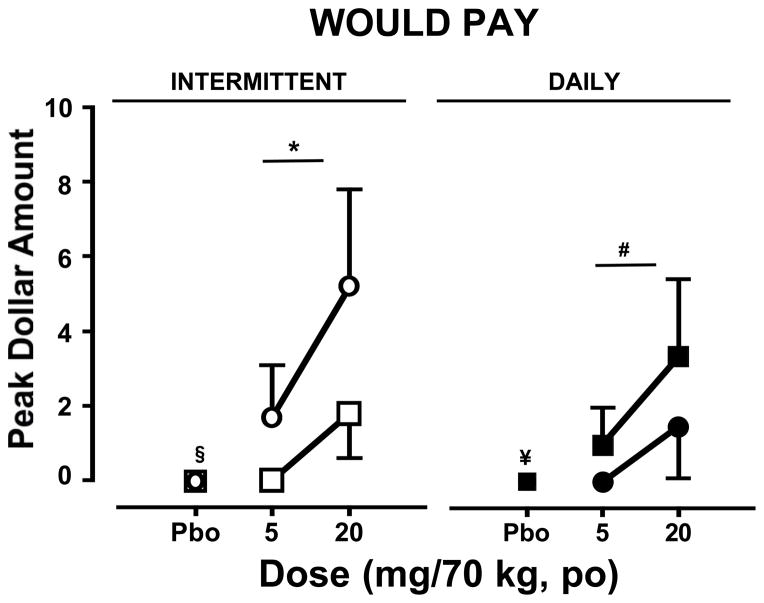
Figure 4 shows the subjective, hypothetical monetary value assigned by participants after placebo and oxycodone administration as determined by the VAS item prompting participants to indicate how much they would pay for the dose. During the intermittent dosing phase (left panel), oxycodone increased subjective ratings for ‘I Would Pay…’ relative to placebo only during the second session (p ≤ 0.01); these ratings were significantly higher during the second session relative to the first (p ≤ 0.01). No differences in placebo effects were detected between the two sessions of this phase.
Figure 4.
Peak participant ratings (mean ± SEM) of the subjective drug value of placebo and oxycodone doses as measured by the VAS during the intermittent (left panels) and daily (right panels) dosing phases during the first and second sessions as a function of cumulative oxycodone dose. See Figure 1 legend for details.
For both sessions of the daily dosing phase (right panel), oxycodone increased ratings of ‘I Would Pay….’ during the first session relative to placebo (p ≤ 0.05) but not the second. No differences in oxycodone or placebo effects on this VAS item were detected between the two sessions of this phase.
A comparison of the subjective effect ratings for ‘I Would Pay….’ between phases demonstrated that oxycodone increased these ratings significantly more during the during the second session of the intermittent phase relative to the second session of the daily dosing phase (p ≤ 0.01). No differences between phases for either session were detected after placebo administration.
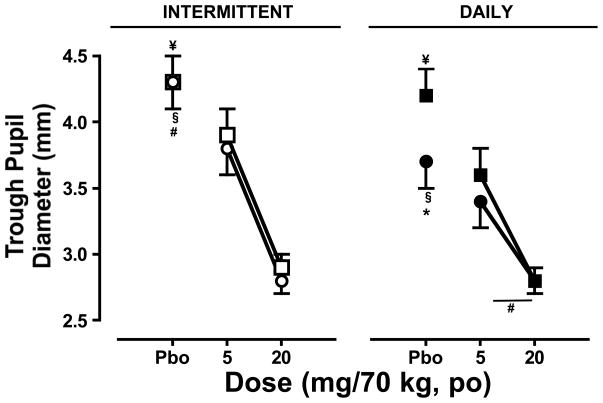
Miotic Effects of Oxycodone
As shown in Figure 5, oxycodone decreased pupil diameter in both sessions during the intermittent dosing phase (left panel; p ≤ 0.0001) and daily dosing phase (right panel; p ≤ 0.001) relative to placebo. The miotic effects of oxycodone did not differ between sessions for the intermittent phase, and pupil diameter after placebo administration did not differ between sessions. During the daily dosing phase, effect of oxycodone on pupil diameter did not differ between phases; however, pupil diameter after placebo administration was significantly smaller during the second session relative to the first (p ≤ 0.001).
Figure 5.
Trough pupil diameter (mean ± SEM) measured during the intermittent (left panel) and daily (right panel) dosing phases during the first and second sessions as a function of cumulative oxycodone dose. See Figure 1 legend for details.
A comparison of pupil diameter between phases demonstrated that though the miotic effects of oxycodone did not differ between the first sessions of both phases, the effects were greater during the second session of the daily dosing phase relative to the intermittent phase (p ≤ 0.05). Similarly, though pupil diameter after placebo administration did not differ between phases for the first session, it was significantly smaller during the second session of the daily dosing phase relative to the intermittent phase (p ≤ 0.001).
DISCUSSION
Under the current conditions, repeated, daily administration of oxycodone did not induce tolerance to the drug’s analgesic effects, but did seem to decrease participant ratings of some positive subjective drug effects. In contrast, the data suggest that intermittent oxycodone administration enhanced its analgesic effects in addition to some of its positive subjective drug effects. These findings suggest that the schedule and frequency of oxycodone administration can potentially impact both its analgesic and subjective effects.
The finding that daily administration did not decrease the analgesic effects of oxycodone was unexpected given the abundance of preclinical findings demonstrating profound induction of tolerance to mu-opioid agonists. However, despite the large body of preclinical literature pointing to this phenomenon, there are reports demonstrating that under a variety of paradigms, tolerance to opioid-induced analgesia is dependent upon a number of factors including dose, duration, and interdose interval of opioid administration, as well as genetic factors and the presence of pre-existing pain (e.g., Cox and Tiffany, 1997; Mas et al., 2000; Javan et al., 2005; Liang et al., 2006). Although tolerance to the analgesic effects of oxycodone was not observed under the given experimental parameters, one should be cautious in generalizing these present results to more extended opioid dosing schedules. Additionally, it is unknown if and how preexisting pain conditions may affect tolerance development to opioid-induced analgesia.
The increase in participant ratings of positive subjective effects of oxycodone observed under the intermittent dosing condition seems to parallel the preclinical literature demonstrating increases in some of the behavioral effects of opioids under similar dosing paradigms. For instance, repeated intermittent exposure to opioids enhanced the positive conditioned effects of morphine using the conditioned place preference model in rats (Lett, 1989; Gaiardi et al., 1991; Shippenberg et al., 1996; Simpson and Riley, 2005). The preclinical findings demonstrating enhanced behavioral effects of opioids as a result of intermittent administration are consistent with the increased positive subjective effects observed under the intermittent dosing phase in the current study. In addition to increases in participant ratings of the subjective effects of oxycodone under the intermittent dosing schedule, the analgesic effects also seemed to increase during this phase, as demonstrated by increased latency to report pain in the CPT. A comparison between the first sessions of the two phases for this measure revealed that latencies to report pain were unexpectedly higher during the daily dosing phase compared to the intermittent phase. Though the study was designed such that half of the participants were exposed to each dosing condition first and a consistent order effect did not emerge, the increased latency to report pain observed during the intermittent dosing phase raises the possibility that exposure to intermittent dosing during the first phase may have impacted the analgesic effects of oxycodone during the subsequent daily dosing phase.
Oxycodone decreased pupil diameter, a reliable physiological endpoint of opioid effects in humans. While this effect did not differ in magnitude between sessions for either phase, baseline pupil diameter was significantly smaller during the second session of the daily dosing phase compared to the first session of the phase and the second session of the intermittent dosing phase. The apparent miosis observed under placebo conditions on the 5th day of the daily dosing phase may be ascribed to the effects of the oxycodone dose that was administered the previous evening. Though the half-life of oral oxycodone has been reported to be about 3.51 ± 1.43 hours (Leow et al., 1992), its miotic effects have been previously shown to persist beyond what would be predicted by its half-life (Zacny and Gutierrez, 2003; Walsh et al., 2008; Stoops et al., 2010). Therefore, the miotic effects of oxycodone during the second session of the daily dosing phase may have been altered by physiologically active concentrations of oxycodone remaining from the previous evening’s dose. It is unlikely that behaviorally active concentrations of the previous evening’s dose affected the analgesic and subjective effects of oxycodone during the second session; no significant differences between baseline (placebo) measures for the other endpoints indicative of opioid effects were observed.
Acknowledgments
This research was supported by US National Institute on Drug Abuse Grant R01DA16759. The authors acknowledge and appreciate the assistance of Janet Murray, RN, Ronnie Shapiro, RN and Susanna Stephens, BS.
References
- Bodnar RJ. Endogenous opiates and behavior: 2007. Peptides. 2008;29:2292–2375. doi: 10.1016/j.peptides.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Kuyps JJ, Niemegeers CJ, Janssen PA. Discriminative stimulus properties of fentanyl and morphine: tolerance and dependence. Pharmacol Biochem Behav. 1976;5:401–408. doi: 10.1016/0091-3057(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J. Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend. 2010;109:130–138. doi: 10.1016/j.drugalcdep.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Kintaudi K, Ling W. Pain responses in methadone-maintained opioid abusers. J Pain Symptom Manage. 2000;20:237–245. doi: 10.1016/s0885-3924(00)00191-3. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST. Associative and nonassociative tolerance: the effects of dose and interdose interval. Pharmacol Biochem Behav. 1997;57:31–6. doi: 10.1016/s0091-3057(96)00125-6. [DOI] [PubMed] [Google Scholar]
- Evans SM, Funderburk FR, Griffiths RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther. 1990;255:1246–55. [PubMed] [Google Scholar]
- Fairbanks CA, Wilcox GL. Acute tolerance to spinally administered morphine compares mechanistically with chronically induced morphine tolerance. J Pharmacol Exp Ther. 1997;282:1408–1417. [PubMed] [Google Scholar]
- Ferguson RK, Adams WJ, Mitchell CL. Studies of tolerance development to morphine analgesia in rats tested on the hot plate. Eur J Pharmacol. 1969;8:83–92. doi: 10.1016/0014-2999(69)90132-0. [DOI] [PubMed] [Google Scholar]
- Gaiardi M, Bartoletti M, Bacchi A, Gubellini C, Costa M, Babbini M. Role of repeated exposure to morphine in determining its affective properties: place and taste conditioning studies in rats. Psychopharmacology. 1991;103:183–186. doi: 10.1007/BF02244201. [DOI] [PubMed] [Google Scholar]
- Galici R, McMahon LR, France CP. Cross-tolerance and mu agonist efficacy in pigeons treated with LAAM or buprenorphine. Pharmacol Biochem Behav. 2005;81:626–634. doi: 10.1016/j.pbb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Jage J. Opioid tolerance and dependence – do they matter? Eur J Pain. 2005;9:157–162. doi: 10.1016/j.ejpain.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Javan M, Ahmadiani A, Motamadi F, Kazemi B. Changes in G proteins genes expression in rat lumbar spinal cord support the inhibitory effect of chronic pain on the development of tolerance to morphine analgesia. Neurosci Res. 2005;53:250–256. doi: 10.1016/j.neures.2005.06.020. [DOI] [PubMed] [Google Scholar]
- King T, Ossipov MH, Vanderah TW, Porreca F, Lai J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals. 2005;14:194–205. doi: 10.1159/000087658. [DOI] [PubMed] [Google Scholar]
- Kumar P, Sunkaraneni S, Sirohi S, Dighe SV, Walker EA, Yoburn BC. Hydromorphone efficacy and treatment protocol impact on tolerance and mu-opioid receptor regulation. Eur J Pharmacol. 2008;597:39–45. doi: 10.1016/j.ejphar.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow KP, Smith MT, Williams B, Cramond T. Single-dose and steady-state pharmacokinetics and pharmacodynamics of oxycodone in patients with cancer. Clin Pharmacol Ther. 1992;52:487–95. doi: 10.1038/clpt.1992.176. [DOI] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine morphine and cocaine. Psychopharmacology. 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–40. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Luginbühl M, Gerber A, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Curatolo M. Modulation of remifentanil-induced analgesia hyperalgesia and tolerance by small-dose ketamine in humans. Anesth Analg. 2003;96:726–732. doi: 10.1213/01.ANE.0000048086.58161.18. [DOI] [PubMed] [Google Scholar]
- Mas M, Sabater E, Olaso MJ, Horga JF, Faura CC. Genetic variability in morphine sensitivity and tolerance between different strains of rats. Brain Res. 2000;866:109–15. doi: 10.1016/s0006-8993(00)02255-1. [DOI] [PubMed] [Google Scholar]
- Medical Economic Staff. PDR: Physicians’ Desk Reference, 2001. PDR Network. 55 2001. [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Kalant H, Birbaumer N. Loss of tolerance to morphine after a change in route of administration: control of within-session tolerance by interoceptive conditioned stimuli. Psychopharmacology. 1996;124:365–372. doi: 10.1007/BF02247442. [DOI] [PubMed] [Google Scholar]
- Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker EA, Yoburn BC. Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of mu-opioid receptors and dynamin-2. Eur J Pharmacol. 2007;563:92–101. doi: 10.1016/j.ejphar.2007.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Opioid discrimination in humans: discriminative and subjective effects of progressively lower training dose. Behav Pharmacol. 1998;9:533–543. doi: 10.1097/00008877-199811000-00009. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. In: Relationship between the discriminative stimulus properties and subjective effects of drugs in Transduction mechanism of drug stimuli. Colpeart FC, Balster RL, editors. Springer-Verlag; 1988. pp. 161–175. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C, Lefevour A. Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. Eur J Pharmacol. 1996;299:33–39. doi: 10.1016/0014-2999(95)00852-7. [DOI] [PubMed] [Google Scholar]
- Simpson GR, Riley AL. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacol Biochem Behav. 2005;80:471–479. doi: 10.1016/j.pbb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Singler B, Tröster A, Manering N, Schüttler J, Koppert W. Modulation of remifentanil-induced postinfusion hyperalgesia by propofol. Anesth Analg. 2007;104:1397–1403. doi: 10.1213/01.ane.0000261305.22324.f3. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Walker EA, Yoburn BC. The analgesic efficacy of fentanyl: relationship to tolerance and mu-opioid receptor regulation. Pharmacol Biochem Behav. 2008;91:115–120. doi: 10.1016/j.pbb.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Modulation of drug-induced sensitization processes by endogenous opioid systems. Behav Brain Res. 1995;70:37–49. doi: 10.1016/0166-4328(94)00176-g. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Hatton KW, Lofwall MR, Luzzo PA, Walsh SL. Intravenous oxycodone, hydrocodone, and morphine in recreational opioid users: abuse potential and relative potencies. Psychopharmacology. 2010;212:193–202. doi: 10.1007/s00213-010-1942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tröster A, Sittl R, Singler B, Schmelz M, Schüttler J, Koppert W. Modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by parecoxib in humans. Anesthesiology. 2006;105:1016–1023. doi: 10.1097/00000542-200611000-00024. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98:191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Steigerwald ES, Makhay MM, Kapitsopoulos G. Onset of tolerance to discriminative stimulus effects of morphine. Pharmacol Biochem Behav. 1991a;39:487–493. doi: 10.1016/0091-3057(91)90213-l. [DOI] [PubMed] [Google Scholar]
- Young AM, Kapitsopoulos G, Makhay MM. Tolerance to morphine-like stimulus effects of mu opioid agonists. J Pharmacol Exp Ther. 1991b;257:795–805. [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Characterizing the subjective psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology. 2003;170:242–54. doi: 10.1007/s00213-003-1540-9. [DOI] [PubMed] [Google Scholar]