Abstract
Background
The function of sPLA2 is site dependent. In tissue, sPLA2 regulates eicosanoid production; in the blood, sPLA2 primes neutrophils; and in the intestinal lumen sPLA2 provides innate bactericidal immunity as a defensin-related protein. Since parenteral nutrition (PN) with lack of enteral stimulation primes leukocytes while suppressing intra-luminal mucosal immunity, we hypothesized that 1) PN would diminish luminal sPLA2 activity, but increase sPLA2 activity in small intestinal (SI) tissue and serum and 2) stress would accentuate these changes.
Methods
Mice received Chow, Complex Enteral Diet (CED), intragastric PN (IG-PN), or PN in Experiment 1, and Chow, Chow + Stress, PN, and PN + Stress in Experiment 2. Tissue, intestinal luminal fluid, and portal and systemic serum were analyzed for sPLA2 activity. IgA was measured in luminal fluid as a marker of acquired mucosal immunity.
Results
Expt1
Luminal fluid sPLA2 activity was greatest in Chow and decreased in CED (p=0.0001), IG-PN (p=0.0002), and PN (p=0.0001) with PN lower than CED (p<0.002) or IG-PN (p<0.0001). Compared to Chow, serum sPLA2 activity dropped after CED (p = 0.042), IG-PN (p<0.0001), and PN (p=0.0004). Serum sPLA2 was higher in portal than systemic serum (p=0.04).
Expt2
PN lowered luminal fluid sPLA2 activity vs Chow (p<0.0001). Stress lowered luminal sPLA2 activity in Chow (p<0.0001), without a change with PN. Following stress, luminal IgA increased in Chow (p=0.0025) but not PN (p=0.18). Serum sPLA2 activity was unchanged after Chow but increased in PN (p<0.03).
Conclusions
Parenteral nutrition with lack of enteral stimulation attenuates sPLA2 activity in intestinal fluid consistent with a suppressed innate mucosal defense. Stress suppresses luminal fluid sPLA2 activity in Chow, but not the IgA response: PN impairs both. Stress significantly elevates serum sPLA2 in PN fed mice consistent with the known increased neutrophil priming with PN. PN reduces innate bactericidal immunity of the gut but up-regulates serum pro-inflammatory products after stress.
Keywords: secretory phospholipase A2, parenteral nutrition, surgical stress, small intestine, defensins, enteral feeding, innate immunity
INTRODUCTION
Parenteral Nutrition (PN) prevents progressive malnutrition in patients with contraindications to enteral feeding. However, PN is associated with increased infectious complications and inflammation compared to enterally fed patients.1–3 These risks may be partially attributable to increased host tissue inflammation and compromised mucosal immune mechanisms when the gut is not stimulated with enteral feeding.
Our laboratory previously demonstrated that route and type of nutrition affect numerous components of mucosal immunity particularly in the gut associated lymphoid tissue (GALT). As enteral stimulation increases from none (PN) to enteral administration of an elemental diet (intragastric PN:IG-PN) to enteral feeding of a complex enteral diet (CED) containing proteins, carbohydrates and fat to ad libitum feeding with chow, mucosal immune function increases with higher levels of intestinal and respiratory IgA; greater lymphocyte counts in Peyer’s patches, the intestinal lamina propria and lung tissue; increasing levels of IgA-stimulating cytokines in the gut; and an ability to respond to injury with increases in gut and airway IgA levels. Systemically, PN increases expression of ICAM-1 and p-selectin in the gut vasculature recruiting polymorphonuclear leukocytes (PMNs) while priming them for a subsequent insult.4,5 Subsequent injury then augments PMN activation and increases tissue damage compared to animal fed enterally.6 Within the GALT itself, PN impairs production and transport of Immunoglobulin-A (IgA),7–10 which functions as an antibacterial and anti-inflammatory molecule. Furthermore, PN also eliminates the ability of the gut mucosa to increase IgA levels in response to injury. While IgA represents an adaptive immune molecule at mucosal surfaces, Paneth cells within gut mucosa also produce innate immune molecules, the defensins and other bactericidal proteins. Secretory phospholipase A2 (sPLA2) is involved in both processes: it functions as a defensin-related protein when released into the gut lumen but activates PMNs when elevated systemically.
sPLA2 is a superfamily of lipolytic enzymes responsible for diverse regulatory functions in mammals and all sPLA2 isoforms depend on millimolar concentrations of calcium for catalytic activity. The catalytic function of sPLA2 hydrolyzes phospholipids from the sn-2 position of the glycerol backbone to release long-chain fatty acids from either bacterial or mammalian cell membranes, but with different outcomes. Paneth cells within the small intestine (SI) produce and secrete defensins, lysozyme P, and the sPLA2-IIA isoform to provide bactericidal activity.11 The cationic sPLA2 enzyme attacks the negatively charged bacterial cell membranes inducing membrane permeability and lysis.12–13 In vitro, sPLA2-IIA kills gram-positive and several gram-negative bacterial species14–17 and may preferentially attack membrane sites involved in cellular growth.18 sPLA2-IIA over-expression in transgenic mice reduced the risk of septic mortality during bacterial challenge compared to PLA2-deficient littermates.19
On the other hand, sPLA2 within host tissue and the vascular system provides a pro-inflammatory function. The outer envelopes of mammalian cellular membranes are largely comprised of uncharged phosphotidylcholine and cholesterol. With its net cationic charge, sPLA2 acts weakly on host tissue under normal circumstances. However, inflammatory conditions such as rheumatoid arthritis, 20, 21 acute pancreatitis,22 and critical illness,23,24 are characterized by rapid elevation in serum sPLA2. Under these conditions elevated sPLA2 correlates with increases in other serum and tissue inflammatory markers, such as TNF-α, IL-1, and IL-6.25–27 Increased sPLA2 releases free fatty acids which are metabolized to eicosanoids, e.g. prostaglandins, thromboxanes, and prostacyclins, which are powerful inflammatory mediators.14 Additionally, sPLA2 activates cell surface receptors on immune cells, such as PMNs, to prime them for augmented inflammatory responses. 28, 29
PN exerts detrimental effects on the adaptive mucosal immune system of the gut and gut inflammatory processes but little is known about its effect on innate immunity. Since sPLA2 is involved in both innate bactericidal activities of the gut lumen and host inflammation systemically through mediators and cell priming, we investigated alterations in sPLA2 activity within the small intestine tissue, lumen, and serum after PN. We compared and contrasted these results with the responses of the adaptive mucosal immune system using our established model of surgical stress. We hypothesized that PN and surgical injury would suppress the defensin-related protein sPLA2 in the intestinal lumen while elevating tissue and serum levels.
MATERIALS AND METHODS
Animals
The Animal Care and Use Committee of the University of Wisconsin-Madison and Middleton Veterans Administration Hospital, Madison approved all animal experimental protocols. Male Institute of Cancer Research (ICR) mice were purchased through Harlan (Indianapolis, IN) and housed in an American Association for Accreditation of Laboratory Animal Care-accredited conventional facility on the V.A Williamson Hospital Campus. Mice were acclimatized for one week in an environment controlled for temperature and humidity with a 12/12-hour light/dark cycle. Mice were housed 5 per covered/filtered box and fed ad libitum chow (LabDiet, PMI Nutrition International, St. Louis, MO) and water for 1 week prior to initiation of study protocol. After entry into study protocol mice were housed individually in metal cages with wire grid floors to prevent coprophagia and bedding ingestion.
Experimental Design
Experiment 1
Male ICR mice, ages 6 to 8 weeks, were randomized to Chow with an IV catheter (n = 11), PN (n = 11), intragastric (IG−) PN (n=11) via gastrostomy, or a complex enteral diet (CED, Nutren® 1.0, Nestle, Deerfield, IL) via gastrostomy (n=11). Animals were anesthetized by intraperitoneal injection of Ketamine (100 mg/kg) and Acepromazine (10 mg/kg). Animals with IV lines were cannulated in the vena cava via the right external jugular vein with a silicone rubber catheter (0.012″ I.D./0.25″ O.D.; Helix Medical, Inc., Carpinteria, CA). Gastric catheters were placed directly via gastrostomy. Catheters were tunneled subcutaneously, from either the right jugular vein or gastrostomy site, over the back and exited mid tail. Mice were partially immobilized by tail fixation to protect the catheter during infusion. This technique does not induce significant physical or biochemical stress as was previously shown.30
Catherized mice were connected to infusion pumps and allowed recovery for 48 hours while receiving 4 mL/day saline (0.9%) and ad libitum chow (Agway Inc., Syracuse, NY) and water. A 2 day recovery was chosen because serum cytokines and corticosteroid levels induced by the surgical stress return to baseline, the airway and gut IgA response is over, and animals in all groups have resumed oral intake in normal amounts. Following the recovery period experimental diets were given. Chow mice received 0.9% saline at 4 mL/day as well as ad libitum chow and water throughout the study. PN and IG-PN fed mice received solution at 4 mL/day (day 1), 7 mL/day (day 2) and 10 mL/day (days 3–5) as well as ad libitum water throughout the study. The PN solution includes 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins, with a non-protein calorie/nitrogen ratio of 126.1 (527.0 kJ/g Nitrogen). Since Nutren® contains 4.0% amino acids, 12.7% carbohydrates, 3.8% lipids, electrolytes, and multivitamins, with a non-protein calorie/nitrogen ratio of 130.4 (545.1 kJ/g Nitrogen), CED fed mice received the formula at 7 mL/day, 10 mL/day, and 14 mL/day (as well as ad libitum water) to provide a regimen which is isocalorical and isonitrogenous to the PN formula. Each formula meets the calculated nutrient requirements of mice weighing 25 to 30 g. 31
After 5 days of feeding (7 days post-catherterization), mice were anesthetized by intraperitoneal injection of Ketamine (100 mg/kg) and Acepromazine (10 mg/kg). Serum samples were taken via exsanguination from a left axillary artery transection. During tissue sacrifice, portal vein serum was taken directly from the vessel. The small intestine was removed and the lumen was washed with 20 ml Hanks Balanced Saline Solution (HBSS, Bio Whittaker, Walkersville, MD). The wash fluid was then centrifuged at 3,000 RPM for 10 minutes and 1 ml supernate was frozen at −80°C for analysis. Small intestinal tissue samples were taken by removing a 3 cm segment from the mid-distal and mid-proximal regions for ileum and jejunum samples, respectively. All tissues were frozen in liquid N2 and stored at −80°C until processing and analysis.
Experiment 2
Male ICR mice, ages 6 to 8 weeks were randomized to Chow with intravenous saline (n = 22) or IV-PN (n = 22), as described above. After 5 days of feeding (7 days post-catherization) animals were randomized to sacrifice or surgical stress (11/group). Surgical stress was delivered as follows. The skin was disinfected using 75% ethanol and 2 wounds were then created. First, a 3.0-cm celiotomy incision was made and the small intestine was gently eviscerated and immediately returned to the peritoneal cavity. The wound was closed in 2 layers with 3 simple interrupted 4-0 silk sutures per layer. Second, a 1.5-cm ventral neck incision was made and blunt dissection carried down to the pretracheal plane. This wound was closed with a single layer of 2 simple interrupted 4-0 silk sutures. Eight hours later mice were again anesthetized (up to half original dose until righting reflex was lost) and sacrificed by exsanguination via left axillary artery transection. This time point was chosen due to previous observations that the maximal increase in respiratory and gut IgA levels in response to surgical stress occurred at 8 hours. Tissues were harvested as previously mentioned with the exception of portal vein serum due to limited splanchnic blood flow which prevented consistent sampling.
sPLA2 Continuous Florescence Assay
Substrate Solution
Assay method followed previously published techniques by Tsao FHC et al 32, with minor modification of substrate preparation. Breifly, substrate was prepared by dissolving phosphotidylglycerol (Sigma, St. Louis, MO) in chloroform (2 mg/ml) and 1 ml aliquots were stored in glass vials with organic resistant cap at −20°C. 10 ul of Bis-BODipy FL C11-PN (Molecular Probes, Eugene, OR) fluorescently labeled probe was added to each 1 ml aliquot of dissolved phosphotidylglycerol. The chloroform was evaporated from the mixture under a stream of nitrogen gas until dry. Then, the chloroform free phospholipids were redissolved in 1 ml ethanol (100%) instead of Sucrose/Tris buffer (1ml; 0.25 mol/l sucrose, 50 mmol/l Tris-HCL, 0.02% sodium azide), modified to enhance assay sensitivity. The substrate solution was vortexed thoroughly and stable for up to a month when stored at −20°C.
Sample Preparation and Analysis
Assay samples were produced by mixing 987 uL Tris-HCL buffer, 10 uL substrate solution, and 3 uL sample to disposable glass culture tubes. Assay blanks were produced by mixing 990 uL 0.01 mol/l Tris-HCL buffer (pH 7.4) and 10 uL substrate solution into disposable glass culture tubes. Culture tubes were stored on ice during preparation. Then, triplicate volumes of 300 uL were aliquoted from the culture tubes to wells in white polystyrene 96-well microplates (Porvair PS White, Perkin Elmer, Norwalk, CT). Microplates were placed in temperature controlled (30°C) microplate reader (Perkin Elmer) and attached to a luminescence Spectometer LS50B (PerkinElmer). Florescence intensity of each well was recorded every 20s for 90 cycles at 488 nm excitation (excitation slit: 2.5 nm) and 530 nm emission (emission slit: 5.0 nm). To confirm calcium dependent sPLA2 activity, samples were also run with EGTA-Buffer (0.01 mol/l Tris-HCL (pH 7.4) containing 10 mmol/l Ca+2 and 20 mmol/l EGTA) which contains ample EGTA for complete calcium sequestration. After the reactions reached equilibrium temperature, the reaction curve was fit to a second-order polynomial equation and the first-degree coefficient was taken as the initial rate of reaction (expressed as change in FI/min/uL sample). Blank wells containing only substrate and buffer were used to find coefficient rates determined as background activity.
Western Blot for sPLA2-IIA
The amount of solubilized protein from each tissue homogenate was determined using the Bradford assay method. 10 ug of tissue homogenate protein, 4 uL of serum, or 4 uL of luminal wash fluid were denatured at 95°C for 10 minutes with sodium dodecylsulfate and beta-mercaptoethanol and separated in 4–15% agarose gel by electrophoresis at 150 V for 40 minutes at room temperature. Proteins were transferred to polyvinylidene fluride membrane using tris-glycine buffer plus 20% methanol at 80 V for 35 minutes at 4°C. Membrane was blocked with 5% nonfat dry milk prepared in TBS-Tween for 1 hour at room temperature with constant agitation. Membranes were incubated with primary antibody, mouse anti-human sPLA2-IIA (sc-58363, Santa Cruz Biotechnology, CA) duluted 1:1,000 over night at 4°C with constant agitation. Membranes were washed and incubated with stabilized goat anti-mouse IgG-HRP conjugate (sc-2005, Santa Cruz Biotechnology, CA) diluted 1:20,000 for 1 hour at room temperature with constant agitation. After washing, membranes were incubated with HRP substrate (Super Signal West Femto maximum sensitivity substrate; Pierce, Rockford, IL) for 5 minutes and bands were detected using photographic film. Molecular weight bands of 14–16 kDa were used to verify sPLA2 concentration.
IgA Antibody Quantitative Analysis
IgA concentration from the SI luminal fluid was measured using a sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates (BD Biosciences, Bedford, MA) were pre-coated with 50 μl of a 10 μg/mL goat anti-mouse IgA, α-chain specific (Sigma-Aldrich, St Louis, MO) in 0.1 mol/L coating buffer (0.1M carbonate-bicarbonate, pH 9.6), and incubated overnight at 4 °C. Plates were washed 3 times and blocked with 100 μl/well of 1% bovine serum albumin in Tris-buffered saline with 0.5% Tween-20 solution for 1 hour at room temperature. SI luminal fluid samples were diluted (diluted 1:100) and 100 ul of diluted sample or IgA standards (seven 2-fold dilutions, from 1,000 → 7.8 ng/mL, Sigma-Aldrich) were added to respective wells and plates were incubated for 1 hour at room temperature. Plates were washed 3 times and 100 μL of a 1:500 dilution of the secondary antibody, goat anti-mouse IgA, α-chain specific-HRP conjugate (Sigma-Aldrich) were added to each well and incubated for 1 hour at room temperature. Plates were washed 3 times, and 100 μL of the substrate solution (H2O2 and o-phenylenediamine) were added for 7 minutes at room temperature. The reaction process was stopped by the addition of 50 μL of 2N H2SO4 and the absorbance read at 490 nm in a Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). SI luminal fluid IgA concentrations were calculated by plotting their absorbance values on the IgA standard curve, which was calculated using a 4-parameter logistic fit with SOFTmax PRO software (Molecular Device, Sunnyvale, CA).
Statistical analysis
Experimental values were compared using analysis of variance (ANOVA) and Fisher protected least significance difference (PLSD) corrected for multiple comparisons, with α = 0.05 (Statview 5.0.1, SAS, Cary, NC). Serum vs Portal vein sPLA2 were analyzed with students paired T-test in each animal, respectively. Numerical results are presented as mean ± standard deviation of the mean.
RESULTS
Experiment 1: Route and type of feeding
Intestinal luminal sPLA2 activity
CED (2.51 ± 0.53, p = 0.0001), IG-PN (3.1 ± 0.79, p = 0.0002), and PN (1.37 ± 0.83, p = 0.0001) significantly reduced SI luminal fluid sPLA2 activity (Fl/min/μL) compared to Chow (4.44 ± 0.96). PN significantly lowered SI luminal fluid sPLA2 activity compared to CED (p < 0.002) or IG-PN (p < 0.0001). CED and IG-PN were statistically similar (p = 0.09) [Figure 1].
Figure 1.
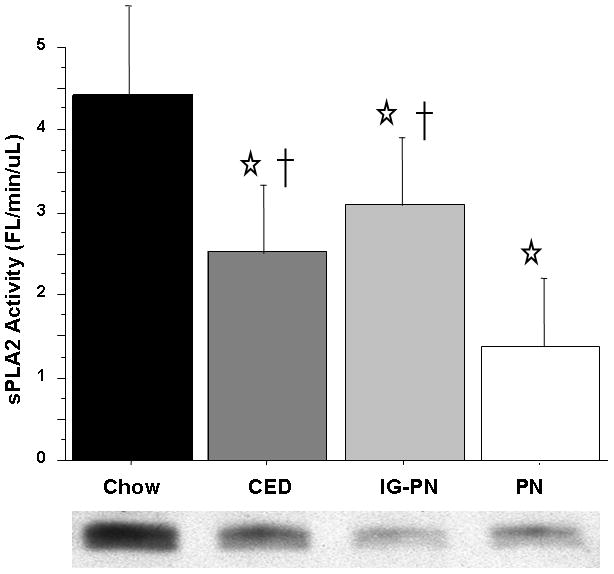
Intestinal Fluid sPLA2 activity.
Intravenous parenteral nutrition (PN) and Intragastric parenteral nutrition (IG-PN) and complex enteral diet (CED) significantly suppress sPLA2 levels compared with Chow. PN sPLA2 activity was significantly less than CED and IG-PN. Representative western blot detected bands at ~14 kDA confirm sPLA2-IIA protein concentration in 10 ug total tissue protein.
Values are means ± SD. *p<0.001 vs. Chow, †p<0.002 vs. PN.
Serum and tissue sPLA2 activity
Compared with Chow (4.46 ± 0.85), serum sPLA2 activity was lower in CED (3.83 ± 0.7, p = 0.042), IG-PN (2.75 ± 0.37, p < 0.0001), and PN (3.3 ± 0.79, p = 0.0004). Compared with CED, IG-PN serum sPLA2 activity was significantly lower (p = 0.0008) while PN failed to reach significance (p = 0.08). IG-PN and PN were not significantly different (p = 0.07) [Figure 2]. Portal vein serum sPLA2 activity was significantly greater than systemic serum (p = 0.04) in the combined groups. Subgroup analysis of portal vs. systemic serum sPLA2 activity showed that IG-PN and PN reached statistical significance (IG-PN: portal vein 2.53 ± 0.54 vs. systemic 1.99 ± 0.66, p < 0.001; PN: 3.07 ± 0.93 vs. 2.29 ± 0.82, p = 0.005) with no significant difference in the chow or CED groups (Chow: 3.924 ± 1.2 vs. 3.66 ± 1.39, p = 0.18; CED: 3.57 ± 0.79 vs. 3.3 ± 1.27, p = 0.09). Neither Jejunum nor Ileum tissue sPLA2 activity was significantly affected by route of feeding.
Figure 2.
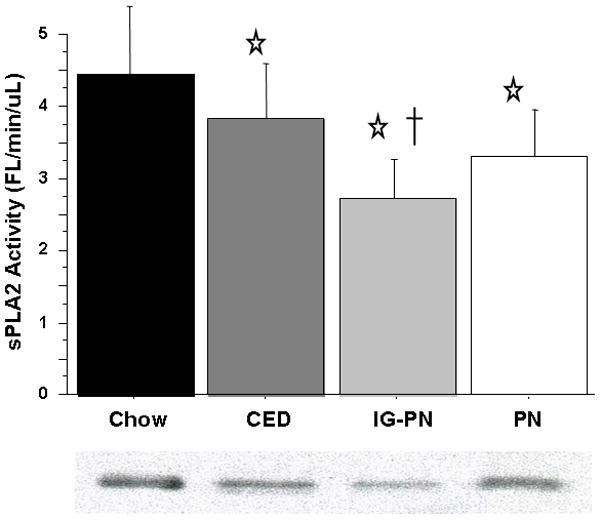
Serum sPLA2 activity.
Intravenous parenteral nutrition (PN) and Intragastric parenteral nutrition (IG-PN) and complex enteral diet (CED) significantly suppress sPLA2 levels compared with Chow. Representative western blot detected bands at ~14 kDA confirm sPLA2-IIA protein concentration in 4 uL serum.
Values are means ± SD. *p<0.05 vs. Chow, †p<0.001 vs. CED.
Experiment 2: Stress and route of nutrition
Intestinal fluid sPLA2 activity and IgA response
Consistent with Experiment 1, SI luminal fluid sPLA2 activity (Fl/min/μL) in PN was significantly lower than Chow (2.47 ± 1.3 vs. 7.71 ± 2.1, p < 0.0001). Following stress, PN luminal fluid sPLA2 activity remained low and unchanged (2.47 ± 1.3 vs. 3.4 ± 1.8, p = 0.3) and remained significantly lower than Chow at baseline (3.4 ± 1.8 vs. 7.71 ± 2.1, p < 0.0001). Stress significantly decreased SI luminal fluid sPLA2 activity in Chow (7.71 ± 2.1 vs. 3.5 ± 2.4, p < 0.0001) [Figure 3]. As previously reported, after stress SI luminal fluid IgA concentrations (ng/ml) significantly increased in Chow (209.3 ± 91 vs 404.7 ± 248, p = 0.0025) while a non-significant elevation was observed in PN (95.5 ± 33 vs 181.9 ± 66, p = 0.18). This slight elevation was due to a single outlier in the PN group. The level of IgA in Chow following stress was significantly greater than in PN at baseline (p < 0.0001) or PN following stress (p = 0.001) [Figure 4].
Figure 3.
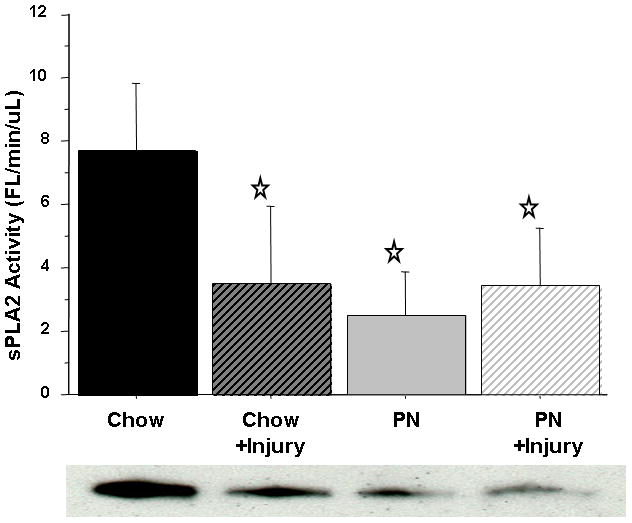
Intestinal Fluid sPLA2 activity.
sPLA2 activity was significantly suppressed in Chow+Stress, Parenteral Nutrition (PN), and PN+Stress, compared with Chow. Representative western blot detected bands at ~14 kDA confirm sPLA2-IIA protein concentration in 4 uL of intestinal fluid.
Values are means ± SD. *p<0.0001 vs. Chow.
Figure 4.
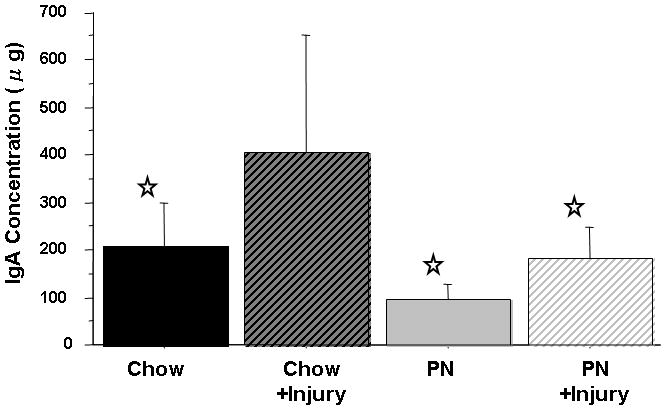
Intestinal Fluid IgA concentration.
Parenteral Nutrition (PN) significantly suppressed IgA levels compared with Chow. Surgical Injury significantly increased IgA in Chow+Stress compared with Chow, while no changes were observed in PN+Stress compared with PN.
Values are means ± SD. *p<0.003 vs. Chow+Stress.
Serum and tissue sPLA2 activity
Serum sPLA2 activity was significantly reduced in PN (3.06 ± 0.76) compared with Chow (4.19 ± 0.74, p < 0.03). Serum sPLA2 activity remained unchanged in Chow (4.19 ± 0.74 vs. 4.41 ± 1) mice after stress but significantly increased in PN compared with baseline (3.06 ± 0.76 vs. 4.18 ± 1.6, p < 0.03). The serum sPLA2 activity in PN following stress (4.18 ± 1.6) was similar to both Chow at baseline (4.19 ± 0.74, p = 0.98) and Chow after stress (4.41 ± 1, p = 0.63) [Figure 5]. Jejunum tissue sPLA2 activity remained unchanged [Figure 6A] with stress in both groups. PN mice exhibited an increase in Ileum sPLA2 activity following stress (3.95 ± 2.9 vs. 6.33 ± 4.3, p = 0.16) but this failed to reach statistical significance [Figure 6B].
Figure 5.
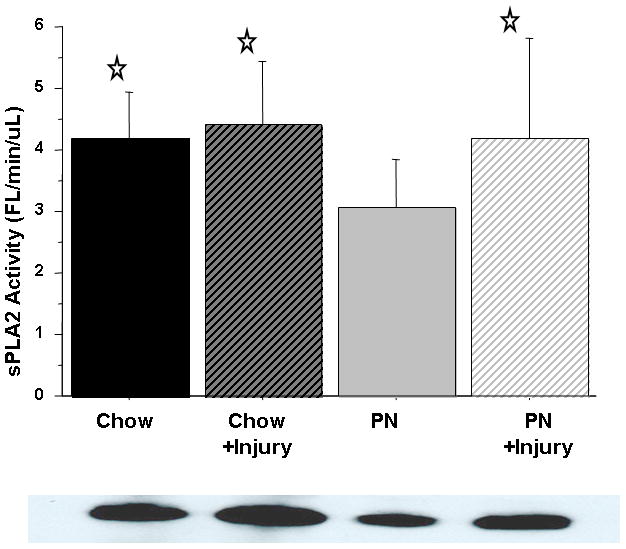
Serum sPLA2 activity.
PN significantly suppressed sPLA2 activity compared with Chow. Surgical Injury significantly increased serum sPLA2 in PN+Stress, but not Chow+Stress, compared with respective baseline levels. Representative western blot detected bands at ~14 kDA confirm sPLA2-IIA protein concentration in 4 uL serum.
Values are means ± SD. *p<0. 03 vs. PN.
Figure 6.
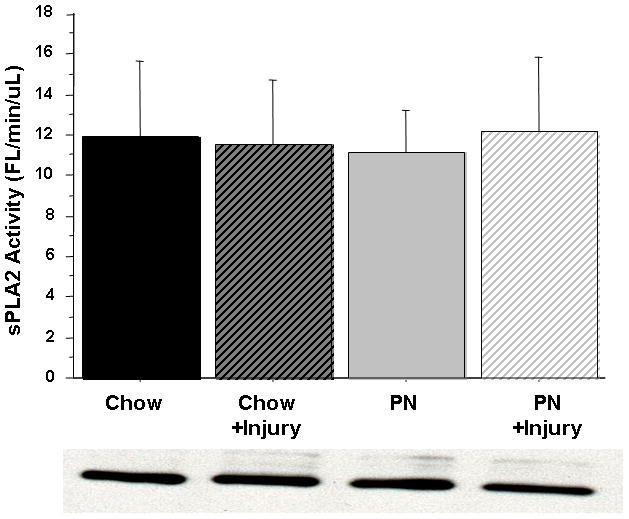
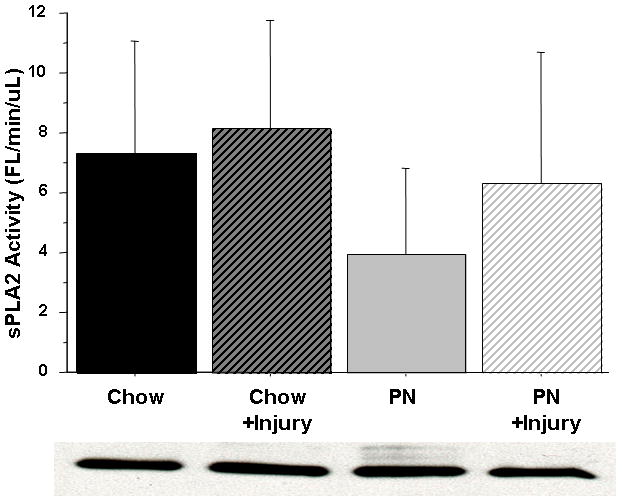
Figure 6A & 6B Small Intestine Tissue sPLA2 activity.
There were no effects on sPLA2 activity with Parenteral Nutrition (PN), Chow, or subsequent Surgical Injury in either group in the Jejunum (A) or Ileum (B) tissue. Representative western blot detected bands at ~14 kDA confirm sPLA2-IIA protein concentration in 10 ug total tissue protein.
Western Blot
sPLA2-IIA iso-form protein concentration determined with western blot was found to be similar to florescent activity measured via continuous florescence assay. These results are shown as sample gels with respective florescent activity results.
DISCUSSION
Both enteral and parenteral nutrition prevent progressive malnutrition in critically ill and critically injured patients compared with starvation, but PN is associated with an increase in infectious complications and multiple organ dysfunction. Although these PN-induced vulnerabilities are multi-factorial, both decreased mucosal defenses and increased tissue and systemic inflammation likely contribute to the observed morbidity. The implicated etiologies of increased infections include decreases in GALT cells, IgA production & transport; increases in bacterial virulence; and alterations in other factors such as GI permeability. Increases in tissue and serum sPLA2 have been implicated in inflammatory regulation of eicosinoid production and neutrophil priming. sPLA2 also plays a key role in innate defense of the small intestinal mucosa as one of the intraluminal bactericidal defensin-related molecules. This work focused on this molecule in experiments designed to investigate two questions. The first experiment explored effects of altering route and complexity of diet on basal serum, tissue and luminal sPLA2 activity. The second experiment investigated the effect of induced surgical stress after enteral and parenteral pretreatment in an established model of murine stress and injury which stimulates an adaptive immune response in the form of increases in airway and intestinal IgA. This adaptive immune response provides a marker of adaptive immune integrity in these experiments. Both experiments analyzed sPLA2 activity in the small intestine luminal fluid to evaluate potential bactericidal activity in the small intestinal and its potential role as a regulator of inflammation in the serum and tissue.
The first experiment proved consistent with our hypothesis that a decrease in enteral stimulation (DES), achieved by reducing complexity of the enteral diet from chow to CED to IG-PN to complete lack of enteral stimulation with PN, reduces gut mucosal defenses. PN resulted in significant suppression of both IgA and sPLA2 activity in the small intestine luminal fluid compared with any enteral stimulation. The IgA changes are identical to our previously published work (and show the reproducibility of our model) while suppression of sPLA2 activity in the intestine luminal fluid with unchanged tissue levels in jejunum or ileum suggests that DES/PN decreases sPLA2 production, secretion, or both producing a previously unrecognized loss of an innate mucosal defense. Since the source of luminal sPLA2 appears to be the Paneth cell, this cell is the likely source of the defect. The suppressed sPLA2 activity also correlates with our previous studies showing that DES/PN reduces IgA protective mechanisms by reducing levels of Th2 IgA-stimulating cytokines in the GALT as well as levels of polymeric immunoglobulin receptor (pIgR), the princpal IgA transport protein in the mucosa7–10. In agreement with these studies of mucosal immunity, the degree of enteral stimulation correlates with sPLA2 activity with Chow levels greater than CED and/or IG-PN, and all three greater than PN. Together these data support our hypotheses that DES/PN reduces bactericidal activity and bacterial binding within the gut, through inhibition of sPLA2 and IgA, respectively. The effects of these DES/PN alterations of this defensin-related molecule upon the microflora environment remain unknown, but other work shows that lower sPLA2 activity correlates with reduced bactericidal activity. Recent work in our laboratory (unpublished) demonstrates that intestinal sPLA2 obtained from PN fed mice is much less bactericidal than sPLA2 obtained from Chow fed mice despite equal concentrations of each. Therefore both the concentration and the form of sPLA2 may be critical in controlling the bacterial population.
The second experiment showed that surgical stress significantly elevates systemic sPLA2 after PN but not Chow feeding. This change in serum sPLA2 is consistent with an acute illness 23,24 and further supports PN induced vulnerability to systemic inflammation. The second experiment also showed that surgical stress did not affect the already low luminal fluid sPLA2 activity after PN, but significantly suppressed sPLA2 activity in chow mice. The change in intestinal tissue levels implicates a decrease in sPLA2 production. However, this may have been compensated for in Chow mice since another gut mucosal defense molecule, IgA, increased in the lumen as sPLA2 levels dropped after injury. No significant elevation in IgA occurred in PN mice. One possible reason for the down regulation of sPLA2 after injury in chow (and in all PN animals) is because of the potential for sPLA2 to damage the host mucosa. Recent work by Dial et al demonstrated that neutralization of sPLA2 in the small bowel reduced mucosal barrier degradation when animals were challenged with intra-peritoneal LPS, a stimulus which induces a rapid increase in luminal sPLA2 33. The mucosa normally heals quickly after injury and a mucus layer secreted by goblet cells protects its surface. Injury and PN reduce the mucus layer possibly rendering the host mucosa more vulnerable to sPLA2 injury. In this instance, IgA, which is not bioactive toward the host, can bind bacteria to prevent their mucosal attachment during injury and recovery. PN suppresses both sPLA2 and IgA after surgical stress implying a loss of both adaptive and innate branches of normal mucosal defense.
Our data also allow a general speculation regarding the source of sPLA2 in these experiments. While Paneth cells lining the lumen are likely the source of luminal sPLA2, the increase in circulation and ileal tissue levels in PN following stress implies that Paneth cells are not the source of this response since stress had no effect on luminal fluid sPLA2 activity. The likely source appears to be the GI tract tissue itself and/or the GI vascular system since sPLA2 activity was higher in the portal vein serum than in systemic serum. Subgroup analysis showed that portal sPLA2 activity exceeded systemic levels in PN and IG-PN mice while CED also almost reached significance. Chow differences failed to reach significance due to increased sample variation and relatively small numbers of animals. We speculate that this may be due to reduced splanchnic blood flow with DES/PN, resulting in slower portal-systemic mixing and greater sPLA2 concentration differences. In the GI tract vasculature, sPLA2 has been shown to ‘prime’ neutrophils in both animal models to produce an augmented inflammatory response to subsequent stress or injury. In humans, sPLA2 has also been implicated in neutrophil priming 28. Since we previously showed that DES/PN primes neutrophils as well, we were surprised that portal vein levels were lowest in PN fed mice. However, the reduced sPLA2 activity may be a protective response since PN increases expression of ICAM-1 and p-selectin 4,5 in the gut vasculature and increases the number of PMNs sequestered onto the gut endothelium for interaction with the tissue and their priming.
There are several limitations to this study. First, we did not directly measure alterations in inflammation in the vascular system and relied on previous work performed in our animal models of diet and injury. Second, we did not differentiate mucosal sPLA2 from other tissue compartments, such as the endothelium. Finally, we did not analyze the bactericidal or structural changes in sPLA2 that could be occurring with altered Paneth cell function. These experiments are currently underway and preliminary results suggest that both are occurring.
In summary, this study strengthens the evidence that DES/PN suppresses mucosal defenses of the small intestine. While the effects of PN upon adaptive immune mechanisms of the intestinal mucosa were extensively studied, to our knowledge this work is the first evidence of suppressed innate immunity with PN. While we did not show direct evidence of altered inflammation by sPLA2 via eicosanoid profiles or PMN priming, we confirmed the intestine is a significant source of this molecule and that injury in PN fed animals induces significant elevation in circulating sPLA2 consistent with PN-induced vulnerability to injury.
Clinical Relevancy.
The dynamics of the bacterial – host relationship results in a symbiotic which is beneficial to both. These relationships deteriorate in illness and following injury as both bacteria and the host adapt to changing environments in attempts to survive. Most of the recent research into these changes have focused on bacterial responses and changes in mucosal immunity (as the functional arm of adaptive immunity). The finding that parenteral feeding negatively affects gut bactericidal molecules after injury implicates impairment of the most basic level of the gut immune system -innate immunity – and failure to maintain the integrity of this basic anti-bacterial defense.
Acknowledgments
This research is supported by National Institute of Health (NIH) Grant R01 GM53439. This material is also based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Service. The contents of this article do not represent the views of the Dept. of Veterans Affairs or the United States Government.
This work was originally presented February 7, 2010 at the American Society of Parenteral and Enteral Nutrition (A.S.P.E.N)/Clinical Nutrition Week in Las Vegas, NV, USA.
References
- 1.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: effects on spetic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH Booth McL, Morgenstein-Wagner TB, Kellum JM, Welling RE, Moore EE. Early enteral feeding, compared with parenteral, reduced postoperative septic complications: the results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, Kuhl MR, Brown RO. Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1991;215:503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukatsu K, Lundberg AH, Hanna MK, et al. Route of nutrition influences ICAM-1 expression and neutrophil accumulation in intestine. Arch Surg. 1999;134:1055–1060. doi: 10.1001/archsurg.134.10.1055. [DOI] [PubMed] [Google Scholar]
- 5.Fukatsu K, Lundberg AH, Hanna MK, et al. Increased expression of intestinal P-selectin and pulmonary E-selectin during IV-TPN. Arch Surg. 2000;135:1177–1182. doi: 10.1001/archsurg.135.10.1177. [DOI] [PubMed] [Google Scholar]
- 6.Fukatsu K, Zarzaur BL, Johnson CD, Lundberg AH, Wilcox HG, Kudsk K. Enteral Nutrition Prevents Remote Organ Injury and Death After a Gut Ischemic Insult. Annals of Surgery. 2000;233(5):660–668. doi: 10.1097/00000658-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132:1303–1309. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Kudsk KA, Gocinsky B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–52. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Zarzaur BL, Kukatsu K, Johnson CJ, Eng E, Kudsk KA. A temporal study of diet-induced changes in Peyer patch Mad-CAM-1 expression. Surg Forum. 2001;52:194–196. [Google Scholar]
- 10.Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229:662–668. doi: 10.1097/00000658-199905000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. Faseb J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 12.Beers SA, Buckland AG, Koduri RS, Cho W, Gelb MH, Wilton DC. The antibacterial properties of secreted phospholipase A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. Jour Biol Chem. 2002;277:1788–1793. doi: 10.1074/jbc.M109777200. [DOI] [PubMed] [Google Scholar]
- 13.Koduri RS, Gronroos JO, Laine VJO, Le Calvez C, Lambeau G, Nevalainen TJ, Gelb MH. Bacteridical properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. Jour biol chem. 2002;277:5849–5857. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- 14.Weinrauch Y, Elsbach, Madsen LM, Foreman A, Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest. 1996 Jan 1;97(1):250–7. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinrauch Y, Abad C, Liang NS, Lowry SF, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J Clin Invest. 1998;102(3):633–8. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwig SS, Tan L, Qu XD, Cho Y, Eisenhauer PB, Lehrer RI. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95(2):603–10. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss J, Inada M, Elsbach P, Crowl RM. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. Biol Chem. 1994;269(42):26331–7. [PubMed] [Google Scholar]
- 18.Foreman-Wykert AK, Weinrauch Y, Elsbach P, Weiss J. Cell-wall determinants of the bactericidal action of group IIA phospholipase A2 against Gram-positive bacteria. J Clin Invest. 1999;103(5):715–21. doi: 10.1172/JCI5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laine VJ, Grass DS, Nevalainen TJ. Protection by Group II Phospholipase A2 against Staohylococcus aureus. Jour of Immunology. 1999;162:7402–7408. [PubMed] [Google Scholar]
- 20.Seilhamer JJ, Pruzanski W, Vadas P, Plant S, Miller JA, Kloss J, Johnson LK. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem. 1989;264(10):5335–8. [PubMed] [Google Scholar]
- 21.Kramer RM, Hession C, Johansen B, Hayes G, McGray P, Chow EP, Tizard R, Pepinsky RB. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989;264(10):5768–75. [PubMed] [Google Scholar]
- 22.Mäkelä A, Sternby B, Kuusi T, Puolakkainen P, Schröder T. Phospholipase A2 activity and concentration in several body fluids in patients with acute pancreatitis. Scand J Gastroenterol. 1990;25(9):944–50. doi: 10.3109/00365529008997616. [DOI] [PubMed] [Google Scholar]
- 23.Anderson BO, Moore EE, Banerjee A. Phospholipase A2 Regulates Critical Inflammatory Mediators of Multiple Organ Failure. Jour of Surgical Research. 1992;56:199–205. doi: 10.1006/jsre.1994.1032. [DOI] [PubMed] [Google Scholar]
- 24.Keuter M, Dharmana E, Kullberg BJ, Schalkwijk C, Gasem MH, Seuren L, Djokomoeljanto R, Dolmans WM, van den Bosch H, van der Meer JW. Phospholipase A2 is a circulating mediator in typhoid fever. J Infect Dis. 1995;172(1):305–8. doi: 10.1093/infdis/172.1.305. [DOI] [PubMed] [Google Scholar]
- 25.Groeneveld ABJ, Tacx AN, Bossink AWJ, van Mierlo GJ, Hack CE. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clinical Immunology. 2003;106:106–115. doi: 10.1016/s1521-6616(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 26.Crowl RM, Stoller TJ, Conroy RR, Stoner CR. Induction of phospholipase A2 gene expression in human hepatoma cells by mediators of the acute phase response. J Biol Chem. 1991;266(4):2647–51. [PubMed] [Google Scholar]
- 27.Nevalainen TJ, Haapamäki MM, Grönroos JM. Roles of secretory phospholipases A(2) in inflammatory diseases and trauma. Biochim Biophys Acta. 2000;1488(1–2):83–90. doi: 10.1016/s1388-1981(00)00112-8. [DOI] [PubMed] [Google Scholar]
- 28.Silliman CC, Moore EE, Zallen G, Gonzalez R, Johnson J, Elzi DJ, Meng X, Hanasaki K, Ishizaki J, Arita H, AO L, England K, Banerjee A. Presence of the M-type sPLA2 receptor on neutrophils and its role in elastase release and adhesion. Am J Physiol Cell Physiology. 2002;283:C1102–1113. doi: 10.1152/ajpcell.00608.2001. [DOI] [PubMed] [Google Scholar]
- 29.Zallen G, Moore EE, Johnson JL, Tamura DY, Barkin M, Stockinger H, Silliman CC. New mechanisms by which secretory phospholipase A2 stimulates neutrophils to provoke the release of cytotoxic agents. Arch Surg. 1998;133:1229–1233. doi: 10.1001/archsurg.133.11.1229. [DOI] [PubMed] [Google Scholar]
- 30.Sitren HS, Heller PA, Bailey LB, Cerda JJ. Total parenteral nutrition in the mouse: development of a technique. JPEN J Parenter Enteral Nutr. 1983;7:582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 31.National Academy of Science. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy of Science; 1978. National Research Publication No. 10. [Google Scholar]
- 32.Tsao FHC, Shanmuganayagam D, Zachman DK, Khosravi M, Folts JD, Meyer KC. A continuous fluorescence assay for the determination of calcium-dependent secretory phospholipase A2 activity in serum. Clinica Chimica Acta. 2007;379:119–126. doi: 10.1016/j.cca.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Zayat M, Lichtenberger LM, Dial EJ. Pathophysiology of LPS-induced gastrointestinal injury in the rat: role of secretory phospholipase A2. Shock. 2008;30(2):206–11. doi: 10.1097/shk.0b013e318160f47f. [DOI] [PubMed] [Google Scholar]


