Abstract
To date, ultrasonography of monkey ovaries is rare and typically of low resolution. The objectives of this study were to use state-of-the-art, high-resolution, transabdominal ultrasonography with real-time Doppler capabilities to: (1) determine whether one can reliably detect in real time the large dominant follicle, the corpus luteum (CL), and small (<2 mm) antral follicles on the ovaries of rhesus monkeys during the natural menstrual cycle; and (2) predict the follicular response of rhesus ovaries to controlled ovarian stimulation (COS) protocols. Rhesus monkeys were selected for transabdominal ultrasonography using a GE Voluson 730 Expert Doppler System at discrete stages of the menstrual cycle. Subsequently, serial ultrasound scanning was employed to observe growth of antral follicles and the CL. Finally, females were scanned to assess follicular growth during COS. The dominant structure and small antral follicles (<2 mm) were reliably visualized in real time. The follicle destined to ovulate could be identified by size differential by day 3 of the follicular phase. The number of small antral follicles present before onset of COS protocol correlated positively with the number of metaphase II-stage oocytes collected after treatment. The results of this study demonstrate that the population dynamics of antral follicle pools can be noninvasively evaluated in monkeys during natural and pharmacologic ovarian cycles.
Keywords: follicular dynamics, menses, ultrasound, rhesus monkey, recombinant human gonadotropin
INTRODUCTION
Studies on the rhesus macaque have been invaluable for understanding the processes of follicular selection and growth in primates during the menstrual cycle. For example, in landmark experiments, Goodman et al. [1977] and diZerega and Hodgen [1981] ablated the largest antral follicle on the ovary between 8 and 9 days after onset of menses and observed the absence of a timely luteinizing hormone (LH) surge or a subsequent luteal phase after follicle ablation. In addition, asymmetry in the estradiol (E) levels in the blood supply from each ovary during the follicular phase was observed 5–7 days before the LH surge [diZerega & Hodgen, 1981]. Thus, the dominant follicle destined to ovulate in the menstrual cycle is irreversibly selected from the pool of growing follicles by day 6 following the onset of menses.
Zeleznik and colleagues also used the rhesus monkey to study the gonadotropin requirements for growth of antral follicles [Zeleznik, 1990]. They hypothesized that small antral follicles have a threshold requirement for follicle stimulating hormone (FSH), owing to fluctuation in FSH levels from the mid-luteal phase to the late follicular phase [Zeleznik, 1990]. To investigate the effect of fluctuating FSH levels, endogenous gonadotropin levels in cynomolgus macaques were suppressed and replaced with FSH for 14 days (the length of a typical follicular phase). In animals receiving low levels (as in the midto late luteal phase and the late follicular phase) of FSH (8–10 and 12–15 mIU/ml), only preantral and small antral follicles were present, and there was no stimulation of estrogen production. In contrast, animals receiving higher levels of FSH (similar to early follicular phase; above 15–20 mIU/ml), displayed a number of large preovulatory antral follicles secreting estrogen. The number of these large antral follicles was related to the duration of exposure to these elevated levels of FSH. Therefore, the authors concluded that initiation of antral follicle development during the early follicular phase is controlled by an increase in FSH levels at that time. Selection of the dominant follicle is controlled by the largest antral follicle suppressing endogenous FSH levels and depriving subordinate follicles of FSH support. By providing exogenous gonadotropins at this time, development of several large, preovulatory antral follicles can be promoted in the primate ovary [Stouffer & Zelinski-Wooten, 2004].
More recently, transvaginal ultrasonography has been used to track follicular growth and development in women throughout the menstrual cycle [Baerwald et al., 2003a, b], as well as the growth and regression of the corpus luteum [CL; Baerwald et al., 2005]. Baerwold and colleagues were the first to longitudinally follow the growth (and atresia) of antral follicles in the human ovary during the luteal phase. This finding is in agreement with earlier data gathered from rhesus monkeys, using radiographic methods, which demonstrated that the granulosa and theca cells of small antral follicles are actively dividing during the luteal phase [Zeleznik et al., 1980]. These studies of follicular dynamics in women presented evidence that growth and atresia of large (>2 mm) antral follicles follows a wave pattern during the cycle. In this study, 68% of women displayed a two-wave pattern: a single antral follicle grows to peak in size around the mid-late to late luteal phase, and then undergoes atresia, followed by a second antral follicle that grows in the early follicular phase and ovulates at mid-cycle [Baerwald et al., 2003a]. A smaller percentage of women displayed a three-wave cycle (32%) during the late luteal and follicular phase of the menstrual cycle.
Ultrasonography is useful in estimating the numbers of antral follicles present in the ovaries of women just before and at onset of menses. This parameter has been predictive of response to controlled ovarian stimulation (COS) in women undergoing in vitro fertilization (IVF) protocols [Kwee et al., 2007]. Women who have very few antral follicles in their ovaries often fail to respond to stimulation regimens, suggesting that this parameter may be of prognostic value in triaging patients who are unsuitable for IVF therapy.
Ultrasonography in the monkey model, in which the ovary can be experimentally manipulated [as in diZerega & Hodgen, 1981; Zeleznik, 1990], would be invaluable to further study the control of antral follicular development and dominant follicle selection in primates. But to date, evaluation of ovaries by ultrasonic imaging techniques of macaques during the menstrual cycle is rare, [Adams & Dierschke, 1992; Morgan et al., 1987], in part because of the small size (≤7 mm) of preovulatory antral follicles and limited access to high-resolution ultrasound systems. The limited analysis of data from ultrasound studies of monkey ovaries was performed retrospectively.
The objectives of this study were to use state-of-the-art, high-resolution ultrasonography with real-time Doppler capabilities to determine whether one can reliably detect in real-time: (1) the large dominant follicle, (2) the CL, and (3) small antral follicles (<2 mm, especially in the absence of a dominant structure), on the ovaries of rhesus monkeys during the natural menstrual cycle. Also, once it was deemed possible to visualize small antral follicles throughout the menstrual cycle, studies were performed to determine whether the number of antral follicles present at menses could predict response to COS in rhesus monkeys. Our two hypotheses were, (1) that use of high-resolution ultrasound would allow identification of the dominant follicle by size differential before day 5 of the follicular phase; and (2) the number of small (<2 mm) antral follicles present on both ovaries at onset of menses would be predictive of response to COS by rhesus monkeys.
METHODS
All studies were performed at the Oregon National Primate Research Center (ONPRC) on the West Campus of Oregon Health & Science University (OHSU), from October 2006 to April 2007, and from September to December 2007. All procedures were approved by the ONPRC/OHSU Institutional Animal Care & Use Committee before experiments, and complied with the United States Animal Welfare Act and Regulations. Adult, female rhesus macaques (Macaca mulatta) with regular menstrual cycles were used for all studies under direct care of the ONPRC Department of Animal Resources.
Natural cycles
Menstruation was monitored, and blood samples were collected daily from day 6 following onset of menses to measure serum levels of estradiol (E) and progesterone (P) by a high-throughput, continuous random access immunoassay analyzer, Immulite® 2000 Advanced Immunoassay System (Siemens Healthcare Diagnostics Inc., Tarrytown, NY). Day of ovulation (day 1 of the luteal phase) was defined as the first day of low serum E (less than 100 pg/ml) following the mid-cycle E surge [Stouffer et al., 1994]. Initially (Phase 1), monkeys were selected for transabdominal scans as described below at discrete stages of the menstrual cycle: late follicular (E above 150 pg/ml to indicate near mid-cycle LH surge), mid-luteal (6–9 days after ovulation), and menses (n = 6/stage). Subsequently (Phase 2), monkeys (n = 5) underwent serial scanning during one inter-ovulatory interval (i.o.i) at the following stages: day of/before onset of mid-cycle E surge to observe the preovulatory follicle; at early, mid, late, and very late stages of the luteal phase to observe the developing, fully formed, and regressing CL and growth of small antral follicles; at early, mid, and late follicular phase to follow the selection, growth and ovulation of the subsequent dominant follicle.
COS
Monkeys (n = 15) were monitored for menses, and then subjected to COS as per the ONPRC Assisted Reproductive Technology (ART) Core's protocol [Meng & Wolf, 1997; Wolf et al., 2004]. Animals received recombinant human FSH (60 IU/day) from day 1 (onset of menses) to day 6, and then they received FSH:LH (1:1 ratio both 60 IU/day) from days 7–8 of the follicular phase. On day 7, animals also received the GnRH antagonist Acyline (75 ug/kg body weight). On day 8, animals received a bolus of hCG (1000 IU recombinant human hCG), and follicles are aspirated via laparoscopic surgery at 36 hr post-hCG. Ultrasonography was performed on day 1 before stimulation (n = 15), and once again on a subset of animals before hCG administration (day 8; n = 9). The total number of antral follicles present was compared with the number and stage of oocytes collected by aspiration.
Ultrasound Imaging
Imaging of the ovaries was performed on sedated (Ketamine HCl, 10 mg/kg, KetaVed® VEDCO, St. Joseph, MO) monkeys using a GE Medical Systems Voluson© 730 Expert Doppler ultrasound instrument (GE Healthcare, Waukesha, WI) with both 2D (4.5–16.5 MHz) and 4D (3.3–9.1 MHz) transabdominal probes. Both probes had real-time power-flow and color-flow Doppler imaging capabilities; the power-flow capability was used extensively to differentiate between artifact, and blood flow through the CL, and between extra-ovarian arterial and venous flow [Jansson et al., 1999]. The 4D probe has an added feature that allows for a scan through the tissue of interest, and software then permits the user to archive the entire scan, and scroll through cross-sections at a later date.
Animals were scanned first with the 2D probe to orientate the image field to the uterus and to identify locations and status of both the dominant and nondominant ovary. Additionally, when exact measurements and counts of antral follicles were desired, animals were then scanned with the 4D transabdominal probe to generate a data file of the ovary where the maximum number of antral follicles was visible, and any artifacts present were minimized, and the entire image was data filed for analysis at a later date. Visible antral follicles were then sized individually in the images generated by the 4D probe by positioning the curser along the longest axis, and noting the sizes given by the software. Size of the ovary as measured by both probes was defined as the diameter along the longest axis.
The 4D probe was more useful for estimating the size of small antral follicles, because the 4D software allows one to scroll through the ovary and orientate the ovary, eliminating artifacts, which can obscure some of the smaller antral follicles in a stimulated ovary. When possible, all measurements of small antral follicles were estimated using the 4D probe.
During the follicular phase of both natural and COS cycles, scans were typically performed with the 4D probe. The 2D probe was used primarily during the luteal phase, unless small antral follicles on the ovary were not visible, and then a scan was performed with the 4D probe to determine the presence of antral follicles.
The numbers of antral follicles were counted by methods similar to the “2D equivalent” technique used for human ovaries [Jayaprakasan et al., 2007], except at the time of menses when only counts from one image per ovary were used because of the small size of the ovaries. When animals were scanned on the day of hCG administration, two images per ovary (the X and Y planes) were counted, and the number of antral follicles (>1 mm) on each image averaged. To decrease inter-observer variation, only one investigator counted antral follicles from all animals in each protocol.
Statistics
The diameter of ovaries at onset of menses, plus the peak levels of E and duration of first and second follicular phases were compared by t-tests performed using SigmaStat© Version 2.0 software (1992–1997). Linear regression analysis of the COS data was performed by the use of Origin© Version 75E (1991–2004). The total number of antral follicles at the start of stimulation (menses) was compared with the total number of oocytes collected, plus the numbers of germinal vesicle (GV; immature)-intact, at metaphase I (MI) stage, and at MII stage. The total number of antral follicles on the day of hCG was compared with the total number of small antral follicles at the onset of menses, plus the numbers of GV-intact, MI-stage, and MII-stage oocytes. Comparisons were considered significant if analysis of variance (ANOVA) P<0.05.
RESULTS
Phase 1: Initial Detection
Initial scanning during the late follicular phase of the menstrual cycle identified the ovary bearing a single, large preovulatory follicle in 5 of 6 animals. In one animal, neither the ovaries nor the uterus could be visualized owing to a large fluid-filled bladder that pushed the reproductive tract beyond the depth of the ultrasonic field for transabdominal imaging. The preovulatory follicle measured 5.9±1.8 (mean±SEM) mm in diameter. The nondominant ovary was found in one of six animals, and that ovary had four visible small antral follicles. Figure 1 shows an example of a preovulatory follicle with power-flow Doppler imaging of blood flow in areas surrounding the ovary.
Fig. 1.
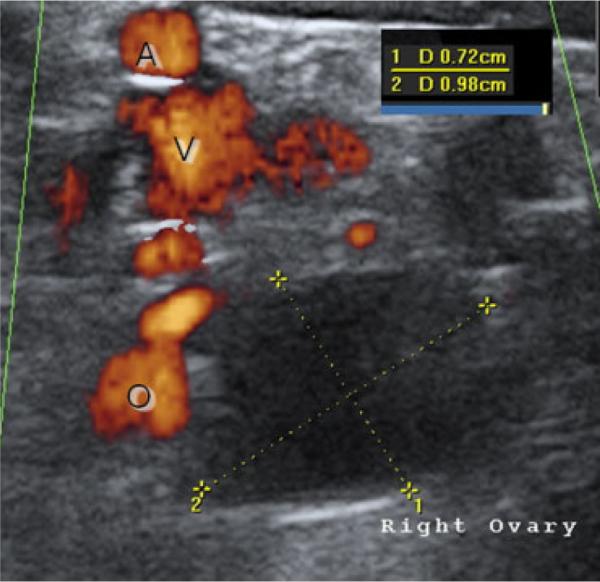
Example of a rhesus ovary at late follicular phase, just before ovulation. The preovulatory follicle is indicated by the yellow lines, and by inset the diameter measures 7.2 × 9.8 mm. Blood flow in the field of view surrounding the ovary is indicated by power-flow Doppler, which was confirmed during real-time imaging by waveform signature; O, ovarian supply A, large artery V, large vein.
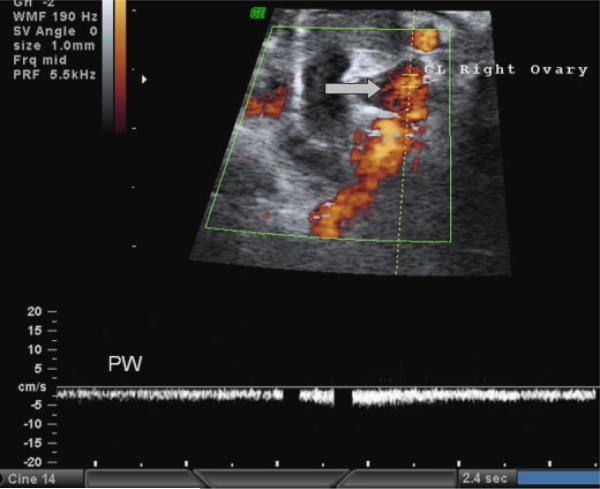
During the mid-luteal phase of the menstrual cycle, the CL was only visible by power-flow Doppler imaging and displayed a distinctive wave pattern (Fig. 2). The CL-bearing ovary was found in four of six animals scanned in the mid-luteal phase. Again, the same animal as above had a large bladder obstructing all abdominal structures. Of the animals that were successfully scanned during the late follicular phase, three were selected for subsequent scanning during the luteal phase; in all these females the CL was found on the same ovary as the preovulatory follicle. The ovary contralateral to the CL was found in three of six animals, and 10±2 small antral follicles were observed. On the CL-bearing ovary an average of 3±2 small antral follicles were detected.
Fig. 2.
Example of a rhesus ovary at mid-luteal phase. The arrow points to the corpus luteum (CL) and real-time pulse-wave analysis (PW) demonstrates a typical waveform for a CL: high volume flow through a small space.
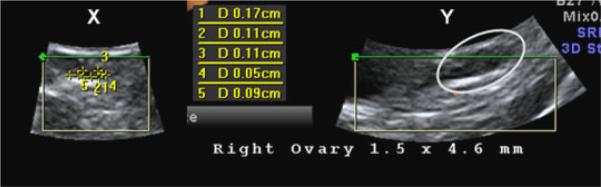
In animals scanned on the first day of menses, the larger ovary tended to have more small antral follicles, but there was no significant difference in the size of the ovaries. Both ovaries were identified in five of six individuals, but in one animal only one ovary was visualized. The larger ovary measured 6.2±1 mm in diameter and 10±3 antral follicles were observed. The smaller ovary measured 4.8±0.8 mm in diameter and 8±2 antral follicles were detected. Figure 3 shows an ovary imaged at the onset of menses with the 4D probe and software.
Fig. 3.
Example of a rhesus ovary on the first day of menses. This is an archived 4D ultrasound scan, with the X and Y planes shown. In the X-plane, five small antral follicles are sized, and the results are listed in the inset. In the Y-plane, the ovary is seen in the boundary of the gray circle.
Aberrant conditions were identified, including one monkey with two large, antral follicles (presumably dominant) at late follicular phase, and one monkey with multiple large ovarian cysts (up to 9 mm) at menses that were not included in the detection analysis. As it was typically possible to visualize both the ovary with and without a dominant structure throughout the menstrual cycle, we proceeded to Phase 2 of the study.
Phase 2: Longitudinal Observations
The average duration of the follicular and luteal phases in monkeys during serial ultrasonography was 14±1 and 15±1 days, respectively. There was no significant difference (P>0.05) between the duration of the follicular phases preceding ovulation and during the inter-ovulatory interval (i.o.i) of ultrasonographic imaging. Also, there was no significant difference (P>0.5) between peak E levels at the beginning and the end of the i.o.i., with the mean level measuring 349.1±34.1 pg/ml. Peak P levels during the luteal phase measured 4.1±0.6 ng/ml.
Small antral follicles (<2 mm diameter) were identified in all individuals throughout the menstrual cycle on both ovary bearing the dominant follicle/CL and the contralateral ovary. Distinct patterns of follicular development were observed. Of five animals scanned, three monkeys were retrospectively identified as displaying an expected pattern based on previous studies of rhesus follicular dynamics [Zeleznik, 2004]. These three animals had a single, large antral follicle that developed and ovulated on one ovary; this follicle could be prospectively identified owing to its size differential, from its cohorts as early as the onset of menstruation. A graph of the follicle growth during the follicular phase of one of these typical animals is presented as Figure 4. There is a distinct size difference between the largest follicle and the other antral follicles on day 1 of the cycle, and growth of this follicle can be tracked to ovulation. As illustrated in Figure 4, in four of five monkeys (excluding the monkey with two large antral follicles; see below), the preovulatory follicle was the only visible antral follicle, i.e. there were no detectable small antral follicles, by the late follicular phase just before the E surge.
Fig. 4.
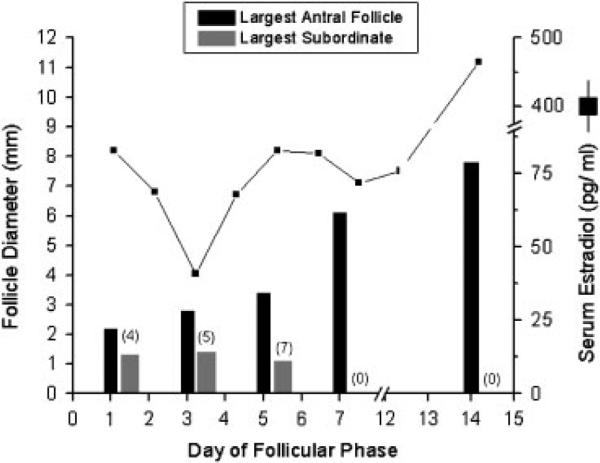
Representative longitudinal analysis of the diameter of antral follicles in one monkey during a spontaneous follicular phase. A single antral follicle grew to dominance and ovulated. The numbers of subordinate antral follicles on the ovary bearing the follicle destined to ovulate are listed in parentheses. Serum estrogen levels during the follicular phase are depicted for reference.
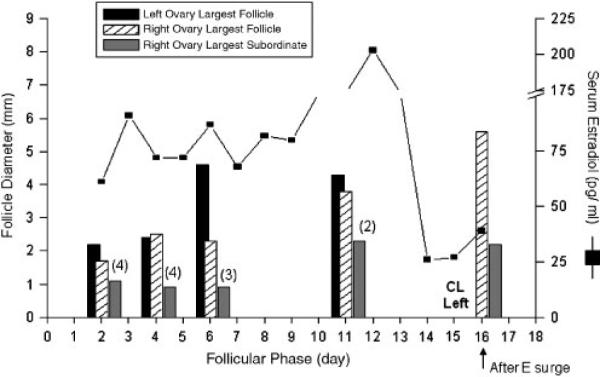
Unexpected patterns were identified in two of five females based on follicular dynamics. One animal had one antral follicle grow to 2.4 mm in diameter by day 3 of the follicular phase, only to shrink to 1.5 mm by day 5. A second, smaller antral follicle was observed to grow slowly throughout the follicular phase. This second antral follicle did not appear larger than its cohorts until day 7, and on the day before ovulation was only 3.4 mm (the smallest preovulatory follicle observed in this study). Another female displayed two larger antral follicles of similar sizes, one on each ovary by the early follicular phase (Fig. 5). The antral follicle on the left side ovulated as evidenced by a fully formed CL in the luteal phase, whereas the right ovary retained its large follicle after the mid-cycle E surge.
Fig. 5.
Longitudinal analysis of antral follicle diameters in one monkey who had two large antral follicles grow, one on each ovary, during a spontaneous follicular phase. The numbers of subordinates on the right ovary are given in parentheses. Estrogen pattern during the follicular phase is given for reference. Data on day 16 illustrate the presence of a large antral follicle on the right ovary following the E surge: this surge resulted in ovulation of the large antral follicle on the left ovary (CL left).
Phase 3: COS Protocols
Of the monkeys selected for COS, 14 of 15 responded to gonadotropin treatment with development of multiple large antral follicles and underwent follicle aspiration. All animals, including the non-responder, were included in the analysis. The number of small antral follicles (<2 mm) visible on both ovaries per animal at menses was 14±1 (SEM). The total number of oocytes collected per animal was 38±5, with 11±2 at MII stage, 12±1 at MI stage, and 10±2 GV-intact.
The number of follicles present at the onset of menstruation positively correlated (n=15, ANOVA P<0.01; r=0.72) with the number of MII-stage oocytes collected 10 days later by follicle aspiration (Fig. 6). The number of large antral follicles present on the day of hCG administration (day 8) positively correlated with the number of total oocytes collected 2 days later by follicle aspiration (n=9, ANOVA P<0.01; r=0.39; Fig. 7). No other parameters investigated were statistically significant.
Fig. 6.
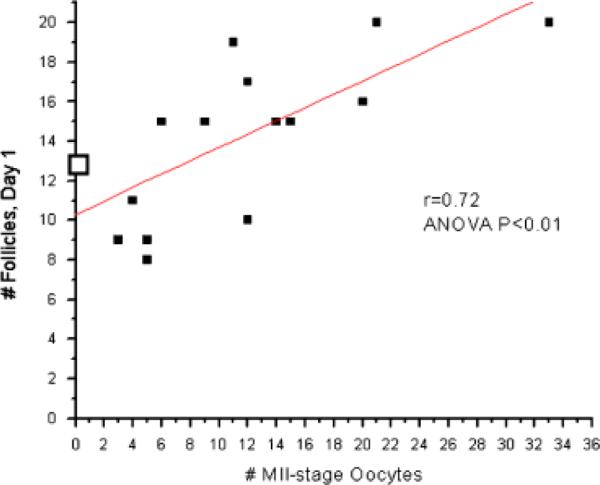
Controlled ovarian stimulation (COS) analysis (n = 15 protocols; closed boxes). The number of small antral follicles at menses positively correlates with the number of metaphase II (MII)-stage oocytes collected on the day of aspiration (10 days later). One animal failed to respond to stimulation and her data is presented as □.
Fig. 7.
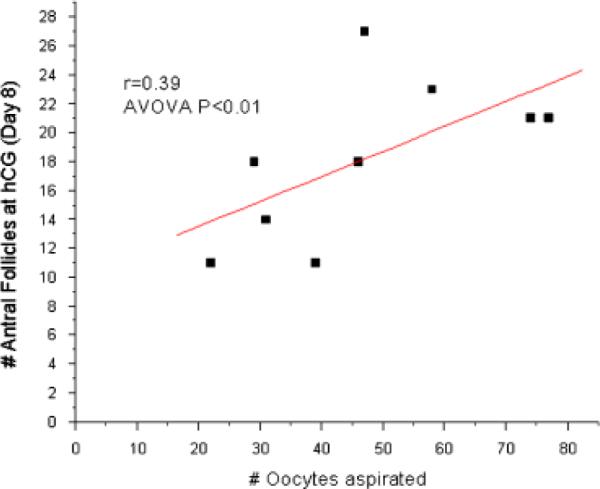
Controlled ovarian stimulation (COS) analysis (n = 9 protocols; closed boxes). The number of antral follicles at the time of hCG (day 8) positively correlates with the number of oocytes collected on the day of aspiration (2 days later).
DISCUSSION
For the first time it is possible to observe small antral follicles (<2 mm) on the ovaries of rhesus monkeys throughout the menstrual cycle by real-time ultrasonography. Previous studies analyzing ultrasound images of rhesus ovaries could not reliably detect small antral follicles; moreover, detection of the large antral follicle destined to ovulate was only possible retrospectively [Adams & Dierschke, 1992; Morgan et al., 1987]. During the initial phase of the study, investigators were training to use the ultrasound instrumentation. By the end of phase 1, both the ovary devoid of the dominant structure, as well as the larger ovary containing the preovulatory follicle or CL, were routinely visualized. The use of Doppler imaging greatly improved the identification of small ovaries without a dominant structure. There were consistently more small antral follicles observed on the nondominant vs. the dominant ovary during scanning in both the follicular and luteal phase of the cycle. Previous instrumentation provided only the capability to observe an (one) ovary in the very late follicular phase when a large preovulatory follicle was present.
In three of five animals studied longitudinally, the dominant follicle (designated as such because one can track its growth trajectory from the early follicular phase until the ovulatory event) appears to be selected based on its size differential from other antral follicles before day 3 of the follicular phase. This is much earlier than 8 days after menses as reported by Adams and Dierschke [1992] and Goodman et al. [1977]; and even earlier than day 6 reported by diZerega and Hodgen [1981]. These previous studies used evidence from ablation of the dominant structure [Goodman et al., 1977], a combination of follicle ablation and catheterization of the ovarian vein [diZerega & Hodgen, 1981] and ultrasound (with less sensitive probes [Adams & Dierschke, 1992]) to estimate the day of selection. Despite an obvious size differential between follicles in the early follicular phase, the largest follicle may not be irreversibly selected and destined to ovulate. It remains to be determined whether ablation of the largest antral follicle on day 3, as per Goodman et al. [1977], allows another appropriately staged follicle to grow and ovulate. This could occur in the early follicular phase if other smaller antral follicles have not yet started the process of atresia. In all three animals displaying this selection pattern, the follicle identified as larger than its cohorts changed shape as it proceeded toward ovulation. The antral follicle destined to ovulate (dominant) was asymetrical in the early follicular phase. It was not until late in the follicular phase that these antral follicles appeared more symmetrical.
One of the unexpected patterns during the menstrual cycle had one larger antral follicle in the early follicular phase grow to a peak of 2.4 mm in diameter on day 3, and then undergo atresia before follicular day 5. Another antral follicle in the cohort on the same ovary grew slowly throughout the follicular phase to reach dominance later in the follicular phase (day 7). This slower growing antral follicle did not become very large (3.4 mm). The observed follicular growth in this animal may follow the three-wave pattern of follicular dynamics similar to those identified by Baerwald et al. [2003a] in women. In fact, the majority of women studied had a two-wave pattern of follicular development, with some showing three waves as discussed above. Women exhibiting three waves displayed growth of a larger antral follicle that started in the luteal phase before menses and peaked early in the follicular phase, whereas the follicle destined to ovulate did not start growing until after menses. These women typically had longer inter-ovulatory intervals than those with two waves. This pattern was seen in 32% of the women studied so it was not rare, but it remains to be seen if these patterns of growth are typical in macaques. A caveat of this study was the limited use of the 4D probe during the luteal phase. Based on our positive results, a more extensive study, employing 4D analysis of the ovaries in the luteal, as well as the follicular phase, is warranted to consider whether growth of small antral follicles in monkeys are similar to those in women.
Our data demonstrate a significant, positive correlation (r=0.72) between the number of mature MII-stage oocytes collected and the number of small antral follicles present in macaque ovaries at the start of COS, similar to the results seen in women [Forabosco & Sforza, 2007; Jayaprakasan et al., 2007; Johnson et al., 2006; Kwee et al., 2007]. The number of antral follicles present before the start of COS was found to be predictive of the response to gonadotropin stimulation in human studies [Dumesic et al., 2001; Jayaprakasan et al., 2007; Johnson et al., 2006; Kwee et al., 2007]. However, there is no correlation between the number of small antral follicles at the start of protocol and the total number of macaque oocytes collected at follicular aspiration, as there is in human studies. This may be owing to differences in the follicular aspiration procedures between rhesus monkeys and women. Women undergo single follicle aspiration, in which transvaginal ultrasonic imaging helps the physician selectively aspirate individual large antral follicles one by one. Rhesus monkey aspiration is done by a laparoscopic procedure as described before [Wolf et al., 2004]; after a single puncture of the ovary many follicles are aspirated one at a time from within the ovary. Because the needle passes into more than one follicle, and must go through the stroma of the ovary to gain access to adjoining antral follicles, there is a high likelihood of immature oocytes from primary and secondary follicles being collected at the same time. Indeed, many (~40%) of the macaque oocytes collected by follicular aspiration are immature, GV-intact oocytes.
There is another confounding factor in considering the number of small antral follicles as predictive of COS success in monkeys: the issue of antigenicity to the recombinant human proteins used in our COS protocols. There is a well-documented immune response to these proteins in macaques [for review, see Stouffer & Zelinski-Wooten, 2004], and after multiple stimulations (n≥2–3), some animals generate antibodies to the proteins. This is associated with the failure of animals to respond to additional gonadotropin treatment with multiple follicular development. This may have been the case for the one animal that failed to respond to hormone treatment in this study. Her antral follicle count at the start of the COS protocol was near average (13, with the average around 14), but it was her third protocol. Therefore, in animals that have less than two protocols, the status of the ovary as measured by ultrasound imaging may be predictive of response to COS. After the second protocol, other factors may confound the results. Recombinant macaque gonadotropins are now available from the National Hormone & Peptide Program (Torrance, CA) and were used at the ONPRC in COS protocols [Sparman et al., 2008], but their widespread use is currently cost prohibitive.
In conclusion, it is now possible to use high-resolution ultrasonic imaging to noninvasively monitor the antral follicle pool, the selected dominant follicle, and the CL in macaque ovaries during the natural menstrual cycle. This technique will be useful for monitoring in real time the response of the small antral follicle pool to various hormonal treatments. This technique also allows the investigator to follow the effect of those treatments on the antral follicle pool across the menstrual cycle. Thus, the population dynamics of the antral follicle pool, and preovulatory follicle/luteal structure function can be evaluated in spontaneous menstrual cycles, as well as in conditions of ovarian dysfunction or pharmacologic treatment. Ultrasound imaging of the antral follicle pool may prove to be a beneficial addition to macaque ART programs, allowing one to screen potential candidates before initiating costly COS stimulation protocols.
ACKNOWLEDGMENTS
The authors thank the animal technicians in the Division of Animal Resources (DAR), ONPRC, for blood collection and hormone administration, and the DAR Surgery staff under the supervision of Dr. Theodore Hobbs, DVM for laparoscopic oocyte retrieval. The authors are also grateful to the ONPRC Endocrine Services Core directed by Dr. David Hess for performing the hormone assays. Thanks to ART Core technicians Cathy Ramsey and Joy Woodward for assistance with ultrasounds of COS monkeys and selecting COS candidates.
The OHSU/ONPRC IACUC follows the United States Animal Welfare Act and Regulations, and the guidelines of the Office of Laboratory Animal Welfare at the National Institutes of Health (OLAW; assurance number A33401); in addition OHSU/ONPRC has full accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). This research was funded by HD18185 (Specialized Cooperative Centers Program in Reproduction and Infertility Research—SCCPIR Project 3), RR00163, T32HD007133 (C. V. B.), and T32DK007674-13 (A. B.).
Contract grant sponsor: Specialized Cooperative Centers Program in Reproduction and Infertility Research—SCCPIR Project 3; Contract grant numbers: HD18185; RR00163; T32HD007133; T32DK007674-13.
REFERENCES
- Adams G, Dierschke D. Ultrasonic imaging of ovarian dynamics during the menstrual cycle in rhesus monkeys. Am J Primatol. 1992;27:13–66. [Google Scholar]
- Baerwald A, Adams G, Pierson R. Characterization of ovarian follicular wave dynamics in women. Biol Reprod. 2003a;69:1023–1031. doi: 10.1095/biolreprod.103.017772. [DOI] [PubMed] [Google Scholar]
- Baerwald A, Adams G, Pierson R. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003b;80:116–122. doi: 10.1016/s0015-0282(03)00544-2. [DOI] [PubMed] [Google Scholar]
- Baerwald A, Adams G, Pierson R. Form and function of the corpus luteum during the human menstrual cycle. Ultrasound Obstet Gynecol. 2005;25:498–507. doi: 10.1002/uog.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- diZerega GS, Hodgen GD. Folliculogenesis in the primate ovarian cycle. Endocr Rev. 1981;2:27–49. doi: 10.1210/edrv-2-1-27. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Damario MA, Session DR, Famuyide A, Lesnick TG, Thornhill AR, McNeilly AS. Ovarian morphology and serum hormone markers as predictors of ovarian follicle recruitment by gonadotropins for in vitro fertilization. J Clin Endocrinol Metab. 2001;86:2538–2543. doi: 10.1210/jcem.86.6.7605. [DOI] [PubMed] [Google Scholar]
- Forabosco A, Sforza C. Establishment of ovarian reserve: a quantitative morphometric study of the developing human ovary. Fertil Steril. 2007;88:675–683. doi: 10.1016/j.fertnstert.2006.11.191. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Nixon WE, Johnson DK, Hodgen GD. Regulation of folliculogenesis in the cycling rhesus monkey: selection of the dominant follicle. Endocrinology. 1977;100:155–161. doi: 10.1210/endo-100-1-155. [DOI] [PubMed] [Google Scholar]
- Jansson T, Persson HW, Lindstrom K. Estimation of blood perfusion using ultrasound. Proc Inst Mech Eng [H] 1999;213:91–106. doi: 10.1243/0954411991534834. [DOI] [PubMed] [Google Scholar]
- Jayaprakasan K, Hilwah N, Kendall NR, Hopkisson JF, Campbell BK, Johnson IR, Raine-Fenning NJ. Does 3D ultrasound offer any advantage in the pretreatment assessment of ovarian reserve and prediction of outcome after assisted reproduction treatment? Hum Reprod. 2007;22:1932–1941. doi: 10.1093/humrep/dem104. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Bagrie EM, Coomarasamy A, Bhattacharya S, Shelling AN, Jessop S, Farquhar C, Khan KS. Ovarian reserve tests for predicting fertility outcomes for assisted reproductive technology: the international systematic collaboration of ovarian reserve evaluation protocol for a systematic review of ovarian reserve test accuracy. Bjog. 2006;113:1472–1480. doi: 10.1111/j.1471-0528.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- Kwee J, Elting ME, Schats R, McDonnell J, Lambalk CB. Ovarian volume and antral follicle count for the prediction of low and hyper responders with in vitro fertilization. Reprod Biol Endocrinol. 2007;5:9. doi: 10.1186/1477-7827-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Wolf DP. Sperm-induced oocyte activation in the rhesus monkey: nuclear and cytoplasmic changes following intracytoplasmic sperm injection. Hum Reprod. 1997;12:1062–1068. doi: 10.1093/humrep/12.5.1062. [DOI] [PubMed] [Google Scholar]
- Morgan P, Hutz R, Kraus E, Cormie J, Dierschke D, Bavister B. Evaluation of ultrasonography for monitoring follicular growth in rhesus monkeys. Theriogenology. 1987;27:769–780. doi: 10.1016/0093-691x(87)90299-8. [DOI] [PubMed] [Google Scholar]
- Sparman ML, Ramsey C, Thomas CM, Parlow AF, Wolf DP, Mitalipov S, Stouffer RL. Controlled ovarian stimulation of rhesus monkeys using macaque recombinant gonadotropins. Abstract: Society for the Study of Reproduction 41st Annual Meeting, #411.2008. [Google Scholar]
- Stouffer R, Zelinski-Wooten M. Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod Biol Endocrinol. 2004;2:32. doi: 10.1186/1477-7827-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffer RL, Dahl KD, Hess DL, Woodruff TK, Mather JP, Molskness TA. Systemic and intraluteal infusion of inhibin A or activin A in rhesus monkeys during the luteal phase of the menstrual cycle. Biol Reprod. 1994;50:888–895. doi: 10.1095/biolreprod50.4.888. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Thormahlen S, Ramsey C, Yeoman RR, Fanton J, Mitalipov S. Use of assisted reproductive technologies in the propagation of rhesus macaque offspring. Biol Reprod. 2004;71:486–493. doi: 10.1095/biolreprod.103.025932. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ. In: Control of follicular growth during the primate menstrual cycle. Mashiach S, editor. Plenum Press; New York: 1990. p. xvii.p. 1077. [Google Scholar]
- Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol. 2004;2:31. doi: 10.1186/1477-7827-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleznik AJ, Wildt L, Schuler HM. Characterization of ovarian folliculogenesis during the luteal phase of the menstrual cycle in rhesus monkeys using [3H]thymidine autoradiography. Endocrinology. 1980;107:982–988. doi: 10.1210/endo-107-4-982. [DOI] [PubMed] [Google Scholar]