Abstract
Mycobacterium tuberculosis (Mtb) is thought to live in an altered phagosomal environment. In this setting, the mechanisms by which mycobacterial antigens access the major histocompatibility class I (MHC-I) processing machinery remain incompletely understood. There is evidence that Mtb antigens can be processed in both endocytic and cytosolic environments, with different mechanisms being proposed for how Mtb antigens can access the cytosol. Recently, electron microscopy was used to demonstrate that Mtb has the potential to escape the phagosome and reside in the cytosol. This was postulated as the primary mechanism by which Mtb antigens enter the MHC-I processing and presentation pathway. In this commentary, we will review data on the escape of Mtb from the cytosol and whether this escape is required for antigen presentation to CD8+ T cells.
Keywords: Mycobacterium tuberculosis, phagosome, MHC-I antigen processing, CD8+ T cells
Introduction
Mycobacterium tuberculosis (Mtb) remains a highly prevalent and successful pathogen worldwide, with co-infection with HIV and emergence of multiple drug-resistant strains compounding the impact. Estimates indicate approximately one-third of world population has immunological evidence of infection with Mtb with over 10 million new cases each year (Dye et al., 1999). Mtb is a successful human pathogen because it is able to subvert the host immune response, often choosing an intracellular lifestyle. At the same time, the human immune response is largely successful at containing Mtb-infection, due to an effective Th1 response, reflecting the coordinated action of CD4+ and CD8+ T cell immunity. While CD4+ T cells play an important role in this process, CD8+ T cells are essential because of their unique ability to recognize intracellular infection, particularly in those cells that are major histocompatibility class (MHC)-II negative (Grotzke and Lewinsohn, 2005). One critical question that remains unanswered is how antigens derived from Mtb gain access to the MHC-I antigen processing and presentation pathway.
Following aerosol exposure, Mtb is taken up by lung-resident macrophages and dendritic cells (DC), where it resides in a phagosomal compartment. DCs have been shown to play an essential role in the immune response in vivo through cross-presentation and cross-priming functions (Mellman and Steinman, 2001; Segura and Villadangos, 2009; Amigorena and Savina, 2010). Although in vivo studies indicate DCs are involved in the immune response following infection with Mtb (Tian et al., 2005; Wolf et al., 2007; Leepiyasakulchai et al., 2012), very little is known about their role in the initiation of CD8+ T cell responses. In vitro, many studies have focused on the nature of the Mtb-containing phagosomal compartment. The consensus in the field is that this compartment does not fuse with lysosomes, but intersects with the endosomal pathways, recruiting molecules and necessary nutrients for survival and replication (reviewed in Russell, 2001; Philips, 2008). With regard to MHC-I antigen presentation and initiation of a CD8+ T cell response, studies indicate the presence of processing machinery on phagosomes, including MHC-I and the transporter associated with antigen processing (TAP; Ackerman et al., 2003; Guermonprez et al., 2003; Houde et al., 2003; Grotzke et al., 2009). Work by other groups demonstrates that Mtb antigens can access both cytosolic and vacuolar antigen processing pathways (Mazzaccaro et al., 1996; Neyrolles et al., 2001; Schaible et al., 2003; Lewinsohn et al., 2006). We recently showed that the Mtb compartment is a competent MHC-I antigen processing and presentation organelle. Loaded HLA-E molecules and TAP are present on the phagosome (Grotzke et al., 2009), and peptide import into the lumen of the Mtb phagosome occurs (Harriff, M. J., unpublished data). An alternative model was also proposed that attributes Mtb antigen presentation on MHC-I molecules and subsequent recognition by CD8+ T cells to the ability of Mtb to escape from its phagosomal membrane and reside in the cytoplasm (van der Wel et al., 2007; Weerdenburg et al., 2010).
In this review, we will revisit the literature analyzing the intracellular localization of Mtb and address potential reasons for the discrepancies seen in various in vitro and in vivo studies. Much of our understanding of the intracellular lifestyle of Mtb is derived from microscopy studies. In particular, electron microscopy (EM) provides sufficient ultrastructural resolution to visualize Mtb within vacuolar structures. In addition, many researchers utilize immuno-fluorescence (IF) microscopy using antibodies against vacuolar membrane proteins to characterize the co-localization of these markers with surface-labeled or fluorescent protein-expressing Mtb. Here, we will focus on studies that document Mtb localization by EM and immuno-EM techniques. We will then look at how these studies correspond to in vivo analyses of Mtb localization within cells. Finally, we will assess whether or not escape from the phagosome is necessary for access of antigen to the MHC-I pathway. If the intracellular lifestyle of Mtb is critical to recognition of infection by CD8+ T cells, it is imperative to characterize Mtb localization in cells for better vaccine design strategies.
Intracellular Localization of Mtb in vitro
Intracellular localization of Mtb within phagocytes has been observed since the late 1960s and early 1970s. In their seminal study, Armstrong and Hart analyzed mouse peritoneal macrophages infected with viable or non-viable M. tuberculosis H37Rv strain and Mycobacterium bovis BCG by EM. They noted that 4 days post infection, “bacteria were never seen to be free in the cytoplasm (i.e., outside phagosomes),” with 23% of these phagosomes exhibiting lysosomal fusion. Macrocyclon was subsequently used to prevent replication of the bacteria. In this case, viable H37Rv remained in membrane-bound phagosomes out to 14 days post infection, with 21% fusing with lysosomes (Armstrong and Hart, 1971).
The first reports of Mtb escape from the phagosome were published in 1980s by the Wright laboratory. Surprisingly, this group reported that at as early as 18–24 h post infection, as many as 60–100% of bacteria had escaped the phagosome and were in the cytosol (Leake et al., 1984; Myrvik et al., 1984). The reasons for this discrepancy remain unclear, however these studies differed dramatically between in the species, source, and cultivation of macrophages, and the preparation of and multiplicity of infection (MOI) of the bacterial inoculum. Specifically, rabbit alveolar macrophages were infected with H37Rv at higher MOI (20–25:1) than was used in the Armstrong and Hart (1971) study (MOI ∼5:1). These studies and others describing the intracellular location of Mtb are summarized in Table 1.
Table 1.
In vitro ultrastructural analyses of Mtb intracellular localization.
| Author and Year | Journal | Cell type | Mycobacterial strain | MOI (if indicated) | Maximum length of infection | % Bacteria free in the cytosol |
|---|---|---|---|---|---|---|
| Armstrong and Hart (1971) | J. Exp. Med. | Mouse peritoneal macrophages | H37Rv | ∼5:1 | 14 d | 0 |
| Myrvik et al. (1984) | Am. Rev. Respir. Dis. | Rabbit alveolar macrophages | H37Rv | 20–25:1 | 24 h | 60–100 |
| H37Ra | 20–25:1 | 24 h | <1 | |||
| Leake et al. (1984) | Infect Immun. | Rabbit alveolar macrophages | H37Rv | 20–25:1 | 18 h | 70–99 |
| Rabbit alveolar macrophages – BCG immunized | H37Rv | 20–25:1 | 18 h | 8–28 | ||
| McDonough et al. (1993) | Infect. Immun. | J774 mouse macrophages | H37Rv | 1–10:1 | 4 d | ∼50 |
| Xu et al. (1994) | J. Immunol. | Mouse bone marrow-derived macrophages | H37Rv | 10–20:1 | 14 d | 0 |
| Clemens and Horwitz (1995) | J. Exp. Med. | Primary human monocytes | Erdman | 0.5:1 | 5 d | 0 |
| Paul et al. (1996) | J. Infect. Dis. | Human monocyte-derived macrophages from PBMC | H37Rv | 1:1 | 6 d | 0 |
| Mazzaccaro et al. (1996) | Proc. Natl. Acad. Scei. U.S.A. | Mouse bone marrow-derived macrophages | Erdman | 3–10:1 | 24 h | 0 |
| Beatty et al. (2000) | Traffic | Mouse bone marrow-derived macrophages | CD1551 (CSU93) | 25:1 | 16 d | 0 |
| Clemens et al. (2002) | Infect. Immun. | Human peripheral blood mononuclear cells, THP-1 monocytes (human) | Erdman | 30:1 | 3 d | 01 |
| van der Wel et al. (2007) | Cell | Human monocyte-derived DC | H37Rv | 10:1 | 4 d | 322 |
| 7 d | 572 | |||||
| Peyron et al. (2008) | PLoS Pathog. | Foamy macrophages (human) | H37Rv | 1:100 | 11 d | 0 |
1Determined by fluorescence microscopy, percentage not given for ultrastructural studies.
2Percentage of DC containing cytosolic mycobacteria.
A series of papers published in the 1990s evaluated the precise intracellular location of Mtb. McDonough et al. (1993) noted that half of the bacteria infecting J774 mouse macrophages had escaped to the cytosol 4 days following infection. The authors qualified their observations by suggesting “one must exercise caution in distinguishing between a tubercle bacillus free in the cytoplasm and one which is encased in a tightly apposed vacuolar membrane.” Subsequent studies by Xu et al. (1994) and Clemens and Horwitz (1995) addressed this concern using H37Rv-infected mouse bone marrow-derived macrophages and primary human monocytes, respectively. Xu et al. observed mycobacteria in a membrane-bound, LAMP1 positive compartment for as long as 14 days post infection. In many cases, the membrane was tightly apposed to the bacteria (Xu et al., 1994). The differences between McDonough et al. and Xu et al. may reflect microscopic technique, since Xu et al. utilized cryopreservation prior to EM, whereas McDonough et al. (1993) used organic solvents to dehydrate the sample. The use of organic solvents has the potential to extract the phagosomal membranes and result in the appearance of cytosolic localization. Studies by Clemens and Horwitz also utilized cryopreservation and immuno-EM to delineate the intracellular location of Mtb. In this study, the intracellular compartment of single bacteria versus multiple bacteria was markedly different. Specifically, single bacteria cell were localized to phagosomes that rarely fused with lysosomes and stained positively for multiple markers, including MHC-I, MHC-II, and the transferrin receptor (TfR). These bacteria were not found free in the cytoplasm out to 5 days post infection. In contrast, organelles containing multiple mycobacteria were observed to fuse with lysosomes, and stained positively for CD63 (Clemens and Horwitz, 1995).
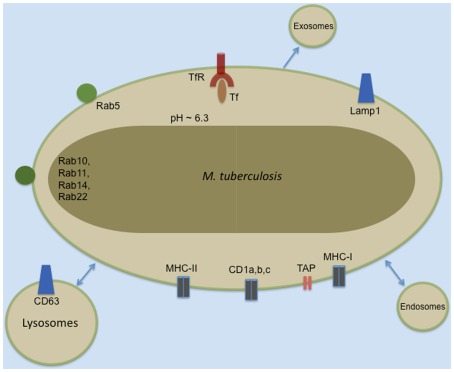
Subsequent EM experiments revealed Mtb surrounded by a phagosomal membrane that had not fused with the lysosome. The studies listed in Table 1 and others have defined the phenotype of this phagosomal membrane. Many proteins are now commonly used markers in the study of host–pathogen interactions of Mtb and other intracellular bacteria (illustrated schematically in Figure 1). Identification of the vacuolar trafficking proteins that are acquired and retained or excluded from the Mtb phagosome defined the point of Mtb phagosome maturation arrest as the retention of Rab5 and failure to acquire Rab7 or CD63. While phagosome maturation is arrested, the Mtb-containing vacuole remains dynamic and fuses with other vacuolar compartments. These interactions result in localization of the TfR and MHC-I to the Mtb phagosome (Clemens and Horwitz, 1995, 1996; Sturgill-Koszycki et al., 1996; Via et al., 1997; Kelley and Schorey, 2003). The kinetics and mechanisms of molecule trafficking to and from the phagosome, the bacterial protein and lipid effectors responsible for preventing fusion of Mtb phagosomes with lysosomes have been extensively reviewed elsewhere (Deretic et al., 1997; Deretic and Fratti, 1999; Pieters, 2001; Russell, 2001; Vergne et al., 2004; Brumell and Scidmore, 2007; Philips, 2008).
Figure 1.
Common markers of the Mtb phagosome.
Recent work by Peter Peters’ group challenged this view and suggested that the Mtb phagosome fuses with lysosomes early after infection and that bacteria translocate to the cytosol by 48 h after infection (van der Wel et al., 2007). In contrast to earlier work that had focused on macrophages and monocytes, human monocyte-derived DC that had been differentiated for 5 days prior to bacterial infection were employed. Cryo-immunogold EM was performed at time points from 2 to 96 h following infection. At early time points Mtb was present in a phagosome characterized by the presence of CD63, Lamp1, Lamp2, and Cathepsin D, while MHC-I, TfR, and EEA1 were absent. In spite of the presence of lysosomal markers, these early organelles did not acidify. At later time points, the authors observed the gradual accumulation of Mtb, but not BCG in the cytosol. By 7 days, more than half of the cells contained cytosolic Mtb. Translocation of Mtb to the cytosol was dependent on the ESX-1 Type VII secretion system and EspA. The ESX-1 system is encoded in the region of difference 1 (RD1) region of the Mtb genome that is missing from BCG. Based on their data, van der Wel et al. (2007) proposed that Mtb escape into the cytosol is the primary mechanism by which Mtb antigens access the MHC-I processing pathway (van der Wel et al., 2007; Weerdenburg et al., 2010).
Preparation of samples for EM, the type and viability of the host cell, and preparation of the bacterial inoculum may explain the conflicting results regarding phagolysosomal fusion as well as the escape of Mtb to the cytosol. However, van der Wel et al. (2007) used state of the art techniques for EM sample preparation and analysis. Alternatively, it is possible that presence of cytosolic mycobacteria reflected the viability of the DC. First, in our experience, Mtb-infected DC cultured for longer than 7 days have high levels of cell death, and consequently diminished capacity for antigen presentation. Second, it has been reported that Mtb-infection is associated with the death of the host cell. It has been previously demonstrated that virulent mycobacteria inhibit apoptosis. Uptake of Mtb within apoptotic bodies by uninfected cells is a mechanism by which Mtb growth is inhibited (Keane et al., 2000). Conversely, the ability to promote macrophage necrosis is a function of mycobacterial virulence (Hsu et al., 2003; Pan et al., 2005). The induction of necrotic cell death is postulated to promote mycobacterial spread. More recently, it has been demonstrated that high intracellular bacterial burden results in a process termed “atypical cell death.” This Mtb-induced cell death is characterized by lysosomal membrane permeabilization followed by degradation of lipid bilayers, and results in morphological characteristics and activation of molecules distinct from apoptosis and necrosis (Lee et al., 2006, 2011; Park et al., 2006). This elevated intracellular mycobacterial burden can result from either a high initial inoculum (Lee et al., 2006), or from ongoing intracellular bacterial replication (Park et al., 2006), and was associated with the disruption of phagosomal membranes and free cytosolic Mtb (Lee et al., 2011). Apoptosis was evaluated by van der Wel et al. (2007) through examination of Caspase 3 levels using fluorescence microscopy in conjunction with analysis of the morphological changes associated with apoptosis by EM. Only 5% of the Mtb-infected DC were apoptotic at 96 h after infection. However, their analysis does not provide an estimate of those cells undergoing atypical and/or necrotic cell death. Although the ability of Mtb to induce atypical cell death is DC is not known, these data suggest that the observation of cytosolic Mtb is a reflection of atypical cell death.
Along with virulence and bacterial burden, the preparation of the inoculum is another possible explanation for discordance between these studies. As discussed previously, both viability and homogeneity of the culture can influence whether or not the Mtb phagosome will undergo lysosomal fusion. For example, it has been previously demonstrated that non-viable mycobacteria are taken up into phagosomes that fuse with lysosomes. As the viability of the inoculum used by van der Wel et al. (2007) was not indicated, this parameter is difficult to evaluate. Furthermore, tight apposition of the phagosomal membrane to the mycobacteria is required to prevent lysosomal fusion (De Chastellier et al., 2009). In this case even modest bacterial clumping will result in lysosomal fusion. The presence of multiple bacteria in a single phagosome at time points shortly after infection in the van der Wel study suggests these mycobacteria-containing phagosomes will likely fuse with lysosomes and may explain the discordant protein co-localization data.
Does Mtb Escape the Phagosome in vivo?
While in vitro models are invaluable in the study of host–pathogen interactions, of central importance is the intracellular location of Mtb in vivo. In humans, surprisingly little is known regarding the intracellular location of Mtb. Following exposure to Mtb, widely divergent outcomes can occur, ranging from no clinical or immunologic evidence of exposure, to a state of latency defined by immunologic evidence of exposure in the absence of clinical or radiographic manifestations of disease. At present, the means by which aerosolized Mtb enters the lung is poorly understood. Furthermore, it is worth noting that in the limited studies using mycobacterial culture to determine the location of Mtb in those thought latently infected, Mtb can be observed in both granulomatous tissue and areas of normal lung (Opie and Aronson, 1927). Ultrastructural analyses of tissues following natural infection of humans are informative, but limited in scope (Table 2). EM of bronchoalveolar lavage samples from infected individuals revealed that Mtb localizes in membrane-bound compartments in infected alveolar macrophages (Russell et al., 2002; Mwandumba et al., 2004). These compartments typically contained a single mycobacterium with the membrane tightly apposed to the organism. In some cases of heavy infection, multiple organisms were observed in a larger compartment. While these studies demonstrate vacuolar Mtb in alveolar macrophages, the intracellular location of Mtb in alternate cells such as DC and epithelial cells in vivo is not clear.
Table 2.
In vivo ultrastructural analyses of Mtb intracellular localization.
| Author and year | Journal | Tissue type | Bacterial strain | MOI (if indicated) | Days after infection | Observations |
|---|---|---|---|---|---|---|
| Merckx et al. (1964) | Am. Rev. Respir. Dis. | Pulmonary, liver, and spleen tissue (mice) | H37Rv | 0.3 mg Mtb/25 g | 31 days–5 months | All bacteria observed in membrane-bound compartments |
| 5 months | Some bacteria appear free in cytosol | |||||
| Kondo et al. (1982) | Jpn. J. Med. Sci. Biol. | Granulomatous lung lesions and lung homogenate (mice) | M. bovis TCT401 | 0.5 mg M. bovis/mouse | 3 weeks | Bacteria in alveolar macrophages are in membrane-bound compartments |
| Bacteria in granulomatous tissue are sometimes observed with disrupted membranes or free in the cytosol | ||||||
| Moreira et al. (1997) | Infect. Immun. | Granulomatous lesions (mice) | M. tuberculosis Erdman | 200 bacteria/mouse | 14–35 days | All bacteria observed in membrane-bound compartments |
| Russell et al. (2002) | J. Cell Biol. | Alveolar macrophages from bronchoalveolar lavage (BAL; human) | UKa | UKa | UKa | Single and multiple bacteria observed in membrane-bound compartments in heavily infected alveolar macrophages |
| Mwandumba et al. (2004) | J. Immun. | Alveolar macrophages from bronchoalveolar lavage (BAL; human) | UKa | UKa | UKa | Bacteria observed in membrane-bound compartments |
| Occasionally in cases of heavy infection, multiple bacteria observed in a large vacuole | ||||||
| Caceres et al. (2009) | Tuberculosis | Foamy macrophages (mouse) | H37Rv | 20–50 bacteria/mouse | 3–9 weeks | Multiple bacteria observed in membrane-bound compartments out to 3 weeks |
| Single bacteria observed in membrane-bound compartments after 3 weeks |
aUnknown.
The mouse model of TB was designed to cause persistent intracellular infection. Nonetheless, the intracellular localization of Mtb has been examined in lung tissue samples from experimentally infected mice (Table 2). While limited in scope, ultrastructural analyses of granulomatous lung lesions and lung homogenates have consistently demonstrated the presence of Mtb and other virulent mycobacteria in a membrane-bound organelle (Merckx et al., 1964; Dumont and Sheldon, 1965; Kondo et al., 1982; Moreira et al., 1997). These studies also revealed heterogeneity in the morphology of the Mtb-containing compartment. In some cases, large membrane-bound vacuoles contained large numbers of bacteria, and in other cases, bacteria were observed singly, in phagosomes with tightly apposed membranes. Moreira et al. (1997) note, however, that multiple mycobacteria per phagosome were only observed in damaged macrophages at inflammatory sites. Within the granuloma, Mtb have been observed in phagosomal compartments in foamy macrophages (Caceres et al., 2009).
Is Escape from the Phagosome Required for Immune Recognition?
It has been postulated that escape of Mtb to the cytosol is the mechanism by which Mtb antigens are processed and presented in the context of MHC-I. Here, we will address the following questions: What is the evidence that MHC-I antigens are exported from the phagosome? Is there enhanced presentation of antigen or recognition by T cells if Mtb escapes to the cytosol?
In contrast to MHC-II, which samples peptide antigens from within the endocytic environment, MHC-I is traditionally considered the primary mechanism by which cytosolically derived antigen can be processed and presented to T cells. The ability of specialized antigen presenting cells such as DC to process and present antigens derived from non-cytosolic sources in the context of MHC-I has been termed cross-presentation. Cross-presentation was originally described by Michael Bevan’s group (Bevan, 1976; Carbone and Bevan, 1990) and has since been extensively characterized (reviewed in Shen and Rock, 2006; Burgdorf and Kurts, 2008; Lin et al., 2008; Amigorena and Savina, 2010; Van Endert, 2011). Kovacsovics-Bankowski and Rock (1995) originally described the presentation of particulate antigens by demonstrating that bead-associated OVA could stimulate IFNγ production by MHC-I restricted T cells. Many of the details of processing and cross-presentation of antigens have since been elucidated using inert particles such as latex beads, and it is clear the physical nature of the antigen has a profound effect on the mechanisms underlying processing and presentation. Particulate antigens taken up in phagosomes are processed and presented on MHC-I molecules early after uptake, prior to complete acidification of the endocytic compartment (Burgdorf and Kurts, 2008). Soluble antigens, on the other hand, are taken up into distinct endocytic compartments, mediated by binding to cell-surface receptors that determine whether or not the antigen will access MHC-I processing pathways. For example, soluble antigens taken up by the mannose, Langerin, or DEC-205 receptors are targeted to early endosomes and presented on MHC-I molecules (Burgdorf and Kurts, 2008). Once taken up by the cell, both particulate and soluble exogenous antigens access the cytosol by an undefined mechanism, where the antigen undergoes proteasomal degradation, TAP-dependent import into the ER or phagosome, and loading onto MHC-I (Shen and Rock, 2006). In some cases, antigen remains in the phagosome/endosome, where it is proteolytically processed for loading on MHC-I molecules (Lin et al., 2008).
Following infection by intracellular bacterial pathogens, bacterially derived particulate antigens present within the phagosome can also access the MHC-I processing and presentation pathway (Ramachandra et al., 2009; Blanchard and Shastri, 2010). This is in contrast to viral antigens that are present at high levels in the cytosol and presented via classical MHC-I processing and presentation in the endoplasmic reticulum. The mechanisms by which antigens from intracellular bacteria are presented have been the focus of numerous subsequent studies. Although many aspects of these mechanisms are still under debate, it is clear that bacterially derived antigens can gain access to the MHC-I pathway in several distinct, non-mutually exclusive pathways. As noted, these pathways have been reviewed extensively and will be discussed below in more detail as they pertain specifically to the Mtb phagosome and processing and presentation of mycobacterially derived antigens from this phagosome.
The study of antigen processing and presentation in the context of an intracellular infection such as Mtb can provide unique insights into these mechanisms. Broadly, these can be divided into cytosolic and non-cytosolic pathways (Figure 2). The cytosolic pathway is defined primarily by a requirement for proteasomal processing, and secondarily for the use of TAP. Direct access of bacterial antigens into the cytosol would allow for proteasomally processed peptides to be transported into the ER by TAP, where they are further processed, loaded onto MHC-I, and subsequently transported to the plasma membrane. Our laboratory and others have used Mtb-specific T cell clones to demonstrate that a number of Mtb antigens access this classical proteasome- and TAP-dependent cytosolic antigen processing pathway in vitro (Lewinsohn et al., 1998, 2006; Canaday et al., 1999; Grotzke et al., 2010). Here, proteasomal blockers including lactacystin, LLnL, and epoxomicin, were used to show that certain secreted Mtb proteins, including CFP10, EsxJ, and Ag85B, access the cytosol and that presentation of these antigens by infected DCs requires the proteasome. Furthermore, the use of virally encoded proteins that block the ability of TAP to import peptides into the ER, such as ICP47, indicated that presentation of these same proteins requires TAP. The DCs used in these studies were fixed and used in ELISPOT assays after less than 18 h of infection with Mtb. Mycobacterial escape from the phagosome has not been reported at this time point, indicating that these secreted antigens access the cytosol without a requirement for bacterial escape. In vivo data also support a role for TAP in MHC-I presentation of Mtb-derived antigens. For example, Sousa et al. (2000) used TAP1(−/−) mice to show that the protective immunity derived from CD8+ T cells is largely TAP-dependent, although TAP-independent mechanisms also contribute to protection. Taken together, these studies suggest a preferential use of the cytosolic pathway for presentation of Mtb antigens on MHC-I.
Figure 2.
Cytosolic and vacuolar pathways for processing and presenting Mtb antigens in the context of MHC-I.
The extent to which particulate phagosomal proteins gain access to the cytosol and can compete with abundant self proteins is unclear. As a result, it has been postulated that the phagosome itself participates in antigen processing and presentation, thus enhancing the display of these proteins (Gagnon et al., 2002; Ackerman et al., 2003; Guermonprez et al., 2003; Houde et al., 2003; Burgdorf et al., 2008). In contrast to the traditional cytosolic pathway, antigens transiently access the cytosol and are proteasomally processed. Peptides are then re-imported via TAP into the phagosome where they are loaded onto MHC-I. Proteins and molecules involved in antigen processing and presentation, including TAP, calnexin, tapasin, calreticulin, ERp57, and MHC-I, are present on bead phagosomes as well as on Mtb phagosomes (Grotzke et al., 2009). Providing direct evidence for this alternative phagosome–cytosolic pathway after Mtb infection, isolated Mtb phagosomes can directly stimulate IFN-γ production by Mtb-specific CD8+ T cells, indicating the presence of loaded MHC-I complexes (Grotzke et al., 2009). Additionally, as demonstrated with latex bead phagosomes (Ackerman et al., 2003), TAP in the Mtb phagosome is functional for peptide translocation (Harriff, M. J., unpublished data). Interestingly, ER molecules have also been observed on phagosomes containing other intracellular pathogens. Goldszmid et al. (2009) first demonstrated the presence of ER markers on the Toxoplasma gondii parasitophorous vacuole (PV). Further studies have identified a role for Sec22b in the recruitment of these ER proteins to the PV. In the absence of Sec22b, ER-derived proteins do not access the PV, and cross-presentation of T. gondii antigens is inhibited (Cebrian et al., 2011). While these studies define a role for the phagosome in cytosolic processing and loading of pathogen-derived antigens, they do not provide an estimate of the extent to which peptides are loaded within the phagosome versus the ER.
What is the direct evidence that mycobacterial antigens can access the cytosol? The Bloom laboratory was among the first to show in vitro that cells infected with Mtb could facilitate the presentation of a soluble antigen. Infection of mouse macrophages with live Mtb led to TAP-dependent presentation of co-administered soluble OVA (Mazzaccaro et al., 1996). Similar experiments by this group with wild-type and listeriolysin (LLO)-deficient Listeria monocytogenes demonstrated LLO-dependent pore formation was required for the MHC-I presentation of soluble OVA antigen. Taken together, these data led the authors to postulate a similar mechanism for Mtb (Mazzaccaro et al., 1996). Subsequently, fluorescent dextrans, ovalbumin, and polystyrene beads were microinjected into the cytosol of macrophages infected with live or heat killed BCG. The BCG phagosome was found to be permeable to molecules up to 70 kDa in size. Uptake of fluorescent molecules was dependent on viable BCG, suggesting that BCG actively generates pores in the phagosome (Teitelbaum et al., 1999). It is unclear, however, what role these pores play in the access of proteins to the cytosol, as this study demonstrated unidirectional transport of molecules into the phagosome. Furthermore, a similar study employing the electroporation of fluorescently labeled 50 kDa Fab fragments indicated that Mtb phagosomes are impermeable to these molecules (Clemens et al., 2002). To evaluate the transport of Mtb-derived antigens from the phagosome to the cytosol, Schaible et al. co-administered Mtb with membrane impermeable fluorescent molecules (HPTS and a FITC-labeled peptide), and found that these molecules did not access the cytosol. Additionally, after infection with radiolabeled Mtb, minimal amounts of radiolabeled, Mtb-derived proteins (<4%) could be found in the cytosol (Schaible et al., 2003).
However, accumulating evidence suggests that certain proteins can gain access to the cytosol. The well-known antigenic proteins secreted by the ESX-1 Type VII secretion system, such as CFP10 and ESAT-6 can be found in the cytosol (Abdallah et al., 2007). Rv1694 (tlyA), a hemolysin and ribosomal RNA methyltransferase, can be exported to the cytosol (Rahman et al., 2010), and epitopes from this protein are recognized by CD8+ T cells in the context of HLA-A*0201 (Shams et al., 2003). The tyrosine phosphatase PtpA can be exported to the cytosol and phagosome membrane, where it disrupts the trafficking of the V-ATPase complex to the phagosome (Bach et al., 2006, 2008; Wong et al., 2011). Zmp1 is an Mtb- and BCG-encoded Zn2+ metalloprotease that interferes with activation of the inflammasome and subsequent maturation of the phagosome. Zmp1 is required for virulence of Mtb in mice and survival of Mtb and BCG in macrophages and has been observed in the cytosol of infected cells (Master et al., 2008). These results suggest that mycobacterial proteins can access the cytosol without a requirement for Mtb escape from the phagosome
Particulate antigens can also be processed and presented in a manner that does not require access of proteins into the cytosol. This pathway has been termed the vacuolar pathway (Figure 2). Here, the generation of antigenic peptides depends on the presence of late endocytic peptidases such as cathepsins normally associated the processing of antigens for MHC-II. It has been postulated that subsequent loading of these peptides occurs through the exchange of peptides with MHC-I molecules found within the relatively acidic endocytic environment (Pfeifer et al., 1993; Song and Harding, 1996; Chefalo and Harding, 2001; Shen et al., 2004). Neyrolles et al. (2001) found that the Mtb 19 kDa lipoprotein is trafficked to distinct compartments from the mycobacteria, and that peptides derived from the 19 kDa lipoprotein are presented to CD8+ T cells by a TAP-independent mechanism. Furthermore, Mtb antigens can also be found in exosomes that are continuously trafficked from the phagosome as well as apoptotic bodies derived from infected cells, allowing for the uptake of antigen by uninfected bystander APCs (Beatty and Russell, 2000; Beatty et al., 2000, 2001; Schaible et al., 2003). The fact that Mtb antigens can be presented to CD8+ T cells without accessing the cytosol suggests that escape to the cytosol is not an absolute requirement.
Is RD1/ESX-1 Necessary for Antigen Presentation?
It is postulated that the ESX-1 secretion system is central to the escape of Mtb into the cytosol and thereby promotes MHC-I antigen presentation. Currently, there is no experimental evidence comparing the efficiency of CD8+ T cell activation in response to cytosolic versus phagosomal Mtb. However, there are studies both in vitro and in vivo that have examined the effect of RD1 deletion on the acquisition and maintenance of mycobacterially reactive CD8+ T cells. From these studies it is clear that presence of ESX-1 is not an absolute requirement for access of Mtb antigens into the cytosol. We have demonstrated that infection of human DC with the MtbΔRD1 mutant was as efficient as the complemented Mtb strain in activating CD8+ T cell clones specific for the TB8.4 antigen (Lewinsohn et al., 2006). Billeskov et al. (2007) similarly observed robust induction of TB10.4 specific CD8+ T cells upon infection of mice with either wild-type Mtb or the MtbΔRD1 mutant. The ability of specific mutants of the RD1 region, including the ΔespA mutant, to induce antigen-specific CD8+ T cells was also unchanged. Woodworth et al. (2008) concluded that Mtb escape is not required for access of antigen to the MHC-I antigen processing pathway.
For in vivo experiments, virulence and the association of Mtb-specific CD8+ T cells with bacterial burden make interpretation of these experiments difficult. It is clear that both the virulence and the induction of CD8+ T cell responses are enhanced when the RD1 region is restored to BCG (Pym et al., 2003; Brodin et al., 2004; Majlessi et al., 2005). A number of experiments have addressed the issue of reduced bacterial burden in BCG-infected animals with regard to CD8+ T cell activation. Russell et al. (2007) used an OVA expressing-BCG strain to show that increasing the dose of BCG leads to a more rapid CD8+ T cell response, due to an increase in the amount of antigen. Ryan et al. (2009) administered greater numbers of BCG than Mtb to mice, such that the lymph node mycobacterial burden was comparable. By establishing equivalent levels of antigen at the site of T cell priming, functionally equivalent, antigen-specific CD8+ T cells were induced (Ryan et al., 2009). Taken together, these data imply that reduced CD8+ T cell frequencies in response to bacteria lacking RD1 reflect decreased availability of antigen due to lower virulence, as opposed to an inability of these bacteria to escape to the cytosol.
Conclusion
While Mtb is not an obligate intracellular pathogen, its ability to co-opt the intracellular environment is central to Mtb to persistence in its human host. Maintaining a poorly acidified phagosomal environment provides protection, nutrients, and many other benefits to this pathogenic mycobacteria. Conversely, egress of Mtb into the cytosol with concomitant induction of apoptosis, autophagy, and atypical cell death are likely critical events in the interrelationship of pathogen and host, and may eventually be key to transmission. Mtb is a successful pathogen yet it is also contained by the host to a large extent. Here, it is important for the immune system to recognize and respond to infected cells. Knowing the intracellular niche of Mtb, and understanding how Mtb antigens are processed and presented to T cells within this context is critical to design of better vaccines. In this review, we have examined the evidence suggesting that Mtb escapes the phagosome, and whether or not this is a critical event in the induction and maintenance of MHC-I dependent immunity.
In vitro, it is clear that although escape of Mtb into the cytosol is possible, we have an incomplete understanding of the circumstances necessary for this to occur. While limited, ultrastructural evidence does not support the escape of Mtb from the phagosome in vivo. The presence of Mtb in cytosol could have consequences both for the host and the microbe. For example, cytosolic Mtb could lead to stimulation of innate intracellular sensors such as NOD2. While the presence of NOD2 has not been associated with mycobacterial growth control in the mouse, it has recently been shown that NOD2 stimulation can inhibit growth of Mtb in human macrophages (Brooks et al., 2011). It is also likely that presence of mycobacteria in the cytosol host cell leads to the death of this cell. At present, whether this event leads to transmission or enhanced control is not known.
At present, the evidence from both in vitro and in vivo studies does not support the case that mycobacterial escape to the cytosol is necessary for CD8+ T cell recognition. In vivo, while CD8+ T cell responses are observed at a higher frequency in the presence of the RD1-associated proteins, it is likely that these differences are a reflection of enhanced antigen load. (Russell et al., 2007; Woodworth et al., 2008; Ryan et al., 2009) rather than escape to the cytosol. While an in vivo system does not exist that models cytosolic Mtb, some insight can be gained from vaccination studies. The laboratory of Stefan Kaufmann has developed a BCG vaccine strain that secretes the LLO protein, a protein whose ability to form pores has been optimized via the deletion of urease leading to decreased phagosomal acidification (Grode et al., 2005). It was postulated that this strain could allow for more efficient egress of mycobacterial proteins and/or the bacterium itself into the cytosol, and thus improved CD8+ T cell responses. Indeed, vaccination of mice with this recombinant BCG provides increased protection against subsequent infection with Mtb, but the mechanism has not been completely defined. In vitro, there is increased cytosolic BCG-derived protein. Furthermore, infection of mouse macrophages with this vaccine strain induces apoptosis, possibly leading to cross-priming by bystander APCs via the uptake of apoptotic vesicles containing mycobacterial antigens (Grode et al., 2005). As such, it remains unclear as to whether or not this vaccine leads to improved frequency and/or quality of the Mtb-specific CD8+ T cell response, and whether or not this is responsible for the improved vaccine efficacy that has been observed.
Based on our understanding of how Mtb antigens are processed and presented in the context of MHC-I to CD8+ T cells in vitro, and the differences in antigen load and subsequent protection between Mtb and BCG in vivo, it is our opinion that Mtb escape to the cytosol is not critical for induction of CD8+ T cell responses. Rationale vaccine design requires that those CD8+ T cells induced during the course of vaccination can recognize those cells infected with Mtb. In this regard, an enhanced understanding of both the mechanisms of antigen processing and presentation as well as the repertoire of those antigens is of more than semantic importance.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abdallah A. M., Gey Van Pittius N. C., Champion P. A., Cox J., Luirink J., Vandenbroucke-Grauls C. M., Appelmelk B. J., Bitter W. (2007). Type VII secretion – mycobacteria show the way. Nat. Rev. Microbiol. 5, 883–891 10.1038/nrmicro1773 [DOI] [PubMed] [Google Scholar]
- Ackerman A. L., Kyritsis C., Tampe R., Cresswell P. (2003). Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc. Natl. Acad. Sci. U.S.A. 100, 12889–12894 10.1073/pnas.1735556100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amigorena S., Savina A. (2010). Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr. Opin. Immunol. 22, 109–117 10.1016/j.coi.2010.01.022 [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. (1971). Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134, 713–740 10.1084/jem.134.3.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach H., Papavinasasundaram K. G., Wong D., Hmama Z., Av-Gay Y. (2008). Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe 3, 316–322 10.1016/j.chom.2008.03.008 [DOI] [PubMed] [Google Scholar]
- Bach H., Sun J., Hmama Z., Av-Gay Y. (2006). Mycobacterium avium subsp. paratuberculosis PtpA is an endogenous tyrosine phosphatase secreted during infection. Infect. Immun. 74, 6540–6546 10.1128/IAI.01106-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty W. L., Rhoades E. R., Ullrich H. J., Chatterjee D., Heuser J. E., Russell D. G. (2000). Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1, 235–247 10.1034/j.1600-0854.2000.010306.x [DOI] [PubMed] [Google Scholar]
- Beatty W. L., Russell D. G. (2000). Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect. Immun. 68, 6997–7002 10.1128/IAI.68.12.6997-7002.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty W. L., Ullrich H. J., Russell D. G. (2001). Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur. J. Cell Biol. 80, 31–40 10.1078/0171-9335-00131 [DOI] [PubMed] [Google Scholar]
- Bevan M. J. (1976). Cross-priming for a secondary cytotoxic resonse to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143, 1283–1288 10.1084/jem.143.5.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeskov R., Vingsbo-Lundberg C., Andersen P., Dietrich J. (2007). Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J. Immunol. 179, 3973–3981 [DOI] [PubMed] [Google Scholar]
- Blanchard N., Shastri N. (2010). Cross-presentation of peptides from intracellular pathogens by MHC class I molecules. Ann. N. Y. Acad. Sci. 1183, 237–250 10.1111/j.1749-6632.2009.05135.x [DOI] [PubMed] [Google Scholar]
- Brodin P., Majlessi L., Brosch R., Smith D., Bancroft G., Clark S., Williams A., Leclerc C., Cole S. T. (2004). Enhanced protection against tuberculosis by vaccination with recombinant Mycobacterium microti vaccine that induces T cell immunity against region of difference 1 antigens. J. Infect. Dis. 190, 115–122 10.1086/421468 [DOI] [PubMed] [Google Scholar]
- Brooks M. N., Rajaram M. V., Azad A. K., Amer A. O., Valdivia-Arenas M. A., Park J. H., Nunez G., Schlesinger L. S. (2011). NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell. Microbiol. 13, 402–418 10.1111/j.1462-5822.2010.01544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell J. H., Scidmore M. A. (2007). Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol. Mol. Biol. Rev. 71, 636–652 10.1128/MMBR.00023-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf S., Kurts C. (2008). Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol. 20, 89–95 10.1016/j.coi.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Burgdorf S., Scholz C., Kautz A., Tampe R., Kurts C. (2008). Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 9, 558–566 10.1038/ni.1601 [DOI] [PubMed] [Google Scholar]
- Caceres N., Tapia G., Ojanguren I., Altare F., Gil O., Pinto S., Vilaplana C., Cardona P. J. (2009). Evolution of foamy macrophages in the pulmonary granulomas of experimental tuberculosis models. Tuberculosis (Edinb) 89, 175–182 10.1016/j.tube.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Canaday D. H., Ziebold C., Noss E. H., Chervenak K. A., Harding C. V., Boom W. H. (1999). Activation of human CD8+ alpha beta TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen-processing pathway. J. Immunol. 162, 372–379 [PubMed] [Google Scholar]
- Carbone F. R., Bevan M. J. (1990). Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J. Exp. Med. 171, 377–387 10.1084/jem.171.2.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrian I., Visentin G., Blanchard N., Jouve M., Bobard A., Moita C., Enninga J., Moita L. F., Amigorena S., Savina A. (2011). Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell 147, 1355–1368 10.1016/j.cell.2011.11.021 [DOI] [PubMed] [Google Scholar]
- Chefalo P. J., Harding C. V. (2001). Processing of exogenous antigens for presentation by class I MHC molecules involves post-Golgi peptide exchange influenced by peptide-MHC complex stability and acidic pH. J. Immunol. 167, 1274–1282 [DOI] [PubMed] [Google Scholar]
- Clemens D. L., Horwitz M. A. (1995). Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181, 257–270 10.1084/jem.181.1.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D. L., Horwitz M. A. (1996). The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp. Med. 184, 1349–1355 10.1084/jem.184.4.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D. L., Lee B. Y., Horwitz M. A. (2002). The Mycobacterium tuberculosis phagosome in human macrophages is isolated from the host cell cytoplasm. Infect. Immun. 70, 5800–5807 10.1128/IAI.70.10.5800-5807.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Chastellier C., Forquet F., Gordon A., Thilo L. (2009). Mycobacterium requires an all-around closely apposing phagosome membrane to maintain the maturation block and this apposition is re-established when it rescues itself from phagolysosomes. Cell. Microbiol. 11, 1190–1207 10.1111/j.1462-5822.2009.01324.x [DOI] [PubMed] [Google Scholar]
- Deretic V., Fratti R. A. (1999). Mycobacterium tuberculosis phagosome. Mol. Microbiol. 31, 1603–1609 10.1046/j.1365-2958.1999.01279.x [DOI] [PubMed] [Google Scholar]
- Deretic V., Via L. E., Fratti R. A., Deretic D. (1997). Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking. Electrophoresis 18, 2542–2547 10.1002/elps.1150181409 [DOI] [PubMed] [Google Scholar]
- Dumont A., Sheldon H. (1965). Changes in the fine structure of macrophages in experimentally produced tuberculous granulomas in hamsters. Lab. Invest. 14, 2034–2055 [PubMed] [Google Scholar]
- Dye C., Scheele S., Dolin P., Pathania V., Raviglione M. C. (1999). Consensus statement: global burden of tuberculosis: estimated incidence, prevalence. and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282, 677–686 10.1001/jama.282.7.686 [DOI] [PubMed] [Google Scholar]
- Gagnon E., Duclos S., Rondeau C., Chevet E., Cameron P. H., Steele-Mortimer O., Paiement J., Bergeron J. J., Desjardins M. (2002). Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110, 119–131 10.1016/S0092-8674(02)00797-3 [DOI] [PubMed] [Google Scholar]
- Goldszmid R. S., Coppens I., Lev A., Caspar P., Mellman I., Sher A. (2009). Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J. Exp. Med. 206, 399–410 10.1084/jem.20082108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grode L., Seiler P., Baumann S., Hess J., Brinkmann V., Nasser Eddine A., Mann P., Goosmann C., Bandermann S., Smith D., Bancroft G. J., Reyrat J. M., Van Soolingen D., Raupach B., Kaufmann S. H. (2005). Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Invest. 115, 2472–2479 10.1172/JCI24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzke J. E., Harriff M. J., Siler A. C., Nolt D., Delepine J., Lewinsohn D. A., Lewinsohn D. M. (2009). The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 5, e1000374. 10.1371/journal.ppat.1000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzke J. E., Lewinsohn D. M. (2005). Role of CD8+ T lymphocytes in control of Mycobacterium tuberculosis infection. Microbes Infect. 7, 776–788 10.1016/j.micinf.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Grotzke J. E., Siler A. C., Lewinsohn D. A., Lewinsohn D. M. (2010). Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway. J. Immunol. 185, 4336–4343 10.4049/jimmunol.1000801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermonprez P., Saveanu L., Kleijmeer M., Davoust J., Van Endert P., Amigorena S. (2003). ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 10.1038/nature01911 [DOI] [PubMed] [Google Scholar]
- Houde M., Bertholet S., Gagnon E., Brunet S., Goyette G., Laplante A., Princiotta M. F., Thibault P., Sacks D., Desjardins M. (2003). Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402–406 10.1038/nature01912 [DOI] [PubMed] [Google Scholar]
- Hsu T., Hingley-Wilson S. M., Chen B., Chen M., Dai A. Z., Morin P. M., Marks C. B., Padiyar J., Goulding C., Gingery M., Eisenberg D., Russell R. G., Derrick S. C., Collins F. M., Morris S. L., King C. H., Jacobs W. R., Jr. (2003). The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. U.S.A. 100, 12420–12425 10.1073/pnas.1635213100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane J., Remold H. G., Kornfeld H. (2000). Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 164, 2016–2020 [DOI] [PubMed] [Google Scholar]
- Kelley V. A., Schorey J. S. (2003). Mycobacterium’s arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol. Biol. Cell 14, 3366–3377 10.1091/mbc.E02-12-0780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E., Yasuda T., Kanai K. (1982). Electron microscopic demonstration of close contact between intracellular mycobacteria and the phagosomal membrane. Jpn. J. Med. Sci. Biol. 35, 197–201 [DOI] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Rock K. L. (1995). A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science 267, 243–246 10.1126/science.7809629 [DOI] [PubMed] [Google Scholar]
- Leake E. S., Myrvik Q. N., Wright M. J. (1984). Phagosomal membranes of Mycobacterium bovis BCG-immune alveolar macrophages are resistant to disruption by Mycobacterium tuberculosis H37Rv. Infect. Immun. 45, 443–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Remold H. G., Ieong M. H., Kornfeld H. (2006). Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J. Immunol. 176, 4267–4274 [DOI] [PubMed] [Google Scholar]
- Lee J., Repasy T., Papavinasasundaram K., Sassetti C., Kornfeld H. (2011). Mycobacterium tuberculosis induces an atypical cell death mode to escape from infected macrophages. PLoS ONE 6, e18367. 10.1371/journal.pone.0018367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leepiyasakulchai C., Ignatowicz L., Pawlowski A., Kallenius G., Skold M. (2012). Failure to recruit anti-inflammatory CD103+ dendritic cells and a diminished CD4+Foxp3+ regulatory T cell pool in mice that display excessive lung inflammation and increased susceptibility to Mycobacterium tuberculosis. Infect. Immun. 80, 1128–1139 10.1128/IAI.05552-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn D. M., Alderson M. R., Briden A. L., Riddell S. R., Reed S. G., Grabstein K. H. (1998). Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J. Exp. Med. 187, 1633–1640 10.1084/jem.187.10.1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn D. M., Grotzke J. E., Heinzel A. S., Zhu L., Ovendale P. J., Johnson M., Alderson M. R. (2006). Secreted proteins from Mycobacterium tuberculosis gain access to the cytosolic MHC class-I antigen-processing pathway. J. Immunol. 177, 437–442 [DOI] [PubMed] [Google Scholar]
- Lin M. L., Zhan Y., Villadangos J. A., Lew A. M. (2008). The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol. Cell Biol. 86, 353–362 10.1038/icb.2008.3 [DOI] [PubMed] [Google Scholar]
- Majlessi L., Brodin P., Brosch R., Rojas M. J., Khun H., Huerre M., Cole S. T., Leclerc C. (2005). Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system. J. Immunol. 174, 3570–3579 [DOI] [PubMed] [Google Scholar]
- Master S. S., Rampini S. K., Davis A. S., Keller C., Ehlers S., Springer B., Timmins G. S., Sander P., Deretic V. (2008). Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 3, 224–232 10.1016/j.chom.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaccaro R. J., Gedde M., Jensen E. R., Van Santen H. M., Ploegh H. L., Rock K. L., Bloom B. R. (1996). Major histocompatibility class I presentation of soluble antigen facilitated by Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U.S.A. 93, 11786–11791 10.1073/pnas.93.21.11786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough K. A., Kress Y., Bloom B. R. (1993). Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect. Immun. 61, 2763–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Steinman R. M. (2001). Dendritic cells: specialized and regulated antigen processing machines. Cell 106, 255–258 10.1016/S0092-8674(01)00449-4 [DOI] [PubMed] [Google Scholar]
- Merckx J. J., Brown A. L., Jr., Karlson A. G. (1964). An electron-microscopic study of experimental infections with acid-fast bacilli. Am. Rev. Respir. Dis. 89, 485–496 [DOI] [PubMed] [Google Scholar]
- Moreira A. L., Wang J., Tsenova-Berkova L., Hellmann W., Freedman V. H., Kaplan G. (1997). Sequestration of Mycobacterium tuberculosis in tight vacuoles in vivo in lung macrophages of mice infected by the respiratory route. Infect. Immun. 65, 305–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwandumba H. C., Russell D. G., Nyirenda M. H., Anderson J., White S. A., Molyneux M. E., Squire S. B. (2004). Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J. Immunol. 172, 4592–4598 [DOI] [PubMed] [Google Scholar]
- Myrvik Q. N., Leake E. S., Wright M. J. (1984). Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am. Rev. Respir. Dis. 129, 322–328 [PubMed] [Google Scholar]
- Neyrolles O., Gould K., Gares M. P., Brett S., Janssen R., O’Gaora P., Herrmann J. L., Prevost M. C., Perret E., Thole J. E., Young D. (2001). Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J. Immunol. 166, 447–457 [DOI] [PubMed] [Google Scholar]
- Opie E. L., Aronson J. D. (1927). Tubercule bacilli in latent tuberculous lesions and in lung tissue without tuberculous lesions. Arch. Pathol. 1–21 [Google Scholar]
- Pan H., Yan B. S., Rojas M., Shebzukhov Y. V., Zhou H., Kobzik L., Higgins D. E., Daly M. J., Bloom B. R., Kramnik I. (2005). Ipr1 gene mediates innate immunity to tuberculosis. Nature 434,767–772 10.1038/nature03419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Tamayo M. H., Gonzalez-Juarrero M., Orme I. M., Ordway D. J. (2006). Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J. Leukoc. Biol. 79, 80–86 10.1189/jlb.0505250 [DOI] [PubMed] [Google Scholar]
- Paul S., Laochumroovorapong P., Kaplan G. (1996). Comparable growth of virulent and avirulent Mycobacterium tuberculosis inhuman macrophages in vitro. J. Infect. Dis. 174, 105–112 10.1093/infdis/174.1.105 [DOI] [PubMed] [Google Scholar]
- Peyron P., Vaubourgeix J., Poquet Y., Levillain F., Botanch C., Bardou F., Daffé M., Emile J. F., Marchou B., Cardona P. J., de Chastellier C., Altare F. (2008). Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 4, e1000204. 10.1371/journal.ppat.1000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. (1993). Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature 361, 359–362 10.1038/361359a0 [DOI] [PubMed] [Google Scholar]
- Philips J. A. (2008). Mycobacterial manipulation of vacuolar sorting. Cell. Microbiol. 10, 2408–2415 10.1111/j.1462-5822.2008.01239.x [DOI] [PubMed] [Google Scholar]
- Pieters J. (2001). Entry and survival of pathogenic mycobacteria in macrophages. Microbes Infect. 3, 249–255 10.1016/S1286-4579(01)01376-4 [DOI] [PubMed] [Google Scholar]
- Pym A. S., Brodin P., Majlessi L., Brosch R., Demangel C., Williams A., Griffiths K. E., Marchal G., Leclerc C., Cole S. T. (2003). Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9, 533–539 10.1038/nm859 [DOI] [PubMed] [Google Scholar]
- Rahman A., Srivastava S. S., Sneh A., Ahmed N., Krishnasastry M. V. (2010). Molecular characterization of tlyA gene product, Rv1694 of Mycobacterium tuberculosis: a non-conventional hemolysin and a ribosomal RNA methyl transferase. BMC Biochem. 11, 35. 10.1186/1471-2091-11-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra L., Simmons D., Harding C. V. (2009). MHC molecules and microbial antigen processing in phagosomes. Curr. Opin. Immunol. 21, 98–104 10.1016/j.coi.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G. (2001). Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell Biol. 2, 569–577 [DOI] [PubMed] [Google Scholar]
- Russell D. G., Mwandumba H. C., Rhoades E. E. (2002). Mycobacterium and the coat of many lipids. J. Cell Biol. 158, 421–426 10.1083/jcb.200205034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. S., Iskandar M., Mykytczuk O. L., Nash J. H., Krishnan L., Sad S. (2007). A reduced antigen load in vivo, rather than weak inflammation, causes a substantial delay in CD8+ T cell priming against Mycobacterium bovis (bacillus Calmette-Guerin). J. Immunol. 179, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A. A., Nambiar J. K., Wozniak T. M., Roediger B., Shklovskaya E., Britton W. J., Fazekas De St Groth B., Triccas J. A. (2009). Antigen load governs the differential priming of CD8 T cells in response to the bacille Calmette Guerin vaccine or Mycobacterium tuberculosis infection. J. Immunol. 182, 7172–7177 10.4049/jimmunol.0801694 [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Winau F., Sieling P. A., Fischer K., Collins H. L., Hagens K., Modlin R. L., Brinkmann V., Kaufmann S. H. (2003). Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 9, 1039–1046 10.1038/nm906 [DOI] [PubMed] [Google Scholar]
- Segura E., Villadangos J. A. (2009). Antigen presentation by dendritic cells in vivo. Curr. Opin. Immunol. 21, 105–110 10.1016/j.coi.2009.03.011 [DOI] [PubMed] [Google Scholar]
- Shams H., Barnes P. F., Weis S. E., Klucar P., Wizel B. (2003). Human CD8+ T cells recognize epitopes of the 28-kDa hemolysin and the 38-kDa antigen of Mycobacterium tuberculosis. J. Leukoc. Biol. 74, 1008–1014 10.1189/jlb.0403138 [DOI] [PubMed] [Google Scholar]
- Shen L., Rock K. L. (2006). Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr. Opin. Immunol. 18, 85–91 10.1016/j.coi.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Shen L., Sigal L. J., Boes M., Rock K. L. (2004). Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity 21, 155–165 10.1016/j.immuni.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Song R., Harding C. V. (1996). Roles of proteasomes, transporter for antigen presentation (TAP), and beta 2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway. J. Immunol. 156, 4182–4190 [PubMed] [Google Scholar]
- Sousa A. O., Mazzaccaro R. J., Russell R. G., Lee F. K., Turner O. C., Hong S., Van Kaer L., Bloom B. R. (2000). Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. U.S.A. 97, 4204–4208 10.1073/pnas.97.8.4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki S., Schaible U. E., Russell D. G. (1996). Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 15, 6960–6968 [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum R., Cammer M., Maitland M. L., Freitag N. E., Condeelis J., Bloom B. R. (1999). Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc. Natl. Acad. Sci. U.S.A. 96, 15190–15195 10.1073/pnas.96.26.15190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Woodworth J., Skold M., Behar S. M. (2005). In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J. Immunol. 175, 3268–3272 [DOI] [PubMed] [Google Scholar]
- van der Wel N., Hava D., Houben D., Fluitsma D., Van Zon M., Pierson J., Brenner M., Peters P. J. (2007). M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129, 1287–1298 10.1016/j.cell.2007.05.059 [DOI] [PubMed] [Google Scholar]
- Van Endert P. (2011). Post-proteasomal and proteasome-independent generation of MHC class I ligands. Cell. Mol. Life Sci. 68, 1553–1567 10.1007/s00018-011-0662-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I., Chua J., Singh S. B., Deretic V. (2004). Cell biology of mycobacterium tuberculosis phagosome. Annu. Rev. Cell Dev. Biol. 20, 367–394 10.1146/annurev.cellbio.20.010403.114015 [DOI] [PubMed] [Google Scholar]
- Via L. E., Deretic D., Ulmer R. J., Hibler N. S., Huber L. A., Deretic V. (1997). Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272, 13326–13331 10.1074/jbc.272.20.13326 [DOI] [PubMed] [Google Scholar]
- Weerdenburg E. M., Peters P. J., Van Der Wel N. N. (2010). How do mycobacteria activate CD8+ T cells? Trends Microbiol. 18, 1–10 10.1016/j.tim.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Wolf A. J., Linas B., Trevejo-Nunez G. J., Kincaid E., Tamura T., Takatsu K., Ernst J. D. (2007). Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 179, 2509–2519 [DOI] [PubMed] [Google Scholar]
- Wong D., Bach H., Sun J., Hmama Z., Av-Gay Y. (2011). Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc. Natl. Acad. Sci. U.S.A. 108, 19371–19376 10.1073/pnas.1018927108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth J. S., Fortune S. M., Behar S. M. (2008). Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect. Immun. 76, 4199–4205 10.1128/IAI.00307-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Cooper A., Sturgill-Koszycki S., Van Heyningen T., Chatterjee D., Orme I., Allen P., Russell D. G. (1994). Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153, 2568–2578 [PubMed] [Google Scholar]