Abstract
A dipodal bis-urea receptor has been synthesized from the reaction of 8-amino quinoline and 1,4-phenylene diisocyanate in dichloromethane, and the anion binding ability of the receptor has been studied using fluoride, chloride, bromide, iodide, perchlorate, nitrate, dihydrogen phosphate and hydrogen sulfate by UV-Vis titrations in DMSO. The results show that the receptor binds each of the anions with a 1:1 stoichiometry, showing high affinity, and moderate selectivity for hydrogen sulfate among the anions studied. Ab initio calculations based on density functional theory (DFT) suggest that an anion (X−) is bonded within the cleft formed by the two arms of the receptor through two NH…X− and two aromatic CH…X− interactions. The results from solution and theoretical studies suggest that binding is predominately influenced by hydrogen bonding interactions and the basicity of anions.
Keywords: Urea receptor, Anion coordination, UV-Vis titrations, Host-guest complex, Anion selectivity
Anion-recognition chemistry emerged in the 1960s with the discovery of diazabicyclic compounds (named katapinands) by Park and Simmons, which were shown to form complexes with halide anions in water by H-bonding interactions [1]. Seven years later, the structure was confirmed crystallographically by Bell et al., showing one encapsulated chloride in the macrocyclic cavity [2]. During the last 40 years, a great deal of attention has been given to polyamine-based hosts for binding anions by virtue of hydrogen bonding and electrostatic interactions. However, the binding of such receptors is greatly affected by the solution pH [3,4]. Molecules with H-bond donors, such as amide, thioamide, sulfonamide, urea and thiourea have been reported to bind anions by hydrogen-bonding interactions under neutral conditions [5a–5f].
In particular, molecules with a urea group can provide two H-bond donors. In 1992, Wilcox reported that simple acyclic urea hosts containing a single urea binding site were able to form complexes with phosphonates, sulfates, and carboxylates in CHCl3 [5b]. In a subsequent report of Hamilton, one receptor with a single urea group, and another with two urea groups were reported to bind acetate (K = 45 M−1), and glutarate (K = 640 M−1) in DMSO [5c]. Recently, Custelcean et al. reported a tren-based urea linked with Ag2SO4 showing a sulfate binding to twelve hydrogen bonds [5f]. Wu et al. have shown a multiple-coordinated sulfate complex with a tris(3-pyridyl)urea [6]. Fabbrizzi and coworkers reported a simple urea containing two nitrobenzene groups which formed a 1:1 complex with an acetate anion [7]. Gale and coworkers reported indole-based acyclic urea ligands, which were able to form a 2:1 complex with carbonate [8a]. In an earlier paper, we reported a heptacoordinated hydrogen sulfate surrounded by three flexible urea-based tripodal receptors [8b]. Albrecht and coworkers reported a quinoline based receptor containing both amide and urea groups, showing high selectivity for fluoride in chloroform [9a]. In particular, the nitrogen atom in either pyridine or quinoline can act as a hydrogen bond acceptor to preorganize the host molecules, enhancing the binding affinity for anions [9a,9b]. Due to the basic nature of quinoline nitrogen, this group can also be protonated to provide an additional binding site for anions [9c]. In an effort to design urea receptors with rigid spacers for hosting anionic guests, we incorporated a difunctional p-xylyl spacer as a linker to quinoline groups. As shown in Figure 1, the receptor forms a cavity in its optimized structure, which could be used to host an anion. Herein, we report a new quinoline based dipodal bis-urea receptor containing two urea binding sites, its anion binding ability in DMSO, and DFT calculations for the anion complexes.
Figure 1.
Structure of the receptor 1 (Optimized structure is shown on the right).
The synthesis of receptor 1, as shown in Scheme 1, was accomplished following the similar strategy employed for the related compounds [7,8a,8b]. The reaction of 1,4-phenylene diisocyanate with two equivalents of 8-amino quinoline in dichloromethane afforded the bisurea host.
Scheme 1.
Synthesis of 1. (i) CH2Cl2 (ii) stirring, r.t, 6 hrs.
The receptor was found to be insoluble in H2O, CH3OH, CH3CN and CHCl3, but fairly soluble in DMSO. Therefore, the anion binding ability of 1 was evaluated in DMSO by UV-Vis spectroscopy. The host (2.5 x 10−5 M) was titrated with a number of anions using [n-Bu4N]+A− (A− = F−, Cl−, Br−, I−, ClO4−, NO3−, HSO4− and H2PO4−), and its absorbance was recorded at room temperature. The receptor showed two absorption bands, one at 281nm and the other at 344 nm, in the absence of an anion.
The addition of an anion to the receptor solution resulted in a gradual increase in the intensity. Figure 2 illustrates the representative titration spectra for fluoride and iodide, derived from the experiments with portionwise additions of the respective anions (0 to 20 equivalents). The host showed a similar spectral change when it was titrated with oxoanions. The representative titration spectra for bisulfate and perchlorate are displayed in Figure 3. This observation is consistent with our previous results for a macrocycle-based dinuclear host with iodide titrations [9d].
Figure 2.
Changes in absorption spectra of 1 (2.5 x 10−5 M) with an increasing amount of fluoride (A) and iodide (B) in DMSO.
Figure 3.
Changes in absorption spectra of 1 (2.5 x 10−5 M) with an increasing amount of bisulfate (A) and perchlorate (B) in DMSO.
However, no appreciable change in λmax was observed for the addition of anions. The relative absorbance (I/I0, where I0 and I represent the absorbance of 1 before and after the addition of an anion, respectively) as a function of anion concentration provided the best fit for a 1:1 association model (Figure 4). The binding data as estimated from the non-linear regression analysis are given in Table 1. The binding data were also verified at λmax = 281 nm, showing almost similar results. As shown in Table 1, the highest binding constant was observed for fluoride among the halide series, showing the binding trend as F− > Cl− > Br− > I−. Among the oxoanion series the highest affinity was observed for bisulfate under similar conditions. The binding order of 1 for oxoanions was observed to be HSO4− > H2PO4− > NO3− >ClO3. The experimental data suggest that the binding is primarily influenced by the relative basicity of the anions [7].
Figure 4.
Titration plots of change in the absorption of 1 with an increasing amount of anions (R = 0 – 25) at λmax = 344 nm.
Table 1.
Binding constants (K, M−1) and binding energies (E) for the anion complexes of 1 as determined from UV-Vis titrations in DMSO.
| Anions | Ka/M−1 | Eb, kcal/mol |
|---|---|---|
| F− | 7500 | 61.8 |
| Cl− | 5640 | 37.0 |
| Br− | 3560 | 32.0 |
| I− | 3030 | c |
| HSO4− | 9840 | 35.9 |
| H2PO4− | 6580 | 43.3 |
| NO3− | 2500 | 37.3 |
| ClO4− | 1485 | 31.8 |
Binding constants determined by UV-Vis titrations in DMSO (error limit is less than 20%);
binding energy obtained from DFT calculations;
the 6-31+G(d,p) basis set is not available for iodide.
Attempts were also made to examine the interactions of 1 with various anions by 1H NMR experiments in DMSO-d6. However, with the exception of F− and H2PO4−, the addition of an anion to the host solution showed no appreciable shift change to warrant the determination of tenable binding constants (see supporting data). In particular, the addition of one equivalent of F− caused broadening of the NH peaks. Such behavior was not observed for any other anion examined in this study. As shown in Figure 5, these peaks almost disappeared upon further addition of F−, which could be due to the possible proton abstract by fluoride from NH groups of 1. A similar effect was reported previously by Fabbrizzi and coworkers for a urea-based host, where F− was shown to convert into HF2− [7]. In addition, for the aromatic CHs of the quinoline ring both downfield and upfield shifts were observed, which are the results of deshielding and shielding effects of C-H bonds. Interestingly, the CH peak (8.57 ppm), which is close to the NHC=O, was observed to shift downfield - an indication of possible CH-anion interactions. This assumption is further supported by the observation of CH anion interactions in the optimized structure. Other CH signals were shown to move upfield.
Figure 5.
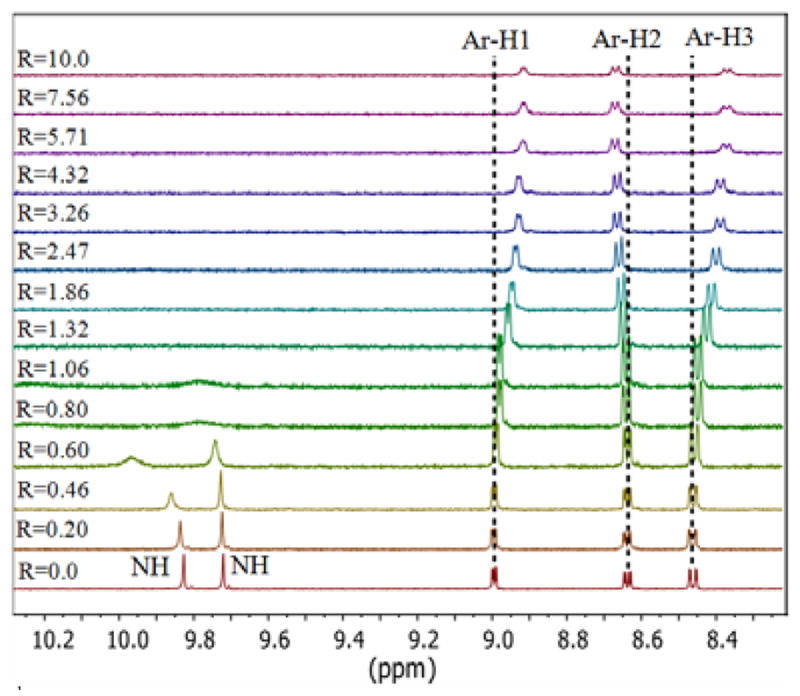
1H NMR spectra of 1 (2 mM) with an increasing amount of TBAF (R = [TBAHF]0/[L]0) in DMSO-d6. [Ar-H1 = ArHN, Ar-H2 = ArHCNH, Ar-H3 = ArHCCH].
In order to investigate the properties of the anion-ligand complexes, with a view to estimating binding energies, we also carried out ab initio calculations based on density functional theory (DFT). Since the anion-ligand systems involve hydrogen- bonding interactions, it is important to choose an exchange-correlation functional which accurately captures these electronic effects. To this end, all DFT calculations were performed using the M06-2X hybrid functional which incorporates an improved description of dispersion energies, an effect which was previously found to be necessary for describing non-covalent interactions [10a]. The optimized structure of the ligand is shown in Figure 1 and the structures of anion complexes are shown in Figures 6 and 7. In the optimized free host, two arms are faced on the same side to form a suitable cavity, and two NH protons are directed towards the cavity. In the DFT-optimized complexes, the host deforms its geometry considerably in order to bind an anion. As seen in Figures 6 and 7, each anion is encapsulated within the cavity and held with four hydrogen bonds from two NHs, and two ArH protons. The CH…anion interactions are well documented in the literature [10b]. The calculated binding energies for the complexes, as shown in Table 1, correlate with the relative basicity, which is also fairly consistent with the binding data within the halide and oxoanion series.
Figure 6.
Optimized structures of the anion complexes of 1 with: fluoride (A), chloride (B), and bromide showing perspective views (left side) and space filling models (right side).
Figure 7.
Optimized structures of the anion complexes of 1 with hydrogen sulfate (A), dihydrogen phosphate (B), nitrate (C) and perchlorate (D) showing perspective views (left side) and space filling models (right side).
In summary, we report a simple bis-urea, which effectively binds oxoanions and halides with high binding for hydrogen sulfate showing good selectivity. DFT calculations suggest that an anion is coordinated not only with the NH groups, but also with aromatic protons. The introduction of two bulky aromatic rings allows the receptor to form a cavity suitable for hosting an anion, and also allows for a spectroscopic analysis for anions in solution. In particular, the binding constant for bisulfate (K = 9840 M−1) is much higher than that observed for the same anion (K = 1000 M−1) with a tren-based ligand measured by 1H NMR studies in DMSO [8b]. However, this value is lower than that reported by Fabbrizzi with 1,3-bis(4-nitrophenyl)urea for bisulfate (K = 18200 M−1) measured by UV-Vis titrations in CH3CN – a comparatively less polar solvent [7]. Clearly, the high affinity for bisulfate and fluoride is likely due to the strong H-bonding interactions between the host and anions.
Experimental
General
All the chemicals were purchased from Aldrich as reagent grade and were used without further purification. Nuclear magnetic resonance (NMR) spectra were recorded at 25°C on a Varian 500 FT-NMR. Chemical shifts for samples measured were expressed in parts per million, and calibrated against TMS as an external reference in a sealed capillary tube. All NMR data were processed and analyzed with MestReNova Version 6.1.1-6384. UV titrations were performed using a spectrophotometer (Solid Spec-3700, 120V, UV-Vis-NIR Spectrophotometer Shimadzu). Mass spectral data were obtained in ESI-MS positive mode on a FINNIGAN LCQDUO. Melting point was determined on a Mel-Temp (Electrothermal 120 VAC 50/60 Hz) melting point apparatus and was uncorrected.
Synthesis
1,4-Phenylene diisocyanate (500 mg, 3.125 mmol) was reacted with 8-amino quinoline (901 mg, 6.34 mmol) in dichloromethane (500 mL) at room temperature under constant stirring. The mixture was refluxed for 6 h. The precipitate was collected by filtration, washed by chloroform, and dried under vacuum to give a white solid (1.195 gm, 85% yield).
White powder.
MP: >200°C.
IR (KBr): νN-H 3335, 3265 cm−1, νN-H 1650 cm−1.
1H NMR (500 MHz, DMSO-d6) δ: 9.77 (2H, s, ArH), 9.66 (2H, s, ArH), 8.93 (2H, d, J = 5.0 Hz, ArH), 8.57 (2H, d, J = 5.0 Hz, ArH), 8.40 (2H, d, J = 10.0 Hz, ArH), 7.65 (2H, dd, J1 = 5.0 Hz, J2 = 5.0 Hz, J3 = 5.0 Hz, ArH) 7.56 (4H, d, J = 10 Hz, ArH), 7.48 (4H, s,).
13C NMR (125 MHz, DMSO-d6) δ: 152.5 (CO), 148.3 (CAr), 137.8 (CHAr), 136.7 (CNAr), 136.0 (CHAr), 134.3 (CAr) 127.9 (CAr), 127.3 (CHAr), 122.1 (CNAr), 119.6 (CHAr), 118.9 (CAr) 114.3 (CAr).
MS (EI, 70 eV): m/z (%) = 449.0 [M + H+] (40), 471.0 [M + Na+] (100), 692.1 (37), 918.7 (80).
Anal. Calcd. for C26H20N6O2: C, 69.63; H, 4.49; N, 18.74. Found: C, 69.33; H, 4.48; N, 18.90.
UV-Vis titration studies
Binding properties of 1 for anions were determined by UV-Vis titrations. All the measurements were performed by titrating 1 with [n-Bu4N]+A− (A− = F−, Cl−, Br−, I−, NO3−, ClO4−, HSO4− and H2PO4−) in DMSO at 25°C. Initial concentrations of the ligand and anions were 2.5 x 10−5 M and 2.5 x 10−3 M, respectively. Each titration was performed by 15 measurements varying [anion]0/[ligand]0 = 0 – 20 equivalents, and the binding constant K was calculated by fitting the change of UV- Vis absorbance (ΔA) with a 1:1 association model using the equation, ΔA= ([G]0 + [H]0 + 1/K − ([G]0+ [H]0 + 1/K)2 − 4[H]0[G]0)1/2) ΔAmax/ΔA [H]0 (where H = ligand and G = anion). The error limit in K was less that 10%.
1H NMR studies
Interactions of 1 for anions (using Bu4N]+A−) were also examined by 1H NMR studies in DMSO-d6. In this case, the initial concentration of 1 was 2.0 x 10−2 M, while the anion solution was prepared at 10 times higher concentration in the same solvent. Sodium salt of 3-(trimethylsilyl)propionic-2,2,3,3,-d4 acid (TSP) in DMSO-d6 was used as an external reference in a sealed capillary tube.
Molecular modeling
All DFT calculations were performed using the M06-2X hybrid functional, which incorporates an improved description of dispersion energies. From the equilibrium geometry, each of the negatively charged anions was added near the two amines at the center of the ligand. The geometries of the anion-urea complexes were then optimized at the M06-2X/6-31g(d,p) level of theory. Single-point energies with a much larger 6-311+G(d,p) basis set was subsequently performed for each of the relaxed geometries.
Supplementary Material
Acknowledgments
The National Science Foundation is acknowledged for a CAREER award (CHE-1056927) to MAH. The work was supported by the National Institute of Health (G12RR013459). The NMR instrument used for this work was funded by the National Science Foundation (CHE-0821357).
Footnotes
Supplementary data: 1H and 13C NMR spectra for 1, mass spectrum, and stacked 1H NMR spectra of binding studies are contained in the supplementary data.
References
- 1.Park CH, Simmons HE. Macrobicyclic amines. III. Encapsulation of halide ions by in, in-1, (k + 2)-diazabicyclo[k.l.m.] alkane-ammonium ions. Journal of the American Chemical Society. 1968;90:2431–2433. [Google Scholar]
- 2.Bell RA, Christoph GG, Fronczek FR, Marsh RE. The cation H13O6+: A short, symmetric hydrogen bond. Science. 1975;190:151–152. [Google Scholar]
- 3.Bowman-James K. Alfred Werner revisited: The coordination chemistry of anions. Accounts of Chemical Research. 2005;38:671–678. doi: 10.1021/ar040071t. [DOI] [PubMed] [Google Scholar]
- 4.Hossain MA. Inclusion complexes of halide anions with macrocyclic receptors. Current Organic Chemistry. 2008;12:1231–1256. [Google Scholar]
- 5.(a) Valiyaveettil S, Engbersen JFJ, Verboom W, Reinhoudt DN. Synthesis and complexation studies of neutral anion receptors. Angewandte Chemie International Edition. 1993;32:900–901. [Google Scholar]; (b) Smith PJ, Reddington MV, Wilcox CS. Ion pair binding by a urea in chloroform solution. Tetrahedron Letters. 1992;33:6085–6088. [Google Scholar]; (c) Fan E, Van Arman SA, Kincaid S, Hamilton AD. Molecular recognition: Hydrogen-bonding receptors that function in highly competitive solvents. Journal of the American Chemical Society. 1993;115:369–370. [Google Scholar]; (d) Inoue Y, Kanbara T, Yamamoto T. Preparation of a new receptor for anions, macrocyclic polythiolactam-Structure and high anion-binding ability. Tetrahedron Letters. 2003;44:5167–5169. [Google Scholar]; (e) Hossain MA, Llinares JM, Powell D, Bowman-James K. Elite new anion ligands: polythioamide macrocycles. Inorganic Chemistry. 2003;42:5043–5045. doi: 10.1021/ic034735r. [DOI] [PubMed] [Google Scholar]; (f) Custelcean R, Moyer BA, Hay BP. A coordinatively saturated sulfate encapsulated in a metal-organic framework functionalized with urea hydrogen-bonding groups. Chemical Communications. 2005:5971–5973. doi: 10.1039/b511809c. [DOI] [PubMed] [Google Scholar]
- 6.Wu B, Liang J, Yang J, Jia C, Yang X-J, Zhang H, Tangb N, Janiak C. Sulfate ion encapsulation in caged supramolecular structures assembled by second-sphere coordination. Chemical Communications. 2008:1762–1764. doi: 10.1039/b719019k. [DOI] [PubMed] [Google Scholar]
- 7.Boiocchi M, Del Boca L, Esteban-Gómez D, Fabbrizzi L, Licchelli M, Monzani E. Nature of urea-fluoride interaction: Incipient and definitive proton transfer. Journal of the American Chemical Society. 2004;126:16507–16514. doi: 10.1021/ja045936c. [DOI] [PubMed] [Google Scholar]
- 8.(a) Caltagirone C, Hiscock JR, Hursthouse MB, Light ME, Gale PA. 1,3-Diindolylureas and 1,3-diindolylthioureas: anion complexation studies in solution and the solid state. Chemistry - A European Journal. 2008;14:10236–10243. doi: 10.1002/chem.200801639. [DOI] [PubMed] [Google Scholar]; (b) Pramanik A, Thompson B, Hayes T, Tucker K, Powell DR, Bonnesen PV, Ellis ED, Lee KS, Yu H, Hossain MA. Seven-coordinate anion complex with a tren-based urea: Binding discrepancy of hydrogen sulfate in solid and solution states. Organic & Biomolecular Chemistry. 2011;9:4444, 4447. doi: 10.1039/c1ob05052d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Albrecht M, Triyanti, Schiffers S, Osetska O, Raabe G, Wieland T, Russo L, Rissanen K. Anion receptors based on a quinoline backbone. European Journal of Organic Chemistry. 2007:2850–2858. [Google Scholar]; (b) Hossain MA, Kang SO, Powell D, Bowman-James K. A new class of amide/quaternized amine macrocycles and the chelate effect. Inorganic Chemistry. 2003;42:1397–1399. doi: 10.1021/ic0263140. [DOI] [PubMed] [Google Scholar]; (c) Kalita D, Deka H, Samanta SS, Guchait S, Baruah JB. Interactions of amino acids, carboxylic acids, and mineral acids with different quinoline derivatives. Journal of Molecular Structure. 2011;990:183–196. [Google Scholar]; (d) Mendy JS, Saeed MA, Fronczek FR, Powell DR, Hossain MA. Anion recognition and sensing by a new macrocyclic dinuclear copper(II) complex: A selective receptor for iodide. Inorganic Chemistry. 2010;49:7223–7225. doi: 10.1021/ic100686m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Wong BM. Noncovalent interactions in supramolecular complexes: A study on corannulene and the double concave buckycatcher. Journal of Computational Chemistry. 2009;30:51–56. doi: 10.1002/jcc.21022. [DOI] [PubMed] [Google Scholar]; (b) Amendola V, Bergamaschi G, Buttafava A, Fabbrizzi L, Monzani E. Recognition and sensing of nucleoside monophosphates by a dicopper(II) cryptate. Journal of the American Chemical Society. 2010;132:147–156. doi: 10.1021/ja9046262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









