Abstract
Importance of the field
Axl and/or Mer expression correlates with poor prognosis in several cancers. Until recently, the specific role of these receptor tyrosine kinases (RTKs) in the development and progression of cancer remained unexplained. Studies demonstrating that Axl and Mer contribute to mechanisms of cell survival, migration, invasion, metastasis, and chemosensitivity justify further investigation of Axl and Mer as novel therapeutic targets in cancer.
Areas covered in this review
Axl and Mer signaling pathways in cancer cells are summarized and evidence validating these RTKs as therapeutic targets in glioblastoma multiforme, non-small cell lung cancer, and breast cancer is examined. A comprehensive discussion of Axl and/or Mer inhibitors in development is also provided.
What the reader will gain
Potential toxicities associated with Axl or Mer inhibition are addressed. We hypothesize that the probable action of Mer and Axl inhibitors on cells within the tumor microenvironment will provide a unique therapeutic opportunity to target both tumor cells and the stromal components which facilitate disease progression.
Take home message
Axl and Mer mediate multiple oncogenic phenotypes and activation of these RTKs constitutes a mechanism of chemoresistance in a variety of solid tumors. Targeted inhibition of these RTKs may be effective as anti-tumor and/or anti-metastatic therapy, particularly if combined with standard cytotoxic therapies.
Keywords: animal models, apoptosis, astrocytoma, breast cancer, cell migration/invasion, cell survival, chemosensitivity, glioblastoma multiforme, glioma, human, metastasis, non-small cell lung cancer, protein kinase inhibitor, receptor tyrosine kinase, signal transduction, targeted therapy
Article Highlights.
Axl and/or Mer receptor tyrosine kinases and their ligands are aberrantly expressed in numerous human cancers. In the absence of described activating mutations, the oncogenic potential of these kinases is thought to arise from autocrine and/or paracrine activation.
Signaling networks downstream of Axl and Mer contribute to a variety of oncogenic mechanisms including cell survival and proliferation, migration and invasion, angiogenesis, chemoresistance, and metastasis.
Axl and Mer inhibition constitutes a novel therapeutic strategy that may enhance the efficacy of standard chemotherapy in glioblastoma multiforme, non-small cell lung cancer, and breast cancer.
Several Axl/Mer inhibitors are currently in development including small molecule tyrosine kinase inhibitors, monoclonal antibodies, and fusion proteins.
1. Introduction
Although age-adjusted cancer death rates in the US have declined over recent decades, cancer remains the second most common cause of death, killing 1,500 Americans every day. Solid tumors of the lung, colon, pancreas, breast, and prostate account for over 50% of all new cancer cases and deaths. While central nervous system tumors are less prevalent, glioblastoma multilforme (GBM) represents the most common brain tumor and is one of the deadliest cancers diagnosed [1]. These sobering facts clearly demonstrate that novel therapeutic strategies must be pursued to reduce cancer-associated morbidity and mortality.
Cancer treatment traditionally involves highly cytotoxic chemotherapeutics, which non-specifically kill any actively dividing cell. These treatments are associated with common toxicities and only short-term efficacy in metastatic disease. New strategies are evolving drugs designed to selectively target oncogenic pathways and molecules dysregulated (e.g., by mutation, aberrant expression, etc.) in specific cancers. Kinases are the most common class of molecules targeted by these new agents. A stunning example is imatinib, approved in 2001 for treatment of chronic myelogenous leukemia (CML), a disease caused by chromosomal translocation (Philadelphia (Ph) chromosome) that results in the BCR-ABL fusion protein, which exhibits constitutive ABL tyrosine kinase activity. Since introduction of this tyrosine kinase inhibitor (TKI), 5-year survival rates for CML have soared to 90%. In addition to its activity against BCR-ABL, imatinib inhibits KIT receptor tyrosine kinase and platelet-derived growth factor receptor alpha (PDGFRα). Nearly all gastrointestinal stromal tumors harbor activating mutations of KIT or PDGFRα and imatinib dramatically improves outcome for patients with this type of solid tumor [2]. Rapid progress over the last decade has lead to FDA approval of numerous biologically targeted anticancer agents [3, 4]. Nearly two-thirds of these novel agents inhibit kinases, including epidermal growth factor receptor (EGFR), Src, and mammalian target of rapamycin (mTOR). In this article, we will introduce the receptor tyrosine kinases (RTKs) Mer and Axl as novel targets for anticancer therapy.
Together with Tyro3, Axl and Mer compose the TAM family of RTKs. Axl and Mer were initially cloned from leukemic cells and the role of these proteins in hematologic malignancies has been discussed elsewhere [5–12]. Axl and Mer share a unique domain structure consisting of extracellular immunoglobulin and fibronectin type III motifs and an intracellular tyrosine kinase domain (Figure 1). Both receptors share the ligand Gas6, which binds Mer with 3–10 fold lower affinity than Axl [13–15]. A second ligand, Protein S, activates Mer but not Axl [16]. Normal functions for Mer and Axl have been described in macrophages and platelets including clearance of apoptotic cells, cytokine secretion, and platelet aggregation (reviewed in [17]). Axl, Mer, and Gas6 are also expressed by erythroid cells and have been implicated in regulation of erythropoiesis [18, 19]. Additionally, natural killer cells require Axl and Mer for differentiation and maturation [20, 21].
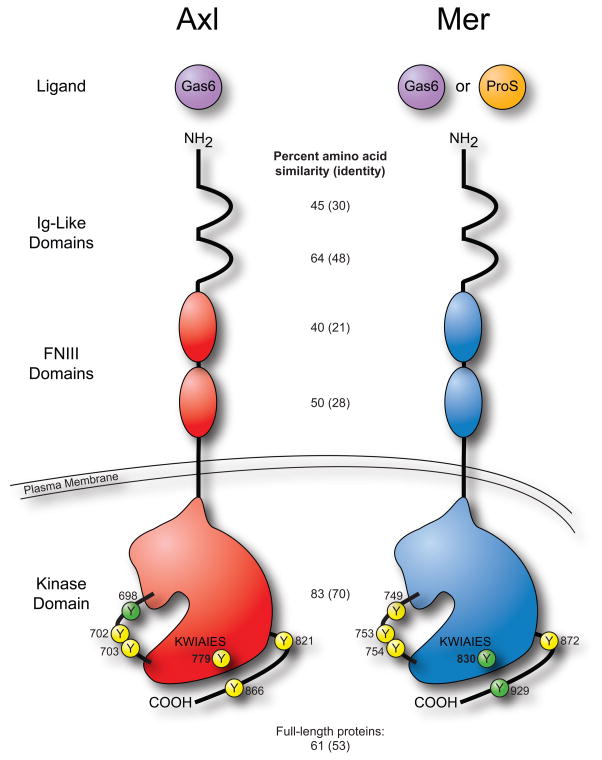
Figure 1. Axl and Mer receptor tyrosine kinases are similar but distinct.
Growth arrest specific gene 6 (Gas6) activates both receptors. Protein S (ProS), a well known anticoagulation factor, activates Mer but not Axl. The domain structure of Axl and Mer is identical, consisting of two immunoglobulin (Ig)-Like domains, two fibronectin type III (FNIII) domains, a single transmembrane domain, and an intracellular kinase domain. The intracellular regions of Axl and Mer contain 17 and 16 tyrosine residues, respectively. Thirteen of them are identical and six of those are symbolized here by a circled Y with the residue number indicated. Residues 698, 702, and 703 of Axl (749, 753, and 754 of Mer) lie within the activation loop. Yellow circles represent tyrosines which have been shown to be phosphorylated while phosphorylation of green tyrosines has not yet been demonstrated [118–122]. Percent amino acid homology within each domain as well as between the full-length proteins was determined using the Basic Local Alignment Search Tool (BLASTP 2.2.23+) to align the sequences of Axl (NP_068713.2) and Mer (NP_006334.2). The conserved sequence (KWIAIES) within the kinase domain distinguishes TAM receptors from other receptor tyrosine kinase families.
Axl and Mer are overexpressed in many human cancers, including various leukemias and numerous solid tumors (Table 1). In non-small cell lung cancer (NSCLC), acute myeloid leukemia (AML), breast cancer, and pancreatic cancer, Axl overexpression is associated with a poor clinical prognosis [12, 22–24]. In gastric cancer, co-expression of Axl and Mer correlated inversely with patient prognosis [25]. The ligands Gas6 and Protein S are also upregulated in some tumors. Furthermore, Gas6 and Protein S are secreted by multiple organs and found in serum [26, 27] suggesting that high levels of Axl and Mer inappropriately expressed on cancer cells may lead to persistent receptor activation. The oncogenic potential of Axl and Mer is attributed to the anti-apoptotic and proliferative signaling pathways (Figure 2) triggered by activation of the tyrosine kinase domain [28, 29]. More specifically, Gas6-dependent signaling through Axl or Mer results in phosphorylation of Akt and Erk 1/2 [8, 30, 31]. Furthermore, anti-apoptotic Bcl-2 family members (e.g., Bcl-2) are upregulated while pro-apoptotic family members (e.g., BAD) are inactivated [8, 31]. Inhibition of either Axl or Mer blocks signaling through the Akt and Erk 1/2 pathways [10, 24, 32], indicating the therapeutic potential of targeting these RTKs. In some instances, complete inactivation of Akt and Erk requires concomitant inhibition of Axl/Mer and other RTKs such as EGFR family members [33, 34], implying cross-talk or alternative activation between the RTK signaling pathways. Consistent with this hypothesis, network modeling of phosphoproteomic data suggests that Axl may be downstream of human epidermal growth factor receptor 2 (HER2) [35]. Furthermore, a recent study indicates that EGFR requires Mer for functional expression in multiple cell lines derived from solid tumors [36]. Agents that target the EGFR family are commonly used to treat specific subsets of NSCLC and breast cancer; Axl and Mer inhibitors may represent additional treatment options in specific patient populations.
Table 1.
Expression of the receptor tyrosine kinases Axl and Mer and the ligands Gas6 and Protein S in solid tumors.
| Tumor Origin | Oncogene(s) | References |
|---|---|---|
| Bone | • | [125] |
| Brain | ••• | [32, 55, 56]a |
| Breast | ••• | [22, 33, 72–74, 76–78]b, c, d |
| Colon & rectum | • | [40, 126] |
| Endometrium | •• | [127] |
| Esophagus | • | [128] |
| Gastrointestinal tract | • | [86]e |
| Kidney | •• | [129, 130]f |
| Liver | • | [131] |
| Lung | •••• | [23, 66–68]b |
| Mantle cell lymphoma | • | [132] |
| Ovary | •• | [106, 133] |
| Pancreas | • | [24]b |
| Pituitary | • | [134] |
| Prostate | •• | [28, 37, 123, 135] |
| Skeletal muscle | • | [136] |
| Skin | •• | [137–139] |
| Stomach | ••• | [25, 29, 140]g, h |
| Thyroid | •• | [141–143] |
indicates upregulation of Axl; • indicates upregulation of Mer; • indicates upregulation of Gas6; and • indicates upregulation of Protein S.
Coexpression of Axl and Gas6 correlates with poor prognosis.
Overexpression of Axlcorrelates with metastatic cancer and poor prognosis.
Overexpression of Axl is a mechanism of lapatinib resistance in vitro.
Axl expression correlates with ER expression; Gas6 expression correlates with ER and PR activation.
Expression of Axl is associated with imatinib resistance.
Serum Gas6 levels negatively predict survival.
Coexpression of Axl and Mer correlates with poor prognosis.
Overexpression of Gas6 correlates with lymph node metastasis.
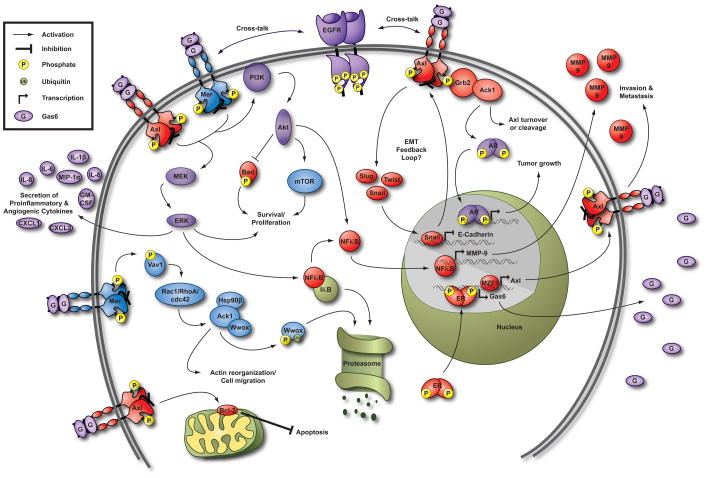
Figure 2. Axl and Mer signaling pathways in cancer cells.
Pathways activated downstream of both Mer and Axl, such as the Phosphatidylinositol 3-kinase (PI3K)/Protein kinase B (Akt) and Mitogen-activated protein kinase (MEK)/Extracellular-signal-regulated kinase (ERK) survival and proliferation pathways, are shown in purple. Blue pathways, including regulation of mammalian target of rapamycin (mTOR) [10], have been described downstream of Mer. Pathways downstream of Axl such as inactivation of Bcl-2-associated death promoter (BAD) via phosphorylation by Akt are shown in red. Mer interacts with, but does not directly phosphorylate, activated cdc42-associated kinase 1 (Ack1). Activation of Ack1 may occur indirectly through the guanine nucleotide-exchange factor Vav1 and cdc42, resulting in degradation of the tumor suppressor protein WW domain containing oxidoreductase (Wwox) and regulation of cell migration [123]. Interestingly, Ack1 also interacts with Axl via Grb2 and may regulate Axl turnover or cleavage [122]. Gas6 stimulates Ack1-dependent phosphorylation of androgen receptor (AR) resulting in proliferation of prostate cancer cells in vitro, presumably via transcriptional regulation of androgen responsive genes [124]. This effect could be mediated by Mer and/or Axl since the cell types evaluated express both receptors. Axl expression is only found in estrogen receptor (ER)-positive patient samples, and ER antagonists reduce Axl expression in breast cancer cells [33, 72]. It has not been determined whether ER binds to the Axl promoter, but Gas6 expression is transcriptionally regulated by ER [75]. See text for explanation of other depicted pathways. Additional signaling pathways have been delineated in non-cancerous cells (reviewed in [17]). Hsp90β, heat shock protein 90β; IκB, inhibitor of kappa-B; Rac1, Ras-related C3 botulinum toxin substrate 1; RhoA, Ras homolog gene family member A.
In addition to the canonical PI3K/Akt and MAPK/Erk pathways, Axl and Mer may play key roles in cancer progression by regulating tumor-stromal cell interactions. For example, Mer or Axl activation induces proinflammatory cytokine (IL-8, CXCL1, CXCL2, and others) secretion from prostate cancer cells. IL-8 expression is regulated at both transcriptional and posttranscriptional levels downstream of Mer via Erk signaling [37]. These findings suggest that important distinctions exist between physiologic and oncogenic signaling pathways downstream of Axl and Mer, as these receptors normally attenuate the inflammatory response in macrophages (reviewed in [17]). Proinflammatory cytokines influence cancer invasion and metastasis by stimulating adhesion molecules and metalloproteases. Murine breast tumors treated with an Axl inhibitor exhibit reduced secretion of pro-inflammatory cytokines (GM-CSF, IL-1β, IL-6, and macrophage inflammatory protein-1α) and fewer metastases [38]. Axl inhibition also blocks angiogenesis in vivo [38, 39]. These studies demonstrate proof of concept that Axl inhibitors may be useful as anti-metastatic agents.
Epithelial-to-mesenchymal transition (EMT) is a normal developmental program but contributes to metastatic potential when dysregulated in cancer. Axl expression can be induced during EMT and Axl signaling may play a role in maintenance of the mesenchymal phenotype. Axl can be transcriptionally regulated by myeloid zinc finger 1 (MZF1) [40], a transcription factor that also regulates expression of the mesenchymal marker N-cadherin [41]. EMT is thought to promote metastasis by giving cells the ability to migrate through extracellular matrix and intravasate into blood vessels. Axl mediates MZF1-induced invasion and metastasis in colorectal cancer [40], effects that may result from extracellular matrix breakdown through Axl activation of matrix metalloproteinase 9 (MMP-9) via MEK-Erk 1/2-NFκB signaling and Brg-1-mediated chromatin remodeling [42]. Additional data supporting roles for Mer and Axl in cellular migration/invasion will be discussed in detail in the following sections.
In addition to the oncogenic signaling pathways downstream of the intracellular kinase domains of Axl and Mer, numerous studies have explored the functional roles of Axl and Mer in various solid tumors. Here, we review recent evidence validating Axl and Mer as therapeutic targets in GBM, NSCLC, and breast cancer. Therapeutic compounds currently in development as Axl and/or Mer antagonists and potential benefits and liabilities associated with their clinical use are discussed subsequently.
2. Mer and Axl in glioblastoma multiforme
GBM is a central nervous system tumor that is difficult to treat because of its profound proliferative and migratory capacities. Despite multimodal aggressive therapy with chemotherapy, radiation and surgery, fewer than 10% of patients survive 5 years beyond diagnosis [43]. Current investigations aim to uncover novel therapeutic approaches by exploring the oncogenic mechanisms and unique potential targets of GBM [44]. Initial research concentrated on amplification, overexpression, and mutation of EGFR in this malignant phenotype [45]. Multiple strategies for EGFR blockade have been successful in vitro, such as small molecule TKIs (gefitinib and erlotinib), downregulation of expression or signaling with the monoclonal antibody cetuximab, or inhibition of the downstream mTOR pathway with sirolimus and temsirolimus. Other biologically targeted agents tested in GBM include the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab and other compounds inhibiting PDGFR, PI3K, PKC, Met, and FGFR kinases. However, to date, there has been only minimal success in clinical trials. The current task entails finding efficacious combinations of biologically targeted and cytotoxic approaches.
The TAM family of RTKs has recently been implicated in gliomagenesis and in the growth, invasion, and chemoresistance of this challenging tumor. TAM RTKs are upstream of the PTEN/PI3K and MAPK pathways, key players in cellular dysregulation and transformation into malignant glioma [1, 46, 47]. Stimulation of these pathways, whether by mutation [48–51] or upstream activation, correlates with higher grade [52] and poor prognosis [53]. Previous investigations found that Axl is constitutively phosphorylated in many glioma cell lines, and the downstream MAPK and PI3K pathways are also activated. Additionally, activated Axl was present in murine xenograft tumors and primary patient tumor samples [54]. Patient samples also highly expressed the ligand Gas6, suggesting the potential for autocrine activation of TAM family receptors overexpressed by the tumor. Furthermore, immunohistochemical analysis of Axl and Gas6 demonstrated that co-expression of these proteins correlates with recurrence and progression of the tumor [55].
Studies to further validate targeting TAM RTKs as a potential therapeutic model have been quite promising. Expression of an Axl dominant-negative mutant in a glioma cell line leads to decreased cell growth in vitro and smaller tumor volumes in xenografts. Additionally, in vivo tumor growth is notably less migratory and invasive, and overall animal survival is prolonged in xenografts containing dominant negative Axl [56]. More recent investigations by Keating et al. [32] confirmed Axl expression in GBM and reported a novel finding, Mer overexpression, with co-expression of both RTKs in the majority of GBM cell lines and patient samples. Downregulation of either Mer or Axl expression using shRNA abrogated signaling through the PI3K and MAPK pathways and functionally increased apoptosis and autophagy. Furthermore, decreased expression of Mer or Axl triggered a profound phenotypic change, with significantly impaired long-term anchorage independent growth, and markedly improved responsiveness to chemotherapy [32]. A major challenge in GBM pathogenesis is the remarkable migratory and invasive phenotype. Recent findings demonstrated that inhibition of Mer significantly reduces migration of GBM cells in vitro [57] suggesting that Mer targeted therapy may diminish GBM invasion. Overall, these data indicate a potential therapeutic benefit for Mer and/or Axl inhibition in the treatment of GBM.
3. Mer and Axl in non-small cell lung cancer
NSCLC is the primary cause of cancer mortality in the US and killed more men and women in 2009 than breast, prostate, and colorectal cancers combined. It is well known that cigarette smoking causes lung cancer, yet 10–25% of patients diagnosed with lung cancer are never smokers [58, 59]. Two-thirds of patients present with advanced stage disease and over 50% are metastatic where the 5-year relative survival is only 3.5% [60]. Treatment in these cases is palliative, rather than curative, and primarily consists of platinum-based doublet chemotherapy, resulting in a median survival of 10.3 months with only 15% of patients surviving 2 years. A handful of biologically targeted agents have received FDA approval for use in NSCLC either alone or in combination with standard chemotherapies. Bevacizumab significantly improves survival for approximately one-third of patients with advanced NSCLC (those with non-squamous histology, < 70 years of age, and no prior hemoptysis) when used in combination with a platinum-based doublet [61]. Erlotinib and gefitinib are now the preferred single agent first-line therapy for patients with somatic activating EGFR mutations [62–64]. The multi-kinase small-molecule inhibitor PF-2341066 is currently being investigated in Phase III trials and has shown benefit in NSCLC tumors harboring echinoderm microtubule-associated protein like-4 (EML4)-anaplastic lymphoma kinase (ALK) chromosomal translocations [65]. While these promising new targeted agents validate RTK inhibition as a therapeutic strategy in NSCLC, activating EGFR mutations and ALK translocations are present in approximately 10% and 5% of lung cancers, respectively, in an unselected Western population. Clearly, further investigation is necessary to identify additional targeted biologic therapies that will improve survival for additional subsets of NSCLC.
Axl, and to a lesser extent Mer, have been the subject of such investigations. Initial studies suggested that high levels of Axl and the ligands Gas6 and Protein S could be found in over 50% of NSCLC cell lines evaluated [66, 67]. Recent data from our laboratory indicate that Mer is also highly expressed in NSCLC cell lines [68]. Much like the scenario in GBM tumors, the expression of both receptors and ligands suggest that Mer and Axl may be continuously activated in NSCLC via autocrine or paracrine mechanisms. Indeed, Axl and Mer are among the most highly phosphorylated RTKs in NSCLC cell lines and tumors [54, 69]. Axl protein expression was observed in 28 of 58 (48.3%) patient samples of lung adenocarcinoma. Furthermore, expression of Axl correlates with lymph node involvement and higher clinical stage indicating that Axl expression is a poor prognostic factor in NSCLC [23]. Mer is also highly expressed in NSCLC tumors and ongoing studies will evaluate the prognostic value of Mer expression in NSCLC [68].
Several studies have begun to elucidate the molecular mechanisms by which Axl and Mer contribute to the development and progression of NSCLC. RNAi-mediated silencing of Axl reduces the viability of NSCLC cells in vitro and inhibits tumor growth in xenograft models [70]. Additional data demonstrate that inhibition of either Axl or Mer reduces long-term growth of NSCLC cells in vitro [68]. Axl expression correlates with NSCLC cell invasiveness and migration in vitro [23, 70], effects that may be mediated by Axl-dependent upregulation of MMP-9 expression [42]. Decreased Mer expression leads to increased induction of cell death [68]. Additional in vitroassays demonstrated that inhibition of Mer or Axl significantly increases the sensitivity of NSCLC cells to numerous chemotherapeutic agents. These data are consistent with the hypothesis that Axl and Mer mediate the proliferation, survival, and migration of NSCLC cells. Taken together, these results suggest that inhibition of Axl and/or Mer may be a viable therapeutic approach to enhance chemotherapy efficacy in NSCLC.
4. Mer and Axl in breast cancer
The most commonly diagnosed tumor in American women is breast cancer. Treatment options include surgery, radiation therapy, chemotherapy, and endocrine therapy. The absence or presence of estrogen receptor (ER), progesterone receptor (PR), and HER2 within the tumor is commonly used to guide treatment selection. The majority of breast cancers are positive for hormone receptors (ER and/or PR). These tumors respond to endocrine therapy and carry a good prognosis. Another 20% of breast cancers exhibit high expression of HER2, a member of the EGF family of RTKs, and until recently these women had a poorer prognosis. Trastuzumab, an anti-HER2 monoclonal antibody, was the first agent targeting a RTK to receive approval as anti-cancer therapy (1998) and improves outcome for patients whose tumors express HER2 in the adjuvant and metastatic setting. The HER2 small-molecule inhibitor lapatinib has shown benefit in patients who progress on trastuzumab. Together with successful screening programs, the currently available therapies have dramatically improved breast cancer survival rates over the past two decades. However, subsets of breast cancer persist for which treatment options are limited and prognosis is grim. For example, as many as 11–20% of breast cancers are described as triple negative, meaning that they do not express ER, PR, or HER2 [71]. Surgery, radiation, and chemotherapy are the only treatment options for these patients. Despite an increased sensitivity to chemotherapy, triple negative breast cancer carries a significantly worse prognosis. Chemotherapy is also the only option for hormone receptor positive tumors that have progressed on hormone therapy. In order to develop new therapies for these types of breast cancer, novel targets must be identified.
Axl is overexpressed in human breast carcinoma cell lines and patient samples and correlates with advanced tumor stage [72, 73]. Furthermore, high Axl expression is statistically associated with poor survival and Axl expression is upregulated in metastases relative to primary tumors [22]. Mer is also upregulated in metastatic relative to non-metastatic breast cancer cells and this upregulation may be due, at least in part, to loss of miR-335 [74]. The prognostic and predictive value of Mer has not been investigated in patient samples of breast cancer. Interestingly, Axl expression is only found in ER+ patient samples, and ER antagonists reduce Axl expression in breast cancer cells [33, 72]. It has not been determined whether ER binds to the Axl promoter, but Gas6 expression is transcriptionally regulated by ER [75]. Gas6 mRNA expression is also upregulated by PR stimulation in breast cancer cells [76] and PR expression correlates with Gas6 mRNA levels in human breast tumors [77]. Much like PR positivity, high Gas6 mRNA levels correlate with favorable prognostic variables; however Gas6 expression does not correlate with survival [77, 78].
The contribution of Axl to the development and progression of breast cancer has been an active area of research in recent years. RNAi-mediated knockdown of Axl reduces viability of triple negative MDA-MB-231 human breast cancer cells and inhibits their orthotopic growth in immunodeficient mice [22, 39, 70]. Axl inhibition also reduces migration and invasion of breast cancer cells in vitro [22, 79]. The effect of Axl on invasion may be mediated by Axl-dependent expression of MMP-9 [42]. In support of this idea, expression of Axl correlates with expression of MMP-9 in human breast tumors.
An early study demonstrated that increased expression of Axl in Ras-transformed breast epithelial cells correlates with anchorage-independent growth and loss of E-cadherin [80], common indicators of EMT. Recent studies have more specifically evaluated the role of Axl in EMT and metastasis of breast cancer cells. Overexpression of EMT-associated transcription factors Twist, Snail, and Slug in premalignant breast epithelial cells that do not express Axl resulted in EMT marked by loss of E-Cadherin and β-catenin, up-regulation of N-cadherin and Vimentin, and de novo expression of Axl on the cell surface [22]. Interestingly, murine breast tumors treated with an Axl inhibitor exhibit reduced Snail expression in vivo [38]. Axl expression and activation also correlate with expression of EMT-associated transcription factors in AML and pancreatic cancer cells [8, 24]. These data suggest that a positive feedback loop between Axl and EMT transcription factors may perpetuate the mesenchymal state in metastatic cancer cells. High levels of Axl expression are found in metastatic breast cancer cell lines and inhibition of Axl reduces growth and metastasis and improves overall survival in multiple animal models [22, 38, 70]. In a syngeneic model where murine breast cancer cells are orthotopically injected into the mammary fat pad of immune competent mice, inhibition of Axl significantly reduces metastatic burden but has little to no effect on primary tumor growth [22, 38]. Taken together, these findings indicate that Axl is an important regulator of breast cancer metastasis and EMT.
Axl has also been shown to play a role in mechanisms of chemoresistance. Chronic exposure of an Axl negative breast cancer cell line to lapatinib resulted in lapatinib resistance, de novo expression of Axl, and increased expression of ER [33]. Lapatinib sensitivity was restored by inhibition of either Axl or ER. An Axl inhibitor also interacted synergistically with cisplatin to reduce liver micrometastases [38]. These data suggest that Axl inhibition may be a novel therapeutic approach in metastatic breast cancer, particularly in recurrent ER positive tumors. Additional studies are necessary to determine the role of Mer in breast cancer.
5. Axl and Mer inhibitors in development
Historically, enthusiasm for development of kinase inhibitors was tempered by the potential toxicity of such compounds. In general, targeted inhibition of kinases presents a less toxic therapeutic strategy than traditional pan-cytotoxic chemotherapeutic agents. To revisit the classical example of targeted therapy in CML, imatinib preferentially inhibits mutated ABL kinase present in Ph+ cancer cells. EGFR TKIs selectively inhibit mutant RTK but also reduce activity of wild type RTK and the toxicity is generally not severe. Unlike ABL and EGFR, activating mutations of Axl and Mer have not been described. Alternatively, the oncogenic potential of these kinases is attributed to chronic activation enabled by aberrant receptor expression and autocrine or paracrine ligand stimulation. As a therapeutic strategy, inhibition of Axl or Mer therefore presents unique clinical challenges since drugs that target them would also inhibit the normal proteins in non-cancerous cells. Clues regarding the potential nature of such adverse effects can be gained through evaluation of TAM receptor knockout mice. Importantly, transgenic mice harboring null mutations of Tyro3, Axl, or Mer are phenotypically normal at birth [81]. However, various phenotypes become apparent during adulthood. The most profound are observed in Mer knockouts and include increased sensitivity to endotoxic shock, splenomegaly, autoimmunity, and blindness. Tyro3 knockouts develop central nervous system dysfunctions including hindlimb paralysis and seizures. These effects are amplified in double and triple knockout mice, a fact that must be considered in the context of ligand sequestration as a therapeutic strategy likely to result in blockade of all three TAM receptors. In addition, triple knockouts exhibit infertility. Despite overt concern regarding the nature of these phenotypes, it is important to note that none of these outcomes become evident until sexual maturation and all of them develop over several months of complete loss of kinase activity. This is in stark contrast to many single protein kinase knockouts which are lethal during embryonic or early postnatal periods [82]. The relatively slow progression of biological abnormality in TAM receptor knockout mice suggests that a short-term therapeutic window likely exists during which inhibition of Axl or Mer results in only mild, tolerable adverse effects. An Axl inhibitor has already demonstrated safety and tolerability in Phase I/II studies [83, 84]. Furthermore, animal studies indicate that at least some adverse effects may be reversible upon cessation of therapy [85]. Given recent evidence that Mer or Axl inhibition enhances chemosensitivity, short-term targeted therapy administered in combination with blocks of chemotherapy might have the potential to significantly impact patient outcome. Therefore, we and others have concluded that the potential toxicities do not represent a significant obstacle for development of Axl and Mer inhibitors.
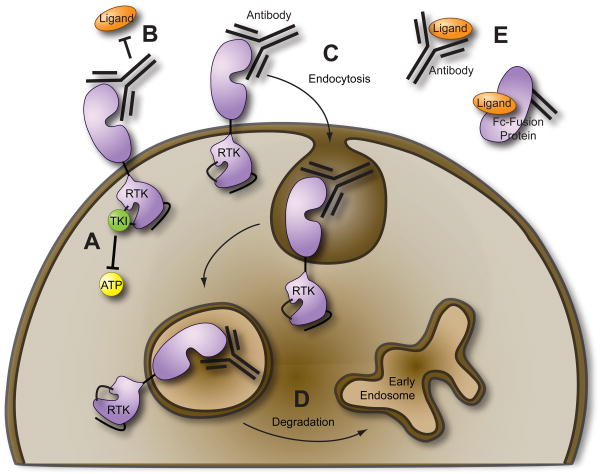
There are several clinically feasible strategies which result in blockade of RTK signaling (Figure 3). The most common approach is small-molecule kinase inhibitors that compete with ATP for binding in the catalytic domain. Another strategy involves monoclonal antibodies that bind the receptor extracellular domain and disrupt RTK signaling by various mechanisms including prevention of ligand binding, receptor endocytosis, or receptor degradation. Finally, monoclonal antibodies or fusion proteins that bind the ligand can be employed as ligand “sinks” preventing ligand-dependent RTK activation (e.g., the anti-VEGF monoclonal antibody bevacizumab). Each of these strategies is more or less favorable given the context under which the oncogenic RTK is activated. For example, monoclonal antibodies or any other method intended to prevent ligand binding would be ineffective against RTKs with somatic mutations which render them constitutively active in a ligand-independent manner. Given the evidence that autocrine or paracrine activation loops may exist for Mer and Axl and the fact that activating mutations of these receptors have not been described, all of the above strategies are currently being explored as methods of Mer and Axl inhibition.
Figure 3. Molecular strategies for therapeutic inhibition of receptor tyrosine kinases (RTKs).
(A) Low molecular weight tyrosine kinase inhibitors (TKIs) compete with adenosine triphosphate (ATP) for binding to the activation loop of the RTK. (B–D) Anti-RTK monoclonal antibodies can prevent binding of ligand (B) and/or cause endocytosis of the RTK (C) which may result in RTK degradation (D). (E) Ligand sequestration via anti-ligand monoclonal antibodies or recombinant fusion proteins such as the extracellular RTK domain fused to the Fc region of human IgG.
Several small-molecule TKIs which exhibit activity against Axl have been described in the literature (Table 2). Most of these agents were developed as inhibitors of other tyrosine kinases such as c-Kit, Src/ABL, and Met and are therefore not specific Axl inhibitors. Furthermore, given the high degree of sequence homology (83% similarity; 70% identity; see Figure 1) between the tyrosine kinase domains of Axl and Mer, it is likely that many Axl inhibitors will exhibit some degree of activity against Mer. Amuvatinib (MP-470, Supergen) was the first compound reported to have Axl inhibitory activity [86]. This TKI was designed as an inhibitor of c-Kit and is several-fold more selective for c-Kit and Met than for Axl. Amuvatinib has also been reported to synergize with erlotinib to inhibit EGFR family members HER1, HER2, and HER3 in preclinical studies of prostate cancer [34]. In addition to c-Kit, Met, and Axl, amuvatinib also exhibits activity against c-Ret, PDGFR, and the DNA repair protein Rad51. This compound has been evaluated in Phase I trials where clinical benefit was observed in neuroendocrine, NSCLC, small cell lung, and endometrial carcinomas. According to the company’s website, Supergen will initiate Phase II studies of amuvatinib in these tumor types in late 2010.
Table 2.
Axl and Mer inhibitors in development.
| Compound (other names) | Axl IC50* | Mer IC50* | Other Known Targets | Phase of Development | Disease Indication | References |
|---|---|---|---|---|---|---|
| Small Molecule Inhibitors | ||||||
| Amuvatinib (MP-470) | 1 μM | Unk | c-Kit, Met, c- Ret, PDGFR, Rad51 | Phase I/II | Neuroendocrine, lung, and endometrial carcinomas | [34, 86] |
| Bosutinib (SKI-606, PF- 5208763) | 0.4 μM | Unk | Src, ABL | Phase II/III | Breast carcinoma, Ph+ CML | [79, 87, 88] |
| Foretinib (GSK1363089, XL880) | 11 nM | Unk | Met, VEGFR2 | Phase II | Renal cell, gastric, and head & neck carcinomas | [33, 83, 84] |
| BMS-777607 | 1.1 nM | 14 nM | Met, Ron, Tyro3, Flt- 3, AuroraB | Phase I/II | Gastroesophageal, prostate, head & neck, and renal cell carcinomas | [89] |
| PF-2341066 | 0.3 μM | Unk | Met, ALK, Ron | Phase III | NSCLC | [65] |
| R428 | 14 nM | 224 nM | Tie-2, Tyro3, Ret, ABL | Preclinical | Breast carcinoma | [38] |
| Receptor Monoclonal Antibodies | ||||||
| α-Axl mAb | Unk | Unk | None | Preclinical | Breast carcinoma and NSCLC | [70, 79] |
| α-Mer mAb | Unk | Unk | None | Preclinical | ALL, AML, and GBM | [30, 57, 92] |
| Fusion Proteins | ||||||
| Axl-Fc | Unk | Unk | Mer, Tyro3 | Preclinical | [94] | |
| Mer-Fc | Unk | Unk | Axl, Tyro3 | Preclinical | Thrombophilia | [93, 94] |
IC50 values for various compounds may not be directly comparable as they were determined by a variety of assays including in vitro kinase assays and cell-based phosphorylation assays. Unk, unknown.
Bosutinib (SKI-606, Wyeth now PF-5208763, Pfizer) was originally developed as a second-generation dual Src/ABL kinase inhibitor. This compound is more potent against BCR-ABL than imatinib and has been reported to inhibit imatinib-resistant BCR-ABL mutations [87, 88]. Sub-micromolar concentrations of bosutinib are sufficient for half maximal inhibition of ligand-dependent Axl phosphorylation in breast cancer cells and inhibition of Axl phosphorylation correlates with reduced motility and invasion of these cells in vitro [79]. Bosutinib is currently in Phase II trials for breast cancer and Phase III trials to compare bosutinib to imatinib as first-line therapy in Ph+ CML.
Foretinib (GSK1363089, GlaxoSmithKline) was originally developed by Exelixis (XL880) as an inhibitor of Met and VEGFR2 and was recently identified as a potent inhibitor of Axl (IC50 = 11 nM) [33]. In lapatinib resistant breast cancer cells, foretinib interacts synergistically with lapatinib to inhibit Akt and Erk phosphorylation resulting in decreased cell growth and increased apoptosis [33]. RNAi experiments suggest that foretinib-mediated inhibition of Axl is sufficient for the observed restoration of lapatinib sensitivity. Foretinib has shown benefit in renal cell carcinoma [84] and is also being investigated in phase II trials of gastric cancer and head and neck squamous cell carcinoma.
BMS-777607 (Bristol-Myers Squibb) and PF-2341066 (Pfizer) were also developed as Met inhibitors (IC50s 3.9 nM and 8 nM, respectively). PF-2341066 exhibits similar potency against ALK (IC50 = 20 nM) and elicited objective responses in NSCLC patients whose tumors harbor activating ALK translocations [65]. PF-2341066 also inhibits Axl (IC50 = 300–320 nM) and is currently in phase III trials of advanced NSCLC as a Met/ALK inhibitor. Interestingly, BMS-777607 is more selective for Axl (IC50 = 1.1 nM) than for its intended target, Met [89]. This agent exhibited efficacy in a xenograft model of human gastric carcinoma and was investigated in a phase I/II trial of advanced or metastatic gastroesophageal cancer, hormone refractory prostate cancer, head and neck squamous cell carcinoma, and type I papillary renal cell carcinoma. Study results have not been reported.
Low-molecular weight TKIs specifically designed to target Axl are uncommon. In early 2009, AstraZeneca published a patent application (PCT/EP2008/058898) identifying novel pyrazine derivatives as inhibitors of Axl with IC50 values in the low nanomolar range. To our knowledge, studies of these compounds have not been published in the scientific literature. The only published report of a highly selective Axl inhibitor is R428 (Rigel), which exhibits potent activity against Axl (IC50 = 14 nM) and 3 to 15 fold selectivity for Axl versus other kinases [38]. This compound exhibited favorable pharmacokinetic and toxicity profiles in preclinical studies and reduced metastatic burden in two different mouse models of breast carcinoma. Furthermore, R428 improved overall survival in the adjuvant setting of an orthotopic mastectomy model and interacted synergistically with cisplatin to reduce liver micrometastases. Based on these data, R428 is an attractive candidate for clinical development.
Although pharmacologic inhibitors of Mer have been reported [90], most of these compounds do not have pharmacokinetic and toxicity profiles necessary for translation to the clinic. As suggested above, it is possible that Axl TKIs will also inhibit Mer. For example, R428 and BMS-777607 exhibit activity against Mer and the third TAM receptor Tyro3 but are approximately 4 to 15-fold more selective for Axl [38, 89]. In addition to the industry-sponsored programs described above, multiple academic institutions are pursuing development of Mer and Axl TKIs. A major objective of these endeavors is to develop dual Axl/Mer inhibitors as well as Axl-selective and Mer-selective compounds. These compounds will be useful in pre-clinical studies to help delineate the individual contributions of Mer and Axl to tumorigenesis and metastasis.
Reports of inhibitory monoclonal antibodies targeting Axl and Mer are also rare. Anti-Axl monoclonal antibodies that bind to the extracellular domain result in reduced expression of Axl [70]. These antibodies decrease viability of Axl-overexpressing cells in vitro and inhibit growth of A549 and H1299 NSCLC xenografts in vivo. A recent follow-up study demonstrated that the anti-Axl monoclonal antibodies reduce metastasis in an MDA-MB-231 xenograft model of breast cancer [91]. Polyclonal anti-Axl antibodies raised against the extracellular domain have also been described that inhibit the motility and invasivity of human breast cancer cells in vitro [79]. Our laboratory has developed anti-Mer monoclonal antibodies that reduce Mer protein expression and downregulate pro-survival signaling in human cancer cells [30, 92]. The anti-Mer antibodies also reduce migration of GBM cells in vitro [57]. Further preclinical testing is necessary before these antibodies are investigated in clinical trials.
Chimeric proteins consisting of the full-length extracellular domains of Mer or Axl fused to the Fc portion of the human IgG (Mer-Fc and Axl-Fc, respectively) are commercially available (R&D Systems, Sigma). While these proteins have yet to be investigated as anti-cancer agents, they have demonstrated promise in other pathologic contexts. In murine macrophages, Mer-Fc prevents Gas6 dependent Mer phosphorylation as well as downstream phosphorylation of Akt [93, 94]. Axl-Fc also binds Gas6 and prevents ligand dependent activation of both Mer and Axl [94]. Mer-Fc inhibits platelet aggregation in vitro and protects mice against fatal thromboembolism suggesting that Mer-Fc may have therapeutic value in the treatment of thrombophilia [94]. Given the concomitant upregulation of Mer or Axl with the ligands Gas6 or Protein S (see Table 1), these fusion proteins may also be effective anti-cancer drugs.
Each method of Axl or Mer inhibition presents different advantages and disadvantages in the clinical setting. Much is known about the mechanism of action of small molecule TKIs and these compounds are most likely to be available in an oral formulation. TKIs are often less specific for the intended target, which may be of benefit if the additional targets contribute to oncogenesis and metastasis or detriment if additional adverse effects are elicited. Similarly, fusion proteins which act via ligand sequestration will likely inhibit all three TAM receptors. Monoclonal antibodies are likely to be the most specific inhibitors of Axl or Mer, but the pharmacokinetic profiles may be less desirable. The mechanism of reduced Axl or Mer expression in response to these antibodies is currently under investigation, but may involve receptor endocytosis and degradation or shedding of the extracellular domain. Understanding these mechanisms may help guide therapeutic application of Axl and Mer monoclonal antibodies. These pros and cons imply that each strategy may be of particular benefit within a specific disease context.
6. Expert Opinion
It is apparent from evidence reviewed in this article that Axl and Mer mediate multiple oncogenic phenotypes including growth, survival, migration, and invasion of tumor cells (Table 3). Of particular interest is a growing body of evidence suggesting a role for these RTKs in chemoresistance and cellular functions which facilitate metastasis, namely migration, invasion, EMT, and angiogenesis. As chemoresistance and metastasis are major barriers to truly curative cancer therapy, these findings strongly suggest investigating the potential therapeutic value of Axl and Mer inhibition in solid tumors. The mechanism(s) of Axl and Mer upregulation in cancer are not well understood has yet to be elucidated. Characterization of the human Axl promoter in cancer cells indicates that at least one mechanism involves transcriptional regulation by Sp(specificity protein)1 and Sp3 as well as promoter methylation [95]. Although the human Mer promoter has not been characterized, a study of the murine Mer promoter in Sertoli cells suggests that Sp1/Sp3 also positively regulate transcription of Mer [96]. Several additional possible mechanisms are currently under investigation including gene amplification, promoter acetylation, and gain or loss of miRNA expression [33, 74, 97]. Similar mechanisms may regulate expression of the ligand Gas6 in cancer cells [78]. A better understanding of how Mer and Axl are overexpressed in cancer cells may aid in determining the best strategy for targeting these RTKs. In some cases, restoration of normal expression levels may be a therapeutic approach of equal or better benefit when compared to the more direct Axl and Mer inhibitors described in the previous section.
Table 3.
Oncogenic phenotypes mediated by Axl and Mer in solid tumors.
| Phenotype | Glioblastoma Multiforme | Non-small cell lung cancer | Breast Cancer |
|---|---|---|---|
| In vitro proliferation | • | •• | • |
| Anchorage-independent growth | •• | • | |
| Xenograft growth | • | • | • |
| Survival signaling (PI3K, MAPK) | •• | ||
| Apoptosis | •• | •• | |
| Autophagy | •• | ||
| Migration | • | • | • |
| Invasion | • | • | • |
| EMT | • | ||
| Metastasis | • | ||
| Angiogenesis* | • | ||
| Chemosensitivity | •• | •• | • |
indicates Axl mediated phenotype; • indicates Mermediated phenotype.
Although angiogenesis has only been specifically evaluated in animal models of breast cancer, this phenotype is due to Axl expression in endothelial cells and therefore may be applicable to all solid tumors.
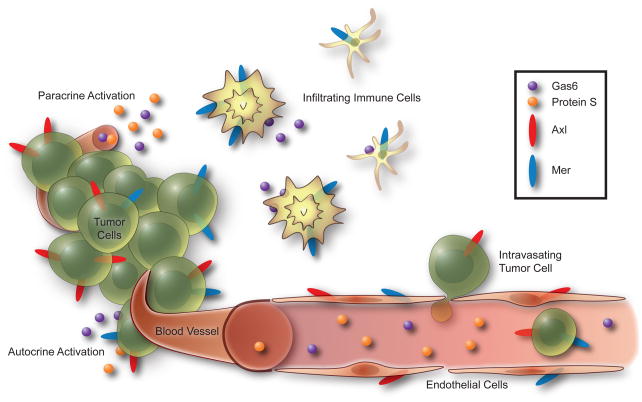
In addition to being expressed by tumor cells, Axl, Gas6, and Protein S are found in the vasculature of multiple solid tumor types [23, 24, 55, 67]. Tissue macrophages are known to express all three TAM receptors and a recent study revealed that tumor-infiltrating leukocytes (including dendritic cells are macrophages) express significantly higher levels of Gas6 than normal tissue macrophages [98]. The same study showed that transplantation of Gas6−/− bone marrow into wild type mice significantly reduces tumor growth in three different syngeneic models. Therefore, an advantage of using direct Axl and Mer inhibitors is the potential for action on both tumor cells as well as cells in the tumor microenvironment (Figure 4). In support of this hypothesis, inhibition of Axl reduces haptotaxis of endothelial cells towards Vitronectin, blocks endothelial tube formation in vitro, and inhibits angiogenesis in vivo [39]. Although inhibition of Axl reduces growth of primary tumors in immune-compromised xenograft models, these results were not recapitulated in a syngeneic mouse model [38]. In the same model, an Axl TKI reduces metastasis and improves survival suggesting that the Axl TKI may in fact be acting on the Axl-expressing stromal cells of the immune-competent host animal. These data suggest that the patient’s immune function may play a role in tumor development as well as therapeutic options. Within this context, Mer/Axl inhibitors may be an effective anti-metastatic therapy even in Mer negative or Axl negative tumors.
Figure 4. Opportunities for therapeutic disruption of Mer and Axl signaling in the tumor microenvironment.
Axl and Mer expressed by tumor cells may be stimulated by autocrine or paracrine activation loops as the ligands Gas6 and Protein S are expressed by tumor cells and found in plasma. Gas6 is also released by infiltrating immune cells such as tumor-associated macrophages and dendritic cells. Blockade of Axl and Mer expressed by endothelial cells may inhibit angiogenesis.
One of the primary challenges to sustained maintenance of complete remission is acquired resistance to targeted therapy. Although therapeutic agents have been identified that produce a robust response in subsets of cancer, many of these tumors eventually develop resistance and recur. Two common mechanisms of TKI resistance have been elucidated: secondary mutation of the targeted RTK or compensatory upregulation of another RTK. For example, mutation of BCR-ABL or EGFR causes resistance to imatinib in CML [99] and erlotinib/gefitinib in NSCLC [100, 101], respectively. Met amplification has also been reported as a mechanism of resistance to erlotinib/gefitinib in NSCLC [102, 103]. Upregulation of Axl has been implicated as a mechanism of resistance to imatinib in CML [104] and gastrointestinal stromal tumors [86] and to lapatinib in breast cancer [33]. Axl expression has also been associated with chemoresistance in AML, NSCLC, and ovarian cancer [8, 105, 106]. In addition to the cancer cell’s exceptional capacity to adapt when one or more RTKs are inhibited, prevalent crosstalk between RTK signaling pathways imparts an additional layer of complexity. Various strategies have been proposed to deal with these issues including development of second-generation agents that are active against new mutations, concomitant administration of multiple TKIs (or multi-targeted agents) to prevent compensatory upregulation of an alternative pathway, and targeted inhibition of downstream effectors that mark a site of RTK pathway convergence [107, 108]. It is noteworthy that similar challenges were encountered decades ago with traditional cytotoxic chemotherapy. In contemporary chemotherapy regimens, this problem is overcome by combination treatment with agents from different classes administered in cycles (e.g., an alkylating agent combined with an anti-metabolite or an anti-mitotic). Based on this historical perspective, it seems reasonable to expect that combinations of targeted therapies applied in conjunction with traditional cytotoxic agents will be necessary to increase therapeutic efficacy and may even make curative treatment a realistic possibility.
Another variable that influences the success or failure of targeted agents is patient selection. In other words, how do we determine which patients will benefit from the drug? In breast cancer, expression of HER2 is a predictive biomarker of response to the anti-HER2 monoclonal antibody, trastuzumab [109]. But patient selection is not always a straightforward task. A clear example of the importance of patient stratification can be learned from clinical trials of EGFR TKIs in NSCLC. Initial studies evaluating the impact of erlotinib or gefitinib when combined with frontline standard chemotherapy were largely unsuccessful showing no survival benefit in unselected populations [110–113]. Although approximately 80% of NSCLC tumors overexpress EGFR, protein levels did not prove to be a sufficient predictor of response to EGFR TKIs in this tumor type [114, 115]. Alternatively, the presence of an EGFR activating mutation is the primary biologic predictor of response to EGFR TKIs [63, 116, 117]. Predictive biomarkers for Axl and Mer remain to be elucidated but may include a tumor gene expression profile or phosphoprotein signature, RTK protein expression levels, or serum Gas6, soluble Axl, or soluble Mer levels.
Recent years have marked the beginning of a new era in cancer therapy revolutionized by the concept of tumor molecular subtype and the possibility of personalized medicine. Therapeutic options are no longer restricted to the one-size-fits-all model. Instead, individual patients are being treated with unique approaches based on a number of clinical, molecular, and histologic features. Targeted agents, including those which inhibit Axl and Mer, will likely be crucial components of the therapeutic toolbox as this new era unfurls.
Acknowledgments
The authors wish to thank Lynn E. Heasley, PhD and Scott A. Kono, DO for helpful comments on the manuscript.
List of Abbreviations
- AML
Acute myeloid leukemia
- ALK
anaplastic lymphoma kinase
- CML
chronic myelogenous leukemia
- EML4
echinoderm microtubule-associated protein like-4
- EGFR
epidermal growth factor receptor
- EMT
epithelial-to-mesenchymal transition
- ER
estrogen receptor
- GBM
glioblastoma multiforme
- HER2
human epidermal growth factor receptor 2
- mTOR
mammalian target of rapamycin
- MMP-9
matrix metalloproteinase 9
- MZF1
myeloid zinc finger 1
- NSCLC
non-small cell lung cancer
- PDGFRα
platelet-derived growth factor receptor alpha
- Ph
Philadelphia chromosome
- PR
progesterone receptor
- RTK
receptor tyrosine kinase
- TKI
tyrosine kinase inhibitor
- TAM
Tyro3, Axl, and Mer
- VEGF
vascular endothelial growth factor
Footnotes
Declaration of interest
This work is supported in part by grants from Uniting Against Lung Cancer: Elliot’s Legacy and the Lung Cancer Research Foundation. RMA Linger is the recipient of a Career Development Award from the University of Colorado Cancer Center SPORE in Lung Cancer (NIH 5P50CA058187).AK Keating is supported by the St. Baldrick’s Foundation. DK Graham is the Damon Runyon-Novartis Clinical Investigator supported by the Damon Runyon Cancer Research Foundation (CI-39-07) and is also supported by the American Cancer Society Research Scholar Grant (RSG-08-291-01-LIB).
Contributor Information
Rachel M.A. Linger, Department of Pediatrics, University of Colorado Denver School of Medicine, Mail Stop 8302, 12800 E. 19th Avenue, Room 4401A, Aurora, CO 80045.
Amy K. Keating, Department of Pediatrics, University of Colorado Denver School of Medicine, Mail Stop 8302, 12800 E. 19th Avenue, Room 4405, Aurora, CO 80045.
H. Shelton Earp, UNC Lineberger Comprehensive Cancer Center, University of North Carolina School of Medicine, 450 West Drive, CB 7295, Chapel Hill, NC 27599.
Douglas K. Graham, Email: doug.graham@ucdenver.edu, Department of Pediatrics, University of Colorado Denver School of Medicine, Mail Stop 8302, 12800 E. 19th Avenue, Room 4408, Aurora, CO 80045, Phone: 303-724-4006, Fax: 303-724-4015.
Annotated Bibliography
- 1.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001 Jun 1;15(11):1311–33. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 2.Sleijfer S, Wiemer E, Verweij J. Drug Insight: gastrointestinal stromal tumors (GIST)--the solid tumor model for cancer-specific treatment. Nat Clin Pract Oncol. 2008 Feb;5(2):102–11. doi: 10.1038/ncponc1037. [DOI] [PubMed] [Google Scholar]
- 3.Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004 Aug;3(8):1035–42. [PubMed] [Google Scholar]
- 4.Sridhara R, Johnson JR, Justice R, et al. Review of oncology and hematology drug product approvals at the US Food and Drug Administration between July and December 2007. J Natl Cancer Inst 2010. 2005 Feb 24;102(4):230–43. doi: 10.1093/jnci/djp515. [DOI] [PubMed] [Google Scholar]
- 5*.Graham DK, Dawson TL, Mullaney DL, et al. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 1994 Jun;5(6):647–57. Reports the discovery of Mer receptor tyrosine kinase. [PubMed] [Google Scholar]
- 6*.O’Bryan JP, Frye RA, Cogswell PC, et al. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991 Oct;11(10):5016–31. doi: 10.1128/mcb.11.10.5016. The first identification of Axl receptor tyrosine kinase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham DK, Salzberg DB, Kurtzberg J, et al. Ectopic expression of the proto-oncogene Mer in pediatric T-cell acute lymphoblastic leukemia. Clin Cancer Res. 2006 May 1;12(9):2662–9. doi: 10.1158/1078-0432.CCR-05-2208. [DOI] [PubMed] [Google Scholar]
- 8*.Hong CC, Lay JD, Huang JS, et al. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 2008 May 23;268(2):314–24. doi: 10.1016/j.canlet.2008.04.017. This is the first report of Twist upregulation following Axl activation and correlates Axl expression with chemoresistance in patient samples of AML. [DOI] [PubMed] [Google Scholar]
- 9**.Keating AK, Salzberg DB, Sather S, et al. Lymphoblastic leukemia/lymphoma in mice overexpressing the Mer (MerTK) receptor tyrosine kinase. Oncogene. 2006 Oct 5;25(45):6092–100. doi: 10.1038/sj.onc.1209633. This is the first study to suggest that Mer contributes to oncogenesis and chemoresistance in leukemia. [DOI] [PubMed] [Google Scholar]
- 10**.Linger RMA, DeRyckere D, Brandao L, et al. Mer receptor tyrosine kinase is a novel therapeutic target in pediatric B-cell acute lymphoblastic leukemia. Blood. 2009 Sep 24;114(13):2678–87. doi: 10.1182/blood-2009-03-209247. This report demonstrates proof-of-concept for Mer inhibition as a therapeutic strategy in leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Lee-Sherick AB, Linger RMA, Gore L, et al. Novel targeted therapies in pediatric acute lymphoblastic leukemia. British Journal of Haematology. 2010 doi: 10.1111/j.1365-2141.2010.08282.x. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochlitz C, Lohri A, Bacchi M, et al. Axl expression is associated with adverse prognosis and with expression of Bcl-2 and CD34 in de novo acute myeloid leukemia (AML): results from a multicenter trial of the Swiss Group for Clinical Cancer Research (SAKK) Leukemia. 1999 Sep;13(9):1352–8. doi: 10.1038/sj.leu.2401484. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Carey K, Godowski PJ. Identification of Gas6 as a ligand for Mer, a neural cell adhesion molecule related receptor tyrosine kinase implicated in cellular transformation. Oncogene. 1997 May 1;14(17):2033–9. doi: 10.1038/sj.onc.1201039. [DOI] [PubMed] [Google Scholar]
- 14.Fisher PW, Brigham-Burke M, Wu SJ, et al. A novel site contributing to growth-arrest-specific gene 6 binding to its receptors as revealed by a human monoclonal antibody. Biochem J. 2005 May 1;387(Pt 3):727–35. doi: 10.1042/BJ20040859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata K, Ohashi K, Nakano T, et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996 Nov 22;271(47):30022–7. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 16.Prasad D, Rothlin CV, Burrola P, et al. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006 Sep;33(1):96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Linger RMA, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelillo-Scherrer A, Burnier L, Lambrechts D, et al. Role of Gas6 in erythropoiesis and anemia in mice. J Clin Invest. 2008 Feb;118(2):583–96. doi: 10.1172/JCI30375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang H, Chen S, Wang H, et al. TAM receptors and the regulation of erythropoiesis in mice. Haematologica. 2009 Mar;94(3):326–34. doi: 10.3324/haematol.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behrens EM, Gadue P, Gong SY, et al. The mer receptor tyrosine kinase: expression and function suggest a role in innate immunity. Eur J Immunol. 2003 Aug;33(8):2160–7. doi: 10.1002/eji.200324076. [DOI] [PubMed] [Google Scholar]
- 21.Caraux A, Lu Q, Fernandez N, et al. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006 Jul;7(7):747–54. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- 22**.Gjerdrum C, Tiron C, Hoiby T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A. 2010 Jan 19;107(3):1124–9. doi: 10.1073/pnas.0909333107. [22] and [38] are the first reports to suggest that in the immune-competent host, Axl inhibitors may act primarily via inhibition of metastasis and angiogenesis. [22] also reports that Axl expression correlates with metastasis and poor prognosis in human breast tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Shieh YS, Lai CY, Kao YR, et al. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005 Dec;7(12):1058–64. doi: 10.1593/neo.05640. This study identifies Axl as a poor prognostic factor in NSCLC and correlates Axl expression with invasive ability of NSCLC cell in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Koorstra JB, Karikari CA, Feldmann G, et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther. 2009 Apr;8(7):618–26. doi: 10.4161/cbt.8.7.7923. Although upregulation of Twist had previously been correlated with Axl activation (see ref 8), this report is the first to suggest a role for Axl in epithelial-to-mesenchymal transition. This study also identifies Axl as a poor prognostic factor in pancreatic cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu CW, Li AF, Chi CW, et al. Clinical significance of AXL kinase family in gastric cancer. Anticancer Res. 2002 Mar–Apr;22(2B):1071–8. [PubMed] [Google Scholar]
- 26.Hafizi S, Dahlback B. Gas 6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. Febs J. 2006 Dec;273(23):5231–44. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- 27.Manfioletti G, Brancolini C, Avanzi G, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993 Aug;13(8):4976–85. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sainaghi PP, Castello L, Bergamasco L, et al. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005 Jul;204(1):36–44. doi: 10.1002/jcp.20265. [DOI] [PubMed] [Google Scholar]
- 29.Sawabu T, Seno H, Kawashima T, et al. Growth arrest-specific gene 6 and Axl signaling enhances gastric cancer cell survival via Akt pathway. Mol Carcinog. 2007 Feb;46(2):155–64. doi: 10.1002/mc.20211. [DOI] [PubMed] [Google Scholar]
- 30.Eisenman K, Sather S, McGranahan A, et al. Mer receptor tyrosine kinase as a potential novel target in acute myeloid leukemia. Pediatric Blood & Cancer. 2010;54(6):790. [Google Scholar]
- 31.Lee WP, Wen Y, Varnum B, Hung MC. Akt is required for Axl-Gas6 signaling to protect cells from E1A-mediated apoptosis. Oncogene. 2002 Jan 17;21(3):329–36. doi: 10.1038/sj.onc.1205066. [DOI] [PubMed] [Google Scholar]
- 32**.Keating AK, Kim GK, Jones AE, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010 May;9(5):1298–307. doi: 10.1158/1535-7163.MCT-09-0707. This study is the first report of Mer overexpression in glioblastoma. Additional evidence indicates both Mer and Axl contribute to mechanisms of chemoresistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Greger J, Shi H, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009 Sep 1;69(17):6871–8. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 34.Qi W, Cooke LS, Stejskal A, et al. MP470, a novel receptor tyrosine kinase inhibitor, in combination with Erlotinib inhibits the HER family/PI3K/Akt pathway and tumor growth in prostate cancer. BMC Cancer. 2009;9:142. doi: 10.1186/1471-2407-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bose R, Molina H, Patterson AS, et al. Phosphoproteomic analysis of Her2/neu signaling and inhibition. Proc Natl Acad Sci U S A. 2006 Jun 27;103(26):9773–8. doi: 10.1073/pnas.0603948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komurov K, Padron D, Cheng T, et al. Comprehensive mapping of the human kinome to EGF receptor signaling. J Biol Chem. 2010 Apr 26; doi: 10.1074/jbc.M110.137828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu YM, Robinson DR, Kung HJ. Signal pathways in up-regulation of chemokines by tyrosine kinase MER/NYK in prostate cancer cells. Cancer Res. 2004 Oct 15;64(20):7311–20. doi: 10.1158/0008-5472.CAN-04-0972. [DOI] [PubMed] [Google Scholar]
- 38**.Holland SJ, Pan A, Franci C, et al. R428, a Selective Small Molecule Inhibitor of Axl Kinase, Blocks Tumor Spread and Prolongs Survival in Models of Metastatic Breast Cancer. Cancer Res. 2010 Feb 15;70(4):1544–54. doi: 10.1158/0008-5472.CAN-09-2997. [22] and [38] are the first reports to suggest that in the immune-competent host, Axl inhibitors may act primarily via inhibition of metastasis and angiogenesis. [38] also describes the first TKI specifically designed to selectively inhibit Axl and demonstrates its safety and tolerability in murine models of breast cancer. [DOI] [PubMed] [Google Scholar]
- 39**.Holland SJ, Powell MJ, Franci C, et al. Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer Res. 2005 Oct 15;65(20):9294–303. doi: 10.1158/0008-5472.CAN-05-0993. This is the first study to suggest a role for Axl in angiogenesis. [DOI] [PubMed] [Google Scholar]
- 40.Mudduluru G, Vajkoczy P, Allgayer H. Myeloid zinc finger 1 induces migration, invasion, and in vivo metastasis through Axl gene expression in solid cancer. Mol Cancer Res. 2010 Feb;8(2):159–69. doi: 10.1158/1541-7786.MCR-09-0326. [DOI] [PubMed] [Google Scholar]
- 41.Le Mee S, Fromigue O, Marie PJ. Sp1/Sp3 and the myeloid zinc finger gene MZF1 regulate the human N-cadherin promoter in osteoblasts. Exp Cell Res. 2005 Jan 1;302(1):129–42. doi: 10.1016/j.yexcr.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 42.Tai KY, Shieh YS, Lee CS, et al. Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-kappaB and Brg-1. Oncogene. 2008 Jul 3;27(29):4044–55. doi: 10.1038/onc.2008.57. [DOI] [PubMed] [Google Scholar]
- 43.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009 May;10(5):459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 44.Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010 May–Jun;60(3):166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985 Jan 10–18;313(5998):144–7. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 46.Hlobilkova A, Ehrmann J, Sedlakova E, et al. Could changes in the regulation of the PI3K/PKB/Akt signaling pathway and cell cycle be involved in astrocytic tumor pathogenesis and progression? Neoplasma. 2007;54(4):334–41. [PubMed] [Google Scholar]
- 47.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002 Jul;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 48.Hartmann C, Bartels G, Gehlhaar C, et al. PIK3CA mutations in glioblastoma multiforme. Acta Neuropathol. 2005 Jun;109(6):639–42. doi: 10.1007/s00401-005-1000-1. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997 Mar 28;275(5308):1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 50.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004 Apr 23;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 51.Wang SI, Puc J, Li J, et al. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res. 1997 Oct 1;57(19):4183–6. [PubMed] [Google Scholar]
- 52.Wang H, Zhang W, Huang HJ, et al. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Lab Invest. 2004 Aug;84(8):941–51. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 53.Mizoguchi M, Betensky RA, Batchelor TT, et al. Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: correlation with EGFR status, tumor grade, and survival. J Neuropathol Exp Neurol. 2006 Dec;65(12):1181–8. doi: 10.1097/01.jnen.0000248549.14962.b2. [DOI] [PubMed] [Google Scholar]
- 54**.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007 Oct 12;318(5848):287–90. doi: 10.1126/science.1142946. To the best of our knowledge, this is the first study to suggest that inhibition of multiple receptor tyrosine kinases may be necessary to effectively inhibit tumor progression. [DOI] [PubMed] [Google Scholar]
- 55*.Hutterer M, Knyazev P, Abate A, et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008 Jan 1;14(1):130–8. doi: 10.1158/1078-0432.CCR-07-0862. This is the first report to identify Axl as a poor prognostic factor in glioblastoma. [DOI] [PubMed] [Google Scholar]
- 56*.Vajkoczy P, Knyazev P, Kunkel A, et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci U S A. 2006 Apr 11;103(15):5799–804. doi: 10.1073/pnas.0510923103. This report demonstrates proof-of-concept for Axl inhibition as a therapeutic strategy in glioblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones AE, Le J, Keating AK. FASEB SRC: Receptor Tyrosine Kinases: Biology and Cancer, 2010. Carefree; Arizona, USA: 2010. Mer receptor tyrosine kinase affects glioblastoma multiforme migration. [Google Scholar]
- 58.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007 Feb 10;25(5):561–70. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 59.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007 Oct;7(10):778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 60.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. based on November 2009 SEER data submission ed. [Google Scholar]
- 61.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006 Dec 14;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 62.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. Feb;11(2):121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 63.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009 Sep 3;361(10):947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 64.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009 Sep 3;361(10):958–67. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 65.Rothenberg ML. ALK: A promising new target in the treatment of NSCLC. AACR-IASLC Joint Conference on Molecular Origins of Lung Cancer: Prospects for Personalized Prevention and Therapy; Coronado, Ca, USA. 2010. [Google Scholar]
- 66.Wimmel A, Glitz D, Kraus A, et al. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer. 2001 Nov;37(17):2264–74. doi: 10.1016/s0959-8049(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 67.Wimmel A, Rohner I, Ramaswamy A, et al. Synthesis and secretion of the anticoagulant protein S and coexpression of the Tyro3 receptor in human lung carcinoma cells. Cancer. 1999 Jul 1;86(1):43–9. doi: 10.1002/(sici)1097-0142(19990701)86:1<43::aid-cncr8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 68.Linger RMA, Middleton DHG, Migdall J, et al. Mer and Axl receptor tyrosine kinases are novel therapeutic targets in NSCLC. AACR-IASLC Joint Conference on Molecular Origins of Lung Cancer - Prospects for Personalized Prevention and Therapy; Coronado, CA. 2010. [Google Scholar]
- 69.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007 Dec 14;131(6):1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 70**.Li Y, Ye X, Tan C, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009 Oct 1;28(39):3442–55. doi: 10.1038/onc.2009.212. This study is the first to suggest a role for Axl in metastasis. It also represents the first report of anti-Axl monoclonal antibodies as therapeutic candidates. [DOI] [PubMed] [Google Scholar]
- 71.Gluz O, Liedtke C, Gottschalk N, et al. Triple-negative breast cancer--current status and future directions. Ann Oncol. 2009 Dec;20(12):1913–27. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 72.Berclaz G, Altermatt HJ, Rohrbach V, et al. Estrogen dependent expression of the receptor tyrosine kinase axl in normal and malignant human breast. Ann Oncol. 2001 Jun;12(6):819–24. doi: 10.1023/a:1011126330233. [DOI] [PubMed] [Google Scholar]
- 73.Meric F, Lee WP, Sahin A, et al. Expression profile of tyrosine kinases in breast cancer. Clin Cancer Res. 2002 Feb;8(2):361–7. [PubMed] [Google Scholar]
- 74*.Tavazoie SF, Alarcon C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008 Jan 10;451(7175):147–52. doi: 10.1038/nature06487. This is the first study to correlate expression of Mer with metastatic potential and suggests that Mer overexpression may result from loss of miRNA expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mo R, Tony Zhu Y, Zhang Z, et al. GAS6 is an estrogen-inducible gene in mammary epithelial cells. Biochem Biophys Res Commun. 2007 Feb 2;353(1):189–94. doi: 10.1016/j.bbrc.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richer JK, Jacobsen BM, Manning NG, et al. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002 Feb 15;277(7):5209–18. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 77.Mc Cormack O, Chung WY, Fitzpatrick P, et al. Growth arrest-specific gene 6 expression in human breast cancer. Br J Cancer. 2008 Mar 25;98(6):1141–6. doi: 10.1038/sj.bjc.6604260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abba MC, Fabris VT, Hu Y, et al. Identification of novel amplification gene targets in mouse and human breast cancer at a syntenic cluster mapping to mouse ch8A1 and human ch13q34. Cancer Res. 2007 May 1;67(9):4104–12. doi: 10.1158/0008-5472.CAN-06-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang YX, Knyazev PG, Cheburkin YV, et al. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res. 2008 Mar 15;68(6):1905–15. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- 80.Zantek ND, Walker-Daniels J, Stewart J, et al. MCF-10A-NeoST: a new cell system for studying cell-ECM and cell-cell interactions in breast cancer. Clin Cancer Res. 2001 Nov;7(11):3640–8. [PubMed] [Google Scholar]
- 81.Lemke G, Lu Q. Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol. 2003 Feb;15(1):31–6. doi: 10.1016/s0952-7915(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 82.Lahiry P, Torkamani A, Schork NJ, Hegele RA. Kinase mutations in human disease: interpreting genotype-phenotype relationships. Nat Rev Genet. 2010 Jan;11(1):60–74. doi: 10.1038/nrg2707. [DOI] [PubMed] [Google Scholar]
- 83.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010 Jul 1;16(13):3507–16. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 84.Srinivasan R, Choueiri T, Vaishampayan U, et al. A phase II study of the dual MET-VEGFR2 inhibitor GSK1363089 (Formerly XL880) in patients with papillary renal carcinoma (PRC). Annual Meeting of the American Society of Clinical Oncology; Chicago, Illinois, USA. 2008. [Google Scholar]
- 85.Vollrath D, Feng W, Duncan JL, et al. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci U S A. 2001 Oct 23;98(22):12584–9. doi: 10.1073/pnas.221364198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86*.Mahadevan D, Cooke L, Riley C, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007 Jun 7;26(27):3909–19. doi: 10.1038/sj.onc.1210173. This study is the first report of a small-molecule tyrosine kinase inhibitor with activity against Axl. It is also the first study to identify upregulation of Axl as a mechanism of resistance to targeted therapy. [DOI] [PubMed] [Google Scholar]
- 87.Quintas-Cardama A, Kantarjian H, Cortes J. Imatinib and beyond--exploring the full potential of targeted therapy for CML. Nat Rev Clin Oncol. 2009 Sep;6(9):535–43. doi: 10.1038/nrclinonc.2009.112. [DOI] [PubMed] [Google Scholar]
- 88.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009 Jan 20;27(3):469–71. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 89.Schroeder GM, An Y, Cai ZW, et al. Discovery of N-(4-(2-Amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluor ophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a Selective and Orally Efficacious Inhibitor of the Met Kinase Superfamily. J Med Chem. 2009 Mar 12;52(5):1251–4. doi: 10.1021/jm801586s. [DOI] [PubMed] [Google Scholar]
- 90.Huang X, Finerty P, Jr, Walker JR, et al. Structural insights into the inhibited states of the Mer receptor tyrosine kinase. J Struct Biol. 2009 Feb;165(2):88–96. doi: 10.1016/j.jsb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ye X, Li Y, Stawicki S, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010 Jul 5; doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 92.Brandao L, Molk D, Sather S, Graham DK. Inhibition of Mer as a novel therapeutic strategy in acute lymphoblastic leukemia. Keystone Symposia: New Paradigms in Cancer Therapeutics; 2010 March 23–28, 2010; Victoria, British Columbia, Canada. 2010. [Google Scholar]
- 93.Anwar A, Keating AK, Joung D, et al. Mer tyrosine kinase (MerTK) promotes macrophage survival following exposure to oxidative stress. J Leukoc Biol. 2009 Jul;86(1):73–9. doi: 10.1189/JLB.0608334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sather S, Kenyon KD, Lefkowitz JB, et al. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007 Feb 1;109(3):1026–33. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mudduluru G, Allgayer H. The human receptor tyrosine kinase Axl gene--promoter characterization and regulation of constitutive expression by Sp1, Sp3 and CpG methylation. Biosci Rep. 2008 Jun;28(3):161–76. doi: 10.1042/BSR20080046. [DOI] [PubMed] [Google Scholar]
- 96.Wong CC, Lee WM. The proximal cis-acting elements Sp1, Sp3 and E2F regulate mouse mer gene transcription in Sertoli cells. Eur J Biochem. 2002 Aug;269(15):3789–800. doi: 10.1046/j.1432-1033.2002.03092.x. [DOI] [PubMed] [Google Scholar]
- 97.Margareto J, Leis O, Larrarte E, et al. DNA copy number variation and gene expression analyses reveal the implication of specific oncogenes and genes in GBM. Cancer Invest. 2009 Jun;27(5):541–8. doi: 10.1080/07357900802563044. [DOI] [PubMed] [Google Scholar]
- 98**.Loges S, Schmidt T, Tjwa M, et al. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010 Mar 18;115(11):2264–73. doi: 10.1182/blood-2009-06-228684. This is the first study to demonstrate that TAM receptors and Gas6 expressed by stromal immune cells contribute to tumor growth and metastasis. [DOI] [PubMed] [Google Scholar]
- 99.Gambacorti-Passerini CB, Gunby RH, Piazza R, et al. Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias. Lancet Oncol. 2003 Feb;4(2):75–85. doi: 10.1016/s1470-2045(03)00979-3. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005 Feb 24;352(8):786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 101.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005 Mar;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007 Dec 26;104(52):20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007 May 18;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 104.Grosso S, Puissant A, Dufies M, et al. Gene expression profiling of imatinib and PD166326-resistant CML cell lines identifies Fyn as a gene associated with resistance to BCR-ABL inhibitors. Mol Cancer Ther. 2009 Jul;8(7):1924–33. doi: 10.1158/1535-7163.MCT-09-0168. [DOI] [PubMed] [Google Scholar]
- 105.Lay JD, Hong CC, Huang JS, et al. Sulfasalazine suppresses drug resistance and invasiveness of lung adenocarcinoma cells expressing AXL. Cancer Res. 2007 Apr 15;67(8):3878–87. doi: 10.1158/0008-5472.CAN-06-3191. [DOI] [PubMed] [Google Scholar]
- 106*.Macleod K, Mullen P, Sewell J, et al. Altered ErbB receptor signaling and gene expression in cisplatin-resistant ovarian cancer. Cancer Res. 2005 Aug 1;65(15):6789–800. doi: 10.1158/0008-5472.CAN-04-2684. To the best of our knowledge, this is the first report to identify Axl as a gene upregulated in chemoresistant cancer cells. [DOI] [PubMed] [Google Scholar]
- 107.Knight ZA, Lin H, Shokat KM. Targeting the cancer kinome through polypharmacology. Nat Rev Cancer. 2010 Feb;10(2):130–7. doi: 10.1038/nrc2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu AM, Huang PH. Receptor tyrosine kinase coactivation networks in cancer. Cancer Res. 2010 May 15;70(10):3857–60. doi: 10.1158/0008-5472.CAN-10-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001 Mar 15;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 110.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007 Apr 20;25(12):1545–52. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 111.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004 Mar 1;22(5):777–84. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004 Mar 1;22(5):785–94. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 113.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005 Sep 1;23(25):5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 114.Cappuzzo F, Gregorc V, Rossi E, et al. Gefitinib in pretreated non-small-cell lung cancer (NSCLC): analysis of efficacy and correlation with HER2 and epidermal growth factor receptor expression in locally advanced or metastatic NSCLC. J Clin Oncol. 2003 Jul 15;21(14):2658–63. doi: 10.1200/JCO.2003.01.039. [DOI] [PubMed] [Google Scholar]
- 115.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003 Oct 15;21(20):3798–807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 116.Fong T, Morgensztern D, Govindan R. EGFR inhibitors as first-line therapy in advanced non-small cell lung cancer. J Thorac Oncol. 2008 Mar;3(3):303–10. doi: 10.1097/JTO.0b013e3181645477. [DOI] [PubMed] [Google Scholar]
- 117.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004 May 20;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 118.Braunger J, Schleithoff L, Schulz AS, et al. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene. 1997 Jun 5;14(22):2619–31. doi: 10.1038/sj.onc.1201123. [DOI] [PubMed] [Google Scholar]
- 119.Fridell YW, Jin Y, Quilliam LA, et al. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Mol Cell Biol. 1996 Jan;16(1):135–45. doi: 10.1128/mcb.16.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Georgescu MM, Kirsch KH, Shishido T, et al. Biological effects of c-Mer receptor tyrosine kinase in hematopoietic cells depend on the Grb2 binding site in the receptor and activation of NF-kappaB. Mol Cell Biol. 1999 Feb;19(2):1171–81. doi: 10.1128/mcb.19.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ling L, Templeton D, Kung HJ. Identification of the major autophosphorylation sites of Nyk/Mer, an NCAM-related receptor tyrosine kinase. J Biol Chem. 1996 Aug 2;271(31):18355–62. doi: 10.1074/jbc.271.31.18355. [DOI] [PubMed] [Google Scholar]
- 122.Pao-Chun L, Chan PM, Chan W, Manser E. Cytoplasmic ACK1 interaction with multiple receptor tyrosine kinases is mediated by Grb2: an analysis of ACK1 effects on Axl signaling. J Biol Chem. 2009 Dec 11;284(50):34954–63. doi: 10.1074/jbc.M109.072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mahajan NP, Whang YE, Mohler JL, Earp HS. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res. 2005 Nov 15;65(22):10514–23. doi: 10.1158/0008-5472.CAN-05-1127. [DOI] [PubMed] [Google Scholar]
- 124.Liu Y, Karaca M, Zhang Z, et al. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene. 2010 Apr 12; doi: 10.1038/onc.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakano T, Tani M, Ishibashi Y, et al. Biological properties and gene expression associated with metastatic potential of human osteosarcoma. Clin Exp Metastasis. 2003;20(7):665–74. doi: 10.1023/a:1027355610603. [DOI] [PubMed] [Google Scholar]
- 126.Craven RJ, Xu LH, Weiner TM, et al. Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer. 1995 Mar 16;60(6):791–7. doi: 10.1002/ijc.2910600611. [DOI] [PubMed] [Google Scholar]
- 127.Sun WS, Fujimoto J, Tamaya T. Coexpression of growth arrest-specific gene 6 and receptor tyrosine kinases Axl and Sky in human uterine endometrial cancers. Ann Oncol. 2003 Jun;14(6):898–906. doi: 10.1093/annonc/mdg257. [DOI] [PubMed] [Google Scholar]
- 128.Nemoto T, Ohashi K, Akashi T, et al. Overexpression of protein tyrosine kinases in human esophageal cancer. Pathobiology. 1997;65(4):195–203. doi: 10.1159/000164123. [DOI] [PubMed] [Google Scholar]
- 129.Chung BI, Malkowicz SB, Nguyen TB, et al. Expression of the proto-oncogene Axl in renal cell carcinoma. DNA Cell Biol. 2003 Aug;22(8):533–40. doi: 10.1089/10445490360708946. [DOI] [PubMed] [Google Scholar]
- 130.Gustafsson A, Martuszewska D, Johansson M, et al. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res. 2009 Jul 15;15(14):4742–9. doi: 10.1158/1078-0432.CCR-08-2514. [DOI] [PubMed] [Google Scholar]
- 131.Tsou AP, Wu KM, Tsen TY, et al. Parallel hybridization analysis of multiple protein kinase genes: identification of gene expression patterns characteristic of human hepatocellular carcinoma. Genomics. 1998 Jun 15;50(3):331–40. doi: 10.1006/geno.1998.5338. [DOI] [PubMed] [Google Scholar]
- 132.Ek S, Hogerkorp CM, Dictor M, et al. Mantle cell lymphomas express a distinct genetic signature affecting lymphocyte trafficking and growth regulation as compared with subpopulations of normal human B cells. Cancer Res. 2002 Aug 1;62(15):4398–405. [PubMed] [Google Scholar]
- 133.Sun W, Fujimoto J, Tamaya T. Coexpression of Gas6/Axl in human ovarian cancers. Oncology. 2004;66(6):450–7. doi: 10.1159/000079499. [DOI] [PubMed] [Google Scholar]
- 134.Evans CO, Young AN, Brown MR, et al. Novel patterns of gene expression in pituitary adenomas identified by complementary deoxyribonucleic acid microarrays and quantitative reverse transcription-polymerase chain reaction. J Clin Endocrinol Metab. 2001 Jul;86(7):3097–107. doi: 10.1210/jcem.86.7.7616. [DOI] [PubMed] [Google Scholar]
- 135.Jacob AN, Kalapurakal J, Davidson WR, et al. A receptor tyrosine kinase, UFO/Axl, and other genes isolated by a modified differential display PCR are overexpressed in metastatic prostatic carcinoma cell line DU145. Cancer Detect Prev. 1999;23(4):325–32. doi: 10.1046/j.1525-1500.1999.99034.x. [DOI] [PubMed] [Google Scholar]
- 136.Khan J, Bittner ML, Saal LH, et al. cDNA microarrays detect activation of a myogenic transcription program by the PAX3-FKHR fusion oncogene. Proc Natl Acad Sci U S A. 1999 Nov 9;96(23):13264–9. doi: 10.1073/pnas.96.23.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Ginkel PR, Gee RL, Shearer RL, et al. Expression of the receptor tyrosine kinase Axl promotes ocular melanoma cell survival. Cancer Res. 2004 Jan 1;64(1):128–34. doi: 10.1158/0008-5472.can-03-0245. [DOI] [PubMed] [Google Scholar]
- 138.Gyorffy B, Lage H. A Web-based data warehouse on gene expression in human malignant melanoma. J Invest Dermatol. 2007 Feb;127(2):394–9. doi: 10.1038/sj.jid.5700543. [DOI] [PubMed] [Google Scholar]
- 139.Quong RY, Bickford ST, Ing YL, et al. Protein kinases in normal and transformed melanocytes. Melanoma Res. 1994 Oct;4(5):313–9. doi: 10.1097/00008390-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 140.Lin WC, Li AF, Chi CW, et al. tie-1 protein tyrosine kinase: a novel independent prognostic marker for gastric cancer. Clin Cancer Res. 1999 Jul;5(7):1745–51. [PubMed] [Google Scholar]
- 141.Tanaka K, Nagayama Y, Nakano T, et al. Expression profile of receptor-type protein tyrosine kinase genes in the human thyroid. Endocrinology. 1998 Mar;139(3):852–8. doi: 10.1210/endo.139.3.5791. [DOI] [PubMed] [Google Scholar]
- 142.Ito M, Nakashima M, Nakayama T, et al. Expression of receptor-type tyrosine kinase, Axl, and its ligand, Gas6, in pediatric thyroid carcinomas around chernobyl. Thyroid. 2002 Nov;12(11):971–5. doi: 10.1089/105072502320908303. [DOI] [PubMed] [Google Scholar]
- 143.Ito T, Ito M, Naito S, et al. Expression of the Axl receptor tyrosine kinase in human thyroid carcinoma. Thyroid. 1999 Jun;9(6):563–7. doi: 10.1089/thy.1999.9.563. [DOI] [PubMed] [Google Scholar]