Abstract
Background
Sublingual nitroglycerin increases exercise duration in patients with stable angina. Brief results from this study were published previously in German. Here, we more fully describe the study methodology, patient characteristics, and detailed results.
Methods
This double-blind, crossover study enrolled 51 patients with stable angina. Patients were randomized to 1 of 5 treatment sequences and were administered placebo or nitroglycerin spray (0.2 mg, 0.4 mg, 0.8 mg, or 1.6 mg). Patients carried out 1 control exercise tolerance test (ETT) and 1 investigational ETT at each visit.
Results
Dose-dependent increases in time to onset of angina, time to onset of moderate angina, and the occurrence of a minimum 1.0-mm ST-segment depression were seen following administration of nitroglycerin spray.
Conclusions
These results support the use of sublingual nitroglycerin spray in patients with stable angina who are being managed with medical therapy and in patients who have persistent angina post-revascularization.
Keywords: chronic stable angina, sublingual nitroglycerin spray, optimal medical therapy
Introduction
Patients with frequent angina (≥1 episode per week) report greater physical limitations and decreased quality of life compared with patients with minimal angina.1 The goals of chronic stable angina management are not only to control symptoms, but also to reduce the risk of adverse clinical outcomes.2–6 As symptom control impacts the patient’s quality of life, maximum consideration should be given to the array of available management options that aim to reduce angina symptoms. For many patients, this would include a physical activity program aimed at improving exercise tolerance in accordance with current secondary prevention guidelines.
Angina management options include anti-anginal drugs and coronary artery revascularization. Several studies have shown that optimal medical therapy, along with aggressive risk factor modification, is as effective as revascularization in reducing the risk of adverse coronary events.7–9 Optimal medical therapy includes anti-anginal or anti-ischemic as well as disease- modifying therapy.8,10 The anti-ischemic agents include short- and long-acting nitrates, beta-blockers, and calcium channel blockers.3,4,11 These agents reduce angina symptoms and prolong exercise duration and/ or time to ST-segment depression.3,11 Frequently, a combination of drugs is necessary for optimal symptom control.3 Nitrates work primarily by venodilation and afford rapid relief of angina.12 A number of placebo-controlled studies have shown that the sublingual application of nitroglycerin increases exercise duration in patients with stable angina.13–17
Published data also suggest that residual and recurrent angina is frequently experienced by patients who have already undergone either a percutaneous or surgical revascularization procedure, and these patients require continued medical therapy with multiple anti-anginal agents, including short- and long-acting nitrates, for control of their symptoms.8,18,19 Increased physical exercise is also important in patients with stable angina to reduce the risk of cardiovascular events, and current recommendations call for the use of sublingual nitroglycerin as part of optimal medical therapy.5,20
This randomized, double-blind, placebo-controlled, crossover, dose-ranging multicenter study in patients with chronic stable angina evaluated the effect of a sublingual nitroglycerin spray formulation on time to onset of angina during exercise, total exercise duration, and time to exercise-induced myocardial ischemia, as evidenced by electrocardiographic ST-segment depression. Brief results were previously published in German.21 The goal of the present article is to more fully describe the study methodology, patient characteristics, and detailed results in the context of current optimal medical therapy.
Methods
Study approval and consent
This study was carried out at four centers in the United States and conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and Food and Drug Administration guidelines. The study was approved by local Investigational Review Boards. All patients provided written informed consent at the screening visit, prior to starting any study-related procedures.
Patients
Adults (≥18 years of age) with coronary artery disease as documented by a ≥50% reduction in arterial lumen diameter in 1 or more major coronary arteries or their primary branches, a previously documented myocardial infarction, or reversible perfusion defect on stress thallium-201 scintigraphy and a diagnosis of chronic stable exertional angina that had been made at least 2 months prior to screening were eligible to be screened. In the 2 months prior to study entry, patients were required to have been on a stable medical treatment regimen for their angina. Patients with unstable angina, myocardial infarction, coronary angioplasty, or cardiac surgery within 3 months of screening were excluded. Additional exclusion criteria included a history of congestive heart failure (NYHA III or IV), significant disease of the cardiac conduction system, uncontrolled hypertension or hypotension, any physical conditions known to affect ST-segment morphology, or severe organ damage or disease of any system. Patients were not permitted to receive concomitant therapy with long-acting nitrates or digitalis preparations during the study. In addition, participation in an investigational drug study within 1 month prior to the screening visit or while participating in the study was prohibited. Patients with clinically significant deviations from normal in either the physical exam or lab parameters and patients with peripheral arterial disease that limited their exercise capacity were also excluded.
Study Design and Treatment
According to the approved protocol, each center was to enroll a minimum of 12 patients to ensure evaluable outcomes for 40 patients at the end of study. Following an initial screening visit, eligible patients returned for a baseline exercise tolerance test (ETTB) according to a standard Bruce protocol.22 Of note, exercise testing is associated with minimal complications; death and myocardial infarction occur at a rate of ≤1 per 2,500 tests performed.15,23 Patients who developed angina within 3–9 minutes of commencing exercise were eligible to continue in the study. Following a 90-minute rest period, these patients were assessed for nitrate response. Patients who were able to exercise for ≥60 seconds longer in this ETT (ETTRES) than in the ETTB were eligible to continue in the study. At their next visit, patients completed a confirmatory ETT (ETTCONF) to establish reproducibility (within 20%) of the time to development of moderate angina when compared to the ETTB. In order to reduce baseline variability, all ETTs were carried out at approximately the same time of day. Non–study-related sublingual nitroglycerin was discontinued 6 hours prior to testing, while other anti-anginal medications were discontinued approximately 24 hours prior to each visit.
After baseline and confirmatory ETTs were completed, patients were randomized to 1 of 5 treatment sequences (Fig. 1). The goal of this crossover study design with each patient serving as his/her own control was to minimize the impact of any training effect on the mean values for each treatment dose. The crossover period consisted of 5 additional visits within 14 days, at which patients were administered placebo or sublingual nitroglycerin spray (0.2 mg, 0.4 mg, 0.8 mg, or 1.6 mg; Nitrolingual® Pumpspray, G. Pohl-Boskamp GmbH & Co. KG, Germany). In order to minimize the impact of any training effect, patients completed a control ETT (ETTC) and an investigational ETT (ETTINV) at each visit. ETTC studies were carried out at approximately the same time of day as the introductory ETT studies (ETTB and ETTCONF). Following a 90-minute rest period, study medication was administered and then ETTINVs commenced within 2–5 minutes.
Figure 1.
Study design.
Notes: aThe baseline ETT was followed by a 90-minute rest period before the nitrate response ETT was performed; bthe control ETT was followed by a 90-minute rest period before the investigational ETT was performed. The investigational ETT was carried out within 2–5 minutes of study drug administration.
Abbreviations: ETTB, Baseline exercise tolerance test (treadmill); ETTRES, Nitrate response exercise tolerance test (treadmill); ETTCONF, Confirmation exercise tolerance test (treadmill); ETTC, Control exercise tolerance test (treadmill); ETTINV, Investigational exercise tolerance test (treadmill).
Outcomes
Primary efficacy endpoints were time to onset of angina symptoms and time to development of moderate angina (defined as the level of activity at which the patient would normally stop activity). The secondary endpoint was time to 1-mm ST-segment depression (myocardial ischemia). To minimize the impact of any training effect, efficacy endpoints are described as the mean difference between ETTINV and ETTC at each visit. The difference between the confirmation and baseline ETTs was then subtracted from this value to further reduce the impact of any training effect.
Safety parameters included evaluation of all changes in physical examination, vital signs, ECG, and laboratory evaluations. All adverse events experienced during the study were summarized.
Results
Patient characteristics
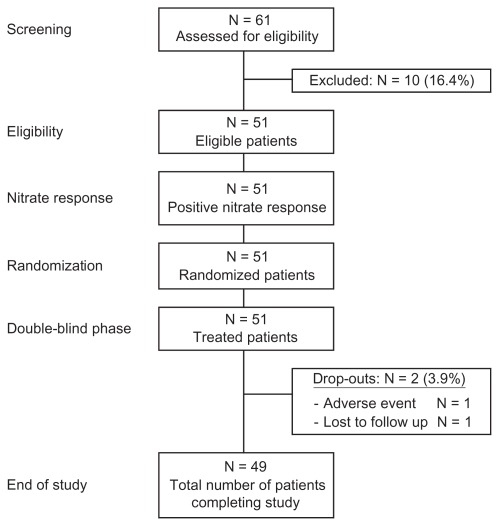
Of the 61 patients assessed for eligibility, 10 patients did not meet the protocol criteria and were therefore excluded. A total of 51 patients performed the baseline exercise test and qualified for continuation. Following a 90-minute rest period, patients underwent a subsequent stress test after receiving 0.4 mg of open-label sublingual nitroglycerin spray. All 51 patients showed an improvement in exercise duration of 60 seconds or greater (range 1.02–4.7 minutes).
A total of 51 patients between the ages of 34 and 77 years continued in the double-blind phase and were randomized to 1 of 5 treatment sequences. Figure 2 provides a flow diagram of patient disposition over the study period. Two patients did not complete the study (1 patient was lost to follow-up and 1 patient discontinued prematurely due to ventricular tachycardia/fibrillation requiring cardioversion after the control exercise test at Visit 5; this event was not considered to be treatment related). The remaining 49 patients completed all portions of the study. Overall patient characteristics including angina and cardiovascular event history, along with concomitant medications, are described in Table 1.
Figure 2.
Patient disposition.
Table 1.
Patient characteristics (N = 51).
| Gender, n | |
| Male | 39 |
| Female | 12 |
| Age, years (median [min–max]) | 65 (34–77) |
| Race, n | |
| Caucasian | 38 |
| Hispanic | 11 |
| Asian | 1 |
| Black | 1 |
| Height, cm (mean) | 172.2 |
| Weight, kg (mean) | 81.7 |
| Angina history, years (median [min–max]) | 6 (0–23) |
| Angina attacks | |
| Duration, minutes (mean [min–max]) | 2.7 (1.0–10.0) |
| Attacks/week, no. (mean [min–max]) | 4.9 (1–35) |
| NTG sprays/week, no. (mean [min–max]) | 4.25 (0–35) |
| Severity of attacks | |
| Mild | 30 |
| Moderate | 19 |
| History of cardiovascular events, n | |
| Myocardial infarction | 23 |
| PTCA | 12 |
| Bypass surgery | 12 |
| Concomitant study medication, % | |
| Disease-modifying therapy | |
| Aspirin | |
| Primary prevention | 64 |
| Secondary prevention | 65 |
| Statins | |
| Primary prevention | 25 |
| Secondary prevention | 17 |
| ACE inhibitors | |
| Primary prevention | 7 |
| Secondary prevention | 13 |
| Beta blockers | |
| Primary prevention | 25 |
| Secondary prevention | 26 |
| Symptomatic treatment for angina/ischemia control | |
| Calcium antagonists | |
| Primary prevention | 82 |
| Secondary prevention | 87 |
| Sublingual NTG spray | |
| Primary prevention | 79 |
| Secondary prevention | 87 |
| Sublingual NTG tablets | |
| Primary prevention | 11 |
| Secondary prevention | 9 |
| Other relevant medications | |
| Anti-hypertensives | 29 |
| Diabetes medication | 26 |
Abbreviations: NTG, nitroglycerin; PTCA, percutaneous transluminal coronary angioplasty.
Efficacy
A dose-dependent increase in time to onset of moderate angina was seen following administration of nitroglycerin spray (Table 2, Fig. 3). In addition, the time to onset of angina was significantly higher in all treatment groups (0.2 mg, 0.4 mg, 0.8 mg, and 1.6 mg) than in the placebo group (Table 2, Fig. 4). The increase in time to onset of angina and to onset of moderate angina was most pronounced in the 1.6-mg group, with dose linearity between 0.4- and 1.6-mg doses of nitroglycerin spray. The occurrence of a minimum 1.0-mm ST-segment depression was later in all treatment groups (0.2 mg, 0.4 mg, 0.8 mg, 1.6 mg) compared with the placebo group (Table 2, Fig. 5).
Table 2.
Exercise tolerance time following administration of nitroglycerin spray.
| Adjusted mean change in time to onset of moderate angina (min)a | Adjusted mean change in time to onset of angina (min)a | Adjusted mean change in time to onset of 1-mm ST-segment depression (min)a | |
|---|---|---|---|
| Placebo | 0.49 | 0.61 | 0.19 |
| 0.2 mg NTG | 0.88c | 1.12b | 0.93b |
| 0.4 mg NTG | 0.87c | 1.05b | 0.8 |
| 0.8 mg NTG | 1.13d | 1.37d | 1.26c |
| 1.6 mg NTG | 1.26d | 1.47d | 1.59c |
Notes:
Investigational ETT minus control ETT; sublingual nitroglycerin spray versus placebo;
P < 0.05;
P < 0.01;
P < 0.001.
Abbreviation: NTG, nitroglycerin.
Figure 3.
Adjusted mean change in time to onset of moderate angina.a
Notes: aInvestigational ETT minus control ETT; sublingual nitroglycerin spray versus placebo; bP < 0.01; cP < 0.001.
Abbreviation: NTG, nitroglycerin.
Figure 4.
Adjusted mean change in time to onset of angina.a
Notes: aInvestigational ETT minus control ETT; sublingual nitroglycerin spray versus placebo; bP < 0.05; cP < 0.001.
Abbreviation: NTG, nitroglycerin.
Figure 5.
Adjusted mean change in time to onset of 1-mm ST-segment depression.a
Notes: aInvestigational ETT minus control ETT; sublingual nitroglycerin spray versus placebo; bP < 0.05; cP < 0.01.
Abbreviation: NTG, nitroglycerin.
Safety
Overall, nitroglycerin spray was generally well tolerated. Of 51 patients, 12 (24%) reported adverse events at the qualification visit (open-label sublingual nitroglycerin response test). These primarily consisted of mild headache (n = 9).
Adverse events considered related to treatment were reported for 2 (4%), 4 (8%), 8 (16%), 9 (18%), and 13 (26%) patients receiving placebo and 0.2 mg, 0.4 mg, 0.8 mg, and 1.6 mg nitroglycerin spray, respectively. The most frequently reported adverse events of this nature were headache and dizziness, all of which were mild to moderate in severity. The incidence of treatment-related adverse events was dose dependent, with the incidence of headache increasing from 0 with placebo to 3 (6%), 5 (10%), 6 (12%), and 8 (16%) after 0.2 mg, 0.4 mg, 0.8 mg, and 1.6 mg nitroglycerin spray, respectively. No serious adverse events related to treatment were reported.
Per protocol, patients with systolic blood pressure (SBP) less than 100 mmHg were excluded from the study. Symptomatic hypotension was not reported as an adverse event, however SBP decreased to a level of 100 mmHg or less in 1 (2%), 0 (0%), 1 (2%), 0 (0%), and 3 (5%) patients after receiving placebo and 0.2 mg, 0.4 mg, 0.8 mg, and 1.6 mg nitroglycerin spray in the double-blind phase.
Discussion
Lifestyle modifications, including supervised exercise therapy, play an important role in the success of optimal medical therapy.24–27 However, patients with stable angina generally have impaired exercise tolerance and reduced daily physical activity.28 Sublingual nitroglycerin increases physical exercise tolerance which, in turn, helps to increase the amount of exertion possible prior to the onset of angina.12,13 Sublingual forms of nitroglycerin are readily absorbed through mucous membranes, and their effect is prompt, reliable, and more effective than other forms of nitroglycerin, including ointments, transdermal patches, and sustained-release preparations.11,29 The results of this study are in line with prior findings and demonstrate a significant improvement in exercise tolerance with sublingual nitroglycerin spray in patients with stable angina. Although sublingual nitroglycerin spray was generally well tolerated, an increase of treatment-related adverse events was observed with higher dose levels (26% of patients with 1.6 mg nitroglycerin spray).
One important issue that is not specifically addressed in this study is the clinical relevance of the nearly 1-minute improvement in exercise duration with sublingual nitroglycerin spray over placebo. It must be recognized that this improvement was experienced by patients who were exercising at a higher work load (higher speed and incline during the treadmill test) after receiving the study medication. As most real-world patients have a much slower walking pace, this finding indicates that prophylactic nitroglycerin spray might allow much greater increases in walking times during normal daily activities. Although this outcome was not formally evaluated in the present study or discussed in previous publications to our knowledge, patients have often reported a marked increase in angina-free walking time and exercise duration after taking prophylactic sublingual nitroglycerin.
Additional Considerations
Since this study of sublingual nitroglycerin spray was conducted, there have been significant changes in optimal medical therapy for stable coronary artery disease. This is particularly apparent in the greater use of beta-blockers compared with calcium channel blockers and long-acting nitrates to treat angina, and the routine use of daily aspirin and a statin to reduce serious cardiovascular outcomes.3,8 Although beta-blockers are currently considered a standard treatment for stable angina, patients receiving these medications may continue to experience angina.30 However, patients with a history of beta-blocker use who are appropriately supplemented with sublingual nitroglycerin or long-acting nitrates show significant improvements in angina-free exercise duration and total walking time compared with placebo.3,5,29
The American College of Cardiology and American Heart Association guidelines for the management of patients with chronic stable angina emphasize the initial use of medical therapy.5,20,31 These guidelines include the recommendation for all patients to undergo a risk assessment with a physical activity history and/or an exercise test to guide prognosis and prescription. ETT is associated with a very low rate of serious adverse events such as death (0.5 per 10,000 tests), myocardial infarction (3.58 per 10,000 tests), or serious arrhythmias (4.78 per 10,000 tests).15 Guideline-compliant medical therapy improves clinical outcome in many patients with stable angina, as evidenced by an observational study in which increasingly guideline-compliant therapy was associated with a reduction in death and myocardial infarction in patients with stable angina during 1 year of follow-up.32 Indeed, implementation of a quality improvement program in Utah to enhance the prescription of appropriate discharge medications among patients with cardiovascular disease was associated with improvements in cardiovascular readmission rates and reductions in mortality.33
A number of studies have compared the effect of optimal medical therapy versus revascularization on symptoms and outcomes in patients with coronary artery disease and stable angina. In general, these studies have shown that optimal medical therapy is effective in controlling symptoms and, along with risk factor modification, can reduce the risk of adverse cardiac events.8,34 Moreover, these studies have shown that there is no significant difference in serious adverse clinical events, such as myocardial infarction and death, between patients who have received medical therapy and those who have undergone revascularization.2,5,8 The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial, which compared the efficacy of percutaneous coronary intervention (PCI) plus optimal medical therapy versus optimal medical therapy alone, demonstrated similar rates of death and nonfatal myocardial infarction in both patient groups over a follow-up period of 55 months.8 Moreover, both groups demonstrated a substantial reduction in angina symptoms, with 74% and 72% of patients in the PCI and optimal medical therapy groups, respectively, angina-free after 5 years of follow-up.8 Further analysis of data from the COURAGE study found that both groups of patients had marked improvements in health status, including reductions in angina symptoms, and improved quality of life. Although the PCI group demonstrated significantly greater health benefits in the short term, these benefits disappeared after longer-term follow-up, and by 36 months there was no significant difference in health status between the 2 groups.9 The Bypass Angioplasty Revascularization Investigation 2 Diabetes trial in patients with diabetes, coronary artery disease, and classic angina compared the effects of revascularization with medical therapy alone on clinical outcomes. During the 5-year follow-up, no difference was found between the medical therapy and revascularization groups on the risk of all-cause death, myocardial infarction, or stroke.34 Furthermore, a meta-analysis of 11 randomized trials comparing PCI with conservative treatment in patients with stable coronary artery disease found no significant difference between the 2 treatment strategies with regard to overall mortality, cardiac death, or myocardial infarction.7
Current recommendations state that patients with stable angina may defer surgical intervention without additional risk to allow for determination of response to optimal medical therapy.5,11 Despite the published outcome data and current guideline recommendations, many patients are still referred for revascularization, mainly due to a belief that these procedures reduce the risk of myocardial infarction or death more effectively than optimal medical therapy alone.2,3,8,35 Additional studies assessing the long-term benefits of exercise in the context of medical therapy compared with revascularization outcomes could help to further inform the clinical decision making process.
Conclusions
In this study, sublingual nitroglycerin spray was shown to increase time to onset of moderate angina during exercise. This improvement in physical exercise tolerance may be of great clinical relevance, as the prophylactic use of sublingual nitroglycerin spray allows patients to face exertion with confidence and permits exercise for a longer amount of time. These results support the use of sublingual nitroglycerin spray as nitrate therapy in patients with stable angina who are being managed with optimal medical therapy. This approach may also be beneficial for patients who have persistent angina post-revascularization. Further studies are needed to evaluate the long-term beneficial effects of using routine prophylactic nitroglycerin spray in patients with stable angina to increase exercise duration and improve quality of life and well-being.
Acknowledgements
Data were compiled and analyzed by Rhone-Poulenc Rorer Clinical Development. Data were provided to the authors by G. Pohl-Boskamp GmbH & Co. KG, Germany through Arbor Pharmaceuticals. Writing and editorial assistance was provided by Claire Gibbons, PhD and Sabrina L. Maurer, PharmD from Fishawack Communications and funded by Arbor Pharmaceuticals.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: UT with writing assistance of Claire Gibbons, PhD and Sabrina L. Maurer, PharmD. Contributed to the writing of the manuscript: UT, TW. Agree with manuscript results and conclusions: UT, TW. Jointly developed the structure and arguments for the paper: UT, TW. Made critical revisions and approved final version: UT, TW.
Funding
This study was sponsored by G. Pohl-Boskamp GmbH & Co. KG, Germany.
Competing Interests
Dr. Thadani has served as a consultant to Merck, Pfizer, Gilead Science, Eli-Lilly, BMS, Forest Labs, MAP 3, A straZeneca, Boehringer Ingelheim, Akrinmax, and Eisai Pharmaceutical Companies, and is a member of the speakers bureau for Eli-Lilly, Daiichi Sankyo, and Gilead Science. Dr. Thadani is a local Principal Investigator for research studies for which he does not receive any personal salary or funds, but the University of Oklahoma HSC and the VA Medical Center do receive grant support to conduct these studies.
As an employee of G. Pohl-Boskamp, Dr. Wittig received no remuneration for his participation in this study, other than the salary that he is entitled to per his employment. This publication reports findings resulting from a clinical trial that was conducted on behalf of G. Pohl-Boskamp. Dr. Wittig was not involved in the study design, analysis, and reporting of these data, but did participate as an author in the aforementioned German publication, and in the preparation of this publication. Dr. Wittig furthermore declares that neither he nor G. Pohl-Boskamp have unduly influenced the generation, analysis, or interpretation of data.
Disclosures and Ethics
Authors have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. Authors have confirmed that the published article is unique and not under consideration by any other publication and that they have consent to reproduce any copyrighted material. The authors disclose that brief results were published previously in German (Wittig and Beuscher, 1999). The peer reviewers declared no conflicts of interest.
Professor Udho Thadani received an honorarium from Arbor Pharmaceuticals for participating as an advisor, and has received grant support for reviewing the data published here. Dr. Thadani was not involved in the conduct of the study, but was provided with the detailed study design, group data, and individual patient data. Dr. Thadani reviewed data for this study and co-authored this manuscript with Dr. Wittig.
References
- 1.Beltrame JF, Weekes AJ, Morgan C, Tavella R, Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices. Arch Intern Med. 2009;169(16):1491–9. doi: 10.1001/archinternmed.2009.295. [DOI] [PubMed] [Google Scholar]
- 2.Deedwania PC, Carbajal EV. Medical therapy versus myocardial revascularization in chronic coronary syndrome and stable angina. Am J Med. 2011;124(8):681–8. doi: 10.1016/j.amjmed.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 3.Thadani U. Current medical management of chronic stable angina. J Cardiovasc Pharmacol Ther. 2004;9(Suppl 1):S11–29. doi: 10.1177/107424840400900103. quiz S98–99. [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47(10):2130–9. doi: 10.1016/j.jacc.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2011 Update A Guideline From the American Heart Association and American College of Cardiology Foundation Endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58(23):2432–46. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 6.Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27(11):1341–81. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 7.Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation. 2005;111(22):2906–12. doi: 10.1161/CIRCULATIONAHA.104.521864. [DOI] [PubMed] [Google Scholar]
- 8.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. New Engl J Med. 2007;356(15):1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. New Engl J Med. 2008;359(7):677–87. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 10.Boden WE, O’Rourke RA, Teo KK, et al. Design and rationale of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial Veterans Affairs Cooperative Studies Program no. 424. Am Heart J. 2006;151:1173–9. doi: 10.1016/j.ahj.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Kones R. Recent advances in the management of chronic stable angina II. Anti-ischemic therapy, options for refractory angina, risk factor reduction, and revascularization. Vasc Health Risk Manag. 2010;6:749–74. doi: 10.2147/vhrm.s11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graboys TB, Lown B. Cardiology patient page. Nitroglycerin: the “mini” wonder drug. Circulation. 2003;108(11):e78–9. doi: 10.1161/01.CIR.0000086629.67552.3A. [DOI] [PubMed] [Google Scholar]
- 13.Kolenda KD. Glycerol trinitrate and coronary heart disease. Value of prophylactic administration within the scope of exercise therapy. Fortschr Med. 1998;116(20–1):41–2. [PubMed] [Google Scholar]
- 14.Kimchi A, Lee G, Amsterdam E, Fujii K, Krieg P, Mason DT. Increased exercise tolerance after nitroglycerin oral spray: a new and effective therapeutic modality in angina pectoris. Circulation. 1983;67(1):124–7. doi: 10.1161/01.cir.67.1.124. [DOI] [PubMed] [Google Scholar]
- 15.Stuart RJ, Jr, Ellestad MH. National survey of exercise stress testing facilities. Chest. 1980;77(1):94–7. doi: 10.1378/chest.77.1.94. [DOI] [PubMed] [Google Scholar]
- 16.Parker JO, Vankoughnett KA, Farrell B. Nitroglycerin lingual spray: clinical efficacy and dose-response relation. Am J Cardiol. 1986;57(1):1–5. doi: 10.1016/0002-9149(86)90940-9. [DOI] [PubMed] [Google Scholar]
- 17.Thadani U, Parker JO. Influence of glyceryl trinitrate during supine and upright exercise in patients with angina pectoris. Br Heart J. 1978;40(11):1229–36. doi: 10.1136/hrt.40.11.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. New Engl J Med. 2001;344(15):1117–24. doi: 10.1056/NEJM200104123441502. [DOI] [PubMed] [Google Scholar]
- 19.Holubkov R, Laskey WK, Haviland A, et al. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. Am Heart J. 2002;144(5):826–33. doi: 10.1067/mhj.2002.125505. [DOI] [PubMed] [Google Scholar]
- 20.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina— summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41(1):159–68. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 21.Wittig T, Beuscher N. Increased physical performance following administration of glycerol trinitrate in spray form. Fortschritte der Medizin. 1999;117:109–13. [Google Scholar]
- 22.Fletcher GF, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards. A statement for health professionals from the American Heart Association. Circulation. 1990;82(6):2286–322. doi: 10.1161/01.cir.82.6.2286. [DOI] [PubMed] [Google Scholar]
- 23.Skalidis EI, Vardas PE. Guidelines on the management of stable angina pectoris. Eur Heart J. 2006;27(21):2606. doi: 10.1093/eurheartj/ehl257. author reply 2606–7. [DOI] [PubMed] [Google Scholar]
- 24.Hambrecht R, Walther C, Mobius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109(11):1371–8. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 25.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682–92. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation. 2003;107(1):e2–5. doi: 10.1161/01.cir.0000048890.59383.8d. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PD. Exercise prescription and proscription for patients with coronary artery disease. Circulation. 2005;112(15):2354–63. doi: 10.1161/CIRCULATIONAHA.104.502591. [DOI] [PubMed] [Google Scholar]
- 28.Gardner AW, Montgomery PS, Ritti-Dias RM, Thadani U. Exercise performance, physical activity, and health-related quality of life in participants with stable angina. Angiology. 2011;62(6):461–6. doi: 10.1177/0003319711399897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thadani U, Lipicky RJ. Short and long-acting oral nitrates for stable angina pectoris. Cardiovasc Drugs Ther. 1994;8(4):611–23. doi: 10.1007/BF00877415. [DOI] [PubMed] [Google Scholar]
- 30.Thadani U. Management of patients with chronic stable angina at low risk for serious cardiac events. Am J Cardiol. 1997;79(12B):24–30. doi: 10.1016/s0002-9149(97)00382-2. [DOI] [PubMed] [Google Scholar]
- 31.Fraker TD, Jr, Fihn SD, Gibbons RJ, et al. 2007 chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. Circulation. 2007;116(23):2762–72. doi: 10.1161/CIRCULATIONAHA.107.187930. [DOI] [PubMed] [Google Scholar]
- 32.Daly C, Clemens F, Lopez-Sendon JL, et al. The impact of guideline compliant medical therapy on clinical outcome in patients with stable angina: findings from the Euro Heart Survey of stable angina. Eur Heart J. 2006;27(11):1298–304. doi: 10.1093/eurheartj/ehl005. [DOI] [PubMed] [Google Scholar]
- 33.Lappe JM, Muhlestein JB, Lappe DL, et al. Improvements in 1-year cardiovascular clinical outcomes associated with a hospital-based discharge medication program. Ann Intern Med. 2004;141(6):446–53. doi: 10.7326/0003-4819-141-6-200409210-00010. [DOI] [PubMed] [Google Scholar]
- 34.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. New Engl J Med. 2009;360(24):2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carasso S, Markiewicz W. Medical treatment of patients with stable angina pectoris referred for coronary angiography: failure of treatment or failure to treat. Clin Cardiol. 2002;25(9):436–41. doi: 10.1002/clc.4960250908. [DOI] [PMC free article] [PubMed] [Google Scholar]