Abstract
Background
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract. More rarely neoplasms with histology and immunohistochemistry similar to GISTs may occur outside the gastrointestinal tract ( omentum, mesentery and retroperitoneum) and are so-called Extra-gastrointestinal Stromal Tumors (EGISTs). EGISTs arising in the retroperitoneum are extremely rare: to date, only 58 cases have been reported in the literature.
Case report
We herein report a case of a primary EGIST of the retroperitoneum surgically treated. The pre-operative radiological evaluation showed a retroperitoneal mass, placed in left paravertebral region.
Results
Morphological and immunohistochemical features led to a diagnosis of extra-gastrointestinal stromal tumor (intermediate-low risk form).
Conclusions
As a result of the rarity of reports of primary EGISTs of retroperitoneum we need to analyze the data of reported cases in order to gain a better understanding about the pathogenesis, prognosis and optimal treatment of this disease.
Keywords: Extra-gastrointestinal Stromal Tumor, retroperitoneum, CD117
Introduction
The gastrointestinal stromal tumors (GISTs) represent less than 1% of all malignancies, but they are the most common mesenchymal neoplasms of the gastrointestinal tract.1–11
GIST arises from the wall of the gastrointestinal (GI) tract and is thought to originate from the Interstitial Cells of Cajal (ICC), which regulate the motility of the gastrointestinal tract.10,19–23
The most specific and important immunohistochemical marker is the KIT (CD117) protein, a tyrosine kinase growth factor receptor expressed in more than 95% of cases.4,7,10,16,23,24
The gastrointestinal tract is the site of onset of elective GIST: 40%–70% originates from the stomach, 20%–40% from small intestine, 5%–15% from the colon and rectum and 5% from the esophagus.10,15,25,26
More rarely neoplasms with histology and immunohistochemistry similar to GISTs may occur outside the gastrointestinal tract (omentum, mesentery and retroperitoneum) and are so-called Extra-gastrointestinal Stromal Tumors (EGISTs).4,10,12,15,18,25–32,58
Pathogenesis, incidence, clinicopathological features and prognosis of EGISTs have not been completely defined yet.4,13–15,18,27–32,58
EGISTs arising in the retroperitoneum are extremely rare: to date there have been only 58 cases described in the literature.4,12,15,18,27–29,33–35,58 These tumors are of general interest both in diagnosis and treatment.
Since the preoperative diagnosis based on clinical and radiological data is very difficult7,12,15,27,49–51 the patient undergoes a surgical operation for the generic diagnosis of “abdominal mass” which causes anxiety in both the surgeon and patient.
Surgical removal is the gold standard treatment for non-metastatic EGISTs and it is important to achieve a complete removal of the mass when possible “en bloc” with the contiguous tissues.7,11,15,48,53–55
The role of imatinib mesylate, which is the inhibitor of the tyrosine kinase activity of KIT in the treatment of EGISTs, is unclear.11,13,57,59
As a result of the rarity of reports of primary EGISTs of retroperitoneum it is necessary to analyze the data of reported cases in order to define clearly the phenotypic and genetic characteristics as well as the prognostic factors and the optimal treatment of these rare tumors.
We herein report a case of a primary EGIST of the retroperitoneum surgically treated and discuss its clinical behavior and treatment through a literature review.
Case Report
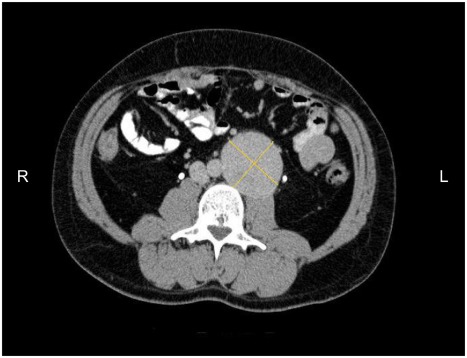
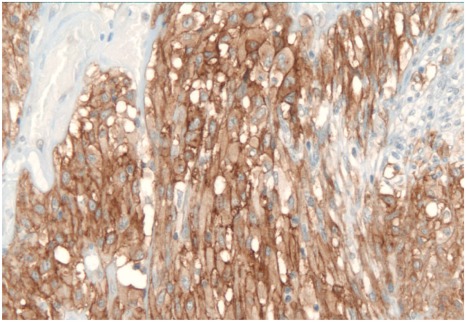
A 39-year-old man was admitted to our institute for abdominal back pain present for 4 months, without any other sign or symptom. The pain over time has gradually increased in intensity and was treated without benefit with analgesic drugs. Before admission, he underwent outpatient ultrasonography and abdominal computed tomography (CT) scan that showed a retroperitoneal solid mass (60 × 60 × 80 mm), placed in left paravertebral region, level L3–L4, on the left side of aorta, ilio-psoas muscle adherent. No adenopathies or local infiltrations were found. The bowel was dislocated without signs of intestinal occlusion (Fig. 1). The patient underwent CT-guided fine needle aspiration (FNA) with the result of inadequate sampling of the mass lesion. As part of clinical and instrumental workup he underwent standard blood tests, electrocardiogram (ECG) and chest X-ray, the results of which were normal. The patient’s abdomen was normal and no mass was palpable. In view of patient’s characteristics (a previous laparotomy for peritonitis due to acute appendicitis) and the dimension of the mass, an open procedure was preferred instead of laparoscopy approach. The laparotomy confirmed the presence of a solid and well defined mass located in the left paramedian region of the retroperitoneum. After sectioning the parietal peritoneum in the transition from the left colon and sigmoid colon, the mass was gradually exposed and removed. The tumor was completely excised (R0 resection). No perioperative complications were recorded and the patient was discharged four day after surgery. Postoperative pathologic evaluation revealed a 139—gram plurinodular mass, encapsulated, measuring 7.5 × 6 × 5.3 cm. After cutting, the tissue appeared brownish—yellow in color and had elastic consistency. Microscopically the tumor border was delineated from surrounding fat and no tumor cells were observed in the surgical margin. Microscopic examination also showed neoplastic proliferation composed by spindle cells type. Mitotic count was <5 per 50 high-power fields (HPF) (low mitotic activity). The MIB-1 labelling index (monoclonal antibody used to determine the Ki-67 labelling index that is correlated with the clinical course of tumor) was 3%–5%. Immunohistochemistry showed positivity for KIT (CD 117) (Fig. 2), focal positivity for S-100 protein and NSE (neuron specific enolase), negativity for CD34, smooth and skeletal muscle actine. A molecular genetic analysis for KIT protein mutation was not performed due to its unavailability at our hospital. Morphological and immunohistochemical features leaded to a diagnosis of gastrointestinal stromal tumor which dimension and number of mitosis indicated an intermediate risk form according to risk assessment proposed by Fletcher et al16 and a low risk form according to the risk assessment proposed by Yamamoto et al.4 The further oncological evaluation did not consider necessary an adjuvant therapy with imatinib mesylate because the tumor was completely excised (resection R0) and it was classified as an intermediated-low risk form. An abdominal CT scan did not show any recurrence of disease in the 2 years after surgery.
Figure 1.
Axial CT-scan shows a retroperitoneal mass (60 × 60 × 80 mm), solid, placed in left paravertebral region, level L3–L4, on the left side of aorta, ilio-psoas muscle adherent.
Figure 2.
Immunohistochemically, the tumor cells test strongly positive for c-kit (×200).
Discussion
The GISTs are the most common mesenchymal tumors of the gastrointestinal tract with an incidence estimated at 7 to 14 per 1 million in the general population.1–11,36,37 The discovery of the mutation of KIT (CD117) in GIST proto-oncogene was fundamental in order to understand the genesis and classification of these tumors.24 The fact that the cells of GISTs show ultrastructural features and cellular markers, typical of Interstitial Cells of Cajal (ICC), has supported the hypothesis that they may originate from these cells.10,19–23 ICC are characterized by expression of KIT (CD117).22 A subset of GISTs has mutation in the KIT-related kinase gene: platelet-derived growth factor receptor alpha (PDGFRA).39,40 Proto-oncogene KIT mutations are found in more than 95% of GISTs, and in 5%–10% of cases mutations of the PDGFRA gene.4,7,10,16,23,24,38 The mutations mainly involving exon 11 of KIT gene are less frequently mutated exons 9, 13, 14 and 17 and exons 12 and 18 of PDGFRA gene.16,22,26 Mutations of KIT lead to a constitutional activation of tyrosine kinase function that determines resistance to cellular proliferation and apoptosis.23 KIT and PDGFRA mutations appear to be mutually exclusive oncogenic mechanisms in GISTs.39,40 It is not clear whether the presence or absence of a mutation in the KIT or PDGFRA genes per se influences prognosis, although delections of the exon 11 have been linked to poor survival.16,22,26,41,42 Staining for other markers is more variable: BCL 2 80%, CD34 70%, muscle specific actin 50%, smooth muscle actine 35%, S100 10% and desmin 5%.10,22,26 GISTs are usually composed of a population of spindle cells (60%–70%) or epithelioid cells (20%) and the reminder consists of a mixture of these two morphologies.10,16,18,22,23 GISTs have been documented in all parts of the gastrointestinal tract: especially in the stomach (60% to 70%) and small intestine (25% to 35%), with rare occurrence in the colon and rectum (5%), esophagus (<2%) and appendix, gallbladder and pancreas.10,15,25,26 Some GISTs, primary in the omentum, mesentery or retroperitoneum, are unrelated to the tubular gastrointestinal tract and they are so-called Extra-gastrointestinal Stromal Tumor (EGISTs).4,10,12,15,18,25–31,58 EGISTs are identical by histological and immunohistochemical features with GISTs.4,18,22,23,25,26,32,58 However, incidence, histogenesis, clinical and prognostic factors of EGISTs are not defined yet.4,13–15-,18,27–32,58 EGISTs show staining of KIT, marker of Interstitial Cells of Cajal which are normally present just in the gut wall.4,18,27,32 The reason why this kind of cells, which normally are in the gut wall, can stay in the retroperitoneum, omentum or mesentery and originate a tumor is not clear yet. It can be hypothesized that these tumors originate from common precursor cells which differentiate into the Interstitial Cells of Cajal type outside from the gut wall.10,18,27,32,43,44 EGISTs are rare tumors. A total of 99 omental and mesenteric EGISTs are reported in four published series from 1999 to 2008.18,44–46 EGISTs arising in the retroperitoneum are uncommon. To date, only 58 cases of EGISTs of the retroperitoneum have been reported in Literature4,12,15,18,27–29,33–35,58 (Table 1). Reith et al18 reported 48 cases of EGISTs and 6 of them originated in the retroperitoneum. 17 cases of EGSTs of the retroperitoneum have been reported by Yamamoto et al4 and by Barreda Bolanos et al.15 These studies were frequently limited by the lack of information available on clinical, pathological and prognostic features.27 The absence of specific symptoms associated with an objective, not always measurable, makes difficult an early diagnosis. These tumors grow silently and consequently they are discovered once they have reached a significant size, causing symptoms of compression.7,15,27 In the case we reported, there were non-specific symptoms (posterior abdominal pain) and there was no palpable mass on physical examination. The CT-scan and MRI are the standard imaging techniques of these tumors,7,12,47–51 but the preoperative diagnosis based on radiological data is very difficult.12,49–51 Small tumors usually appear like a well-defined homogeneous mass, large tumors tend to be ill defined and inhomogeneous, sometimes with calcification and necrosis. Malignant forms can be described like large lesions (>10 cm) with wall invasion or hepatic metastasis.52 Radiological findings in EGSTs are often similar to those of other retroperironeal tumors (sarcomas, malignant fibrous histiocytoma, liposarcoma, leiomyosarcoma, fibrosarcoma).12,50,51 In our case the CT scan showed a periaortic mass (diameter 6 × 6 × 8 cm). The retroperitoneal mass was placed in the paravertebral region, level L3–L4, on the left side of the aorta, in front of the left ilio-psoas muscle adherent to it. The lesion showed heterogeneous and persistent enhancement even in scans acquired in the late phase after administration of contrast. No adenopathies or local infiltration were found (Fig. 1). Contrary to what was reported by other authors12 the CT-scan did not show in our case the presence of peripheral calcifications or central necrosis. The biopsy eco- or CT-guided help to plan the best treatment, and risk of peritoneal dissemination is negligible. 7 Limitations of pre-operative image-guided FNA may be caused by inadequate sampling of the target mass lesion, as in our case, or by the difficulty in performing a mitotic count on aspiration cytology smears.7 The microscopic evaluation in the case we studied showed a neoplastic cellular proliferation composed of spindle cells by type. The immunohistochemical study showed positive for CD117, and focal positivity for S-100 protein and NSE, negative for CD34, smooth muscle and skeletal actin (Fig. 2). Surgical removal is the gold standard treatment for non metastatic EGISTs.7,11,15,48,53,54 Adjacent organs adherent to the mass should be resected en bloc with the tumor, in order to avoid capsule rupture and intra-abdominal spillage7,11,15,53–55 Laparoscopic surgery should be avoided in large tumors because of the high risk of tumor rupture and consequent peritoneal seeding. A laparoscopic resection might be accepted in case of small tumors (<2–5 cm).55,56 Prognostic factors in the case of EGISTs are not well encoded in the literature.4,13–15,18,27–32 The location, size, cellularity, mitotic activity and necrosis are reported to be the most accurate predictors of a poor outcome.4,16,18,30,44 Omental EGISTs have a better prognosis than those in the mesenteric location.45 In respect to GIST size, it is generally accepted as one of the main prognostic factors (low risk cut off <5 cm in diameter),16 although other authors15 found no correlation between tumor size and survival. Some authors15 have reported a shorter survival in patients younger than 50 years. Ruiz-Tovar et al29 revealed in a multivariate analysis that masculine gender, constitutional syndrome, abdominal mass at diagnosis, small bowel and retroperitoneum location and actin- negative tumors are bad prognostic factors. High cellularity, mitotic activity (>2 mitoses/50 HPF) and the presence of necrosis are significantly associated with an adverse outcome.18 For Barreda- Bolanos et al15 the low mitotic index associated with metastases did not reflect a good prognosis of disease. On the basis of a combination of the mitotic rate and MIB-1 labeling index, three risk categories can be identified: the high-risk group (≥5/50 HPF with ≥10% Ki-67); the intermediate-risk group (≥5/50 HPF with <10% Ki-67, or, <5/50 HPF with ≥10% Ki-67), and the low-risk group (<5/50 HPF with <10% Ki-67).4 The exon 11 mutation causes a more aggressive biological behavior.16,22,26 In our case, morphological and immunohistochemical features of tumor leaded to a diagnosis of EGIST as an intermediate risk form according to the risk assessment proposed by Fletcher et al16 and a low risk form according to risk assessment proposed by Yamamoto et al.4 The introduction of drugs that inhibit tyrosine kinase activity of receptors (imatinib mesylate) has resulted in a significant increase in survival from 18 to 60 months in patients with mesenchymal tumors with mutations of the KIT gene in advanced stage.57
Table 1.
Case review of the primary EGIST of the retroperitoneum.
| Authors | Age/Gender | Size (cm) | Pattern | Cellularity | Mitoses no./50 HPF | Ki-67 LI | c-kit mutation | Imatinib | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Reith et al18 | 44/M | 32 | S | Low | 1 | NA | NA | NA | NED/11 mo |
| 41/F | 26 | E | Low | 0 | NA | NA | NA | NED/37 mo | |
| 45/F | 16.5 | E | High | 49 | NA | NA | NA | DOD/5 mo | |
| 67/F | 9 | E | Low | 2 | NA | NA | NA | NED/40 mo | |
| 45/F | 18 | E | Low | 2 | NA | NA | NA | NED/67 mo | |
| 82/F | 12.5 | S | Low | 1 | NA | NA | NA | NED/24 mo | |
| Takao et al12 | 70/F | 16 × 15 × 18 | S | Low | NA | NA | NA | NA | NED/12 mo |
| Park et al58 | 32/M | 13.3 × 10.2 × 9.4 | S | High | 6–10 | NA | NA | Yes | NED/6 mo |
| Ferchichi et al34 | 54/M | 10 | S | NA | 6 | NA | NA | NA | NA |
| Takizawa et al35 | 67/M | 3.7 × 5.5 | S | NA | 10–15 | 3–5 | NA | NA | NED/18 mo |
| Yamamoto et al4 | 80/F | 15 | S | Moderate | 9 | 3.9 | NA | NA | NED/9 mo |
| 46/F | 7 | E | High | 1 | 2.3 | Exon 11 | NA | NED/86 mo | |
| 69/M | 18 | S | High | 12 | 17.2 | NA | NA | DOD/37 mo | |
| 68/F | 15 | S | High | 5 | 32 | ND | NA | DOD/136 mo | |
| 45/F | 10 | S | High | 19 | 10 | NA | NA | DOD/50 mo | |
| 63/M | 6 | S | Moderate | 4 | 4.1 | Exon 11 | NA | DOD/96 mo | |
| 45/M | 3 | E | Moderate | 1 | 2 | Exon 11 | NA | NED/86 mo | |
| 50/F | 14 | S | High | 10 | 1 | NA | NA | DOD/68 mo | |
| 30/F | 14 | E | Low | 9 | 1.8 | Exon 11 | NA | DOD/21 mo | |
| 67/F | 10 | S | Moderate | 20 | 33.9 | Exon 11 | NA | AWD/6 mo | |
| 56/F | 12 | S | High | 20 | 12.1 | ND | NA | DOD/48 mo | |
| 48/F | 11 | S | High | 50 | 1 | Exon 11 | NA | DOD/28 mo | |
| 52/F | 10 | S | High | 25 | 11.5 | NA | NA | DOD/60 mo | |
| 54/M | 35 | S | High | 0 | 2 | NA | NA | NED/94 mo | |
| 57/F | 9 | S | Moderate | 5 | 4.2 | ND | NA | DOD/9 mo | |
| 74/F | 14 | S | Moderate | 4 | 1.9 | ND | NA | DOD/24 mo | |
| 75/F | 8 | S | Moderate | 10 | 2.7 | ND | NA | NA | |
| Goh BKP et al27 | 57/F | 22 | S | High | >10 | NA | NA | NA | AWD/42 mo |
| Fuertes J et al33 | 41/M | 13 | Mixed | Moderate | 6 | 40 | NA | Yes | NED/24 mo |
| Vij M et al28 | 9 cases | NA | NA | NA | NA | NA | NA | NA | NA |
| Ruiz-Tovar J et al29 | 3 cases | NA | NA | NA | NA | NA | NA | 1 patient received treatment | AWD/12 mo |
| Barreda Bolanos F et al15 | 17 cases | >10 (16 cases) | NA | High (2 cases) Low (3 cases) |
NA | NA | NA | 2 cases | 1 AWD/29 mo 1 AWD/60 mo |
| Present case 2011 | 39/M | 7.5 | S | Low | 5 | 5 | 7 | NO | NED/24 mo |
Abbreviations: S, spindle; E, epitheliod; NA, not available; NED, no evidence disease; mo, month; AWD, alive with disease; DOD, dead of disease; ND, not done; LI, labeling index.
At present, there is no consensus in the literature about the use of imatinib mesylate as standard treatment for patients with localized cancer KIT.11,13 A randomized study showed that administration of these drugs for a period of one year prolongs relapse free-survival in localized tumors with KIT, completely removed, with diameter >3 cm, when compared with placebo.59 The use of imatinib mesylate is recommended for ≥1 year in patients with KIT resectable at high risk of recurrence.59 Li et al13 have also shown that adjuvant imatinib can improve 1-, 2- and 3-years relapse-free survival rates in patients at intermediate or high risk of recurrence after complete tumor resection.
Not all authors who describe the EGISTs of the retroperitoneum reported the use of imatinib mesylate after surgery (Table 1). Our patient underwent a R0 section and the tumor was classified as an intermediate and low-risk according to the classification of Fletcher et al16 and Yamamoto et al4 respectively, and therefore there was no need to proceed with the use of imatinib mesylate. The mean follow-up of patients reported in several case studies often do not exceed 24 months and this could lead to bias. Our patient is alive and disease free at 24 months after surgery.
In summary, CD117 positive stromal tumors, although extremely rare, can involve retroperitoneum. This should be considered when imaging studies reveal an abdominal tumor involving the retroperitoneum without a connection to the gastrointestinal tract. In most cases a preoperative diagnosis is not possible. Complete surgical removal of the mass and, when possible, “en bloc” with contiguous tissues and regional lymph nodes, is the gold standard therapy for non metastatic EGISTs. There is no consensus about the use of inhibitors of receptors of tyrosine kinase activity in these tumors. More studies are necessary to evaluate the role of molecular biology in targeted therapy. It is difficult to assess their malignant potential and their overall prognosis although some Authors4,16 reported criteria that appear to be useful in predicting the risk of recurrence.
As a result of the rarity of reports of primary EGISTs of retroperitoneum we need to analyze the data of reported cases in the literature in order to gain a better understanding about the pathogenesis, clinicopathological features, prognosis and optimal treatment of this disease.
Footnotes
Author Contributions
CC, VV, FD, MC and BS were responsible for data collection/entry/analysis and assistance with manuscript preparation. CC was responsible for the study design and preparation of the manuscript. All authors contributed to the writing of the manuscript. All authors reviewed and approved the final manuscript.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Koh JS, Trent J, Chen L, et al. Gastrointestinal stromal tumors: overview of pathologic features, molecular biology, and therapy with imatib mesylate. Histol Histopathol. 2004;19:565–74. doi: 10.14670/HH-19.565. [DOI] [PubMed] [Google Scholar]
- 2.Tryggvason G, Gislanson HG, Magnusson MK, Jonasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: the Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005;117:289–93. doi: 10.1002/ijc.21167. [DOI] [PubMed] [Google Scholar]
- 3.Tzen CY, Wang JH, Huang YJ, Wang MN, Pin PC, Lai gL, et al. Incidence of gastrointestinal stromal tumor: a retrospective study based on immunohistochemical and mutational. Dis Sci. 2007;52:792–7. doi: 10.1007/s10620-006-9480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto H, Oda Y, Kawaguchi K, et al. C-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue) Am J Surg Pathol. 2004;28:479–88. doi: 10.1097/00000478-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Perez EA, Livingstone AS, Franceschi D, et al. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. J Am Coll Surg. 2006;202:623–9. doi: 10.1016/j.jamcollsurg.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655–64. doi: 10.1016/s1470-2045(02)00899-9. [DOI] [PubMed] [Google Scholar]
- 7.Logrono R, Jones DV, Faruqi S, Bhutani MS. Recent advances in cell biology, diagnosis and therapy of gastrointestinal stromal tumor (GIST) Cancer Biol Ther. 2004;3:251–8. doi: 10.4161/cbt.3.3.615. [DOI] [PubMed] [Google Scholar]
- 8.Von MM. The role of adjuvant and neoadjuvant therapy in gastrointestinal stromal tumors. Curr Opin Oncol. 2008;20:428–32. doi: 10.1097/CCO.0b013e328302ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohenberger P. Gastrointestinal stromal tumors. Med Prax. 2007;96:29–33. doi: 10.1024/1661-8157.96.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Lasota J. Gastrointestinal stromal tumors: definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 11.Casali PG, Blay JY On behalf of the ESMO/CONTICANET/EUROBONET Consensus Panel of Experts. Gastrointestinal stromal tumours: ESMO Clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):98–102. doi: 10.1093/annonc/mdq208. [DOI] [PubMed] [Google Scholar]
- 12.Takao H, Yamahira K, Doi I, Watanabe T. Gastrointestinal stromal tumor of the retroperitoneum: CT and MR findings. Eur Radiol. 2004;14:1926–9. doi: 10.1007/s00330-004-2404-3. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Gong JF, Wu AW, Shen L. Post-operative imatinib in patients with intermediate or high risk gastrointestinal stromal tumor. Eur J Cancer Surg. 2001;37:319–24. doi: 10.1016/j.ejso.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg BL, Trent JC. Adjuvant and neoadjuvant imatinib therapy: current role in the management of gastrointestinal stromal tumors. Int J Cancer. 2011;129:2533–42. doi: 10.1002/ijc.26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barreda-Bolanos F, Liu Bejarano H, Sanchez Lihon J, Landeo Aliaga I, Sanchez Rodriguez Z. Survival factors in 152 patients with gastrointestinal stromal tumors. Rev Gastroenterol Peru. 2010;30:305–23. [PubMed] [Google Scholar]
- 16.Fletcher CDM, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–65. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 17.Yan H, Marchettini P, Acherman YIZ, Gething SA, Brun E, Sugarbaker PH. Prognostic assessment of gastrointestinal stromal tumor. Am J Clin Oncol. 2003;26:221–8. doi: 10.1097/01.COC.0000018296.45892.CE. [DOI] [PubMed] [Google Scholar]
- 18.Reith JD, Goldblum JR, Lyles RH, Weiss SW. Extra-gastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 2000;13(5):577–85. doi: 10.1038/modpathol.3880099. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Mori I, Tang W, Utsunomiya H, Nakamura M, Nakamura Y, et al. Gastrointestinal stromal tumors: are they of Cajal cell origin? Exp Mol Pathol. 2002;72:172–7. doi: 10.1006/exmp.2001.2419. [DOI] [PubMed] [Google Scholar]
- 20.Kindblom LG, Remotti HE, Aldenborg F. Gastrointestinal pacemaker cell tumor (GIPACT). Gastrointestinal stromal tumors show phenotypic characteristics of interstitial cell of Cajal. Am J Pathol. 1998;152(5):1259–69. [PMC free article] [PubMed] [Google Scholar]
- 21.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal tumors. Am J Surg Pathol. 1999;23:377–89. doi: 10.1097/00000478-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2005;22(18):3813–25. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 23.Connolly EM, Gaffney E, Reynolds JV. Gastrointestinal stromal tumors. Br J Surg. 2003;90:1178–86. doi: 10.1002/bjs.4352. [DOI] [PubMed] [Google Scholar]
- 24.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain of function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen MB, Larsen KE, Fristrup CW, Nielsen HO. Gastrointestinal stromal tumor: clinical and pathological presentation. Ungeskr Laeger. 2007;169:2776–9. [PubMed] [Google Scholar]
- 26.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38(Suppl 5):39–51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 27.Goh BK, Chow PK, Kesavan SM, Yap WM, Chung YF, Wong WK. A single-institution experience with eight CD117-positive primary extragastrointestinal stromal tumors: critical appraisal and a comparison with their gastrointestinal counterparts. J Gastrointest Surg. 2009;13(6):1094–8. doi: 10.1007/s11605-009-0828-4. [DOI] [PubMed] [Google Scholar]
- 28.Vij M, Agrawal V, Kumar A, Pandey R. Gastrointestinal stromal tumors: a clinicopathological and immunohistochemical study of 121 cases. Indian J Gastroenterol. 2010;29:231–6. doi: 10.1007/s12664-010-0079-z. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Tovar J, Diez-Tabernilla M, Housari G, Martinez-Molina E, Sanjuanbenito A. Gastrointestinal stromal tumors: actin expression, a new prognostic factors? Am Surg. 2010;76:1244–9. [PubMed] [Google Scholar]
- 30.Franzini C, Alessandri L, Piscioli I, et al. Extra-gastrointestinal stromal tumor of the greater omentum: report of a case and review of the literature. World J Surg Oncol. 2008;6:25. doi: 10.1186/1477-7819-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todokori T, Sano T, Sakurai S, Segawa A, Saitoh T, Fujikawa K, et al. Primary omental Gastrointestinal stromal tumor (GIST) World J Surg Oncol. 2007;5:66. doi: 10.1186/1477-7819-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agaimy A, Wunsch PH. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. review of 200 cases to critically re-evaluate the concept of so-called extragastrointestinal stromal tumors. Langenbecks Arch Surg. 2006;391:322–9. doi: 10.1007/s00423-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 33.Fuertes MJ, Navarro DC, Lopez-Andujar R, Pallardo JM, Medina JAV. Retroperitoneal GIST: an unusual location for a rare tumour. Cir Esp. 2010;87(4):252–64. doi: 10.1016/j.ciresp.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Ferchichi L, Kourda N, Zermani R, Aouem J, Zaouche A, Abdjellil Z, et al. Extragastrointestinal stromal tumors: a report of 4 cases. Ann de Chir. 2006;131:271–6. doi: 10.1016/j.anchir.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Takizawa I, Morishita H, Matsuki S, Komeyama T, Emura I, Hara N. Primary gastrointestinal stromal tumor in the retroperitoneum. Int J Urol. 2006;13:1245–8. doi: 10.1111/j.1442-2042.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 36.Goettsch WG, Bos SD, Breekveldt-Postma N, Casparie M, Herings RM, Hogendoorn PC. Incidence of gastrointestinal stromal tumours is underestimated. Results of a nation-wide study. Eur J Cancer. 2005;41:2868–72. doi: 10.1016/j.ejca.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Mucciarini C, Rossi G, Bertolini F, Valli R, Cirilli C, Rashid I, et al. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. Br Med Cancer. 2007;7:230. doi: 10.1186/1471-2407-7-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008;3:557–86. doi: 10.1146/annurev.pathmechdis.3.121806.151538. [DOI] [PubMed] [Google Scholar]
- 39.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 40.Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660–7. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 41.Fletcher JA, Corless CL, Dimitrijevic S, et al. Mechanisms of resistance to imatinib mesylate (IM) in advanced gastrointestinal stromal tumor (GIST) (Abstract) Proc Am Soc Clin Oncol. 2003;22:815. [Google Scholar]
- 42.Demetri GD, Desai J, Fletcher JA, et al. SU 11248, a multi-targted tyrosine kinase inhibitor, can overcome imatinib (IM) resistance caused by diverse genomic mechanisms in patients (pts) with metastatic gastrointestinal stromal tumor (GIST) (Abstract 3001) Proc Am Soc Clin Oncol. 2004:195. [Google Scholar]
- 43.Li Zy, Huan XQ, Liang XJ, Li ZS, Tan AZ. Clinicopatological and immunohistochemical study of extra-gastrointestinal stromal tumours arising from the omentum and mesentery. Zhonghua Bing Li Xue Za Zhi. 2005;34:11–4. [PubMed] [Google Scholar]
- 44.Sakurai S, Hishima T, Takazawa Y, Sano T, Nakajima T, Saito K, et al. Gastrointestinal stromal tumors and KIT-positive mesenchymal cells in the omentum. Pathol Int. 2001;51:524–31. doi: 10.1046/j.1440-1827.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 45.Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–8. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Castillo-Sang M, Mancho S, Tsang aW, Gociman B, Almaroof B, Ahmed MA. A malignant omental extra-gastrointestinal stromal tumor on a young man: a case report and review of the literature. World J Surg Oncol. 2008;6:50. doi: 10.1186/1477-7819-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bucher P, Villiger P, Egger JF, Buhler LH, Morel P. Management of gastrointestinal stromal tumors: from diagnosis to treatment. Swiss Med Wkly. 2004;134:145–53. doi: 10.4414/smw.2004.10530. [DOI] [PubMed] [Google Scholar]
- 48.De Matteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors. Recurrence patterns and prognostic factors for survival. Ann Surg. 2010;321(1):51–8. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radio Graphics. 2003;23:283–304. doi: 10.1148/rg.232025146. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi N, Haraikawa T, Tsuda T, Mochizuki T, Ikezoe J. CT and MRI findings in gastrointestinal stromal tumor (GIST) and malignant lymphoma. Eur Radiol. 2003;13(SI):379. [Google Scholar]
- 51.Chourmouzi D, Sinakos E, Papalavrentios L, Akriviadis E, Drevelegas A. Gastrointestinal stromal tumors: a pictorial review. J Gastrointestin Liver Dis. 2009;18(3):379–83. [PubMed] [Google Scholar]
- 52.Ghanem N, Altehoefer C, Furtwangler A, Winterer J, Schafer O, Kotter E, et al. Computed Tomography in gastrointestinal stromal tumors. Eur Radiol. 2003;13(7):1669–78. doi: 10.1007/s00330-002-1803-6. [DOI] [PubMed] [Google Scholar]
- 53.Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992;215:68–77. doi: 10.1097/00000658-199201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Matteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors. Before and after STI-571. Hum Pathol. 2002;33:446–77. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 55.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tosa P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of March 20–21, 2004, under the auspices of ESMO. Ann Oncol. 2005;16(4):566–78. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 56.Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST). Update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5(Suppl 2):1–29. [PubMed] [Google Scholar]
- 57.Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard – versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–5. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 58.Park SS, Min BW, Kim WB, Choi JW, Lee JH, Chae YS, et al. Malignant extragastrointestinal stromal tumor of retroperitoneum. Acta Oncol. 2005;44(5):497–9. doi: 10.1080/02841860510029897. [DOI] [PubMed] [Google Scholar]
- 59.De Matteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localized primary gastrointestinal stromal tumour: a randomized, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]