Abstract
Coordinated interactions between T and B cells are crucial for inducing physiological B cell responses. Mutant mice in which tyrosine 136 of linker for activation of T cell (LAT) is replaced by a phenylalanine (LatY136F) exhibit a strong CD4+ T cell proliferation in the absence of intended immunization. The resulting effector T cells produce high amounts of TH2 cytokines and are extremely efficient at inducing polyclonal B cell activation. As a consequence, these LatY136F mutant mice showed massive germinal center formations and hypergammaglobulinemia. Here, we analyzed the involvement of different costimulators and their ligands in such T–B interactions both in vitro and in vivo, using blocking antibodies, knockout mice, and adoptive transfer experiments. Surprisingly, we showed in vitro that although B cell activation required contact with T cells, CD40, and inducible T cell costimulator molecule-ligand (ICOSL) signaling were not necessary for this process. These observations were further confirmed in vivo, where none of these molecules were required for the unfolding of the LAT CD4+ T cell expansion and the subsequent polyclonal B cell activation, although, the absence of CD40 led to a reduction of the follicular B cell response. These results indicate that the crucial functions played by CD40 and ICOSL in germinal center formation and isotype switching in physiological humoral responses are partly overcome in LatY136F mice. By comparison, the absence of CD80–CD86 was found to almost completely block the in vitro B cell activation mediated by LatY136F CD4+ T cells. The role of CD80–CD86 in T–B cooperation in vivo remained elusive due to the upstream implication of these costimulatory molecules in the expansion of LatY136F CD4+ T cells. Together, our data suggest that CD80 and CD86 costimulators play a key role in the polyclonal B cell activation mediated by LatY136F CD4+ T cells even though additional costimulatory molecules or cytokines are likely to be required in this process.
Keywords: costimulation, T–B synapse, humoral immunity
Introduction
The humoral immune response that is responsible for providing long-lasting immunity against pathogens depends in large on T-dependent B cell activation. Since B cells are less stringently selected than T cells during their development (Adelstein et al., 1991), their activation in the periphery has to be tightly regulated when they give rise to extrafollicular short-lived plasmablasts, germinal center B cells, high affinity long-lived plasma cells and memory B cells (Wardemann et al., 2003; McHeyzer-Williams and McHeyzer-Williams, 2004). Accordingly, complex interactions between B and T cells are required to trigger fully fledged humoral responses, but also to avoid the generation of antibodies against self-components. The generation of effector B cells requires the formation of an immune synapse between antigen-primed B cells and T helper cells (Fazilleau et al., 2009). This synapse, which is stabilized by intercellular adhesion molecules such as LFA-1 and its ICAM1 ligand, comprises an antigen-specific component that involves peptide loaded MHC class II (MHCII) molecules on the surface of the antigen-primed B cells and the T cell antigen receptor (TCR) expressed on the surface of T cells. This last interaction is essential for triggering the clonal expansion of antigen-specific B cells. However, a multitude of additional signals provided by costimulatory molecules and cytokines may modify the outcome of the B cell activation process (Bishop and Hostager, 2001).
One of the most important costimulatory molecule involved in the T–B immune synapse is CD40, a member of the tumor necrosis factor (TNF) receptor family. Upon interaction with CD40L (CD154), CD40 triggers signals that are crucial for many aspects of B cell activation. For instance, in its absence, isotype switching, germinal center formation, and memory B cell response are defective (Kawabe et al., 1994; Xu et al., 1994). By engaging CD28 on naïve T cells, CD80 and CD86 are essential for naive T cell activation (Jenkins et al., 1991). Some experiments provide evidence that signaling through CD80 and CD86 may also influence the outcome of the B cell response and in particular enhances IgG secretion (Suvas et al., 2002; Rau et al., 2009). Another member of the CD28 family, the inducible T cell costimulator molecule (ICOS) is highly expressed on the surface of follicular helper T cells (TFH), a subset of T cells found within B cell follicles and responsible for the activation of germinal center B cells (Hutloff et al., 1999; Greenwald et al., 2004). Its interaction with ICOS-Ligand (ICOSL) on the surface of B cells has been implicated in many aspects of the humoral response. Indeed, ICOSL deficient mice present a deficit in isotype switching, antibody secretion, and germinal center formation (Mak et al., 2003). OX-40, a member of the TNFR family seems also to be specifically required for the generation of an effective secondary B cell activation (Stuber and Strober, 1996).
Strikingly, when linker for activation of T cell (LAT) is mutated, the T cell mediated control of B cell activation is disrupted. LAT is a transmembrane adaptor protein that constitutes a major transduction hub of the TCR signaling cassette. Upon TCR engagement, different tyrosines present in the LAT cytoplasmic tail become phosphorylated and give rise to the formation of a multiprotein complex called the LAT signalosome and that is responsible for triggering downstream signaling events (Malissen et al., 2005; Fuller and Zhang, 2009) The thymus of mice in which the tyrosine residue found at position 136 has been replaced by a phenylalanine (LatY136F) presents a severe but partial blockade in αβ T cell development (Aguado et al., 2002; Sommers et al., 2002). However, the few CD4+ T cells able to reach the periphery of LatY136F mice embark on a rapid and uncontrolled expansion, giving rise to a massive enlargement of the secondary lymphoid organs. These expanding T cells exhibit an activated-effector and memory phenotype (CD25−, CD44high, CD62Llow, CD69+) and produce large amounts of type 2 (TH2) cytokines, particularly IL-4 and IL-10 and to a lesser extent, IL-5, IL-13, and IFN-γ. Recent experiments suggested that the generation of the LATY136F CD4+ T cell pathological expansion likely occurs in two phases (Roncagalli et al., 2010). During the initiation phase, TCR and CD28 engagement are important to trigger the activation of the CD4+ T cells and the onset of the disease (Mingueneau et al., 2009). In the presence of LATY136F molecules, the activated CD4+ T cells undergo a conversion into cells that express low amount of TCR and are hypo-responsive to TCR signaling. During a second phase called the perpetuation phase, the converted CD4+ T cells continuously proliferate at a slower rate in an MHCII independent-, IL-7 dependent-manner (Wang et al., 2008).
This population of TH2 activated LatY136F CD4+ T cells leads to the generation of an exaggerated yet normal sequence of B cell activation. Indeed, all the typical B cell subsets induced during a physiological T-dependent B cell activation, such as germinal center B cells, antibody-secreting cells, and memory B cells are evenly expanded in LatY136F mutant mice (Genton et al., 2006). This activation results in a massive increase of IgG1 and IgE leading to autoimmune disorders and inflammatory diseases (Aguado et al., 2002; Genton et al., 2006). Given that both the kappa and lambda light chain concentrations increase proportionally, this hypergammaglobulinemia is likely due to a polyclonal, antigen-independent driven B cell proliferation (Genton et al., 2006). Further experiments have shown that, although early B cell progenitors express LAT (Oya et al., 2003), the polyclonal B cell activation observed in LatY136F mutant mice does not require the expression of the LatY136F mutation in B cells. The LatY136F mutation has only an indirect effect on B cells that is due to the abnormal CD4+ T cells that develop in its presence (Genton et al., 2006). Consistent with this view, when LatY136F CD4+ T cells are adoptively transferred in Cd3εΔ5/Δ5 mice that lack T cells but contain normal numbers of B cells, the host B cells become activated, further demonstrating that the LatY136F mutation is not required in B cells to render them susceptible to activation by LatY136F CD4+ T cells (Wang et al., 2008). Importantly, transfer of LatY136F CD4+ T cells into Cd3εΔ5/Δ5 host that lack MHCII molecules showed that they are still capable of activating MHCII deficient B cells (Genton et al., 2006). The mechanism leading to the B cell activation in the context of the LAT pathology remains, however, elusive and it is of interest to understand how LatY136F CD4+ T cells activate B cells in absence of MHCII:TCR interaction. This study provides an insight into the different costimulatory molecules that are required to trigger the massive B cell activation mediated by LatY136F CD4+ T cells both in vitro and in vivo.
Materials and methods
Mice
LatY136F mice and Cd3εΔ5/Δ5 mice have already been described (Malissen et al., 1995; Aguado et al., 2002). Cd80−/−/Cd86−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA), Cd40−/− mice (Kawabe et al., 1994) from A. Rolink (Developmental and Molecular immunology Department, Klinisch Biologische Wissenschaften, Basel, Switzerland), and Icosl−/− mice (McAdam et al., 2001) from M. Bachmann (Cytos Biotechnology AG, Zurich, Switzerland). Cd28−/− (Shahinian et al., 1993) and Icos−/− (Tafuri et al., 2001) mice were from T. Mak and A. Tafuri (Ontario Cancer Institute, Toronto, Canada). LatY136F and Cd3εΔ5/Δ5 mice deficient for CD28, ICOS, CD80–CD86, CD40, or ICOSL were derived in parallel using CD28, ICOS, CD80–CD86, CD40, and ICOSL deficient mice, respectively. All mice were maintained at CIML and the Swiss Institute for Experimental Cancer Research specific pathogen free animal facility. All experiments were done in agreement with Institutional and Swiss regulations and with French directives.
Adoptive transfer
CD4+ T cells were isolated from spleen and lymph nodes of 5- to 8-week-old WT or LatY136F mice using CD4 conjugated magnetic microbeads (Miltenyi Biotech) according to manufacturer instructions. In order to reduce the contamination with WT B cells, LatY136F CD4+ T cells were isolated from young LatY136F B cell deficient mice. A total of 3 × 106 CD4+ T cells (purity > 95%) was injected i.v. into 6- to 8-week-old Cd3εΔ5/Δ5, Cd3εΔ5/Δ5 Cd80–/–/Cd86–/–, Cd3εΔ5/Δ5 Cd40–/–, or Cd3εΔ5/Δ5 Icosl–/– mice. When indicated, purified CD4+ T cells were labeled during 10 min at 37°C in PBS containing CFSE (5 μM; Molecular Probes, Invitrogen) before injection. Peripheral blood lymphocytes (PBL) were harvested at indicated time points and CFSE content was analyzed by flow cytometry after gating on CD4+CD5+ T cells.
In vitro co-culture assay
CD4+ T cells were first enriched by MACS (Miltenyi Biotech) using anti-CD4 beads and then sorted by FACS for maximal purity (at least 99%). A total of 3 × 104 CD4+ cells was cultured with 9 × 104 splenocytes of the indicated genotype in 200 μl of DMEM (Invitrogen Life Technologies) complemented with 10% FCS (Brunschwig), 10 mM HEPES buffer (Invitrogen Life Technologies), 200 μg/ml gentamicin (Invitrogen Life Technologies), and 50 μM 2-ME (Invitrogen Life Technologies). When indicated, anti-CD80 (5 μg/ml), anti-CD86 (10 μg/ml), anti-OX-40L (10 μg/ml), anti-IL-4 (10 μg/ml), anti-CD4 (25 μg/ml), and anti-LFA-1 (25 μg/ml), were added to the culture medium. At the specified time points, IgG1 was quantified in culture supernatants by ELISA.
Flow cytometry
Single-cell suspensions from spleen, lymph nodes, thymus, and bone marrow were stained for 20 min with the indicated Abs in presence of 50% 2.4G2 (anti-Fc) supernatant in PBS–FCS 3% EDTA 2 mM. The following antibodies were used: anti-B220 (RA3-6B2; eBioscience), anti-ICOS (15F9; eBioscience), anti-MHCII (2G9; BD Pharmingen), anti-CD138 (281-2; BD Pharmingen), anti-GL-7 (Ly77; BD Pharmingen), PNA (Sigma-Aldrich), anti-CD3 (145-2C11; eBioscience), anti-CD4 (GK1.5; BD Pharmingen), anti-CD5 (53-7-3; Biolegend), anti-CD8 (53-6.7; Biolegend), anti-CD25 (PC61; Biolegend), anti-CD28 (37.51; BD Pharmingen), anti-CD40L (MR1; Biolegend), anti-CD44 (IM7 eBioscience), anti-CD62L (MEL-14; eBioscience), anti-CD69 (H1.2F3; BD Pharmingen), anti-CD95 (JO2; BD Pharmingen), anti-CD134 (OX-86; BD Pharmingen), anti-CXCR5 (2G8, BD Pharmingen), and anti-PD-1 (RPM1-30; Biolegend). Biotinylated Abs were visualized with streptavidin–APC (Caltag Laboratories) or streptavidin–PE-Cy5.5 (eBioscience). For intracellular staining, cells were subsequently fixed with paraformaldehyde 1% and stained in PBS–FCS 3% saponin 0.5% with the antibody of interest. Cells were analyzed on a FACSCanto® cell analyzer (BD Bioscience). FACS data were subsequently analyzed on FlowJo (Tree Star).
Immunohistological analyses
Lymph nodes or spleen were embedded in OCT paraffin (TissueTek). Immunostainings were performed on acetone-fixed frozen sections using standard methods with biotinylated anti-B220 (RA3-6B2; Caltag Laboratories), anti-CD4 (RM4-5; eBioscience) culture supernatant, anti-CD8 (53-6.7; eBioscience), and biotinylated peanut agglutinin (PNA; Sigma-Aldrich). Biotinylated anti-B220 was detected using alkaline phosphatase (AP)-conjugated streptavidin (Boehringer Mannheim) and subsequent incubation with Fast Blue BB salt (Sigma-Aldrich). Biotinylated PNA and CD45.2 were revealed using HRP-conjugated streptavidin and rat Abs using HRP-conjugated goat anti-rat Abs (Biosource International). The substrate used for HRP was 3,3′-diaminobenzidine/urea tablets (Sigma-Aldrich) complemented with hydrogen peroxide. When required, counterstaining was done using Mayer’s hematoxylin.
Analysis of antibody titers
Levels of total IgM, IgG1, and IgE Abs present in mouse sera or in co-culture supernatant were quantified by ELISA using biotinylated polyclonal goat Abs specific for mouse Ig isotypes (Caltag Laboratories). Detection was performed using Streptavidin–HRP and Sigma Fast o-phenylenediamine (OPD; Sigma-Aldrich). Immunoglobulin concentration was determined by comparing a test sample dilution series with that using a isotype control standard (Biolegend).
Results
Costimulatory molecules are differentially required for LatY136F CD4+ T cell mediated B cell activation in vitro
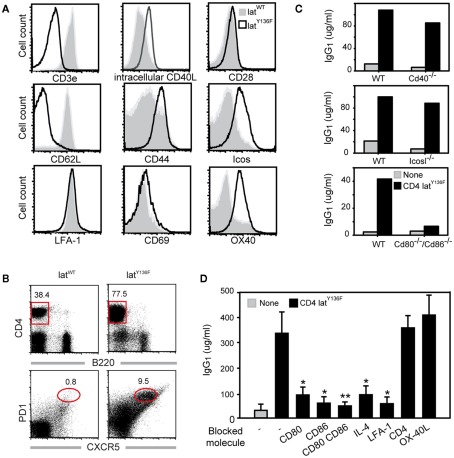
Recent experiments have shown that CD4+ T cells isolated from LatY136F mice and cultured in the presence of WT splenocytes are able to induce a strong B cell activation as monitored by immunoglobulin production (Genton et al., 2006). Moreover, this activation occurs regardless of the occurrence of TCR:MHCII interactions since MHCII deficient splenocytes could still be activated by LatY136F T cells (Genton et al., 2006). The LatY136F CD4+ T cells harbored a typical activated phenotype characterized by a strong up-regulation of CD44 and a down-regulation of CD62L whereas the level of TCR–CD3 complexes was greatly reduced (Aguado et al., 2002; Wang et al., 2008; Figure 1A).
Figure 1.
Costimulatory molecules required for in vitroLatY136F T cell mediated B cell activation. (A) Expression of costimulatory molecules on the surface of WT (gray) and LatY136F (solid line; CD8/CD4) lymph node T cells investigated by flow cytometry. (B) FACS analyses of lymph node from WT and LAT mutant mice using CD4 and B220. The frequency of TFH defined as PD-1+ CXCR5+ was also investigated among CD4 positive T cells. (C) IgG1 secretion was investigated by ELISA 6 days after in vitro culture of LatY136F CD4 T cells in the presence of WT splenocytes or of splenocytes deficient for the specified costimulatory molecules. Data are representative of at least three independent experiments. (D) Effects of blocking antibodies on IgG1 secretion were assessed by ELISA 6 days after co-culture of LatY136F CD4 T cells and of WT splenocytes. Bar graph corresponds to mean ± SD from three independent experiments. Mann Whitney test was performed to determine whether differences were significant. *p-Value < 0.1; **p-value < 0.05.
To better characterize the process by which LatY136F T cells efficiently activate B cells in the absence of TCR:MHCII interaction, the expression profile of molecules present on the surface of T cells and known to be involved in T–B cell interaction was investigated by flow cytometry. Both WT and LatY136F CD4+ T cells expressed similarly high level of LFA-1. The levels of CD28, CD69, and CD40L were slightly increased on LatY136F CD4+ T cells as compared to WT T cells whereas the expression of ICOS and OX-40 was strongly increased in LatY136F CD4+ T cells (Figure 1A).
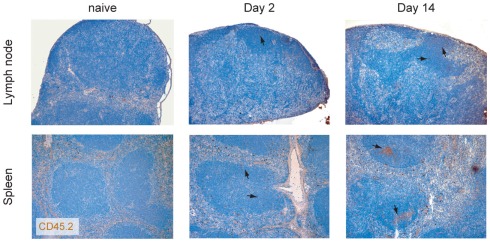
Given the high expression level of ICOS on the surface of LatY136F CD4+ T cells and their potency to induce high level of IgG1 and IgE, we determined whether these cells have a TFH cell phenotype characterized by PD-1 and CXCR5 co-expression (Breitfeld et al., 2000; Haynes et al., 2007). FACS analysis on lymph node cell suspension revealed that the frequency of CXCR5+PD-1+ cells was increased in LatY136F CD4+ T cells as compared to the WT CD4+ T cells (Figure 1B). Moreover, after transfer into WT recipient, the LatY136F CD4+ T cells migrated preferentially into B cell follicles, where they were able to survive for over a month without proliferating (Figure A1 in Appendix and data not shown).
To further investigate which costimulatory molecules are required to induce B cell activation by LatY136F CD4+ T cells, the latter cells were co-cultured with splenocytes deficient for CD40, ICOSL, or for both CD80 and CD86. Analyses of the levels of IgG1 present in the supernatant after 6 days of culture showed that CD80 and CD86 were important for B cell activation whereas CD40 and ICOSL were dispensable (Figure 1C). The differential requirement of those molecules was further confirmed by using blocking antibodies (Figure 1D). Inhibition of either CD80 or CD86 led to a partial blockade of IgG1 secretion whereas simultaneous blockade of both CD80 and CD86 led to a more drastic inhibition. Blocking the adhesion molecule LFA-1 abolished antibody production and thus suggests that T–B cell contacts are likely required for the B cell helper activity of LatY136F CD4+ T cells. IL-4, which is important for isotype switching toward IgG1 was also required. Conversely, OX-40 inhibition did not prevent B cell activation. Altogether, these in vitro data indicate that LatY136F CD4+ T cells activate B cells through a synapse involving in particular CD80–CD86 and cytokines such as IL-4, but this process is independent of CD40 and ICOSL signaling.
The LatY136F mice lymphoproliferative disorder unfolds irrespective of ICOS, ICOSL, and of CD40 expression
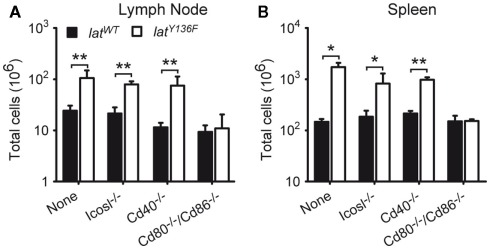
To determine whether the CD28, CD80, CD86, CD278 (ICOS), and CD40 costimulatory molecules and the ICOSL costimulatory ligand were involved in the unfolding of the LATY136F pathology, LatY136F mutant mice were crossed with Cd28−/−, Cd80−/−/Cd86−/−, Icos−/−, Icosl−/−, and Cd40−/− mice to generate LatY136F mice deprived of CD28, CD80–CD86, ICOS, ICOSL, or CD40. The size of the lymph nodes and spleen of the various mutant mice was assessed at 8 weeks of age, a time when the pathology is fully installed in LatY136F mice. Strikingly, the absence of CD28 or of CD80–CD86 completely abrogated the lymphadenopathy and splenomegaly that characterize LatY136F mutant mice, whereas the absence of ICOS, ICOSL, or CD40 did not affect significantly the magnitude of the lymphoproliferative disorder resulting from the presence of LATY136F molecules (Figures 2A,B and data not shown). Therefore, in contrast to ICOS/ICOSL and to CD40, the CD28 receptor, and its CD80–CD86 ligand appear mandatory for the development of the LAT pathology.
Figure 2.
Analysis of the lymphoproliferation occurring in LatY136F mutant mice deficient for a given costimulatory molecule. Total cell number present in pooled inguinal, brachial, and axillary lymph nodes (A) and spleen (B) of 8-week-old WT (black bar) or LatY136F mutant mice (open bar) that were additionally deficient for the costimulatory molecule specified below the bars. Bars represent the mean ± SD from at least three different mice analyzed in independent experiments. t-Test or Mann Whitney test was performed to determine whether differences were significant. *p-Value < 0.05; **p-value < 0.01.
Peripheral LatY136F CD4+ T cell expansion is blocked in the absence of CD28 signaling
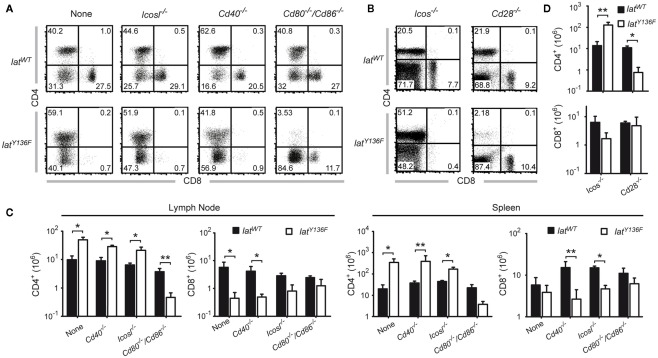
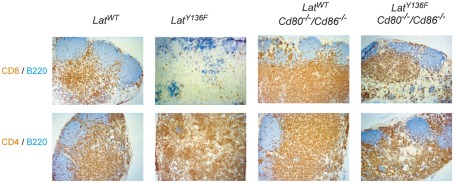
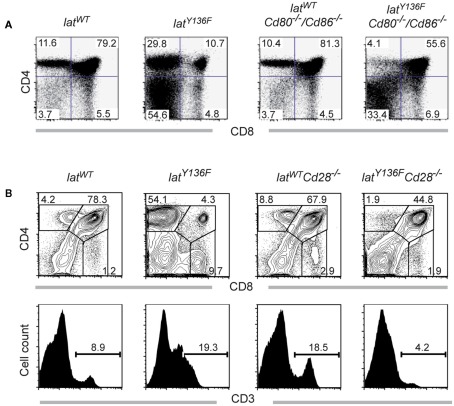
We next compared the frequencies of CD4+ and CD8+ T cells present in the spleen of 8-week-old LatY136F Icos−/−, LatY136F Icosl−/−, LatY136F Cd40−/−, LatY136F Cd28−/−, and LatY136F Cd80−/−/Cd86−/− mice (Figure 3). The absence of ICOS, ICOSL, and CD40 did not modify the aberrant CD4+–CD8+ T cell subset distribution observed in secondary lymphoid organs of LatY136F mutant mice. Indeed, the expanding T cells were dominated by CD4+ T cells whereas the frequency of CD8+ T cells was reduced to almost undetectable levels (Figures 3A,B). The absolute number of CD4+ T cells found in LatY136F Icosl−/−, LatY136F Icos−/−, and LatY136F Cd40−/− was increased about 10-fold, which correspond to the increase observed in LatY136F mutant mice (Figures 3C,D). Strikingly, in the absence of CD28 or of CD80–CD86, the frequency of LatY136F mutant CD4+ T cells was reduced to very low levels, reaching values lower than those observed in WT mice deficient for CD80–CD86. Conversely, LatY136F Cd28−/−, LatY136F Cd80−/−/Cd86−/− mice showed percentages of CD8+ T cells that were increased as compared to LatY136F, LatY136F Icosl−/−, and LatY136F Cd40−/− mutant mice (Figures 3A,B). In term of absolute number, both LatY136F Cd28−/− and LatY136F Cd80−/−/Cd86−/− mice presented a 10-fold reduction of CD4+ T cells compared to WT counterpart whereas the absolute CD8+ T cell number was similar to the number present in WT (Figures 3C,D). The higher proportion of CD8+ T cells in LatY136F mutant spleen in the absence of Cd80−/−/Cd86−/− was further confirmed by immunohistochemistry (Figure A2 in Appendix). Analysis of thymi from 8-week-old LatY136F Cd28−/− and LatY136F Cd80−/−/Cd86−/− mice revealed that small numbers of CD4 single positive T cells were still able to develop in the absence of CD28/CD80–CD86 signaling (Figures 3A,B in Appendix). However, consistent with data based on a model where CD4+ T cells expressed LATY136F mutant molecules only once they have fully matured and reached the periphery (Mingueneau et al., 2009; Roncagalli et al., 2010), the CD4+ T cells of both LatY136F Cd28−/− and LatY136F Cd80−/−/Cd86−/− mice failed to proliferate extensively in secondary lymphoid organs. Importantly, LatY136F mutant mice that were genetically deprived of B cells were still able to develop a fully fledged lymphoproliferative disorder involving CD4+ Th2 effectors (our unpublished observations). Therefore, the expansion of LatY136F CD4+ T cells in the periphery requires CD28 crosslinking by CD80–CD86 through a process that is independent on B cells and most likely involves dendritic cells.
Figure 3.
Effect of costimulatory molecule deficiency on the presence of CD4 and CD8 T cell in LatY136F mutant mice. (A) The frequency of CD4 and CD8 T cells present in the lymph node of 8-week-old WT (upper row) or of LatY136F mice (lower row) lacking the costimulatory molecule specified above the dot plots was assessed by flow cytometry (B) T cells from the spleen of the specified 8-week-old mice were analyzed for the expression of CD4 and CD8. The percentage of cells is shown in each quadrant [upper row (C)]. Total number of CD4 and CD8 T cells present in pooled inguinal, brachial, and axillary lymph nodes (left column) and in the spleen (right column) of WT (black bar) and LatY136F mice (open bar) lacking the indicated costimulatory molecules on the surface of B cells. (D) Absolute numbers of CD4 and CD8 T cells recovered per spleen of 8-week-old mice lacking the indicated costimulatory molecule on the surface of T cells. Histogram corresponds to the mean ± SD of at least three mice analyzed in independent experiments. t-Test or Mann Whitney test was performed to determine whether differences were significant. *p-Value < 0.05; **p-value < 0.01.
Surface phenotype of the T cells expanding in the periphery of LatY136F in absence of costimulatory molecules
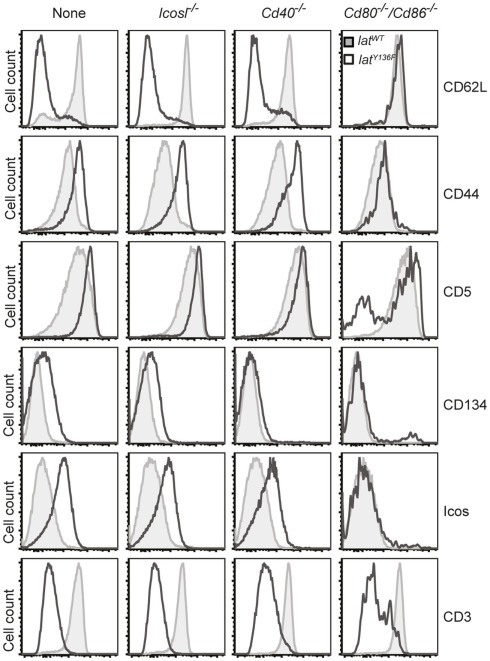
The phenotype of the T cells found in the periphery of LatY136F Icos−/−, LatY136F Icosl−/−, LatY136F Cd40−/−, LatY136F Cd28−/−, and LatY136F Cd80−/−/Cd86−/− mice was next compared to that found in age-matched LatY136F mice (Figure 4 and data not shown). No major differences were found when the CD4+ T cells that expand in LatY136F, LatY136F Icosl−/−, and LatY136F Cd40−/− were analyzed for the presence of CD62L, CD44, CD5, CD134 (OX-40), and ICOS and only a slight defect in the up-regulation of CD134 was observed in the absence of CD40 (Figure 4). Absence of CD80–CD86 led to a more drastic modification of the phenotype of the few CD4+ T cells that are found in the periphery of LatY136F Cd80−/−/Cd86−/− mice. Although those cells showed reduced levels of TCR–CD3 complex at their surface, an attribute that typifies LatY136F CD4+ T cells, they failed to up-regulate CD44, CD134, and ICOS and to down regulate CD62L (Figure 4). The CD8+ T cells present in the periphery of LatY136F Cd28−/− also showed reduced levels of TCR–CD3 complexes at their surface (data not shown). Therefore, the presence of CD28 or of CD80–CD86 is required both for the expansion of the CD4+ T cells and the induction of an activated phenotype.
Figure 4.
Phenotype of LatY136F CD4 T cells in the absence of costimulatory molecules. FACS analysis of the surface phenotype of CD4 T cell expanding in the lymph nodes of 2-month-old WT (gray histogram) and of LatY136F mice (open histogram) deficient for the costimulatory molecules specified above the histograms. FACS plot are representative of at least three independent experiments.
Effect of costimulatory molecule deficiency on B cell activation
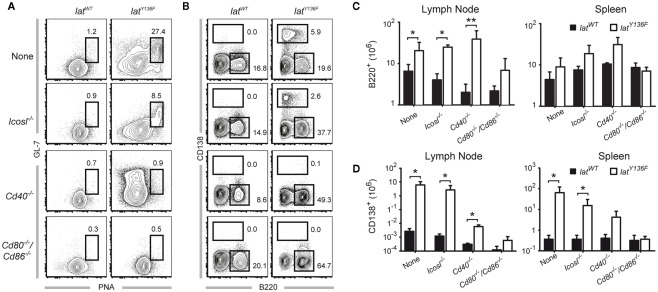
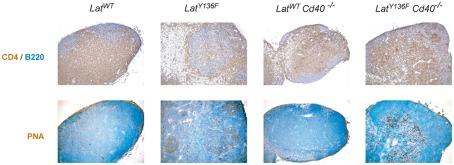
We next sought to determine whether the absence of the ICOS, ICOSL, CD40, CD28, and CD80–CD86 costimulatory molecules affects the massive B cell activation that is triggered by LatY136F CD4+ T cells. As expected, the lymph nodes and the spleen of 8-week-old LatY136F mice are characterized by a strong induction of germinal center B cells characterized by a high expression level of both PNA and GL-7 (Figure 5A and data not shown). Such cells were also observed in LatY136F Icosl−/− mutant mice although with a lower frequency. In marked contrast, no germinal center B cells were identified in LatY136F mice lacking either CD40 or CD80–CD86 (Figure 5A; Figure A4 in Appendix). The more differentiated population of antibody-secreting cells present in WT lymph nodes is characterized by a high expression of CD138 and reduced levels of B220. This population was strongly increased in LatY136F and in LatY136F Icosl−/− mice (Figure 5B). In the absence of CD40 or of CD80–CD86, such CD138+ B220− cells were almost completely absent. In LatY136F mice, the absolute number of CD138+ B220− cells showed a 1000-fold increase when compared to WT mice and such massive increase was also observed in LatY136F Icosl−/− mice (Figures 5C,D, left graph). The increase in antibody-secreting cell population due to the LatY136F mutation was strongly reduced in the absence of CD40, where only a 10-fold increase in the absolute number of CD138+ B220− could be observed and such increase was almost completely abolished in the absence of CD80–CD86. The same trend was observed when antibody-secreting cells were enumerated in the spleen. However, the differences observed between the different mice analyzed were of a lower magnitude than those observed in lymph nodes (Figures 5C,D, right graph). Altogether, these results show that the whole B cell activation process is impeded in the absence of CD80–CD86, an observation consistent with the absence of activated CD4+ T cells. In the absence of CD40, the total lack of germinal center formation coexists with a small increase in the number of antibody-secreting cells present in the spleen and lymph node. This suggests that only the follicular pathway of B cell differentiation is abrogated. Finally, the absence of ICOSL only marginally affects the capacity of LatY136F CD4+ T cells to induce massive B cell activation.
Figure 5.
B cell activation observed in the periphery of LatY136F mutant mice deficient for a given costimulatory molecule. (A) Frequency of B220+ GL-7+ PNA+ cells found in pooled inguinal, axillary, and brachial lymph nodes from 8-week-old WT (left column) or LatY136F mutant mice (right column) deficient for the indicated costimulatory molecule. (B) Frequency of CD138+ and B220+ cells found in pooled inguinal, axillary, and brachial lymph nodes from 8-week-old WT (left column) or LatY136F mutant mice (right column) deficient for the indicated costimulatory molecule. (C) Total number of B220+ cells present in pooled inguinal, brachial, and axillary lymph nodes (left column) and in the spleen (right column) of WT (black bar) and LatY136F mice (open bar) lacking the indicated costimulatory molecule. (D) Total number of CD138+ cells present in pooled inguinal, brachial, and axillary lymph nodes (left column) and in the spleen (right column) of WT (black bar) and LatY136F mice (open bar) lacking the indicated costimulatory molecule. Histogram corresponds to the mean ± SD of at least three mice analyzed in independent experiments. t-Test or Mann Whitney test was performed to determine whether differences were significant. *p-Value < 0.05; **p-value < 0.01.
Antibody secretion in the absence of costimulatory molecules
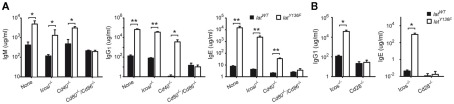
The LATY136F pathology is characterized by a massive increase in antibody secretion, especially in immunoglobulins of the IgE and IgG1 isotypes (Aguado et al., 2002; Genton et al., 2006). The level of IgM, IgG1 and IgE was assessed by ELISA in the blood of 8-week-old LatY136F Icos−/−, LatY136F Icosl−/−, LatY136F Cd40−/− LatY136F Cd28−/−, and LatY136F Cd80−/−/Cd86−/−, mice. Increased levels of IgM secretion were noted in LatY136F, LatY136F Cd40−/−, and LatY136F Icosl−/− LatY136F Cd40−/− mice. In LatY136F mice lacking CD80–CD86, no increase over basal levels of IgM was observed (Figure 6A). The level of IgG1 increased at least 100-fold in LatY136F Icosl−/− and in LatY136F Cd40−/− mice and reached values close to those observed in LatY136F mice. In contrast, LatY136F Cd80−/−/Cd86−/− showed levels of IgG1 similar to WT mice (Figure 6A). Finally, IgE levels were massively increased in LatY136F and in LatY136F Icosl−/− mice, moderately increased in LatY136F Cd40−/− mice and undetectable in LatY136F Cd80−/−/Cd86−/− (Figure 6A). Consistent with the above results, the lack of ICOS had no effect on the LatY136F IgG1 and IgE hypergammaglobulinemia whereas the lack of CD28 prevented it (Figure 6B). In conclusion, ICOS and ICOSL are dispensable for the induction of hypergammaglobulinemia G1 and E in LatY136F mice whereas CD40 partially decreased the IgE hypergammaglobulinemia while leaving the IgG1 hypergammaglobulinemia unaffected. On the other hand, absence of CD80–CD86 completely abolished the induction of immunoglobulin secretion in LatY136F mice, an observation consistent with the absence of an expanded population of CD4+ T cells.
Figure 6.
Analysis of antibody production in WT and LatY136F mutant mice lacking a given costimulatory molecule. (A) The level of IgM, IgG1, and IgE was investigated by ELISA in the sera of 8-week-old WT (black bar) and LatY136F mutant mice (open bar) deficient for CD40, CD80–CD86, or ICOSL. (B) The concentration of IgG1 and IgE found in the serum of 8-week-old WT (black bar) and LatY136F mutant mice (open bar) deficient for CD28 or ICOS. Bars represent the mean ± SD from three different mice. t-Test or Mann Whitney test was performed to determine whether differences were significant. *p-Value < 0.05; **p-value < 0.01.
Unfolding of the LatY136F pathology upon adoptive transfer into hosts lacking costimulatory ligands
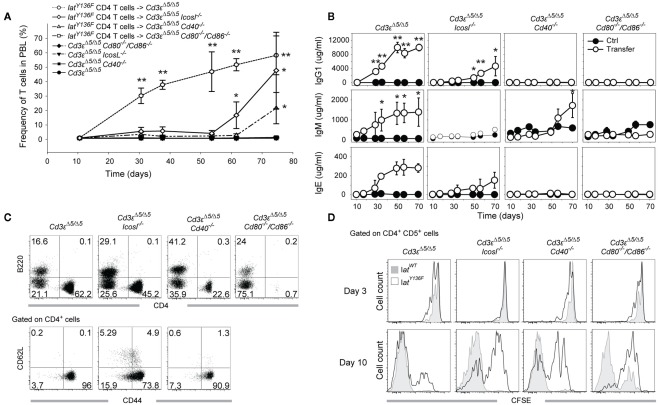
LatY136F CD4+ T cells were not able to expand correctly in the absence of CD28 or of CD80–CD86 molecules (Mingueneau et al., 2009), and such block prevented to analyze in vivo the role played by the CD28–CD80–CD86 receptor–ligand pair during LatY136F CD4+ T cell mediated B cell activation. To circumvent this problem and to determine whether converted LatY136F CD4+ T cells require costimulatory molecules to activate B cells, LatY136F CD4+ T cells were isolated from LatY136F mice using MACS purification and adoptively transferred into Cd3εΔ5/Δ5 mice that were made additionally deficient for a given costimulatory molecule. Following adoptive transfer, the frequency of LatY136F CD4+ T cells was monitored in the blood. A massive expansion of the LatY136F CD4+ T cells ensued during the first month after transfer into Cd3εΔ5/Δ5 recipient (Figure 7A). Transfer into both Cd3εΔ5/Δ5 Icosl−/− and Cd3εΔ5/Δ5 Cd40−/− hosts resulted in an expansion that followed a protracted kinetics and lagged by a 40- and 50-day-delay, respectively, behind the kinetics observed after transfer into Cd3εΔ5/Δ5 recipient. Importantly, when LatY136F CD4 T cells were transferred into Cd3εΔ5/Δ5 Cd80−/−/Cd86−/− mice, no expansion ensued (Figure 7A). Immunoglobulin levels were monitored over the same time period and compared to the basal levels of immunoglobulins present in control hosts that have received no LatY136F CD4+ T cells (Figure 7B). As expected, IgM, IgG1, and IgE levels increased rapidly after transferring LatY136F CD4+ T cells into Cd3εΔ5/Δ5 hosts. In Cd3εΔ5/Δ5 Cd40−/− mice, IgM secretion was strongly delayed whereas IgG1 and IgE were not induced. In Cd3εΔ5/Δ5 Cd80−/−/Cd86−/− mice, no increase over basal immunoglobulin levels was noted. In Cd3εΔ5/Δ5 Icosl−/− mice reconstituted with LatY136F CD4 T cells, a late increase in IgG1 and IgE levels was noted whereas IgM levels were only slightly increased.
Figure 7.
Expansion and B cell activation potential of LatY136F CD4 T cells transferred into T cell deficient recipient lacking a given costimulatory molecule. (A) MACS purified LatY136F CD4 T cells (3 × 106) were adoptively transferred into Cd3eΔ5/Δ5, Cd3eΔ5/Δ5Cd40−/−, Cd3eΔ5/Δ5Cd80/Cd86−/−, and Cd3eΔ5/Δ5Icosl−/− recipient mice. Kinetic of LatY136F CD4 T cells expansion was monitored by flow cytometry in the blood of transferred mice at the indicated time points. Each point corresponds to the mean ± SD of three mice. t-Test was performed to determine whether differences between transferred and non-transferred mice of the same genotype were significant. *p-Value < 0.05; **p-value < 0.01. (B) IgM, IgG1, and IgE levels were monitored in the sera of transferred (open circle) and control mice (black circle) at the indicated time points. Each point corresponds to the mean ± SD of three mice. t-Test was performed to determine whether differences were significant. *p-Value < 0.05; **p-value < 0.01. (C) FACS analyses of B220+ and CD4+ cells observed in the blood of transferred mice (upper row) 70 days after transfer. Expression of CD62L and CD44 was subsequently investigated by flow cytometry at the surface of CD4+ cells (lower row). (D) WT or LatY136F CD4+ T cells (3 × 106) were CFSE labeled and transferred into mice lacking T cells and deficient for CD40, CD80/86, or ICOSL. CFSE profile of WT (gray) and LatY136F transferred cells (open histogram) identified as CD4+ CD5+ cells was established in the blood 3 and 10 days after transfer in the indicated recipients.
When recipient mice were sacrificed 8 weeks after transfer, both Cd3εΔ5/Δ5, Cd3εΔ5/Δ5 Icosl−/−, and Cd3εΔ5/Δ5 Cd40−/− mice had enlarged spleens and lymph nodes (data not shown) whereas Cd3εΔ5/Δ5 Cd80−/−/Cd86−/− had normal sized organs. LatY136F CD4+ T cells were readily found in the enlarged spleens and lymph nodes of Cd3εΔ5/Δ5, and Cd3εΔ5/Δ5 Icosl−/− mice and their phenotype corresponded to activated T cells with a CD44high and CD62Llow phenotype (Figure 7C). Therefore, this adoptive transfer model reproduced in a general manner what was observed in the double mutant mice. However, since the transferred LatY136F CD4+ T cells failed to expand in the absence of CD80–CD86, such system precluded the definitive conclusion to be reached on the role played by the CD28–CD80–CD86 receptor–ligand pair during LatY136F CD4 T cell mediated B cell activation.
Cell division of transferred cells at early time points after transfer
To determine how the absence of a given costimulatory molecule might affect the initial expansion of LatY136F CD4+ T cells that follows their adoptive transfer into Cd3εΔ5/Δ5 mice, WT, and LatY136F CD4+ T cells were labeled with the CFSE division tracking dye before i.v. injection into Cd3εΔ5/Δ5 hosts that were deficient in such costimulatory molecules. The number of divisions was assessed 3 and 10 days after transfer. No major difference in CFSE dilution was observed in the absence of ICOSL, CD40, or CD80–CD86 molecules 3 days after transfer. As previously described (Wang et al., 2008), 10 days after transfer, WT CD4+ T cells went already through a large number of divisions in a process which has been shown to be MHCII-dependent and results in a single peak of CFSE negative cells (Wang et al., 2008; Figure 7D, lower row, first graph, gray histogram). Upon transfer, a fraction of converted LatY136F CD4+ T cells underwent a slower, MHCII independent, and IL-7 dependent division process (Wang et al., 2008), and as a result, remained CFSE positive 10 days after transfer (Figure 7D, lower row, first graph, open histogram). The absence of CD80–CD86 impeded this explosive, MHCII-dependent division process and a substantial number of CD4+ T cells remained CFSE positive. In the absence of CD40 or of ICOSL, a major CFSE positive peak was still observed 10 days after transfer. Therefore, the absence of ICOSL or of CD40 delayed the rate at which the transferred LatY136F CD4+ T cells enter into cell cycle whereas the lack of CD80–CD86 almost completely abolished it.
Discussion
Although numerous studies have investigated the development of LatY136F CD4+ T cells and their role in generating a TH2 lymphoproliferative disorder, little is known of the mechanism leading to the massive B cell activation associated with such disorder. However, it is known that the underlying mechanisms do not require TCR:MHCII interactions (Genton et al., 2006). This study aimed therefore at elucidating which TCR-independent signals are important to trigger the B cell activation induced by LatY136F CD4+ T cells both in vitro and in vivo. Firstly, we demonstrated in vitro that prolonged cell–cell interaction between B and T cells is required since LFA-1 blockade impedes B cell activation. Next, we assessed the role of molecules suspected of being involved in the synapse between LatY136F CD4+ T cells and B cells. CD40 was a possible candidate since anti-CD40 antibodies is widely used in vitro as a potent signal to induce B cell activation in the absence of MHCII signaling. Another candidate, ICOS, which is essential for an efficient TH2 response and high IgG1 and IgE secretion, is strongly up regulated on the surface of LatY136F CD4+ T cells. We hypothesize therefore that CD40 and ICOSL would be required to enable LatY136F CD4+ T cells to trigger B cell activation. However, in our in vitro settings, B cell activation monitored by IgG1 secretion was not affected by the absence of CD40 signaling. Also, the inhibition of OX-40, another molecule involved in late B cell differentiation failed to block the B cell activation triggered by LatY136F CD4+ T cells. This suggests that in the LatY136F pathology, alternative T cell molecules may be involved in B cell activation and differentiation.
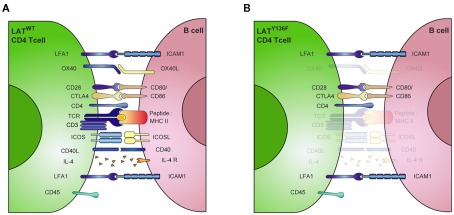
The cell surface molecules CD80 and CD86 are known to play an important role in T cell activation by engaging the CD28 costimulatory molecule. But their actual role during B cell activation remains less well established. It has been shown that monoclonal antibodies directed against CD80 block the immunoglobulin secretion of LPS-stimulated B cells whereas anti-CD86 antibodies have an opposite effect (Suvas et al., 2002). More recently, another study showed that CD86 engagement induces XBP-1 expression and promotes IgG secretion (Rau et al., 2009), suggesting that CD86 could be the primary target of LatY136F CD4+ T cells at the surface of B cells. Strikingly, in our in vitro settings, the use of anti-CD80 or anti-CD86 blocking antibodies significantly decreased the immunoglobulin secretion induced by LatY136F CD4+ T cells, while the combination of both of these antibodies almost completely blocked antibody production. Moreover, this result was further confirmed using CD80–CD86 deficient B cells. In this case, however, and in all subsequent in vivo experiments, the function of these molecules was not assessed individually. Although it has already been shown that CD86 is involved in B cell activation, the level of magnitude of the inhibition observed here in the absence of the CD80–CD86 costimulation represents a previously unexpected role for these molecules during B cell activation. Coupled to the absence of role for ICOSL and CD40, this observation suggests that LatY136F CD4+ T cells rely on an unusual combination of costimulatory molecules to trigger B cell activation (Figure 8). However, the role played by the CD28–CD80–CD86 receptor–ligand pair in the LatY136F pathology may be more complex. For example, it is possible that by engaging CD28, CD80–CD86 could induce the up-regulation of other essential molecules on the surface of T cells that lead to complete B cell activation.
Figure 8.
Unusual combination of costimulatory molecules involved in LATY136F CD4+ T cell mediated B cell activation. (A) Schematic representation of ligand–receptor pairs required for the regulation of B cell function by T lymphocytes. (B) In presence of LATY136F mutant CD4+ T cells, many of these signals are bypassed. B cell activation can occur in absence of antigen-specific interaction mediated by MHCII:TCR recognition. The resulting polyclonal B cell activation is only slightly affected by the absence of CD40, IL-4, OX-40 signaling, while CD80/86 and LFA-1 are critical in this process. CTLA4, cytotoxic T lymphocyte antigen 4, ICAM1, intercellular adhesion molecule; LFA-1, Lymphocyte function associated antigen 1; SMAC, supramolecular activation complex; TCR, T cell receptor. (Adapted from Huppa and Davis, 2003).
To confirm these unexpected findings in vivo, LatY136F mutant mice were crossed with mice deficient for costimulatory molecules. Importantly, this system is not affected by the fact that these mice also display the LatY136F mutation in B cells, since it has been shown that LAT is undetectable at later stages of B cell development and its absence has no measurable effect on B cell activation or differentiation (Oya et al., 2003; Su and Jumaa, 2003; Wang et al., 2005). However, this system was not as tractable as originally expected since in the absence of CD28 or of CD80–CD86, LatY136F CD4+ T cell development and activation was compromised. Indeed, CD4+ T cells did not expand in the periphery and on the contrary, a population of CD8+ T cells was observed. This confirms previous observation suggesting that the very low number of CD4+ T cells able to reach the periphery in LatY136F mice need to receive a signal through CD28 for the onset of the initiation phase of the LAT pathology (Mingueneau et al., 2009; Roncagalli et al., 2010).
Nevertheless, our experimental model was suitable to investigate the function of both CD40 and ICOSL since their absence did not affect the expansion of LatY136F CD4+ T cells. In the absence of CD40 and ICOSL, LatY136F CD4+ T cells still managed to perform tasks that are generally attributed during physiological immune responses to the presence of CD40 and ICOSL. Although CD40 remained crucial for the generation of germinal center induced by LatY136F CD4+ T cells, its absence did not inhibit isotype switching. Indeed, the immunoglobulin production was not limited to IgM, as it is the case in CD40 deficient mice that are known to exhibit a hyper IgM syndrome and very low amount of IgG and IgE. Strikingly, both IgG and IgE were strongly induced, indicating that CD40 deficient B cells were still able to efficiently switch their isotype once in the presence of LatY136F CD4+ T cells. This CD40 independent isotype switching may be due to an excess of BAFF and April, two molecules that are known to induce isotype switching in the presence of IL-4 and IL-10 (Litinskiy et al., 2002), although this possibility has not been addressed directly yet. The origin of these immunoglobulins remains elusive since very low levels of CD138+ antibody-secreting cells were found in the lymph nodes and in the spleen. This suggests that either these cells harbor a different phenotype or that they migrate to or are induced in other organs such as bone marrow. By comparison, LatY136F Icosl−/− mice exhibit a lower frequency of germinal center cells when compared to LatY136F mice. This could be explained by the important role played by ICOS in IL-21 production, which is required for the expansion and persistence of follicular T helper cell generation and function (Vogelzang et al., 2008; Linterman et al., 2010; Zotos et al., 2010). The large plasma cell accumulation and the hypergammaglobulinemia that occur in LatY136F mutant mice were not affected by the absence of ICOSL, indicating that this molecule is not critical for these processes.
Transfer of converted LatY136F CD4+ T cells into Cd3εΔ5/Δ5 mice that are deficient for T cells but harbor a normal compartment of B cells enabled the measurement of the effects of mutant T cells on B cells and revealed that MHCII is not required for the perpetuation of the LatY136F pathology (Wang et al., 2008). Nevertheless, upon transfer into Cd3εΔ5/Δ5 Cd80−/−/Cd86−/− mice, LatY136F CD4+ T cells failed to expand. This indicated that CD80 and CD86 molecules are required not only for the initiation phase of the LatY136F T cell expansion in the periphery but also for the slow, MHCII independent CD4+ T cell expansion occurring in the post-conversion, perpetuation phase. As a consequence of this impeded T cell expansion, determining the direct role played by CD80–CD86 on the massive polyclonal B cell activation in vivo remains intractable.
In conclusion, this study helped in understanding how a single point mutation in the LAT adaptor protein is able to induce a polyclonal, “quasi mitogenic” B cell activation. As previously suggested, LAT is expected to operate a negative regulatory loop that controls conventional CD4+ T cell activation (Mingueneau et al., 2009). When equipped with LATY136F molecules, peripheral CD4+ T cells acquire the capacity to massively activate B cell in the absence of TCR:MHCII interaction. Here, we showed that the need for ICOSL and CD40 is overcome in the context of the LatY136F pathology. Conversely, CD80 and CD86 molecules are mandatory for both in vitro immunoglobulin production and the two phases of LatY136F CD4+ T cell expansion in the periphery. As such, CD80 and CD86 stand as key players in the generation of the LAT pathology. The possibility that other molecules are involved remains, however, undetermined. In this context, the signaling lymphocyte activating molecule (SLAM) was recently shown to be involved in T–B cell interaction and SLAM associated protein (SAP) is required to establish long duration T–B cell contact required for the formation of the germinal center reaction (Qi et al., 2008; Schwartzberg et al., 2009). IL-21, which is now known to play an important role for both TFH cell generation and function, is also strongly implicated in B cell activation and plasma cell differentiation. This raises the intriguing possibility that these molecules are also involved in the B cell activation induced by LatY136F CD4+ T cell and it could be of interest to investigate their role in order to provide new insights into the pathways that trigger B cell activation in the LatY136F mutant context. Indeed, despite its artificial aspect, the LAT pathology could be a useful model to help understand similar lymphoproliferative disorders occurring in human, such as the idiopathic hypereosinophilic syndrome that results from a primitive T cell disorder involving abnormal CD4+ T cells (Roufosse, 2009).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank M. Bachmann, A. Tafuri, and T. Mak for mice, Fabienne Tacchini-Cottier, Floriane Auderset, Sylvie Richelme, Amandine Sansoni, and Aurelie Malzac for technical help and Dianne Emslie for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation to Hans Acha-Orbea, and by CNRS, INSERM, ANR (ADAPT Project), and the European Community’s Seventh Framework Programme Sybilla and Masterswitch under grant agreement HEALTH-F2-2008-223404 to Bernard Malissen and Marie Malissen.
Appendix
Figure A1.
Preferential migration of LAT CD4 T cells into the follicles. LAT CD4 T cells were transferred into WT recipient and their localization in the spleen and in the lymph node was investigated at day 2 and 14 using the congenic marker CD45.2 (brown). Nuclei were counter-stained with Mayer’s hematoxylin (blue). Arrows indicate the location of transferred cells present in the B cell follicles.
Figure A2.
Immunohistological analyses of lymph node sections from 8-week-old WT, LatY136F, Cd80−/−Cd86−/−, and LatY136F Cd80−/−Cd86−/− mice. Anti-B220 (blue) and anti-CD8 (brown; upper row) or anti-B220 (blue) and anti-CD4 (brown) staining (lower row) show the B and T cell distribution.
Figure A3.
Thymocyte development in LatY136FCD80−/−CD86−/− mice. (A) CD4 and CD8 thymocyte distribution was investigated in the thymus from 8-week-old WT and LatY136F mice deficient for CD80 and CD86. (B) Cells from thymi from 8-week-old WT, LatY136F, Cd28−/−, and LatY136F Cd28−/− mice were analyzed for expression of CD4 and CD8. The percentage of cells is shown in each quadrant. Also shown is the expression of CD3 on the surface of total thymocytes of WT, LatY136F, Cd28−/−, and LatY136F Cd28−/− mice. Percentages of CD3high T cells are indicated. Data are representative of three different mice.
Figure A4.
Germinal center formation in LatY136F mutant mice lacking CD40. Lymph node sections from mice of indicated genotype were analyzed by immunohistology for the presence of B220 (blue) and CD4 (brown) cells (upper row). Lymph node sections were also stained with PNA (brown) and nuclei were counter-stained with Mayer’s hematoxylin (blue; lower row).
Abbreviations
ICOSL, inducible T cell costimulator ligand; LAT, linker for activation of T cells; SLAM, signaling lymphocyte activation molecule.
References
- Adelstein S., Pritchard-Briscoe H., Anderson T. A., Crosbie J., Gammon G., Loblay R. H., Basten A., Goodnow C. C. (1991). Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science 251, 1223–1225 10.1126/science.1900950 [DOI] [PubMed] [Google Scholar]
- Aguado E., Richelme S., Nunez-Cruz S., Miazek A., Mura A. M., Richelme M., Guo X. J., Sainty D., He H. T., Malissen B., Malissen M. (2002). Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science 296, 2036–2040 10.1126/science.1069057 [DOI] [PubMed] [Google Scholar]
- Bishop G. A., Hostager B. S. (2001). B lymphocyte activation by contact-mediated interactions with T lymphocytes. Curr. Opin. Immunol. 13, 278–285 10.1016/S0952-7915(00)00216-8 [DOI] [PubMed] [Google Scholar]
- Breitfeld D., Ohl L., Kremmer E., Ellwart J., Sallusto F., Lipp M., Forster R. (2000). Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 10.1084/jem.192.11.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N., Mark L., Mcheyzer-Williams L. J., Mcheyzer-Williams M. G. (2009). Follicular helper T cells: lineage and location. Immunity 30, 324–335 10.1016/j.immuni.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller D. M., Zhang W. (2009). Regulation of lymphocyte development and activation by the LAT family of adapter proteins. Immunol. Rev. 232, 72–83 10.1111/j.1600-065X.2009.00828.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton C., Wang Y., Izui S., Malissen B., Delsol G., Fournie G. J., Malissen M., Acha-Orbea H. (2006). The Th2 lymphoproliferation developing in LatY136F mutant mice triggers polyclonal B cell activation and systemic autoimmunity. J. Immunol. 177, 2285–2293 [DOI] [PubMed] [Google Scholar]
- Greenwald R. J., Freeman G. J., Sharpe A. H. (2004). The B7 family revisited. Annu. Rev. Immunol. 23, 515–548 10.1146/annurev.immunol.23.021704.115611 [DOI] [PubMed] [Google Scholar]
- Haynes N. M., Allen C. D., Lesley R., Ansel K. M., Killeen N., Cyster J. G. (2007). Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 179, 5099–5108 [DOI] [PubMed] [Google Scholar]
- Huppa J. B., Davis M. M. (2003). T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3, 973–983 10.1038/nri1245 [DOI] [PubMed] [Google Scholar]
- Hutloff A., Dittrich A. M., Beier K. C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R. A. (1999). ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 10.1038/16717 [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. (1991). CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J. Immunol. 147, 2461–2466 [PubMed] [Google Scholar]
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. (1994). The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1, 167–178 10.1016/1074-7613(94)90095-7 [DOI] [PubMed] [Google Scholar]
- Linterman M. A., Beaton L., Yu D., Ramiscal R. R., Srivastava M., Hogan J. J., Verma N. K., Smyth M. J., Rigby R. J., Vinuesa C. G. (2010). IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 10.1084/jem.20091738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litinskiy M. B., Nardelli B., Hilbert D. M., He B., Schaffer A., Casali P., Cerutti A. (2002). DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 10.1038/nrg940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak T. W., Shahinian A., Yoshinaga S. K., Wakeham A., Boucher L. M., Pintilie M., Duncan G., Gajewska B. U., Gronski M., Eriksson U., Odermatt B., Ho A., Bouchard D., Whorisky J. S., Jordana M., Ohashi P. S., Pawson T., Bladt F., Tafuri A. (2003). Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat. Immunol. 4, 765–772 10.1038/ni947 [DOI] [PubMed] [Google Scholar]
- Malissen B., Aguado E., Malissen M. (2005). Role of the LAT adaptor in T-cell development and Th2 differentiation. Adv. Immunol. 87, 1–25 10.1016/S0065-2776(05)87001-4 [DOI] [PubMed] [Google Scholar]
- Malissen M., Gillet A., Ardouin L., Bouvier G., Trucy J., Ferrier P., Vivier E., Malissen B. (1995). Altered T cell development in mice with a targeted mutation of the CD3-epsilon gene. EMBO J. 14, 4641–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam A. J., Greenwald R. J., Levin M. A., Chernova T., Malenkovich N., Ling V., Freeman G. J., Sharpe A. H. (2001). ICOS is critical for CD40-mediated antibody class switching. Nature 409, 102–105 10.1038/35051107 [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams L. J., McHeyzer-Williams M. (2004). Antigen-specific memory B cell development. Annu. Rev. Immunol. 23, 487–513 10.1146/annurev.immunol.23.021704.115732 [DOI] [PubMed] [Google Scholar]
- Mingueneau M., Roncagalli R., Gregoire C., Kissenpfennig A., Miazek A., Archambaud C., Wang Y., Perrin P., Bertosio E., Sansoni A., Richelme S., Locksley R. M., Aguado E., Malissen M., Malissen B. (2009). Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity 31, 197–208 10.1016/j.immuni.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Oya K., Wang J., Watanabe Y., Koga R., Watanabe T. (2003). Appearance of the LAT protein at an early stage of B-cell development and its possible role. Immunology 109, 351–359 10.1046/j.1365-2567.2003.01671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Cannons J. L., Klauschen F., Schwartzberg P. L., Germain R. N. (2008). SAP-controlled T-B cell interactions underlie germinal centre formation. Nature 455, 764–769 10.1038/nature07345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau F. C., Dieter J., Luo Z., Priest S. O., Baumgarth N. (2009). B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J. Immunol. 183, 7661–7671 10.4049/jimmunol.0803783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncagalli R., Mingueneau M., Gregoire C., Malissen M., Malissen B. (2010). LAT signaling pathology: an “autoimmune” condition without T cell self-reactivity. Trends Immunol. 31, 253–259 10.1016/j.it.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Roufosse F. (2009). Hypereosinophilic syndrome variants: diagnostic and therapeutic considerations. Haematologica 94, 1188–1193 10.3324/haematol.2009.010421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P. L., Mueller K. L., Qi H., Cannons J. L. (2009). SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat. Rev. Immunol. 9, 39–46 10.1038/nri2456 [DOI] [PubMed] [Google Scholar]
- Shahinian A., Pfeffer K., Lee K. P., Kundig T. M., Kishihara K., Wakeham A., Kawai K., Ohashi P. S., Thompson C. B., Mak T. W. (1993). Differential T cell costimulatory requirements in CD28-deficient mice. Science 261, 609–612 10.1126/science.7688139 [DOI] [PubMed] [Google Scholar]
- Sommers C. L., Park C. S., Lee J., Feng C., Fuller C. L., Grinberg A., Hildebrand J. A., Lacana E., Menon R. K., Shores E. W., Samelson L. E., Love P. E. (2002). A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 296, 2040–2043 10.1126/science.1069066 [DOI] [PubMed] [Google Scholar]
- Stuber E., Strober W. (1996). The T cell-B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J. Exp. Med. 183, 979–989 10.1084/jem.183.2.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y. W., Jumaa H. (2003). LAT links the pre-BCR to calcium signaling. Immunity 19, 295–305 10.1016/S1074-7613(03)00202-4 [DOI] [PubMed] [Google Scholar]
- Suvas S., Singh V., Sahdev S., Vohra H., Agrewala J. N. (2002). Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J. Biol. Chem. 277, 7766–7775 10.1074/jbc.M105902200 [DOI] [PubMed] [Google Scholar]
- Tafuri A., Shahinian A., Bladt F., Yoshinaga S. K., Jordana M., Wakeham A., Boucher L. M., Bouchard D., Chan V. S., Duncan G., Odermatt B., Ho A., Itie A., Horan T., Whoriskey J. S., Pawson T., Penninger J. M., Ohashi P. S., Mak T. W. (2001). ICOS is essential for effective T-helper-cell responses. Nature 409, 105–109 10.1038/35051113 [DOI] [PubMed] [Google Scholar]
- Vogelzang A., Mcguire H. M., Yu D., Sprent J., Mackay C. R., King C. (2008). A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 10.1016/j.immuni.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Wang Y., Horvath O., Hamm-Baarke A., Richelme M., Gregoire C., Guinamard R., Horejsi V., Angelisova P., Spicka J., Schraven B., Malissen B., Malissen M. (2005). Single and combined deletions of the NTAL/LAB and LAT adaptors minimally affect B-cell development and function. Mol. Cell. Biol. 25, 4455–4465 10.1128/MCB.25.11.4455-4465.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kissenpfennig A., Mingueneau M., Richelme S., Perrin P., Chevrier S., Genton C., Lucas B., Disanto J. P., Acha-Orbea H., Malissen B., Malissen M. (2008). Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T Cells. J. Immunol. 180, 1565–1575 [DOI] [PubMed] [Google Scholar]
- Wardemann H., Yurasov S., Schaefer A., Young J. W., Meffre E., Nussenzweig M. C. (2003). Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 10.1126/science.1086907 [DOI] [PubMed] [Google Scholar]
- Xu J., Foy T. M., Laman J. D., Elliott E. A., Dunn J. J., Waldschmidt T. J., Elsemore J., Noelle R. J., Flavell R. A. (1994). Mice deficient for the CD40 ligand. Immunity 1, 423–431 10.1016/1074-7613(94)90073-6 [DOI] [PubMed] [Google Scholar]
- Zotos D., Coquet J. M., Zhang Y., Light A., D’costa K., Kallies A., Corcoran L. M., Godfrey D. I., Toellner K. M., Smyth M. J., Nutt S. L., Tarlinton D. M. (2010). IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 10.1084/jem.20091777 [DOI] [PMC free article] [PubMed] [Google Scholar]