Abstract
ADAM17 (a disintegrin and metalloproteinase 17) is a cell-surface metalloproteinase that regulates signaling via the epidermal growth factor receptor (EGFR) and has important roles in diseases such as cancer and rheumatoid arthritis. ADAM17 can be activated by stimulation of several tyrosine kinase receptors, raising questions about whether oncogenic tyrosine kinases could also enhance EGFR signaling and activation of ERK via stimulation of ADAM17. The main goal of this study was to evaluate the role of Src in activating ADAM17. We provide evidence that a constitutively active transforming form of Src, the E378G mutant, as well as v-Src enhance ADAM17-mediated shedding of the EGFR-ligand TGFα. Moreover, we demonstrate that constitutive shedding of TGFα can be reduced by inhibition of Src in several cell lines, including COS7, MCF7, PAE and HaCaT cells. Src(E378G)-stimulated shedding of TGFα is abolished in Adam17−/− cells, but can be rescued by wild type ADAM17 and a mutant ADAM17 lacking its cytoplasmic domain. These findings demonstrate that ADAM17 is the principal TGFα sheddase that is activated by Src in a manner that does not require the cytoplasmic domain of ADAM17. Finally, we show that stimulation of ADAM17 by Src(E378G) leads to enhanced paracrine signaling via release of EGFR-ligands into the culture supernatant. These results raise the possibility that activation of ADAM17 by oncogenic forms of Src can aid in promoting tumorigenesis by enhancing signaling via the EGFR and ERK in an autocrine and paracrine manner. Enhanced autocrine signaling could further activate tumor cells expressing oncogenic mutants of Src, whereas paracrine signaling could stimulate EGFR and ERK signaling in surrounding non-transformed cells such as stromal cells, thereby contributing to crosstalk between tumor cells and stromal cells.
Keywords: ADAM17, Src, EGF-receptor, EGF-receptor ligands, Protein ectodomain shedding, ERK
Cell-cell interactions are critical for development and for the maintenance of an adult organism, but they can also contribute to pathologies such as cancer when they become dysregulated. Membrane-anchored metalloproteinases have emerged as key regulators of cell-cell interactions, because of their ability to cleave and release growth factors, cytokines and various other membrane-proteins in a process referred to as protein ectodomain shedding (Blobel, 2005; Murphy, 2008). The membrane-anchored metalloproteinase ADAM17 (a disintegrin and metalloproteinase 17, also referred to as TNFα converting enzyme or TACE) is considered a principal sheddase for many membrane proteins, and is essential for regulating the bioavailability of ligands of the EGF-receptor such as TGFα and HB-EGF, and of soluble TNFα (Horiuchi et al., 2007a; Horiuchi et al., 2007b; Peschon et al., 1998; Sahin et al., 2004; Sunnarborg et al., 2002). Recently, the stimulation of the tyrosine kinase receptor VEGF-receptor-2 was shown to activate ADAM17 but not ADAM10 via the extracellular signal-regulated kinase (ERK) and mitogen-activated protein (MAP) kinase pathways (Swendeman et al., 2008). In addition, ADAM17 can be activated by several other cellular signaling pathways, including G-protein coupled receptors (Fischer et al., 2003; Prenzel et al., 1999) and LPS-induced septic shock (Horiuchi et al., 2007a).
The ability of various cellular signaling pathways to activate ADAM17 raises questions about the potential role of oncogenic tyrosine kinases in stimulating ADAM17, and whether this could enhance autocrine and paracrine signaling via the release of ligands of the EGFR. Previous studies have provided evidence for a role of Src family kinases in the activation of ADAM17 (Van Schaeybroeck et al., 2008; Zhang et al., 2006). In addition, Src has been reported to associate with ADAM17, and this is accompanied by phosphorylation of Src and ADAM17 and their translocation to the cell membrane (Zhang et al., 2006). Since ADAM17 has been implicated in the progression of different types of cancer (McGowan et al., 2008; Tanaka et al., 2005; Zhou et al., 2006), the goal of the current study was to determine whether transforming mutants of Src could enhance the activity of ADAM17, as this could be a pathologically relevant aspect of the mechanism underlying oncogenic transformation of cells by Src.
To evaluate the role of a transforming Src-kinase in regulating the catalytic activity of ADAM17, we tested whether the constitutively active Src mutant Src(E378G) (Levy et al., 1986) stimulated the shedding of the ADAM17 substrate TGFα in cell-based assays. We first compared how the transforming Src(E378G) or the inactive mutant Src(K295A) (Levy et al., 1986; Ma et al., 2000) affected the cell-associated levels of alkaline phosphatase-tagged form of the ADAM17 substrate TGFα(Horiuchi et al., 2007b; Sahin et al., 2004) in mouse embryonic fibroblasts (mEFs). When Src(E378G) was co-transfected with TGFα, we observed a depletion of TGFα in the cell lysates compared to cells co-expressing TGFα and the inactive Src(K295A) as a control (Fig. 1A, B, see also supplementary figure 1). The Src family kinase inhibitor PP2 (10μM, Fig. 1A, supplementary figure 1A and C) and the metalloproteinase inhibitor Marimastat (MM, 5μM Fig. 1B and supplementary figure 1B and D) prevented the depletion of TGFα-AP in cells expressing Src(E378G), suggesting that the depletion of TGFα depended both on the mutant Src and on a metalloproteinase, most likely ADAM17. When we evaluated the release of TGFα into the culture supernatant of cells that were co-transfected with Src(E378G) or v-Src, we observed an approximately two-fold increase of TGFα shedding compared to cells co-expressing the inactive Src(K295A) or MAD2, used here as an additional control (Fig. 1C). In these experiments, cells were pre-incubated with 5μM Marimastat over night to normalize the levels of TGFα at the outset of this experiment (see Fig. 1B), and then the Marimastat was washed out in order to monitor the shedding of TGFα over 4 hours following washout. These results suggest that ADAM17 was significantly more active under the influence of the transforming variants of Src (Src(E378G), or v-Src compared to the two controls (Src(K295A) and MAD2). However, Src(E378G) or v-Src did not stimulate shedding of the ADAM10 substrate betacellulin (BTC (Horiuchi et al., 2007b; Sahin et al., 2004)) compared to the inactive Src(K295A) or MAD2 (Fig. 1D), so Src(E378G) appeared to activate ADAM17, but not ADAM10. Because v-Src and the constitutively active Src(E378G) stimulated ADAM17 to a similar extent, we focused on characterizing the effects of Src(E378G) on ADAM17 for the remainder of this study. When we tested whether Src(E378G) also increased the shedding of other substrates of ADAM17 following pre-incubation and washout of Marimastat as described above, we found that it stimulated the shedding of ICAM-1, amphiregulin (AMP) and TNFα, which have all been characterized as substrates of ADAM17 (Horiuchi et al., 2007a; Sahin et al., 2004; Weskamp et al., 2010) (Fig. 1E). Taken together, these results demonstrate that two transforming mutant forms of Src, Src(E378G) and v-Src significantly stimulate ADAM17, as evidenced by the increased shedding of TGFα and other substrates of ADAM17.
Figure 1. Overexpression of an oncogenic mutant form of Src(E378G) increases the shedding of ADAM17 substrates in mouse embryonic fibroblasts.
(A, B) Immortalized wild type mouse embryonic fibroblasts (mEFs) on six-well plates were transiently transfected with an alkaline phosphatase (AP)-tagged TGFα and the inactive Src(K295A) or the constitutively active Src(E378G) (Levy et al., 1986; Ma et al., 2000; Sun et al., 2007). Six hours after transfection the cells were incubated with the Src inhibitor PP2 (10μM, Calbiochem, San Diego, CA) (A) or the metalloproteinase inhibitor Marimastat (MM, 5μM, kindly provided by Dr. O. Ouerfelli, MSKCC, New York) (B) for 8 hours. Cells were lysed on ice in TBS Triton X-100 (1%), 1 mmol/L EDTA and glycoproteins were precipitated with the lectin Con-A overnight and eluted the next day with 0.5 mM Methyl α-D Manno-Pyranoside. All reagents were from Sigma-Aldrich, St. Louis, MO, unless otherwise indicated. For the evaluation of the AP activity in the cell lysates, equal amounts of total protein were separated by 8% SDS-PAGE and then incubated with NBT/BCIP (Roche, San Francisco, CA) substrate solution for 10 min (Sahin et al., 2006). Cells expressing Src(E378G) showed strongly decreased levels of TGFα in comparison to cells transfected with Src(K295A). The expression of TGFα in cells co-transfected with Src(E378G) could be restored by incubation with PP2 (A) or Marimastat (B). (C, D) Shedding of TGFα (C) or betacellulin (BTC) (D) into the supernatant of wild type mEFs was analyzed by colorimetric assay for alkaline phosphatase activity. For photometric quantitation of the AP-activity in the culture supernatant, 100 μl of conditioned medium was mixed with 100 μl of a 2 mg/ml solution of the alkaline phosphatase substrate 4-nitrophenyl phosphate in 100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 20 mM MgCl2. After incubation at 37°C for 30 minutes, the amount of product was quantitated by determining the A405. The shedding of TGFα into the supernatant of mEFs was enhanced by co-transfection of Src(E378G) and v-Src compared to co-transfection with the inactive Src(K295A) or MAD2, used as a control. (D) The shedding of BTC, used here as a readout for the activity of ADAM10 (Sahin et al., 2004), was not affected by the co-expression of Src(E378G) or v-Src compared to Src(K295A) or MAD2. (E) When we compared shedding of the ADAM17 substrates ICAM-1 (Weskamp et al., 2010), amphiregulin (AMP) (Sahin et al., 2004) or TNFα (Black et al., 1997; Horiuchi et al., 2007a; Moss et al., 1997) in the presence of Src(K295A) or Src(E378G), we found that the transforming form of Src increased the shedding of all three ADAM17 substrates. Results were obtained from three independent experiments and expressed as means ± SEM. Pairwise comparison was performed by Mann-Whitney rank sum test, multiple comparisons versus a control group by Dunnett’s method using SigmaStat 3.1 software (SYSTAT, Erkrath, Germany). P values of <0.05 were considered statistically significant, and samples with significant changes compared to controls are marked by an asterisk.
In order to determine whether endogenous Src has a role in the constitutive activity of ADAM17, we tested whether Src-kinase inhibitors affected the release of TGFα from COS7 cells as well as other cell types. As shown in figure 2A, B and C, addition of the Src-kinase family inhibitors PP1, PP2, or Desatinib reduced shedding of the ADAM17 substrate TGFα in a dose-dependent manner. However, the constitutive shedding of the ADAM10 substrate BTC was not significantly affected by 10 μM PP1 or PP2, and only slightly decreased by Desatinib, demonstrating that the constitutive activity of ADAM10 did not depend on Src or related family members. The structurally similar but inactive PP3 did not influence the activity of ADAM17 or ADAM10 (Fig. 2D). Similar results were obtained with three different cell lines, the human MCF7 breast cancer cell line, a pig aortic endothelial cell line (PAE) and the human keratinocyte cell line HaCaT. In all three cell lines, 10 μM Desatinib blocked shedding of transfected TGFα by ~50%, whereas the inactive PP3 had no significant effect (Fig. 2E). Importantly, we found that treatment with 10 μM Desatinib did not reduce shedding of TGFα from Src−/− cells, which corroborates the selectivity of this reagent (Fig. 2E). In addition, Desatinib also blocked the release of other alkaline phosphatase-tagged ADAM17 substrates from COS7 cells, including ICAM-1, TNFα, and amphiregulin (AMP) (Fig. 2F). When we tested whether Desatinib blocks the shedding of TGFα in the presence of various stimuli, we found that it reduced VEGF-stimulated shedding of TGFα from PAE cells, but did not affect thrombin-stimulated shedding, and only weakly affected PMA-stimulated shedding of TGFα (supplementary figure 2A – C). Desatinib also had no effect on shedding of BTC following stimulation with ionomycin (supplementary figure 2D).
Figure 2. ADAM17-mediated shedding is blocked by inhibitors of Src-family kinases.
(AD) The ADAM17-dependent shedding of TGFα from COS7 cells was sensitive to the Src-family kinase inhibitor PP1 (A, Biomol, Plymouth Meeting, PA), PP2 (B, Calbiochem, San Diego, CA) or Desatinib (C, kindly provided by Mark Moasser UCSF Medical Center, San Francisco, CA), but not to the inactive PP3 (D, Calbiochem, San Diego, CA) at concentrations between 1 and 10 μM, as indicated, whereas shedding of the ADAM10 substrate BTC was not affected by 10 μM PP1, 2, or 3, and only weakly reduced by Desatinib at 10 μM (A–D). (E) Treatment of other cell lines with 10 μM Desatinib also reduced shedding of transfected TGFα, whereas treatment with the inactive PP3 did not (MCF7 breast cancer cells, ATCC, PAE-KDR porcine aortic endothelial cells expressing the human VEGFR2/KDR, referred to as PAE cells throughout, provided by Dr. S. Rafii, Weill Cornell Univ., New York, and the HaCaT human keratinocyte cell line, kindly provided by Dr. T. Ludwig, Columbia University, New York). Similar experiments with Src−/− cells showed no effect of Desatinib in the absence of Src. (F) Shedding of the AP-tagged ADAM17 substrates ICAM-1, AMP, and TNFα from COS7 cells could also be reduced by Desatinib. All results were obtained from three independent experiments and expressed as means ± SEM. Pairwise comparisons were performed by Mann-Whitney rank sum test, multiple comparisons versus a control group by Dunnett’s method, all pairwise multiple comparison procedures by Tukey Test using SigmaStat 3.1 software. P values of <0.05 were classified as statistically significant, and corresponding samples are marked by an asterisk.
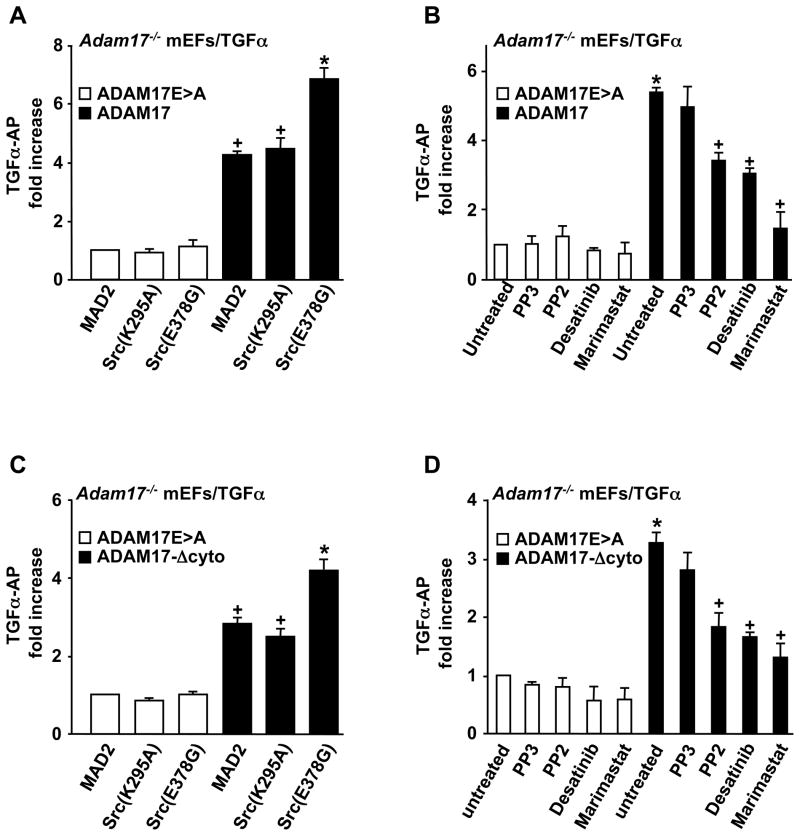
To corroborate that ADAM17 is critical for the Src-stimulated shedding of TGFα, we performed rescue experiments in Adam17−/− mEFs (Horiuchi et al., 2007a). The low amount of shedding of TGFα from Adam17−/− cells transfected with the catalytically inactive ADAM17E>A mutant and MAD2, Src(K295A) or Src(E378G) was significantly increased when these cells were rescued by co-transfection with wild type (wt) ADAM17. More importantly, the constitutively active Src(E378G) further increased TGFα shedding in cells rescued with wt ADAM17 compared to MAD2 or Src(K295A) (Fig. 3A). Furthermore, we found that the increased constitutive shedding of TGFα from cells expressing ADAM17 was sensitive to treatment with the Src-family inhibitors PP2 and Desatinib as well as the hydroxamate Marimastat, whereas none of these compounds significantly affected the low levels of background shedding of TGFα from Adam17−/− cells expressing the inactive ADAM17E>A mutant (Fig. 3B).
Figure 3. Activation of TGFα shedding by Src(E378G) is mediated by ADAM17 and does not require the cytoplasmic domain of ADAM17.
(A) Shedding of TGFα from Adam17−/− cells co-expressing wild type ADAM17 or the inactive ADAM17E>A mutant (Le Gall et al., 2009), and Src(K295A) or Src(E378G). The increased shedding of TGFα in the presence of Src(E378G) depended on ADAM17. (B) Adam17−/− cells transfected with TGFα and either wild type ADAM17 or the inactive ADAME>A were treated with 10 μM of PP3, PP2 or Desatinib or 5μM Marimastat. All inhibitors were pre-incubated for 10 minutes, and AP-activity in the supernatants (conditioned for 2 hours) and cell lysates was measured by colorimetry (Sahin et al., 2006). The effect of PP2, Desatinib and Marimastat depended on the presence of ADAM17. (C) Shedding of TGFα from Adam17−/− cells co-expressing inactive ADAM17E>A or the cytoplasmic deletion mutant of ADAM17 (ADAM17-Δcyto) (Le Gall et al., 2009), and Src(K295A) or Src(E378G). The activation of ADAM17 by Src(E378G) did not require the cytoplasmic domain of ADAM17. (D) Adam17−/− cells rescued with either wild type ADAM17 or ADAM17-Δcyto were treated with or without 10 μM of PP3 or PP2, Desatinib or 5 μM Marimastat. The effect of these inhibitors on TGFα shedding also did not depend on the presence of the cytoplasmic domain of ADAM17. Results were from three independent experiments and are expressed as means ± SEM. All pairwise multiple comparison procedures were performed by Tukey Test using SigmaStat 3.1 software. (A,C) + indicates a P-value of <0.05 with respect to the effect of ADAM17 or ADAM17-Δcyto on the shedding of TGFα compared to ADAM17E>A. * indicates a P-value <0.05 for the effect of Src(E378G) compared to Src(K295A) on the shedding of TGFα by overexpressed ADAM17. (B,D) * indicates a P-value of <0.05 for the effect of ADAM17 or ADAM17-Δcyto on the shedding of TGFα relative to ADAM17E>A, and + indicates a P-value of <0.05 for the effect of Src kinase inhibitors PP2 or Desatinib or the metalloprotease inhibitor Marimastat on the shedding of TGFα compared to untreated cells.
Previous studies have implicated tyrosine phosphorylation of the cytoplasmic domain of ADAM17 in its response to activation by gastrin-dependent peptide and Src (Zhang et al., 2006). Here we found that a mutant form of ADAM17 with an intact transmembrane domain but lacking a cytoplasmic domain (ADAM17-Δcyto (Le Gall et al., 2009)) could rescue the shedding of TGFα from Adam17−/− cells as well as wt ADAM17, and that its activity could be further enhanced by Src(E378G) (Fig. 3C). In addition, the increased shedding of TGFα from Adam17−/− cells rescued with ADAM17-Δcyto could be inhibited with PP2, Desatinib and Marimastat to a similar degree as in Adam17−/− cells rescued with wt ADAM17 when compared to untreated cells or to cells treated with the inactive PP3 (Fig. 3D). These results demonstrate that the Src-stimulated TGFα shedding is independent of phosphorylation of the ADAM17 cytoplasmic tail, which is consistent with previous reports that the activation of ADAM17 by phorbol 12-myristate 13-acetate also does not depend on the presence of its cytoplasmic domain (Horiuchi et al., 2007b; Reddy et al., 2000).
To corroborate that ADAM17 is also responsible for Src-dependent shedding in the other cell lines tested here, we assessed the ability of the ADAM17-selective hydroxamate inhibitors SP26 (Mazzola et al., 2008) and DPC333 (Qian et al., 2007) to block shedding of TGFα from MCF7, HaCaT, PAE and Src−/− cells at concentrations where these inhibitors block ADAM17 with little or no effect on ADAM10 (2.5 μM SP26, 0.25 μM DPC333, see supplementary figure 3A – D for titration curves of SP26 and DPC333 in cell-based assays in COS7 cells). At these concentrations, both inhibitors blocked the shedding of TGFα in all four cell lines, whereas they did not affect shedding of the ADAM10 substrate BTC, corroborating that shedding of TGFα also depends on ADAM17 in these cell lines (supplementary figure 3G – N). As an additional control, we demonstrated that the ADAM10 selective inhibitor GI254023X (Hundhausen et al., 2003) did not significantly affect the stimulation of TGFα shedding by Src(E378G), even though it strongly reduced shedding of BTC in all cell lines tested here at a concentration of 1 μM (the titration curve for GI254023X in cell-based assays is shown in supplementary figure 3E, F). Moreover, treatment of the two human cell lines HaCaT and MCF7 with siRNA against human ADAM17 strongly reduced the expression of ADAM17 and the shedding of TGFα (supplementary figure 4A, B). Finally, in order to rule out a contribution of other ADAMs to Src(E378G)-stimulated shedding of TGFα in mEFs, we tested how lack of ADAM8, ADAM9/12/15, ADAM10, ADAM17 or ADAM19 affected the activation of TGFα shedding by Src(E378G). We found no difference in the Src(E378G)–dependent stimulation of TGFα shedding in Adam8−/−, Adam9/12/15−/−, Adam10−/− or Adam19−/− mEF cells compared to wild type controls (supplementary figure 4C), whereas constitutive shedding of TGFα was strongly reduced in Adam17−/− cells, with no further stimulation by co-transfection with Src(E378G). Taken together, these results corroborate that Src kinases play an important role in mediating the constitutive release of substrates of ADAM17, but not of ADAM10 substrates. In addition, the use of ADAM17 selective inhibitors and siRNA as well as mEF from mice lacking other widely expressed and catalytically active ADAMs support the interpretation that ADAM17 is the main sheddase that responds to activation by endogenous Src and the transforming mutant Src(E378G).
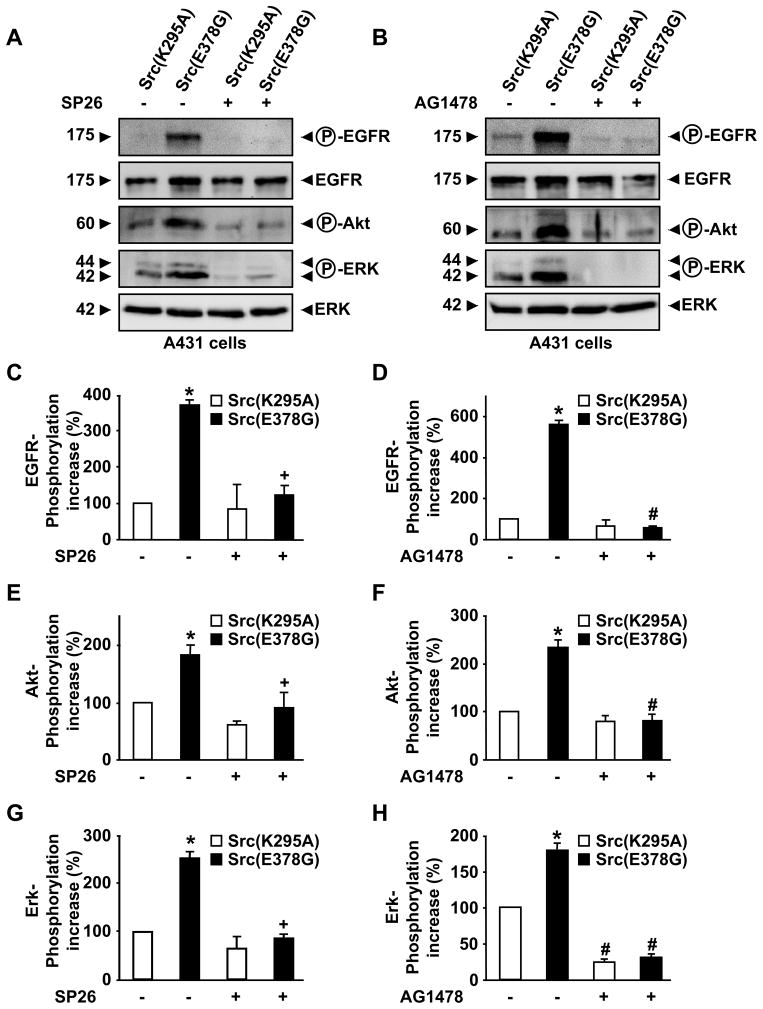
ADAM17 is essential for activating the EGFR during mouse development (Blobel, 2005; Jackson et al., 2003; Peschon et al., 1998; Sternlicht et al., 2005), and the release of EGFR ligands by ADAM17 is known to promote tumor progression (Blanchot-Jossic et al., 2005; Merchant et al., 2008; Nakagawa et al., 2009; Zhou et al., 2006). In order to determine whether Src(E378G) could affect paracrine EGFR/ERK activation via stimulation of ADAM17, we collected supernatants from COS7 cells transfected with Src(E378G) or the inactive Src(K295A), and then added these conditioned supernatants to serum-starved A431 human carcinoma cells, which expresses high levels of the EGFR/ErbB1 (Cooper et al., 1983). A Western blot analysis revealed increased phosphorylation of the EGFR as well as Akt and ERK1/2 in extracts of A431 cells treated with conditioned medium from COS7 cells overexpressing Src(E378G) compared to supernatants from COS7 cells transfected with Src(K295A) (Fig. 4A). The increased phosphorylation of ERK1/2 by supernatants from COS7 cells expressing Src(E378G) could be blocked if the supernatants were conditioned in the presence of 5 μM Marimastat (data not shown) or the ADAM17-selective inhibitor SP26 (Fig. 4A). In addition, the phosphorylation of the EGFR, Akt and ERK in A431 cells in response to adding conditioned media from Src(E378G)-expressing COS7 cells could be completely prevented by the EGFR-selective tyrosine kinase inhibitor AG1478 (Fig. 4B). These results are consistent with a model in which Src(E378G) stimulates ADAM17 to release ligands of the EGFR from COS7 cells that are then able to activate the EGFR in A431 cells in a paracrine manner. Over-expression of EGFR-ligands and inappropriate activation of the EGFR are known to contribute to tumorigenesis (Yarden & Sliwkowski, 2001; Zhou et al., 2006), and the increased stimulation of ADAM17 and release of EGFR-ligands by an activated form of Src is likely to also cause activation of ERK signaling in cells that do not express the mutant Src oncogene. Thus ADAM17 could function to exacerbate Src-dependent transformation and tumorigenesis by producing soluble EGFR-ligands that enhance paracrine EGFR signaling on adjacent non-transformed cells. The increased stimulation of these cells by EGFR-ligands could aid in their transformation, and could be an important component of the interactions between tumor cells and stromal cells, which are known to make critical contributions to tumorigenesis (Egeblad et al., 2005; Page-McCaw et al., 2007).
Figure 4. Supernatants from COS7 cells expressing the transforming mutant Src(E378G) can activate the EGFR and ERK in A431 cells.
(A–B) COS7 cells on 10cm plates were transiently transfected with either Src(K295A) or Src(E378G) and incubated in the presence or absence of 2.5 μM of the ADAM17-selective hydroxamate inhibitor SP26 (A) for 4 hours in Optimem medium. 5ml of these conditioned supernatants were removed and incubated for 10 minutes with A431 cells, a cell line that expresses high levels of the EGFR/ErbB1 (Cooper et al., 1983). The A431 cells had been serum-starved over night before the addition of the conditioned supernatants from COS7 cells. For the experiments with the EGFR tyrosine kinase inhibitor AG1478 shown in panel B, 0.5 μM AG1478 were pre-incubated with A431 cells for 10 minutes in Optimem before adding the conditioned media from COS7 cells, which was adjusted to 0.5 μM AG1478 as indicated. The A431 cells were then lysed on ice in TBS Triton X-100 (1%), 1 mmol/L EDTA and protease inhibitor cocktail (Roche, San Francisco, CA). Identical amounts of protein were separated on 10% SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (BioTrace, Pall Corporation, Ann Arbor, MI). Membranes were blocked in 5% skim milk in TBS, incubated with Rabbit anti-phospho EGFR or anti-total EGFR, anti-phospho-Akt, Rabbit anti-phosphoERK1/2 antibodies (Cell Signaling Technology, Danvers, MA) or anti-total-ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA) and then with peroxidase-conjugated goat anti-rabbit secondary antibody (Promega, Madison, WI), and and bound antibodies were visualized using the enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ) and a Chemdoc image analyzer (Bio-Rad, Hercules, CA). One representative out of three immunoblots with essentially identical results is shown in A and B. Supernatants from COS7 cells expressing Src(E378G) activated ERK1/2 more strongly than supernatants from COS7 cells expressing Src(K295A), and this activation depended on a metalloproteinase, most likely ADAM17, in COS7 cells, and on activation of the EGFR and Akt in A431 cells. (C – H) The results of densitometric quantification of Western blots from three separate experiments + SEM show how treatment with 2.5 μM SP26 (C, E, G) or 0.5 μM AG1478 (D, F, H) affected the phosphorylation of the EGFR (C, D), Akt (E, F), or ERK1/2 (G, H). All pairwise multiple comparison procedures were performed by Tukey Test using SigmaStat 3.1 software. * indicates a P-value of <0.05 for the effect of Src(E378G) on the phosphorylation of the EGFR, Akt or ERK1/2 compared to the effect of Src(K295A). + indicates a P-value of <0.05 for the effect of SP26 on the phosphorylation of ERK1/2 by overexpressed Src(E378G). # indicates a P-value of <0.05 for the effect of AG1478 on phosphorylation of the EGFR, Akt or ERK1/2 by overexpressed SRC(K295A) or Src(E378G) respectively.
In summary, this study provides the first evidence, to our knowledge, that a transforming mutant form of Src can stimulate the catalytic activity of ADAM17. Our findings suggest that activated forms of Src might stimulate the EGFR and ERK in the Src-expressing tumor cells as well as in surrounding non-transformed stromal cells by activation of ADAM17 and release of ligands of the EGFR. ADAM17 has been implicated in proliferation and invasion of tumor cells (McGowan et al., 2008; Tanaka et al., 2005; Zhou et al., 2006), so its dysregulation by oncogenic mutant Src could play an important role during cancer development. Furthermore these results suggest that inhibitors of ADAM17 might be useful for treating cancers in which the function of ADAM17 is dysregulated by activating mutations in Src.
Supplementary Material
Acknowledgments
This study was supported by NIH R01 GM64750, and TM was supported by the Emerald Foundation, W.Z. was supported by the Tri-Institutional MD/PhD Program Gateway Program. We are grateful to Drs. Daniel Lundell and Xioada Niu from the Schering Plough Research Institute in Kenilworth, NJ, for providing SP26, and to Dr. Robert Waltermire from Bristol-Myers Squibb for DPC333, and to Andreas Ludwig at the University of Aachen, Germany, for providing GI254023X.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Black R, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloprotease disintegrin that releases tumour-necrosis factor-a from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Blanchot-Jossic F, Jarry A, Masson D, Bach-Ngohou K, Paineau J, Denis MG, Laboisse CL, Mosnier JF. Up-regulated expression of ADAM17 in human colon carcinoma: co-expression with EGFR in neoplastic and endothelial cells. J Pathol. 2005;207:156–63. doi: 10.1002/path.1814. [DOI] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key players in EGFR-signaling, development and disease. Nat Rev Mol Cell Bio. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Scolnick EM, Ozanne B, Hunter T. Epidermal growth factor receptor metabolism and protein kinase activity in human A431 cells infected with Snyder-Theilen feline sarcoma virus or harvey or Kirsten murine sarcoma virus. J Virol. 1983;48:752–64. doi: 10.1128/jvi.48.3.752-764.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Littlepage LE, Werb Z. The fibroblastic coconspirator in cancer progression. Cold Spring Harb Symp Quant Biol. 2005;70:383–8. doi: 10.1101/sqb.2005.70.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31:1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP. Cutting Edge: TNF-{alpha}-Converting Enzyme (TACE/ADAM17) Inactivation in Mouse Myeloid Cells Prevents Lethality from Endotoxin Shock. J Immunol. 2007a;179:2686–9. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Le Gall S, Schulte M, Yamaguchi T, Reiss K, Murphy G, Toyama Y, Hartmann D, Saftig P, Blobel C. Substrate Selectivity of EGF-Receptor Ligand Sheddases and Their Regulation by Phorbol Esters and Calcium Influx. Mol Biol Cell. 2007b;18:176–188. doi: 10.1091/mbc.E06-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. Embo J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall S, Bobe P, Reiss K, Horiuchi K, Niu X-D, Lundell D, Gibb D, Conrad D, Saftig P, Blobel C. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as TGFα, L-Selectin and TNFα. Mol Biol Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JB, Iba H, Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986;83:4228–32. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–46. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Mazzola RD, Jr, Zhu Z, Sinning L, McKittrick B, Lavey B, Spitler J, Kozlowski J, Neng-Yang S, Zhou G, Guo Z, Orth P, Madison V, Sun J, Lundell D, Niu X. Discovery of novel hydroxamates as highly potent tumor necrosis factor-alpha converting enzyme inhibitors. Part II:Optimization of the S3′ pocket. Bioorg Med Chem Lett. 2008 doi: 10.1016/j.bmcl.2008.09.045. [DOI] [PubMed] [Google Scholar]
- McGowan PM, McKiernan E, Bolster F, Ryan BM, Hill AD, McDermott EW, Evoy D, O’Higgins N, Crown J, Duffy MJ. ADAM-17 predicts adverse outcome in patients with breast cancer. Ann Oncol. 2008;19:1075–81. doi: 10.1093/annonc/mdm609. [DOI] [PubMed] [Google Scholar]
- Merchant NB, Voskresensky I, Rogers CM, Lafleur B, Dempsey PJ, Graves-Deal R, Revetta F, Foutch AC, Rothenberg ML, Washington MK, Coffey RJ. TACE/ADAM-17: a component of the epidermal growth factor receptor axis and a promising therapeutic target in colorectal cancer. Clin Cancer Res. 2008;14:1182–91. doi: 10.1158/1078-0432.CCR-07-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss ML, Jin S-LC, Milla ME, Burkhart W, Cartner HL, Chen W-J, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Lessnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su J-L, Warner J, Willard D, Becherer JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-recrosis factor-a. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;12:929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Nabeshima K, Asano S, Hamasaki M, Uesugi N, Tani H, Yamashita Y, Iwasaki H. Up-regulated expression of ADAM17 in gastrointestinal stromal tumors: coexpression with EGFR and EGFR ligands. Cancer Sci. 2009;100:654–62. doi: 10.1111/j.1349-7006.2009.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russel WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Qian M, Bai SA, Brogdon B, Wu JT, Liu RQ, Covington MB, Vaddi K, Newton RC, Fossler MJ, Garner CE, Deng Y, Maduskuie T, Trzaskos J, Duan JJ, Decicco CP, Christ DD. Pharmacokinetics and pharmacodynamics of DPC 333 ((2R)-2-((3R)-3-amino-3{4-[2-methyl-4-quinolinyl) methoxy] phenyl}-2-oxopyrrolidinyl)-N-hydroxy-4-methylpentanamide)), a potent and selective inhibitor of tumor necrosis factor alpha-converting enzyme in rodents, dogs, chimpanzees, and humans. Drug Metab Dispos. 2007;35:1916–25. doi: 10.1124/dmd.107.015933. [DOI] [PubMed] [Google Scholar]
- Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem. 2000;275:14608–14614. doi: 10.1074/jbc.275.19.14608. [DOI] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Zheng Y, Chesneau V, Horiuchi K, Blobel CP. Epidermal Growth Factor: Methods and Protocols. In: Patel TB, Bertics Pj, editors. Methods in Molecular Biology. Vol. 327. Humana Press Inc; Totowa, NJ: 2006. pp. 99–113. [DOI] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Zhou HM, Higashiyama S, Peschon JJ, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR-ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–33. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Huang J, Xiang Y, Bastepe M, Juppner H, Kobilka BK, Zhang JJ, Huang XY. Dosage-dependent switch from G protein-coupled to G protein-independent signaling by a GPCR. Embo J. 2007;26:53–64. doi: 10.1038/sj.emboj.7601502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Lee DC. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem. 2002;277:12838–12845. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- Swendeman S, Mendelson K, Weskamp G, Horiuchi K, Deutsch U, Scherle P, Hooper A, Rafii S, Blobel CP. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ Res. 2008;103:916–8. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Miyamoto S, Suzuki SO, Oki E, Yagi H, Sonoda K, Yamazaki A, Mizushima H, Maehara Y, Mekada E, Nakano H. Clinical significance of heparin-binding epidermal growth factor-like growth factor and a disintegrin and metalloprotease 17 expression in human ovarian cancer. Clin Cancer Res. 2005;11:4783–92. doi: 10.1158/1078-0432.CCR-04-1426. [DOI] [PubMed] [Google Scholar]
- Van Schaeybroeck S, Kelly DM, Kyula J, Stokesberry S, Fennell DA, Johnston PG, Longley DB. Src and ADAM-17-mediated shedding of transforming growth factor-alpha is a mechanism of acute resistance to TRAIL. Cancer Res. 2008;68:8312–21. doi: 10.1158/0008-5472.CAN-07-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K, Blobel CP. Pathological Neovascularization Is Reduced by Inactivation of ADAM17 in Endothelial Cells but Not in Pericytes. Circ Res. 2010;106:932–940. doi: 10.1161/CIRCRESAHA.109.207415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Thomas SM, Lui VW, Xi S, Siegfried JM, Fan H, Smithgall TE, Mills GB, Grandis JR. Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proc Natl Acad Sci U S A. 2006;103:6901–6. doi: 10.1073/pnas.0509719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Peyton M, He B, Liu C, Girard L, Caudler E, Lo Y, Baribaud F, Mikami I, Reguart N, Yang G, Li Y, Yao W, Vaddi K, Gazdar AF, Friedman SM, Jablons DM, Newton RC, Fridman JS, Minna JD, Scherle PA. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.