Abstract
Leuprolide is a gonadotropin-releasing hormone (GnRH) analog which has been shown to reduce symptoms in patients with irritable bowel syndrome (IBS) and chronic intestinal pseudo-obstruction (CIPO). The mechanism is not known, but one hypothesis is through down-modulation of luteinizing hormone (LH) secretion, a hormone whith antagonistic effect on gastrointestinal motility. However, presence of LH receptors in the gastrointestinal tract has never been described. The aim of this study was to find one possible way of action for leuprolide by examining the presence of the LH receptor, and if present, to see whether there was different expression in patients with or without dysmotility. Full-thickness biopsies from the bowel wall of patients with and without severe dysmotility were examined using immunohistochemistry staining. Biopsies showed expression of LH receptors on myenteric neurons and in glial cells, neutrophils, endothelial cells and mast cells. There was no difference in expression between patient groups.
Keywords: luteinizing hormone (LH), gastrointestinal dysmotility, enteric neurons
Introduction
Chronic intestinal pseudo-obstruction (CIPO) is a disorder affecting gastrointestinal motor activity, producing symptoms and signs resembling those of mechanical obstruction.1–3 Enteric dysmotility (ED) encompasses patients with abnormal intestinal motor activity but no signs of obstruction.4 The etiologies of these two disorders are largely unknown.5 Leuprolide, a gonadotropin-releasing hormone (GnRH) analog, have been used in the treatment of these diseases as well as in patients with irritable bowel syndrome (IBS).6–8 The mechanism of action behind the reduction of symptoms is not known. One hypothesis is that leuprolide continuously stimulates the hypothalamic- pituitary-gonadal axis and thereby down-modulates the secretion of gonadotropines and gonadal products.9,10 These are in turn known to be neural antagonists of gastrointestinal motility11–13 and absence of these hormones could explain the improvements seen. The effect on the gastrointestinal tract demands receptors for the substance, and receptors for progesterone and estrogen have been described in the gastrointestinal tract.14,15 The luteinizing hormone (LH) receptor was first described in gonadal tissues. During the last years it has also been described in several non-gonadal tissues and on cancer cells, but its expression in the gastrointestinal tract has never been examined,16 although LH has been shown to affect intestinal motility in rat.11
To establish a possible way of action for the reported effect of GnRH analogs and LH on gastrointestinal symptoms and motility, the aim of the present study was to examine the presence of LH receptors in the gastrointestinal tract, and if present, to compare the expression in patients with and without severe gastrointestinal dysmotility.
Material and Methods
This study was performed according to the Helsinki declaration and approved by the Ethics Committee of Lund University. All patients gave their informed consent before entering the study.
Subjects
The criterion for inclusion in the dysmotility group was the diagnosis of either CIPO or ED, together with a gastrointestinal specimen available for staining of LH receptors.
Consecutive patients subjected to laparoscopic full-thickness biopsy at the Departments of Surgery or Gastroenterology, Skåne University Hospital, Malmö, between 1998 and 2009 because of severe gastrointestinal pain and dysmotility were identified and included in a retrospective manner. Thorough investigation comprising radiological and/or endoscopic investigation to rule out organic disease or mechanical obstruction had been performed. Gastrointestinal examination was completed with esophageal manometry, gastric emptying scintigraphy, antroduodenojejunal manometry and/or colonic transit time when appropriate. Laparoscopy was performed for diagnostic purposes to exclude mechanical obstruction and to obtain a full-thickness biopsy. A previously described laparoscopy-assisted technique for taking full-thickness biopsies, preparing the biopsies and protocol for CIPO analysis was used.17 In addition, patients with severe dysmotility who underwent intestinal resection (including small or large bowel, or both) within the same time-frame were included, but were not considered for laparoscopic biopsy because full-thickness intestinal wall tissue was already available for analysis.
In order to set a CIPO diagnosis, patients had to fulfill 3 criteria: a medical history compatible with pseudo-obstruction, documented events or chronic signs mimicking mechanical obstruction (bowel dilatation and/or air/fluid levels) and absence of mechanical obstruction or other organic cause for these symptoms and findings.1–3 The criteria for ED were documented abnormal contractile activity, but no past history of episodes, or current signs, mimicking mechanical obstruction and absence of any medication that could lead to the observed motor abnormalities.2,4 These patients represent the majority of cases of suspected CIPO/ED in the most southern part of Sweden. Thirty-five patients fulfilled the inclusion criteria. Apart from the histochemical staining for CIPO analysis,17 representative sections were also available for staining of the LH receptor in 15 patients. The median age of the 15 patients (13 women) was 44 (range 18–96) years at the time of investigation. Ten patients were diagnosed with ED and 5 patients with CIPO. Seven of the patients had been treated by opioids, and 3 had some sort of nutritional supplements. Histopathological analysis revealed that inflammatory neuropathy was most common in our cohort, either as an independent disease or in combination with myopathy (9/15 patients, 60%), whereas degenerative neuropathy or combined myoneuropathy occurred in 40% of the patients (6/15). Twelve small bowel specimen and 7 large bowel specimen were available for LH receptor staining with presence of ganglia, reflecting material from resections with both small and large bowel specimens present in 4 patients. For further patient characteristics, see Table 1.
Table 1.
Characteristics of the patients in the dysmotility group.
| Patient characteristics | Number of patients | Number of patients with available information |
|---|---|---|
| Age, years | ||
| 44 (range 18–96) | 15 | 15 |
| Concurrent diseases* | 5 | 15 |
| Epilepsia | 1 | |
| Cardiovascular diseases | 1 | |
| Borderline psychosis | 1 | |
| Diabetes mellitus | 1 | |
| Ehler Danlos syndrome | 1 | |
| Gynecological problems** | 8 | 13 |
| Endometriosis | 2 | |
| Extra uterine pregnancy | 2 | |
| Salpingo oophorectomized | 2 | |
| Conization | 1 | |
| In vitro fertilization | 2 | |
| Prior surgery** | 10 | 15 |
| Gynecological surgery | 8 | |
| Abdominal surgery | 8 | |
| Multiple prior surgery | 8 | |
| Employment | ||
| Able to work full time | 3 | 11 |
| Partly employed | 1 | 11 |
| Unable to work | 4 | 11 |
| Retired | 2 | 11 |
| Deceased | 1 | 11 |
Notes:
Excluding gynecological diseases;
some patients have more than one gynecological problem or prior surgery.
As controls for LH-receptor +/− neurons in small bowel, sections from 6 cases of small bowel resection due to non-obliterating adenocarcinoma of the jejunum and ileum, and 2 cases of colonic carcinoma were used, median age 69 (range 53–85) years. Three were women. Regarding large bowel, the control group was 8 cases (5 women) with bowel resection due to diverticulosis, median age 74 (range 46–87) years. The samples were taken from areas with normal macro- and microscopic appearance 10 cm above the tumor in the small bowel and from diverticulum-free normal parts of the large bowel specimen. All controls were found to have otherwise normal histology.
Full-thickness biopsy of the bowel
Full-thickness slices perpendicular to each other were cut from the specimen and embedded in paraffin for conventional, transversal sections. The remaining, larger part of the biopsy was embedded in toto for tangential sectioning. Serial sections from all blocks were stained according to a protocol for CIPO analysis with both classical staining (haematoxylin & eosin, PAS, PS-diastase, Giemsa, kresylviolet, trichrome) and immunostaining.17 Apart from the histochemical staining for CIPO analysis, sections were also stained for the LH receptor. The polyclonal rabbit anti-LH receptor antibody (anti-LH/CG receptor; Sigma- Aldrich, Stockholm, Sweden) was applied to sections at 1:100 dilutions. As negative controls the antibodies were replaced by serum.
The length of the biopsy was measured and the number of the LH receptor +/− neurons per mm length of intestinal muscle in the transversal sectioning was counted and expressed as percentage of neurons both in the dysmotility group and controls. Using protein G product (PGP) 9.5 staining, the percentages of labeled cells from the initial countings were verified. We found a strong correlation between the two methods, with a Spearman correlation coefficient of 0.873, P = 0.000001 for the LH receptor in the small bowel. For the large bowel, the coefficient was 0.965 and P value was P = 0.000000001, for LH receptor content.
Statistical methods
Values are expressed as median and interquartile range (IQR) and range. Correlations were made by Spearman’s correlation test. The Mann Whitney U-test was used to compare differences between groups, and P < 0.05 was considered statistical significant.
Results
The median length of counted biopsies for the small bowel was 16.00 (IQR 11.43–24.85, range 4.80–65.00) mm in the dysmotility group and 13.50 (IQR 10.25–16.75, range 10.00–35.00) mm in controls. In the large bowel, the median lenght was 30.00 (IQR 23.00–41.00, range 9.00–60.40) mm in the dysmotility group and 17.5 (IQR 11.00–19.00, range 10.00–23.00) mm in controls. The number of myenteric neurons per mm in the small bowel was 6.45 (IQR 5.25–7.38, range 2.40–21.00) in the dysmotility group compared to 8.21 (IQR 5.72–9.19, range 5.34–15.23) in the control group (P = 0.43). Number of neurons per mm in the large bowel was 5.50 (IQR 4.70–5.90, range 4.30–7.70) in the dysmotility group compared to 8.76 (IQR 5.77–9.08, range 5.00–10.71) in the control group (P = 0.04).
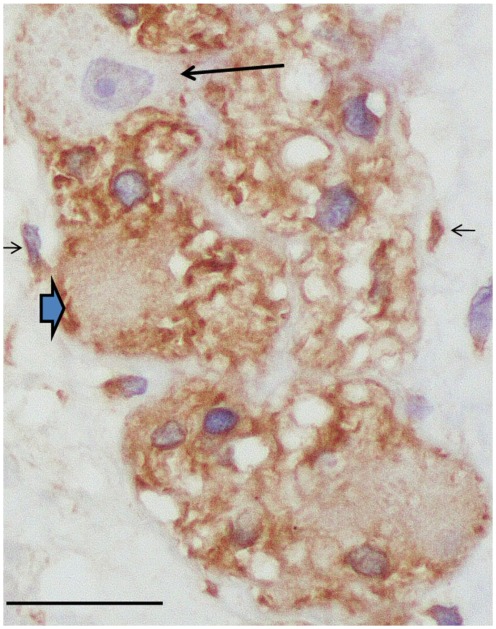
All specimens in both the dysmotility group and the control group had positive LH receptors. The LH receptor was positive in cytoplasm of approximately 50% of myenteric neurons and in glial cells, neutrophils, endothelial cells and mast cells for both the dysmotility group and the controls (Fig. 1). A group of submucosal neurons were labeled for LH receptors, but they were not counted as these neurons are not affected in patients suffering from dysmotility. All other cell types of the bowel wall were negative. No immunostaining was seen in the negative control sections. The percentage of labeled neurons in the dysmotility group was 42.50 (IQR 38.25–48.00, range 26.00–60.01)% in the small bowel and 50.00 (IQR 23.00–51.00, range 12.00–59.00)% in the large bowel. In controls, the median value was 47.14 (IQR 42.69–49.49, range 31.69–52.99)% and 43.40 (IQR 42.14–46.48, range 32.53–47.44)% in the small and large bowel, respectively, which was not significant different between the groups (P = 0.25 and 0.68, respectively).
Figure 1.
Control myenteric ganglion with luteinizing hormone (LH) receptor positive neurons (thick, blue arrow) and one negative neuron (arrow). Glial cells within the ganglion and interstitial cells of Cajal (thin arrows) are immunoreactive (LH receptor immunohistochemistry; bar: 20 μm).
Discussion
This is to the best of our knowledge the first time LH receptor expression in the human gastrointestinal tract has been described. The results suggest no difference in the receptor expression between patients with and without gastrointestinal dysmotility, or between genders. The dysmotility group had significantly lower amount of enteric neurons per mm examined large bowel.
The physiological effect of LH on gastrointestinal tract has previously only been rudimentary examined and has to be further investigated. So far, its antagonistic effect on gastrointestinal motility in rat has been described,11 which theoretically could be explained by the presence of LH receptors on myenteric neurons. Further, a protective and antioxidant effect by LH in rat gastric tissue has been described, but the importance of this is unclear.14
The presence of the receptor on endothelial cells, neutrophils, mast cells and glial cells suggests circulatory, immunological and neuroprotective effects as well. Luteinizing hormone has been described to exert a wide range of effects on other tissues in different species. The LH receptor is a member of a subfamily of G protein coupled receptors (GPCRs). It is well described for these receptors, that they are downregulated by continuous stimulation and up-regulated after intermittent stimulation.18 In a rat model, presence of LH receptors located in the vascular smooth muscle and endothelial cells of uterine blood vessels are hypothesized to be responsible for the reduced uterine blood flow after human chorionic gonadotropin (hCG)/LH stimulation.19 Exogenous administration of LH increased the number of degranulated mast cells in the ovarian complex of mice.20 This is interesting as mast cells have been discussed in the pathophysiology of IBS and visceral hypersensitivity,21 and are expressed in an increased number in patients with severe constipation.22 Glial cells which are important to support neuronal elements, have been shown to be closely related to degranulated mast cells, and are expessed in reduced number in patients with constipation.22,23 Fasting and refeeding affects the LH secretion in monkeys through cholecystokinin (CCK) stimulation.24 As mutations of the LH receptor have been described in Leydig cell hypoplasia,25 mutations could theoretically also affect gastrointestinal function.
The GnRH analog leuprolide has been shown to reduce symptoms in disorders such as functional bowel diseases.7,8 Further, leuprolide has been reported to restore motor function in the gastrointestinal tract in female ovariectomized rats and in a patient suffering from CIPO.6,26 The mechanism is unclear, but one hypothesis is that leuprolide by continuous stimulation, and thereby desensitization of the pituitary GnRH receptor, down-modulates the secretion of gonadotropines and gonadal products,9,10 which are known neural antagonists of gastrointestinal motility.11–13 Thus, continuous stimulation by leuprolide exerts antagonistic GnRH effects, leading to absence of LH secretion and stimulation.9,10 The present finding of LH receptors on enteric neurons gives a morphological explanation to this hypothesis, and absence of LH stimulation on the neural receptors could explain the reduction of symptoms.11
At the same time as continuous stimulation of leuprolide acetate improves patients with gastrointestinal complaints,7,8 repeated intermittent treatment with the analog buserelin in the setting of repeated in vitro fertilization (IVF) has been linked to the development of CIPO in one patient.17 Gonadotropin- releasing hormone and its receptor have been found on enteric neurons,17,27 why the effect evoked by GnRH analogs may be direct on enteric neurons as well, in addition to an effect on the hypothalamic-pituitary-gonadal axis.
The present study demonstrates the presence of LH receptors in the gastrointestinal tract, making it theoretically possible for this hormone to have a direct effect on this organ, and a possibility for GnRH analogs to affect the gasrointestinal tract. The role for regulation of expression and function of the LH receptor in the gastrointestinal tract has to be further investigated, as well as its possible involvement in the development of gastrointestinal disorders.
Acknowledgments
Maria Nilsson and Annika Jönsson are acknowledged for excellent technical support in performing the immunostaining.
Footnotes
Author Contributions
Conceived and designed the experiments: OH, BV, AM, BO. Analysed the data: OH, BV. Wrote the first draft of the manuscript: OH, BO. Contributed to the writing of the manuscript: BV, AM. Made critical revisions: OH, AM, BO. All authors approved final version.
Funding
Crafoord Foundation, Bengt Ihre Foundation and Development Foundation of Region Skane.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.De Giorgio R, Sarnelli G, Corinaldesi R, Stanghellini V. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut. 2004;53:1549–52. doi: 10.1136/gut.2004.043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanghellini V, Camilleri M, Malagelada JR. Chronic idiopathic intestinal pseudo-obstruction: clinical and intestinal manometric findings. Gut. 1987;28:5–12. doi: 10.1136/gut.28.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann SD, Debinski HS, Kamm MA. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut. 1997;41:675–81. doi: 10.1136/gut.41.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingate D, Hongo M, Kellow J, Lindberg G, Smout A. Disorders of gastrointestinal motility: towards a new classification. J Gastroenterol Hepatol. 2002;(Suppl 17):S1–14. doi: 10.1046/j.1440-1746.17.s1.7.x. [DOI] [PubMed] [Google Scholar]
- 5.Stanghellini V, Cogliandro RF, de Giorgio R, Barbara G, Salvioli B, Corinaldesi R. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19:440–52. doi: 10.1111/j.1365-2982.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathias JR, Baskin GS, Reeves-Darby VG, Clench MH, Smith LL, Calhoon JH. Chronic intestinal pseudoobstruction in a patient with heart-lung transplant. Therapeutic effect of leuprolide acetate. Dig Dis Sci. 1992;37:1761–8. doi: 10.1007/BF01299872. [DOI] [PubMed] [Google Scholar]
- 7.Mathias JR, Clench MH, Reeves-Darby VG, et al. Effect of leuprolide acetate in patients with moderate to severe functional bowel disease. Double-blind, placebo-controlled study. Dig Dis Sci. 1994;39:1155–62. doi: 10.1007/BF02093778. [DOI] [PubMed] [Google Scholar]
- 8.Palomba S, Orio F, Jr, Manguso F, et al. Leuprolide acetate treatment with and without coadministration of tibolone in premenopausal women with menstrual cycle-related irritable bowel syndrome. Fertil Steril. 2005;83:1012–20. doi: 10.1016/j.fertnstert.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Rabin D, McNeil LW. Pituitary and gonadal desensitization after continuous luteinizing hormone-releasing hormone infusion in normal females. J Clin Endocrinol Metab. 1980;51:873–6. doi: 10.1210/jcem-51-4-873. [DOI] [PubMed] [Google Scholar]
- 10.Hazum E, Conn PM. Molecular mechanism of gonadotropin releasing hormone (GnRH) action. I. The GnRH receptor. Endocr Rev. 1988;9:379–86. doi: 10.1210/edrv-9-4-379. [DOI] [PubMed] [Google Scholar]
- 11.Ducker TE, Boss JW, Altug SA, et al. Luteinizing hormone and human chorionic gonadotropin fragment the migrating myoelectric complex in rat small intestine. Neurogastroenterol Motil. 1996;8:95–100. doi: 10.1111/j.1365-2982.1996.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 12.Mathias JR, Clench MH. Relationship of reproductive hormones and neuromuscular disease of the gastrointestinal tract. Dig Dis. 1998;16:3–13. doi: 10.1159/000016844. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Zheng TZ, Li W, Qu SY, He DY. Action of progesterone on contractile activity of isolated gastric strips in rats. World J Gastroenterol. 2003;9:775–8. doi: 10.3748/wjg.v9.i4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumtepe Y, Borekci B, Karaca M, Salman S, Hakan Alp H, Suleyman H. Effect of acute and chronic administration of progesterone, estrogen, FSH and LH on oxidant and oxidant parameters in rat gastric tissue. Chem Biol Interact. 2009;182:1–6. doi: 10.1016/j.cbi.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Schleipen B, Hertrampf T, Fritzemeier KH, et al. ERβ-specific agonists and genistein inhibit proliferation and induce apoptosis in the large and small intestine. Carcinogenesis. 2011;32:1675–83. doi: 10.1093/carcin/bgr188. [DOI] [PubMed] [Google Scholar]
- 16.Ziecik AJ, Kaczmarek MM, Blitek A, Kowalczyk AE, Li X, Rahman NA. Novel biological and possible applicable roles of LH/hCG receptor. Mol Cell Endocrinol. 2007;269:51–60. doi: 10.1016/j.mce.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Ohlsson B, Veress B, Janciauskiene S, Montgomery A, Haglund M, Wallmark A. Chronic intestinal pseudo-obstruction due to buserelin-induced formation of anti-GnRH antibodies. Gastroenterology. 2007;132:45–51. doi: 10.1053/j.gastro.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. doi: 10.1016/j.yfrne.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Rao CV, Alsip NL. Use of the rat model to study hCG/LH effects on uterine blood flow. Semin Reprod Med. 2011;19:75–85. doi: 10.1055/s-2001-13914. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal K, Krishna A. Effects of hormones on the number, distribution and degranulation of mast cells in the ovarian complex of mice. Acta Physiol Hung. 1996;84:183–90. [PubMed] [Google Scholar]
- 21.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Bassotti G, Villanacci V, Nascimbeni R, et al. Increase of colonic mast cells in obstructed defecation and their relationship with enteric glia. Dig Dis Sci. 2012;57:65–71. doi: 10.1007/s10620-011-1848-y. [DOI] [PubMed] [Google Scholar]
- 23.Bassotti G, Villanacci V, Maurer CA, et al. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41–6. doi: 10.1136/gut.2005.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreihofer DA, Golden GA, Cameron JL. Cholecystokinin (CCK)-induced stimulation of luteinizing hormone (LH) secretion in adult male rhesus monkeys: examination of the role of CCK in nutritional regulation of LH secretion. Endocrinology. 1993;132:1553–60. doi: 10.1210/endo.132.4.8462453. [DOI] [PubMed] [Google Scholar]
- 25.Kossack N, Simoni M, Richter-Unruh A, Themmen AP, Gromoll J. Mutations in a novel, cryptic exon of the luteinizing hormone/chorionic gonadotropin receptor gene cause male pseudohermaphroditism. PLoS. 2008;22:5 e88. doi: 10.1371/journal.pmed.0050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna R, Browne RM, Heiner AD, Clench MH, Mathias JR. Leuprolide acetate affects intestinal motility in female rats before and after ovariectomy. Am J Physiol. 1992;262:G185–90. doi: 10.1152/ajpgi.1992.262.1.G185. [DOI] [PubMed] [Google Scholar]
- 27.Huang W, Yao B, Sun L, Pu R, Wang L, Zhang R. Immunohistochemical and in situ hybridization studies of gonadotropin releasing hormone (GnRH) and its receptor in rat digestive tract. Life Sci. 2001;68:1727–34. doi: 10.1016/s0024-3205(01)00968-7. [DOI] [PubMed] [Google Scholar]