Abstract
Background
Assessment of hemoglobin is one of the most reliable indicators for anemia, and is widely used to screen for anemia among pregnant women. The HemoCue® has been widely used for as a point-of-care device for hemoglobin estimation in health facilities. Previous studies showed contradictory results regarding the accuracy of HemoCue®.
Methods
This was a hospital-based cross sectional study carried- out among pregnant women at Khartoum hospital in Sudan to find out whether the measurement of hemoglobin concentration by HemoCue® using venous or capillary samples was comparable to that of the automated hematology analyzer as standard. Bland and Altman method was used to compare the measurements with an acceptable difference of ± 1.0 g/dl.
Results
Among the 108 subjects in this study the mean (SD) level of hemoglobin level using HemoCue® venous sample, HemoCue® capillary sample and automated hematology analyzer were 12.70 (1.77), 12.87 (2.04) and 11.53 (1.63) g/dl, respectively. Although the correlations between the measurements were all significant there was no agreement between HemoCue® and automated hematology analyzer. The bias + SD (limits of agreement) for HemoCue® venous versus hematology analyzer was 1.17 ± 1.57 (-1.97, 4.31) g/dl, HemoCue® capillary versus hematology analyzer was 1.34 ± 1.85 (-2.36, 5.04) g/dl, and HemoCue® venous versus HemoCue® capillary samples was 017 ± 1.90 and (3.97-3.63) g/dl.
Conclusion
Hemoglobin concentration assessment by HemoCue® using either venous or capillary blood samples has shown unacceptable agreement with automated hematology analyzer.
Virtual slides
The virtual slide(s) for this article can be found here: http://www.diagnosticpathology.diagnomx.eu/vs/8797022296725036
Introduction
Anemia is one of the most important causes of morbidity and mortality in developing countries, especially among pregnant women [1]. The world prevalence of anemia in pregnant women and non-pregnant women is 41.8% and 30.2% respectively [2]. Pregnant Sudanese women are susceptible to anemia regardless to their age and parity and anemia is one of the leading causes of maternal and perinatal morbidity and mortality [3-5].
Assessment of hemoglobin is one of the most reliable indicators for anemia, and is widely used to screen for anemic individuals, and to evaluate responses to interventions [6]. Hemoglobin concentration is routinely measured using automated hematology analyzers. Although, these are very accurate and reliable, they are expensive and problems of samples transport to the laboratory may delay treatment resulting in preventable deaths [7]. In poor resources where automated hematology analyzer is not available other methods of low price and that require less skill are highly needed. Although Cyanmethemoglobin method is cheaper and often used, it takes more time. The semi-quantitative gravimetric copper sulfate method which is used in blood donation is very easy and inexpensive; it does not provide an acceptable degree of accuracy.
The HemoCue® hemoglobin photometer has been widely used for as a point-of-care device for hemoglobin estimation in mobile blood donations and critical care areas in health facilities [8]. Previous studies showed contradictory results regarding the accuracy of HemoCue®; some of research reported a high accuracy of HemoCue® compared with standard laboratory methods [9,10]. However, others did not recommend this device in general practice [11-13]. Few published data exist on the accuracy of HemoCue® in the measurement of hemoglobin during pregnancy [14,15]. The aim of this study was to compare the accuracy of HemoCue® using venous and capillary samples with that of the automated hematology analyzer in the measurement of hemoglobin among pregnant Sudanese women at Khartoum Hospital.
Material and methods
This was a cross sectional study carried- out among pregnant women at Khartoum hospital in Sudan during the period of October through December 2011. Convenient sampling method was used in the study in which all available subjects who fulfilled the inclusion criteria at the Khartoum hospital antenatal care clinic were recruited in the study every day until total number of sample size was achieved. Sample size of 108 subjects was calculated based on a 2-sided hypothesis tests using Epiinfo with 80% power and confidence interval of 95%. After signing an informed consent venous and capillary blood samples were taken from each woman to be measured by HemoCue® (venous and capillary) and the automated analyzer.
Capillary blood samples were collected by finger prick in the middle finger of left hand, after cleaning and massaging the finger to facilitate blood flow. Venous blood samples were collected into vacutainer tubes and were analyzed immediately by HemoCue® and the tubes were sent to the medical laboratory of the hospital for the analyses by automated analyzer.
HemoCue® portable photometer
The HemoCue® B-Hemoglobin system (HemoCue® AB, Ängelholm, Sweden) consists of disposable microcuvettes containing reagent in a dry form and a single purpose designed photometer. The microcuvettes were stored in a dry place at room temperature. Once opened, they were tightly closed and stored at the same conditions to maintain their integrity and shelf life. The reaction in the microcuvette is a modified azide-methemoglobin reaction. Sodium deoxycholate haemolyses erythrocytes and hemoglobin is released. Sodium nitrite converts hemoglobin to methemoglobin which, together with sodium azide, gives azidemethemoglobin. The absorbance is measured at two wavelengths (570 nm and 880 nm) in order to compensate for turbidity in the sample. The test was performed as stated by the manufacturer [16].
Automated hematology analyzer
The Sysmex KX21N (Sysmex Corporation, Kobe, Japan) is an automated blood cell counter intended for in vitro diagnostic use in clinical laboratories. It is a compact, fully automated hematology analyzer with simultaneous analysis of 18 parameters in whole blood mode and capillary blood mode. It measures the hemoglobin concentration using a non-cyanide hemoglobin method (STROMATOLYSER WH). The instrument has been proven to provide accurate and reliable results including hemoglobin concentrations [17,18]. The test was performed as stated in the manufacturer's manual [19].
Quality control
The HemoCue® photometer was checked on a daily basis using the control cuvette and a standard of known concentration. A three set controls were run daily to ensure the function of the Sysmex.
Statistics
All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA, version 16.0). Hemoglobin levels were measured using two instruments; HemoCue® and automated hematology analyzer. HemoCue® used two types of samples; venous as well as capillary samples. Pairs of hemoglobin measurements were compared as follows: HemoCue® venous sample versus automated hematology analyzer, HemoCue® capillary sample versus automated hematology analyzer, HemoCue® venous sample versus HemoCue® capillary sample. Pearson Correlation analysis was performed and correlation coefficient (r) was calculated. The Mean of differences (bias), standard deviation of differences (SD), and limits of agreement (Mean ± 2 × SD) were calculated according to the Bland and Altman method [20]. Limits of agreement not exceeding ±1 g/dl between any two pairs of methods were considered to be clinically acceptable.
Ethics
This study was completely conducted voluntarily and all respondents were given brief explanations about the purpose and procedures of the study to be used. Voluntarily consent was signed by each subject before taking the blood. Ethical clearance was obtained from the institute Board.
Results
A total number of 108 pregnant women were recruited in this study. Their mean (SD) of the age and gestational age were 27.6 (6.8) years and 24.9 (10.2) weeks.
The mean (SD) hemoglobin level was 12.70 (1.77), 12.87 (2.04) and 11.53 (1.63) g/dl using HemoCue® venous sample, HemoCue® capillary sample and automated hematology analyzer, respectively (Table 1).
Table 1.
Hemoglobin level measured using HemoCue® venous sample, HemoCue® capillary sample, and automated hematology analyzer (g/dl)
| Method of measurement | Mean ± SD | Median | (min; max) |
|---|---|---|---|
| HemoCue® (venous) | 12.70 ± 1.77 | 12.80 | (8.90; 17.90) |
| HemoCue® (capillary) | 12.87 ± 2.04 | 12.90 | (8.20; 17.70) |
| Hematology analyzer | 11.53 ± 1.63 | 11.40 | (8.10; 15.00) |
HemoCue® venous versus automated hematology analyzer
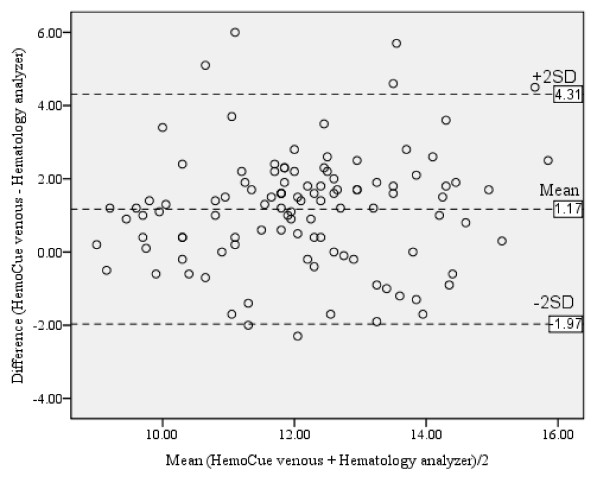
There was a positive correlation (r = 0.58, P < 0.001) between hemoglobin levels by using HemoCue® venous sample versus automated hematology analyzer. The mean difference with limits of agreement between the two reading was 1.17 (-1.97, 4.31) g/dl (Table 2 and Figure 1).
Table 2.
Correlation, bias, and limits of agreement between hemoglobin level using HemoCue® and automated hematology analyzer
| Comparison of methods | Correlation Coefficient | Bias ± SD (95%CI) | Limits of agreement |
|---|---|---|---|
| HemoCue® (venous) vs. Hematology analyzer | 0.58 | 1.17 ± 1.57 (0.87; 1.47) | -1.97 to 4.31 |
| HemoCue® (capillary) vs. Hematology analyzer | 0.51 | 1.34 ± 1.85 (0.99; 1.69) | -2.36 to 5.04 |
| HemoCue® (venous) vs. HemoCue® (capillary) | 0.51 | -0.17 ± 1.90 (-0.53; 0.19) | -3.97 to 3.63 |
Figure 1.
Bland and Altman plot for hemoglobin level by using HemoCue® venous sample versus automated hematology analyzer (g/dl).
HemoCue® capillary versus automated hematology analyzer
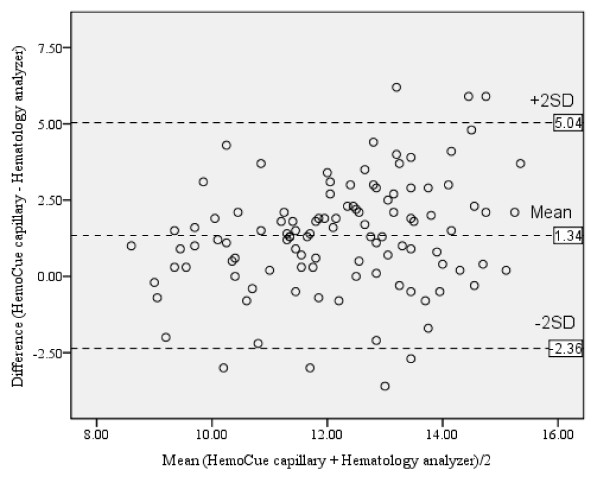
There was a positive correlation between hemoglobin levels by using HemoCue® capillary sample versus automated hematology analyzer (r = 0.51, P < 0.001). The mean difference with limits of agreement between hemoglobin levels by using HemoCue® capillary sample versus automated hematology analyzer was 1.34 (-2.36, 5.04) g/dl (Table 2 and Figure 2).
Figure 2.
Bland and Altman plot for hemoglobin level by using HemoCue® capillary sample versus automated hematology analyzer (g/dl).
HemoCue® venous versus HemoCue® capillary sample
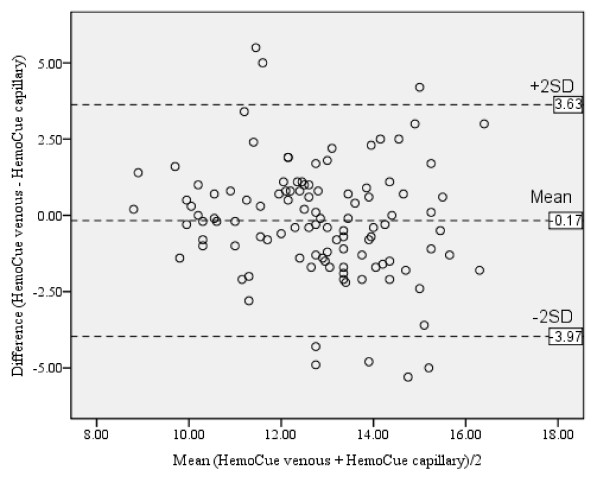
There were positive correlation between hemoglobin levels by using HemoCue® venous sample versus HemoCue® capillary sample (r = 0.51, P < 0.001). The mean difference with limits of agreement between hemoglobin levels by using HemoCue® venous sample versus HemoCue® capillary sample was -0.17 (-3.97, 3.63) g/dl (Table 2 and Figure 3).
Figure 3.
Bland and Altman plot for hemoglobin level by using HemoCue® venous sample versus HemoCue® capillary sample (g/dl).
According to the previously pre-defined clinical acceptable limits of ± 1 g/dl, the 2 methods could not be considered as interchangeable.
Discussion
Recently HemoCue® portable hemoglobin photometer using venous or capillary blood samples has been widely used for quick assessment of hemoglobin concentrations; especially in poor settings where skills and resources are limited.
The accuracy of HemoCue® for measuring hemoglobin in clinical settings is still a matter of controversy. The results of this study have shown that the hemoglobin concentration of HemoCue® using either venous or capillary blood samples have lower level of precision and was not comparable with that of automated hematology analyzer; the limits of agreement were larger than the predefined clinically acceptable limits of ±1 g/dl. This is goes with the previous findings of the studies conducted among pregnant women at high altitude [14] and adult patients hospitalized in surgical intensive care unit [21]. The difference between the readings has been explained by the use of only one microcuvette with HemoCue® especially when using capillary blood samples and laboratory values, and advised loading multiple microcuvettes and averaging the hemoglobin values obtained has been proposed [22].
However, other studies showed that the results obtained by using HemoCue® for hemoglobin assessment among pregnant women were comparable to that of automated hematology analyzer as a standard [9]. Bernard et al., found that the results of hemoglobin concentration among pregnant and non-pregnant populations using HemoCue® were comparable to that of automated hematology analyzer and Cyanmethemoglobin methods [23]. Other studies which were conducted in different settings and populations such as patients with gastrointestinal bleeding, surgical patients repeated measurement of one sample, urban general practice, neonates, patients undergoing aortic surgery in the theatre and blood donors recommended HemoCue® for the hemoglobin estimation [8,24-31]. Paiva et al. found that HemoCue® was more appropriate for capillary compared to venous blood samples [32]. However, there was within-subject variability of capillary blood hemoglobin values that might explain the unreliability of the method, and it has been shown that two capillary samples taken from different fingers of the same subjects had hemoglobin concentrations differing by/ more than two g/dL using the HemoCue® [6].
In spite of the non-acceptable agreement of HemoCue® with automated hematology analyzer in this study, the HemoCue® is however simple to use, need minimum training, cheap, and gives an immediate result. Furthermore, it is useful in clinical and epidemiological settings where finger puncture allows capillary blood sampling as an easy technique which is less resource-intensive than vein puncture, and is more acceptable to patients and the community.
Conclusion
Hemoglobin concentration assessment by HemoCue® using either venous or capillary blood samples does not have an acceptable agreement with automated hematology analyzer. Therefore this study does not recommend this device for hemoglobin concentration assessment among pregnant women.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
IA and MIY designed the study and participated in the statistical analyses, SA and MH conducted the clinical work. MH conducted the lab work. All the authors approved the draft and the final paper.
Contributor Information
Ishag Adam, Email: ishagadam@hotmail.com.
Samah Ahmed, Email: samah289@gmail.com.
Mahmoud H Mahmoud, Email: mheljaaly2020@hotmail.com.
Mohammed I Yassin, Email: dr_yassinibrahim@yahoo.com.
Acknowledgements
This work was funded by University of Khartoum, Khartoum, Sudan.
References
- WHO. The world health report 2002: reducing risks, promoting healthy life. Geneva. 2002. [DOI] [PubMed]
- McLean E, Egli I, Cogswell M, Benoist Bd, Wojdyla D. In: Nutritional anemia. Kraemer K, Zimmermann MB, editor. Basel: Sight and Life Press; 2007. Worldwide prevalence of anemia in preschool aged children, pregnant women and non-pregnant women of reproductive age; pp. 1–12. [Google Scholar]
- Adam I, Elhassan EM, Haggaz AE, Ali AA, Adam GKA. Perspective of the epidemiology of malaria and anaemia and their impact on maternal and perinatal outcomes in Sudan. J Infect Dev Ctries. 2011;5:83–87. doi: 10.3855/jidc.1282. [DOI] [PubMed] [Google Scholar]
- Ali AA, Adam I. Anaemia and stillbirth in Kassala Hospital, Eastern Sudan. J Trop Pediatr. 2011;57:62–64. doi: 10.1093/tropej/fmq029. [DOI] [PubMed] [Google Scholar]
- Elhassan EM, Abbaker AO, Haggaz AD, Abubaker MS, Adam I. Anaemia and low birth weight in Medani. Hospital Sudan. BMC Res Notes. 2010;3:181. doi: 10.1186/1756-0500-3-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SS, Ruel MT, Cohen RJ, Dewey KG, de la Briere B, Hassan MN. Precision, accuracy, and reliability of hemoglobin assessment with use of capillary blood. Am J Clin Nutr. 1999;69:1243–1248. doi: 10.1093/ajcn/69.6.1243. [DOI] [PubMed] [Google Scholar]
- Jahr JS, Lurie F, Driessen B, Davis JA, Gosselin R, Gunther RA. The HemoCue®, a point of care B-hemoglobin photometer, measures hemoglobin concentrations accurately when mixed in vitro with canine plasma and three hemoglobin-based oxygen carriers (HBOC) Can J Anesth. 2002;49:243–248. doi: 10.1007/BF03020522. [DOI] [PubMed] [Google Scholar]
- Bridges N, Parvin RM, Van Assendelft OW. Evaluation of a new system for hemoglobin measurement. Am Clin Products Rev. 1987;6:22–25. [Google Scholar]
- Von Schenck H, Falkensson M, Lundberg B. Evaluation of HemoCue, a new device for determining hemoglobin. Clin Chem. 1986;32:526–529. [PubMed] [Google Scholar]
- Morris SS, Ruel MT, Cohen RJ, Dewey KG, de la Briere B, Hassan MN. Precision, accuracy, and reliability of hemoglobin assessment with use of capillary blood. Am J Clin Nutr. 1999;69:1243–1248. doi: 10.1093/ajcn/69.6.1243. [DOI] [PubMed] [Google Scholar]
- Chen PP, Short TG, Leung DH, Oh TE. A clinical evaluation of the HemoCue hemoglobinometer using capillary, venous and arterial samples. Anaesth Intensive Care. 1992;20:497–500. doi: 10.1177/0310057X9202000419. [DOI] [PubMed] [Google Scholar]
- Rippmann CE, Nett PC, Popovic D, Seifert B, Pasch T, Spahn DR. HemoCue, an accurate bedside method of hemoglobin measurement? J Clin Monit. 1997;13:373–377. doi: 10.1023/A:1007451611748. [DOI] [PubMed] [Google Scholar]
- Neville RG. Evaluation of portable hemoglobinometer in general practice. Br Med J (Clin Res Ed) 1987;294:1263–1265. doi: 10.1136/bmj.294.6582.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yan H, Xing Y, Dang S, Zhuoma B, Wang D. Evaluation of a portable hemoglobin photometer in pregnant women in a high altitude area: a pilot study. BMC Public Health. 2009;9:228. doi: 10.1186/1471-2458-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek NR, Ntonya C, Mhango E, White SA. Diagnosing anaemia in pregnancy in rural clinics: assessing the potential of the Haemoglobin Colour Scale. Bull World Health Organ. 1999;77:15–21. [PMC free article] [PubMed] [Google Scholar]
- HemoCue® Blood Hemoglobin Photometer Operating Manual. http://www.HemoCue®.com/files/900138_B.pdf
- Gamperling N, Mast B, Hagbloom R, Houwen B. Performance Evaluation of the Sysmex KX-21 [TM] Automated Hematology Analyzer. Sysmex J Int. 1998;8:96–101. [Google Scholar]
- United Kingdom National External Quality Assessment Scheme for Haematology (UK NEQAS (H)), WGHV, Watford, WD1 8FJ, UK. An evaluation of the Sysmex KX-21 automated hematology analyzer. Sysmex J Int. 1998;8:102–109. [Google Scholar]
- Sysmex KX-21N Operator's Manual. Sysmex Corporation. 2006.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Seguin P, Kleiber A, Chanavaz C, Morcet J, Mallédant Y. Determination of capillary hemoglobin levels using the HemoCue system in intensive care patients. J Crit Care. 2011;26:423–427. doi: 10.1016/j.jcrc.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Rippmann CE, Nett PC, Popovic D, Seifert B, Pasch T, Spahn DR. HemoCue, an accurate bedside method of hemoglobin measurement? J Clin Monit. 1997;13:373–377. doi: 10.1023/A:1007451611748. [DOI] [PubMed] [Google Scholar]
- Nkrumah B, Nguah SB, Sarpong N, Dekker D, Idriss A, May J, Adu-Sarkodie Y. Hemoglobin estimation by the HemoCue® portable hemoglobin photometer in a resource poor setting. BMC Clinical Pathology. 2011;11:5. doi: 10.1186/1472-6890-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Schenck H, Falkensson M, Lundberg B. Evaluation of "HemoCue", a new device for determining hemoglobin. Clin Chem. 1986;32:526–529. [PubMed] [Google Scholar]
- Van de Louw A, Lasserre N, Drouhin F, Thierry S, Lecuyer L, Caen D, Tenaillon A. Reliability of HemoCue in patients with gastrointestinal bleeding. Intens Care Med. 2007;33:355–358. doi: 10.1007/s00134-006-0461-6. [DOI] [PubMed] [Google Scholar]
- Rippmann CE, Nett PC, Popovic D, Seifert B, Pasch T, Spahn DR. HemoCue, an Accurate Bedside Method of Hemoglobin Measurement? J Clin Monit Comput. 1997;13:373–377. doi: 10.1023/a:1007451611748. [DOI] [PubMed] [Google Scholar]
- Neville RG. Evaluation of portable haemoglobinometer in general practice. BMJ (Clinical research ed) 1987;294:1263–1265. doi: 10.1136/bmj.294.6582.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechner IJ, Twigg A, Davies AF, Imong S. Evaluation of the HemoCue compared with the Coulter STKS for measurement of neonatal haemoglobin. Arch Dis Child Fetal Neonatal. 2002;86:188–189. doi: 10.1136/fn.86.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardi AM, Hirst C, Mortimer AJ, McCollum CN. Evaluation of the HemoCue for measuring intra operative haemoglobin concentrations: a comparison with the Coulter Max M®. Anaesthesia. 1998;53:349–352. doi: 10.1046/j.1365-2044.1998.00328.x. [DOI] [PubMed] [Google Scholar]
- Sari M, dePee S, Martini E, Herman S, Bloem MW, Yip R. Estimating the prevalence of anaemia: a comparison of three methods. Bull World Health Org. 2001;79:506–511. [PMC free article] [PubMed] [Google Scholar]
- Radtke H, Polat G, Kalus U, Salama A, Kiesewetter H. Hemoglobin screening in prospective blood donors: comparison of different blood samples and different quantitative methods. Transfus Aph Sci. 2005;33:31–35. doi: 10.1016/j.transci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Paiva Adriana de A, Rondó Patrícia HC, Silva Silmara S, de B, Latorre Maria do RDO, de Paiva AA, Rondó PHC, de Silva SSB, Latorre MRDOdo. Comparison between the HemoCue® and an automated counter for measuring hemoglobin. Rev Saude Publica. 2004;38:585–587. doi: 10.1590/S0034-89102004000400017. [DOI] [PubMed] [Google Scholar]