Abstract
The genetic nature of sensorineural hearing loss (SNHL) has so far been studied for many ethnic groups in various parts of the world. The single-nucleotide guanine deletion (35delG) of the GJB2 gene coding for connexin 26 was shown to be the main genetic cause of autosomal recessive deafness among Europeans. Here we present the results of the first study of GJB2 and three mitochondrial mutations among two groups of Belarusian inhabitants: native people with normal hearing (757 persons) and 391 young patients with non-syndromic SNHL. We have found an extremely high carrier frequency of 35delG GJB2 mutation in Belarus −5.7%. This point deletion has also been detected in 53% of the patients with SNHL. The 312del14 GJB2 was the second most common mutation in the Belarus patient cohort. Mitochondrial A1555G mt-RNR1 substitution was found in two SNHL patients (0.55%) but none were found in the population cohort. No individuals carried the A7445G mutation of mitochondrial mt-TS1. G7444A as well as T961G substitutions were detected in mitochondrial mt-RNR1 at a rate of about 1% both in the patient and population cohorts. A possible reason for Belarusians having the highest mutation carrier frequency in Europe 35delG is discussed.
Introduction
The genetic mechanisms of hearing loss have been extensively analyzed during the last two decades. Over 100 loci, both nuclear and mitochondrial, are already implicated in the development of this most common sensory disorder of humans [1]. The GJB gene family (GJB2, GJB3 and GJB6) encoding gene gap junction proteins – connexins – was found to be the main “storage place" of mutations causing non-syndromic sensorineural hearing loss (SNHL) [2]–[4]. More than 50% of cases of autosomal recessive non-syndromic hearing loss in many world populations are attributed to mutations in GJB2 gene encoding connexin 26 (OMIM: 121011) [5].
The most frequent GJB2 anomaly causing deafness in Europeans and white Americans is deletion of one guanine within the six-guanine string at the beginning of the second GJB2 exon (positions 30–35), the so-called 35delG mutation (rs80338939) [6], [7]. Japanese people carry another recessive major GJB2 mutation, 235delC (rs80338943); its prevalence in Japan exceeds 73% [8]. V37I (rs72474224) is frequently found among Taiwan natives [5] and 167delT (rs80338942) in Ashkenazi Jews [9]. Besides the point mutations and small deletions, several large deletions were detected on the 13th chromosome within the region where GJB2 and GJB6 genes are positioned close to each other. These are the 309 kb (GJB6-D13S1830) and 232 kb deletions (GJB6-D13S1854) located more than 100 kb upstream of the transcriptional start sites of the GJB2 and GJB6 genes. Both deletions are frequently detected in compound heterozygosity with point GJB2 mutations [10], [11].
The average carrier rate of 35delG GJB2 mutation in Europe is about 2% [12]. In some Mediterranean countries (Italy (Sardinia) Greece and Malta) it exceeds 3%. A moderately high rate (4.5%) was reported for Estonia, one of the Baltic countries [3], [5]. For one of the Volga-middle Ural ethnic group, Mordvins, the 35delG GJB2 carrier frequency is even higher (6.2%) [13]. All these values are surprisingly high and point either to the “founder effect" or selective advantage for heterozygotes, or both. Indeed, according to a recent publication [14], the epidermal thickness is significantly higher among European individuals heterozygous or homozygous for 35delG compared to wild-type. These data stand in line with the previously reported fact that Africans who carry the mutant GJB2 allele R134W (rs80338948) have a thicker epidermis than the wild-type. In vitro studies have demonstrated that cells expressing the R134W allele are significantly less susceptible to cellular invasion of the enteric pathogen Shigella flexneri than wild-type cells [15], [16]. These data support the hypothesis that skin phenotype might counterbalance the evolutionary disadvantage caused by deafness [14].
Besides the nuclear genes, a proper set of mitochondrial (mt) genes is involved in the control of normal hearing [17], [18], [19]. Mutations of mitochondrial genes have some peculiarities: they are maternally inherited; they can be both in homo- and heteroplasmic states. Though definite mitochondrial point mutations and large deletions usually lead to syndromic deafness [20], [21], some point mutations, notably in mt-RNR1 and mt-TS1 (tRNASer) genes, may cause SNHL [17]. A certain range of mutations in the mt-RNR1 gene (A1555G, C1494T, T961G) was found to be associated with aminoglycoside-induced deafness [22]. The incidence of these mutations varies also among different populations; so far nothing has been reported about Belarusian patients with SNHL.
As far as we know, the only data on the 35delG GJB2 carrier rate among Belarusians (97 people with normal hearing) were published in 2010 [13]. The carrier frequency was reported to be more than 6%, the highest rate for European countries studied so far. Here we present, firstly, results of an extensive analysis of 35delG/GJB2 carrier frequency among more than 750 modern Belarusians from six ethno geographic regions of Belarus. Secondly, we summarize the results of the analysis of GJB2 gene structural changes along with the 309 kb deletion truncating the neighboring GJB6 gene del(GJB6-D13S1830) in a large Belarus patient cohort with SNHL (391 patients). Finally, both the Belarus population and patient cohorts were screened for four mitochondrial mutations associated with deafness.
We provide here the genetic evidence that the carrier frequency of the 35delG deafness mutation in the Belarus population is the highest compared to other countries. The same GJB2 defect is the main reason for SNHL in Belarus patients. Additionally our study reveals that the second most common GJB2 mutation in the Belarus patient cohort with SNHL is the 312del14 deletion.
Results and Discussion
Carrier rate of 35delG GJB2 mutation in Belarus
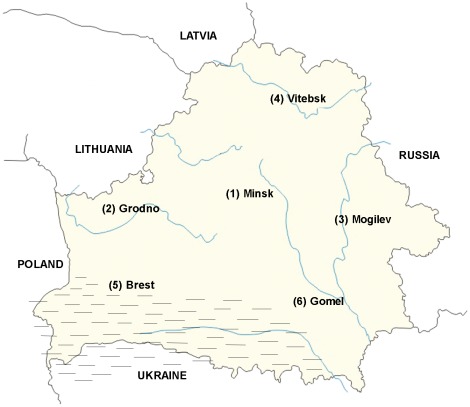
The number of persons genotyped in six regions of Belarus (Figure 1) for estimation of the deafness major mutation carrier rate is presented in table 1. The mutation was found in 43 out of 757 persons with normal hearing, i.e. 5.7% - a high rate of incidence. We have found three regions of Belarus with an extremely high carrier rate: the south-west of the country (west Polessie) where it equals 10.2%, the northern part of Belarus (Vitebsk region) −6.1% and south-east (east Polessie) −5.9%. The northern part of Belarus is geographically proximal to Estonia, where the carrier rate of 35delG was reported to be 4.5% [3]. Counter to this, in the western part of Belarus a carrier rate of 2% was revealed that is comparable to the mean European frequency for this mutation. The differences between 35delG carrier rate in west Polessie and other regions of the country are therefore significant.
Figure 1. Map of Belarus.
Figures indicate the regions where the population samples have been collected; the Polessie region is shown dashed.
Table 1. The rate of 35delG heterozygotes in the population cohort (native inhabitants from six regions of Belarus).
| Belarus regions | Detected 35delG heterozygotes/number of persons genotyped (%) |
| 1 - center | 5/126 (4.0) |
| 2 - west | 2/97 (2.0) |
| 3 - east | 5/127 (3.9) |
| 4 - north | 8/131 (6.1) |
| 5 - south-west | 16/157 (10.2) ** |
| 6 - south-east | 7/119 (5.9) |
| Total | 43/757 (5.7) |
The difference in rates between south-west and all the other regions is significant (P<0,02).
According to the results obtained, the map of the European distribution of 35delG carrier frequencies [23] should be corrected since Belarusians are the population with the highest carrier rate so far reported, not only for Europe, but for the other parts of the world as well.
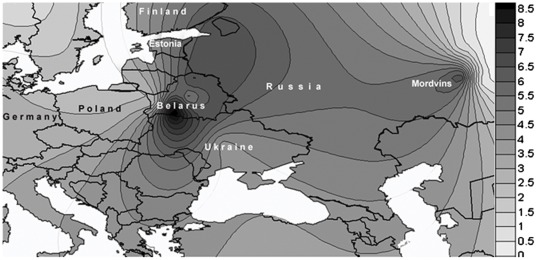
The high 35delG carrier rate in the Belarus population as a whole, together with the uneven distribution of the mutation carrier rate, seems surprising for this small country having no geographically-isolated zones. Some previous studies have assumed that the high frequency of the 35delG mutation reflects the presence of a mutational hot spot [24], [25], while others support the theory of a common founder [26], [27]. Greece is among the countries with the highest carrier frequency of the mutation (3.5%) and, according to the idea of a common founder, the 35delG appeared about 10000 years ago in the territory of contemporary Greece and expanded throughout Europe [28], [29]. The alternative model that a high frequency of the 35delG mutation reflects the presence of a mutational hot spot seems a more probable explanation of the results obtained here. To prove or reject any of these hypotheses, single nucleotide polymorphisms adjacent to GJB2 (haplotypes) should be analyzed and genotyping of the native inhabitants from nearby Ukrainian and Polish Polessie and the regions of Russia adjoining the northern part of Belarus should be performed. A high 35delG carrier frequency (6.2%) was reported recently for Mordvins, a small ethnic group that belongs to the Finno-Ugric linguistic group and inhabits the Volga-middle Ural region of Russia [13]. Taken together, Belarus as well as Estonia and Mordovia are three European regions with a 35delG carrier frequency almost three times higher than that generally found in Europe and twice as high as that found in south-western Europe and it would be fascinating to know whether these three peaks are unrelated or have “common history" (Figure 2).
Figure 2. Geographic distribution of 35delG carrier rate.
The spatial frequency map was obtained by plotting the incidence of the 35delG allele in 4346 individuals representing 20 populations including 6 sub-populations from Belarus together with others taken from the literature [12], [13]. The frequency data were converted to the isofrequency map using Surfer software (version 7, Golden Software Inc., Golden, CO, USA). The right panel shows how the color scale corresponds to 35delG allele frequency (%).
Data on the selective advantage of heterozygotes [14] should be taken into account not only to explain the high rate of 35delG mutation among Belarusians but also to interpret its uneven distribution within the country depending on various ecological conditions and the resulting unevenness in the distribution of skin pathogens. Belarus Polessie (especially its western part, that was found to have the highest carrier rate of 35delG mutation) is situated in the south of Belarus where the humidity is extremely high due to numerous swamps and dense woods, creating a favorable environment for skin pathogens (Figure 1).
Analysis of the Belarus patient cohort with SNHL
35delG mutation
All 391 patients with SNHL were screened for the 35delG GJB2 mutation. 178 patients (45.5%) were homozygous for this genetic defect, 51 (13.1%) had only one mutant allele and in 162 (41.4%) patients the major GJB2 mutation was not found (Table 2). Thus, as for other European SNHL patient cohorts, the 35delG point deletion is the major cause of prelingual SNHL among the Belarus patients studied.
Table 2. Genotype/phenotype correlations for the patients with (D) and without (N) 35delG mutation.
| SNHL degree | Patients analyzed | DD | DN | NN | %DD/%NN |
| moderate | 18 | 3 | 2 | 13 | 16.7/72.2** |
| moderate/severe | 23 | 7 | 1 | 15 | 30.4/65.2 |
| severe | 88 | 36 | 11 | 41 | 40.9/46.6 |
| severe/profound | 45 | 27 | 7 | 11 | 60.0/24.4 |
| profound | 217 | 105 | 30 | 82 | 48.4/37.8 |
| Total | 391 | 178 | 51 | 162 | 45.5/41.4 |
The difference of DD % with other patient groups is significant (P<0,02).
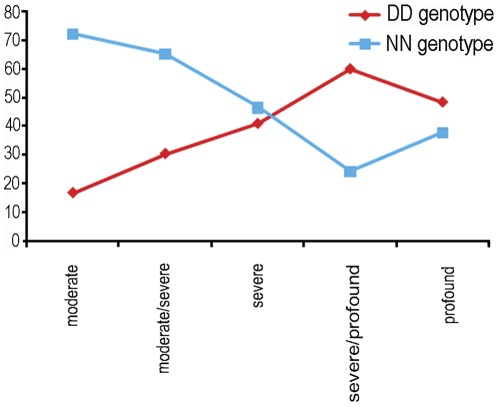
The degree of SNHL varies among the studied Belarus patient cohort from moderate to severe (five grades in total). Among patients with moderate degree of hearing loss (HL) 72.2% lack the mutation and only 16.7% have 35delG/35delG genotype whereas among the patients with severe HL 36 out of 88 (40,9%) were 35delG homozygotes, with severe/profound HL 27 out of 45 (60%) and with profound HL 105 out of 217 (48,4%) (Table 2, Figure 3). Only 3 out of 178 homozygous 35delG patients have moderate HL while 105 are totally deaf. Thus, based on the revealed phenotype-genotype association we conclude that among the Belarus patient cohort the 35delG/35delG genotype results mainly in severe and profound hearing loss, which is similar to the other ethnic groups previously studied [5], [30].
Figure 3. Percentages of DD and NN 35delG genotypes among Belarus SNHL patients with different degrees of hearing loss.
Other mutations of the GJB2 gene and the del(GJB6-D13S1830)
SSCP analysis of the GJB2 coding exon was carried out for all DNA samples heterozygous or lacking the 35delG mutation. The samples with dissimilar mobility patterns were sequenced. All the patients except 35delG homozygotes were screened for the presence of GJB6 309 kb deletion: del(GJB6-D13S1830). The results are summarized in table 3.
Table 3. The rates of GJB2 and del(GJB6- D13S1830) mutations in Belarus SNHL patient cohort.
| GJB2 genotype | Number of mutation carriers in patient cohort (%) | Range of SNHL degree* |
| 35delG/35delG | 178 (45.5) | 3 mod, 7 mod/s, 35 s, 27 s/pr, 106 pr |
| 35delG/312del14 | 20 (5.1) | 2 mod, 1 mod/s, 3 s, 3 s/pr, 8 pr |
| N/312del14 | 5 (1.28) | mod/s, s, s/pr, 2 pr |
| 35delG/del(GJB6-D13S1830) | 3 (0.77) | pr |
| 312del14/312del14 | 2 (0.51) | pr |
| 35delG/235delC | 3 (0.77) | s |
| 167delT/235delC | 2 (0.51) | 1 s, 1 s/pr |
| 35delG/I82M | 2 (0.51) | 1 mod, 1x |
| 35delG/167delT | 2 (0.51) | 1 s, 1 s/pr |
| N/167delT | 2 (0.51) | 1 pr, 1 s/pr |
| N/V27I | 1 (0.26) | pr |
| 35delG/V27I+E114G | 1 (0.26) | s |
| 35delG/N | 20 (5.1) | |
| Total | 241 (61.6) |
Degree of HL: mod – moderate; s – severe, pr – profound, x - no audiological data.
For 31 out of 51 patients bearing one 35delG allele, either another mutation in GJB2 gene or the 309 kb deletion (GJB6-D13S1830) was found; for the remaining 20 patients, only 35delG was detected. According to our data, the second most common GJB2 mutation causing SNHL in Belarus patient cohort is 312del14. We have detected 27 individuals bearing this mutation: two patients without 35delG were homozygous for 312del14, 20 were 35delG/312del14 heterozygotes, and 5 patients lacking 35delG had one deleted 312del14 allele. The overall 312del14 allele frequency among SNHL patients is 3.7%. Six other single nucleotide deletions or substitutions previously reported to cause SNHL [3], [5], [31] were found in the Belarus patient cohort, all of them with a frequency of less than 1% (0.77%–0.26%) (Table 3). Notably, among 241 patients with SNHL that bear various GJB2 mutations, only four individuals were found with nucleotide substitutions, while the vast majority bear deletions: single-nucleotide (207 patients), deletion of 14 nucleotides (27 patients) and 309 kb (3 patients).
Mitochondrial mutations in population and patient cohorts
Two mitochondrial mutations, T961G and A1555G (both are located in mt-RNR1), were screened in the sample of 300 normal hearing ethnic Belarusians. 595 individuals with normal hearing were genotyped for the 7445/7444 (mt-TS1) mutations. All individuals from the patient cohort were genotyped for the four mtDNA mutations. The results are presented in table 4.
Table 4. Rates of mitochondrial mutations in the Belarus population and patient cohorts with SNHL.
| Mitochondrial mutation (%) | ||||
| T961G MT-RNR1 | A1555G MT-RNR1 | G7444A MT-TS1 | A7445G MT-TS1 | |
| Population Detected/studied | 3/300 (1.0) | 0/300 (0) | 6/595 (1.01) | 0/595 (0.0) |
| Patients Detected/studied | 6/391 (1.5) | 2/391 (0.51) | 3/391 (0.77) | 0/391 (0.0) |
No individuals from the population cohort bear the A1555G mutation. Two patients with hearing loss possess this mitochondrial mutation (0.53%). Surprisingly, this rate is similar to that found among 513 Greek children with hearing loss (0.4%) [32] but is much less than the 3.6% found among SNHL patients of neighboring Poland [33].
Two other mitochondrial mutations were found both in the population and patient groups with rates 0.77–1.5%. There are various opinions concerning T961G mutation: it is often considered as a non-pathogenic polymorphism, but there are reports about its association with aminoglycoside-induced deafness and SNHL [19]. 6 representatives of our patient cohort have T961G (1.5%), five of them are also 35delG homozygotes with a profound (4 persons) or severe (1 person) degree of hearing loss and one of these 6 patients has a non-mutant coding exon 2 of the GJB2 gene. Thus, the cause of deafness in these five patients is probably the 35delG GJB2 pathogenic mutation, rather than the mitochondrial T961G. In the population cohort, T961G substitution was found in 3 persons out of 300 analyzed individuals (1%), and all of them still have normal hearing. Taking into consideration these results we suppose that T961G is either a non-pathogenic polymorphism, or possibly a pathogenic mutation with extremely low penetrance among the Belarusian patients cohort studied.
The pathogenic role of G7444A is also questionable. It is considered to be defining for mitochondrial haplogroup V [34], but its role in aggravating the expression of the A1555G mutation was also proposed [35]. G7444A incidence in the Belarusian population and patient cohorts studied is about 1% (Table 4). A high carrier rate of this mutation (1/62 or 1.6%) was found in Poland and as a result this substitution was suggested to be a nonpathogenic polymorphism in the Polish population [33]. In the present study, three patients with this substitution do not bear any mutation in either GJB2 or in the mt-RNR1 mitochondrial gene, and the genetic origin of their profound hearing loss remains uncertain.
In summary, the results from genotyping the large Belarus population cohort for the 35delG mutation proves that the frequency of this mutation (5.7%) is the highest among all European countries studied. In one of the Belarus regions (west Polessie) the mutation frequency exceeds 10%. We show that the same single-nucleotide deletion, 35delG in GJB2, is also the main genetic cause of SNHL in Belarus as it is detected in the homo- or heterozygous state in 53% of the patients with moderate-to-profound hearing loss. The higher the degree of hearing loss the higher the proportion of 35delG homozygotes detected. Six other mutations in the GJB2 gene were found: 312del14 in 7% of patients and the rest in less than 1.5% of patients. The mitochondrial A1555G mutation was revealed in less than 1% of patients. No patient with A7445G was detected but three (0.77%) were found to carry G7444A substitution. We note that the rate of G7444A substitution in the population cohort is about 1%, evidence of its non-pathogenic state among Belarusians.
Materials and Methods
Ethics Statement
Both population and patient samples reported in the study were collected after obtaining informed written consent; written informed consent was obtained from the parents of the minors who took part in this study. The study has been considered and approved specifically by the Bioethics Committee of the Belarusian State Medical University (Minsk, Belarus) and the Scientific Board of the Institute of Genetics and Cytology of National Academy of Sciences (Minsk, Belarus).
The research consisted of two parts as follows:
Analysis of the carrier rate of 35delG GJB2 mutation, as well as mtDNA mutations among native Belarusian people with normal hearing (referred to as the “population cohort" in the main text)
Detection of 35delG GJB2, del(GJB6-D13S1830) deletions, revealing other structural changes in the GJB2 gene along with analysis of mtDNA mutations in the cohort of Belarus patients with SNHL (referred to as the “patient cohort" in the main text).
Subjects under study
Part 1- Population cohort study
To characterize the Belarusian population, the country (9.5 million inhabitants) was divided into six sampling regions according to the ethno geographic approach; samples were collected in 2–4 small towns from each region (Figure 1). Blood samples from 757 native inhabitants of Belarus were collected after obtaining written informed consent. Each person filled in the questionnaire concerning his (her) ethnicity and place of birth, as well as ethnicity and place of birth of his (her) parents and grandparents. Only those volunteers who had normal hearing and lived in the particular region of the country for three or more generations were included in the study.
Part 2- Patient cohort study
391 young patients, mostly school- and preschool- children with sensorineural hearing loss (non-relatives, aged 1–25) with or without family history of SNHL, permanently living in Belarus, were taken into analysis after obtaining a written consent to be involved into the study from their parents or from themselves for adults. All hearing impaired patients have passed otoscopic examination and different audiological tests depending on their age: pure-tone audiometry, impedance audiometry, distortion-product otoacoustic emission, brainstem evoked response audiometry. Only those with bilateral hearing loss were included in the study. Additionally, all patients were assigned to groups according to the degree of SNHL (from moderate to profound) based on the audiological tests. We note that the patient cohort samples have been collected in the same six regions of the country as the population cohort samples.
DNA
For the population cohort study 5 ml of venous blood was collected, whereas 50–100 mcl (1–2 drops) of peripheral blood was collected for patients. DNA was isolated with proteinase K and phenol-chloroform purification [36].
Strategy of genotyping
1) Population cohort study: the 35delG GJB2 mutation was genotyped in 757 samples over six regions of Belarus. Smaller sample sets (while still representing all the regions) were analyzed for the presence of mitochondrial mutations. In particular, 300 samples were screened for mt-RNR1 mutations while mt-TS1 mutations were genotyped among 595 samples.
2) Patients cohort study
a) GJB genes: the 35delG GJB2 mutation was analyzed for all patients with SNHL. Further, for the patients without the 35delG mutation or heterozygous individuals, the SSCP technique was used to detect the structural changes in the coding part of the GJB2 gene. Direct sequencing of dissimilar patterns was used to specify the nucleotide sequence change. All the patients except the 35delG homozygotes were screened for the 309 kb deletion: del(GJB6-D13S1830) using a multiple PCR protocol [37].
b) Mitochondrial mutations A1555G, T961G, A7445G and G7444A were detected after amplification with specific primers and cleavage by endonucleases, all mitochondrial mutations found were also confirmed by sequencing. See table 5 for all primers and conditions of genotyping.
Table 5. Primers, PCR conditions and endonucleases for genotyping.
| Gene | Mutation | Primers (nucleotide sequence or range) | Ta | Product size | Enzyme | Fragment size | Reference | |
| Norm | Mut | |||||||
| GJB2 | 35delG | F gctggtggagtgtttgttcacacccgc | 600 | 89 | MvaI | 60; 29 | 89 | [38] |
| R tcttttccagagcaaacggc | ||||||||
| 309 kb deletion | del(GJB6-D13S1830) | F1 gccatgcatgtggcctacta | 580 | 480, 441 | - | 480 | 441 | [37] |
| F2 cattgttgtgaactaacctcca | ||||||||
| R1 actatctgaaatcagctcattc | ||||||||
| mt-RNR1 | A1555G | F 1009–1032 | 550 | 566 | HaeIII | 455; 111 | 455; 91;20 | [39] |
| R 1575–1556 | ||||||||
| mt-RNR1 | T961G | F 592–613 | 530 | 544 | AciI | 306; 246; 2 | 306; 186;60;2 | * |
| R 1164–1145 | ||||||||
| mt-TS1** | A7445G/G7445A | F 7402–7420 | 620 | 266 | XbaI | 226;40 | 266 | * |
| R 7667–7649 | ||||||||
Notes
- self-designed primers;
- for each sample without XbaI recognition site sequencing was carried out; Norm – normal, Mut – mutant alleles; the nucleotide positions and substitutions of mtDNA are given relative to the rCRS [40].
Acknowledgments
We are thankful to L. Sivitskaja, V. Pankratov and E. Venskaja for technical help and discussing the results. We thank all the patients and Belarusian volunteers for their participation in the study. We also thank reviewers for their constructive comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was supported by Belarus government program Health of mother and child - well-being of family and state (2010–2012) assignment No. 03.03.10. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hilgert N, Smith RJ, Van Camp G. Function and expression pattern of nonsyndromic deafness genes. Curr Mol Med. 2009;9:546–564. doi: 10.2174/156652409788488775. doi: 10.2174/156652409788488775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Camp G, Willems PJ, Smith RJ. Nonsyndromic hearing impairment: unparalleled heterogeneity. Am J Hum Genet. 1997;60:758–764. [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen MB, Willems PJ. Non-syndromic, autosomal-recessive deafness. Clin Genet. 2006;69:371–392. doi: 10.1111/j.1399-0004.2006.00613.x. doi: 10.1111/j.1399–0004.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 4.Kokotas H, Grigoriadou M, Korres GS, Ferekidou E, Giannoulia-Karantana, et al. Are GJB2 mutations an aggravating factor in the phenotypic expression of mitochondrial non-syndromic deafness? J Hum Genet. 2010;55:265–269. doi: 10.1038/jhg.2010.23. doi: 10.1038/jhg.2010.23. [DOI] [PubMed] [Google Scholar]
- 5.Snoeckx RL, Huygen P, Feldmann D, Marlin S, Denoyelle F, et al. GJB2 mutations and degree of hearing loss: a multicenter study. Am J Hum Genet. 2005;77:945–957. doi: 10.1086/497996. doi: 10.1086/497996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estivill X, Fortina P, Surrey S, Rabionet R, Melchionda S, et al. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet. 1998;351:394–398. doi: 10.1016/S0140-6736(97)11124-2. doi: 10.1016/S0140-6736(97)11124–2. [DOI] [PubMed] [Google Scholar]
- 7.Green GE, Scott DA, McDonald JM, Woodworth GG, Sheffield VC, et al. Carrier rates in the midwestern United States for GJB2 mutations causing inherited deafness. JAMA. 1999;281:2211–2216. doi: 10.1001/jama.281.23.2211. doi: 10.1001/jama.281.23.2211. [DOI] [PubMed] [Google Scholar]
- 8.Abe S, Usami S, Shinkawa H, Kelley P, Kimberling W. Prevalent connexin 26 gene (GJB2) mutations in Japanese. J Med Genet. 2000;37:41–43. doi: 10.1136/jmg.37.1.41. doi: 10.1136/jmg.37.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morell RJ, Kim HJ, Hood LJ, Goforth L, Friderici K, et al. Mutations in the connexin 26 gene (GJB2) among Ashkenazi Jews with nonsyndromic recessive deafness. N Engl J Med. 1998;339:1500–1505. doi: 10.1056/NEJM199811193392103. doi: 10.1056/NEJM199811193392103. [DOI] [PubMed] [Google Scholar]
- 10.Del Castillo I, Moreno-Pelayo MA, Del Castillo FJ, Brownstein Z, Marlin S, et al. Prevalence and evolutionary origins of the del(GJB6-D13S1830) mutation in the DFNB1 locus in hearing-impaired subjects: a multi-center study. Am J Hum Genet. 2003;73:1452–1458. doi: 10.1086/380205. doi: 10.1086/380205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilch E, Azaiez H, Fisher RA, Elfenbein J, Murgia A, et al. A novel DFN1 deletion allele supports the existence of a distant cis-regulatory region that controls GJB2 and GJB6 expression. Clin Genet. 2010;78:267–274. doi: 10.1111/j.1399-0004.2010.01387.x. doi: 10.1111/j.1399-0004.2010.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasparini P, Rabionet R, Barbujani G, Melçhionda S, Petersen M, et al. High carrier frequency of the 35delG deafness mutation in European populations. Eur J Hum Genet. 2000;8:19–23. doi: 10.1038/sj.ejhg.5200406. doi: 10.1038/sj.ejhg.5200406. [DOI] [PubMed] [Google Scholar]
- 13.Dzhemileva LU, Barashkov NA, Posukh OL, Khusainova RI, Akhmetova VL, et al. Carrier frequency of GJB2 gene mutations c.35delG, c.235delC and c.167delT among the populations of Eurasia. J Hum Genet. 2010;55:749–754. doi: 10.1038/jhg.2010.101. doi: 10.1038/jhg.2010.101. [DOI] [PubMed] [Google Scholar]
- 14.Guastalla P, Guerci VI, Fabretto A, Faletra F, Grasso DL, et al. Detection of epidermal thickening in GJB2 carriers with epidermal ultrasonography. Radiology. 2009;251:280–286. doi: 10.1148/radiol.2511080912. doi: 10.1148/radiol.2511080912. [DOI] [PubMed] [Google Scholar]
- 15.Meyer CG, Amedofu GK, Brandner JM, Pohland D, Timmann C, et al. Selection for deafness? Nat Med. 2002;8:1332–1333. doi: 10.1038/nm1202-1332. doi: 10.1038/nm1202-1332. [DOI] [PubMed] [Google Scholar]
- 16.Man YK, Trolove C, Tattersall D, Thomas AC, Papakonstantinopoulou A, et al. A deafness-associated mutant human connexin 26 improves the epithelial barrier in vitro. J Membr Biol. 2007;218:29–37. doi: 10.1007/s00232-007-9025-0. doi: 10.1007/s00232-007-9025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischel-Ghodsian N. Mitochondrial deafness mutations reviewed. Hum Mutat. 1999;13:261–270. doi: 10.1002/(SICI)1098-1004(1999)13:4<261::AID-HUMU1>3.0.CO;2-W. doi: 10.1002/(SICI)1098–1004(1999)13:4. [DOI] [PubMed] [Google Scholar]
- 18.Bitner-Glindzicz M. Hereditary deafness and phenotyping in humans. Br Med Bull. 2002;63:73–94. doi: 10.1093/bmb/63.1.73. doi: 10.1093/bmb/63.1.73. [DOI] [PubMed] [Google Scholar]
- 19.Li R, Greinwald J, Yang L, Choo DI, Wenstrup RJ, et al. Molecular analysis of the mitochondrial 12S rRNA and tRNASer(UCN) genes in paediatric subjects with nonsyndromic hearing loss. J Med Genet. 2004;41:615–620. doi: 10.1136/jmg.2004.020230. doi: 10.1136/jmg.2004.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanske AL, Shanske S, DiMauro S. The other human genome. Arch Pediatr Adolesc Med. 2001;155:1210–1216. doi: 10.1001/archpedi.155.11.1210. [DOI] [PubMed] [Google Scholar]
- 21.Finsterer J. Mitochondriopathies. Eur J Neurol. 2004;11:163–186. doi: 10.1046/j.1351-5101.2003.00728.x. doi: 10.1046/j.1351-5101.2003.00728. [DOI] [PubMed] [Google Scholar]
- 22.Fischel-Ghodsian N, Prezant TR, Chaltraw WE, Wendt KA, Nelson RA, et al. Mitochondrial gene mutation is a significant predisposing factor in aminoglycoside ototoxicity. Am J Otolaryngol. 1997;18:173–178. doi: 10.1016/s0196-0709(97)90078-8. doi: 10.1016/S0196-0709(97)90078-8. [DOI] [PubMed] [Google Scholar]
- 23.Lucotte G, Dieterlen F. The 35delG mutation in the connexin 26 gene (GJB2) associated with congenital deafness: European carrier frequencies and evidence for its origin in ancient Greece. Genet Test. 2005;9:20–26. doi: 10.1089/gte.2005.9.20. doi: 10.1089/gte.2005.9.20. [DOI] [PubMed] [Google Scholar]
- 24.Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, et al. Prelingual deafness: high prevalence of a30delG mutation in the connexin 26 gene. Hum Mol Genet. 1997;6:2173–2177. doi: 10.1093/hmg/6.12.2173. [DOI] [PubMed] [Google Scholar]
- 25.Rabionet R, Gasparini P, Estivill X. Molecular genetics of hearing impairment due to mutations in gap junction genes encoding beta connexins. Hum Mutat. 2000;16:190–202. doi: 10.1002/1098-1004(200009)16:3<190::AID-HUMU2>3.0.CO;2-I. doi: 10.1002/1098–1004(200009)16:3. [DOI] [PubMed] [Google Scholar]
- 26.Van Laer L, Coucke P, Mueller RF, Caethoven G. A common founder for the 35delG GJB2 gene mutation in connexin 26 hearing impairment. J Med Genet. 2001;38:515–518. doi: 10.1136/jmg.38.8.515. doi: 10.136/jmg.38.8.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothrock CR, Murgia A, Sartorato EL. Connexin 26 35delG does not represent a mutational hotspot. Hum Genet. 2003;113:18–23. doi: 10.1007/s00439-003-0944-2. doi 10.1007/s00439-003-0944–2. [DOI] [PubMed] [Google Scholar]
- 28.Antoniadi T, Rabionet R, Kroupis C, Aperis GA, Economides J, et al. High prevalence in the Greek population of the 35delG mutation in the connexin 26 gene causing prelingual deafness. Clin Genet. 1999;55(5):381–382. [PubMed] [Google Scholar]
- 29.Kokotas H, Van Laer L, Grigoriadou M, Iliadou V, Economides J, et al. Strong linkage disequilibrium for the frequent GJB2 35delG mutation in the Greek population. Am J Med Genet A. 2008;146A:2879–2884. doi: 10.1002/ajmg.a.32546. doi: 10.1002/ajmg.a.32546. [DOI] [PubMed] [Google Scholar]
- 30.Cryns K, Orzan E, Murgia A, Huygen PL, Moreno F, et al. A genotype-phenotype correlation for GJB2 (connexin 26) deafness. J Med Genet. 2004;41:147–154. doi: 10.1136/jmg.2003.013896. doi: 10.1136/jmg.2003.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmada M, Schmalisch K, Böhmer C, Schug N, Pfister M, et al. Loss of function mutations of the GJB2 gene detected in patients with DFNB1-associated hearing impairment. Neurobiology of Disease. 2006;22:112–118. doi: 10.1016/j.nbd.2005.10.005. doi: 10.1016/j.nbd.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Kokotas H, Grigoriadou M, Korres GS, Ferekidou E. Detection of deafness-causing mutations in the Greek mitochondrial genome. Dis Markers. 2011;30:283–289. doi: 10.3233/DMA-2011-0786. doi: 10.3233/DMA–2011-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rydzanicz M, Wróbel M, Pollak A, Gawecki W, Brauze D, et al. Mutation analysis of mitochondrial 12S rRNA gene in Polish patients with non-syndromic and aminoglycoside-induced hearing loss. Biochem Biophys Res Commun. 2010;395:116–121. doi: 10.1016/j.bbrc.2010.03.149. doi: 10.1016/j.bbrc.2010.03.149. [DOI] [PubMed] [Google Scholar]
- 34.Van Oven M, Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum Mutat. 2009;30:E386–394. doi: 10.1002/humu.20921. doi: 10.1002/humu.20921. [DOI] [PubMed] [Google Scholar]
- 35.Yang AF, Zhu Y, Lu JX, Yang L, Zhao JY, et al. [Mitochondrial DNA G7444A mutation may influence the phenotypic manifestation of the deafness-associated 12S rRNA A1555G mutation] [Article in Chinese]. Yi Chuan. 2008;30:728–734. doi: 10.3724/sp.j.1005.2008.00728. [DOI] [PubMed] [Google Scholar]
- 36.Mathew CC. Walker JMNJ, editor. The isolation of high molecular weight eucaryotic DNA. Methods in Molecular Biology, Clifton: Human Press, vol. 2. 1984. pp. 31–34. [DOI] [PubMed]
- 37.Toth T, Kupka S, Haack B, Fazakas F, Muszbek L, et al. Coincidence of mutations in different connexin genes in Hungarian patients. Int J Mol Med. 2007;20:315–321. [PubMed] [Google Scholar]
- 38.Wilcox SA, Saunders K, Osborn AH, Arnold A, Wunderlich J, et al. High frequency hearing loss correlated with mutations in the GJB2 gene. Hum Genet. 2000;106:399–405. doi: 10.1007/s004390000273. doi: 10.1007/s004390000273. [DOI] [PubMed] [Google Scholar]
- 39.Schon EA, Naini A, Shanske S. Copeland WC, Clifton NJ, editors. Identification of mutations in mtDNA from patients suffering mitochondrial diseases. Mitochondrial DNA. 2002. pp. 55–74. Methods and protocols. [Methods Mol Biol, vol. 197].Humana Press. [DOI] [PubMed]
- 40.Andrews R, Kubacka I, Chinnery P, Lightowlers R, Turnbull D, et al. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;2:147. doi: 10.1038/13779. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]