Abstract
The NIMA-related serine/threonine kinases (Neks) function in the cell cycle and regulate ciliary and flagellar length. The Giardia lamblia genome encodes 198 Neks, of which 56 are predicted to be active. Here we believe that we report the first functional analysis of two Giardia lamblia Neks. The GlNek1 and GlNek2 kinase domains share 57% and 43% identity to the kinase domains of human Nek1 and Nek2, respectively. Both GlNeks are active in vitro, have dynamic relocalization during the cell cycle, and are expressed throughout the life cycle, with GlNek1 being upregulated in cysts. Over-expression of inactive GlNek1 delays disassembly of the parental attachment disk and cytokinesis, while over-expression of either wild type GlNek1 or inactive mutant GlNek2 inhibits excystation.
Keywords: Giardia, Nek, Kinase, Mitosis, Excystation
1. Introduction
Giardia lamblia (synonymous with Giardia intestinalis and Giardia duodenalis) remains a major cause of water-borne diarrheal disease and food-borne illness worldwide (Kramer et al., 1996; Adam, 2001; Savioli et al., 2006; Budu-Amoako et al., 2011). The pathogenesis of this protozoan parasite depends on its biphasic lifecycle (Boucher and Gillin, 1990). Upon ingestion, dormant giardial cysts pass through the stomach where they are activated by gastric acid, although the parasites are protected by the cyst wall. Once the parasites reach the small intestine they rapidly exit the cyst wall, producing four trophozoites after two rounds of cell division. Trophozoites are characterized by their half-pear shape, two nuclei, eight flagella, each anchored by a basal body, and ventral attachment disk. While maintaining infection of the small intestine, parasites divide by binary fission. This cell division involves nuclear duplication, flagellar maturation and segregation, parental disk disassembly and daughter disk assembly (Nohynkova et al., 2006; Tumova et al., 2007; Dawson and House, 2010). Cell division is believed to be a rapid process as a functional cytoskeleton is required for attachment. Parasites that are swept downstream can sense the change in the environment and begin to encyst, which involves cytoskeletal rearrangements, reduced metabolism and motility, and formation of encystation secretary vesicles (ESVs) that export cyst wall components to the plasma membrane (Reiner et al., 1990; Marti et al., 2003; Palm et al., 2005). Mature cysts exit the host in feces and survive in fresh water, waiting to complete the infection cycle.
The molecular signaling pathways that ensure accurate spindle formation, chromosome segregation and final cell division during the giardial cell and life cycle, remain incompletely understood (Abel et al., 2001; Nohynkova et al., 2006; Dawson et al., 2007; Lauwaet et al., 2007; Davids et al., 2008; Alvarado and Wasserman, 2010). The four major mitotic kinase families that control eukaryotic chromosomal segregation during the cell cycle are Never in Mitosis Gene A-related (Nek), Aurora, Polo and cyclin-dependent kinases, (all with members that localize to the centrosomes/basal bodies of mammalian cells) (Malumbres and Barbacid, 2007). To date Aurora Kinase (AK) is the only kinase shown to be involved in the giardial cell cycle. AK is activated and localizes to the basal bodies and spindle microtubules during mitosis (Davids et al., 2008). The lone giardial member of the Polo kinase family, Plk1, was localized to the flagellar basal bodies (Morrison et al., 2007; Lauwaet et al., 2011; Manning et al., 2011).
Neks are known as regulators of the eukaryotic cell cycle and certain members of this family have been implicated in driving G2-M progression (Fry, 2002; Quarmby and Mahjoub, 2005; O’Regan et al., 2007; Liu et al., 2010; Chen et al., 2011a). Human Nek1 is reported to have a role in controlling meiotic events and mutations are implicated in polycystic kidney disease (Liu et al., 2002; Mahjoub et al., 2004; Shalom et al., 2008). The mammalian Nek1 is also involved with DNA damage sensing and repair, mitotic chromosome segregation and cytokinesis (Chen et al., 2011a, 2011b). Human Nek2 is the best-characterized member of the mammalian Nek family and has been described to regulate mitotic commitment, centriole splitting and promote proper kinetochore-microtubule connections (Chen et al., 2002; Fry, 2002; DeLuca et al., 2003; Faragher and Fry, 2003; Lou et al., 2004; Du et al., 2008). Over-expression of active HsNek2 in 293T cells leads to premature splitting of mother and daughter centrioles before the G2/M transition, resulting in the inhibition of mitotic onset (Fry, 2002; Liu et al., 2010). Additionally, during mitosis HsNek2 is essential for efficient kinetochore microtubule attachment and faithful chromosome segregation (Faragher and Fry, 2003; Lou et al., 2004; Fu et al., 2007; Du et al., 2008). Humans have 11 members of the Nek family, while Plasmodium falciparum, Chlamydomonas reinhardtii, Trypanosoma brucei, Leishmania major and Tetrahymena thermophila encode four, 11, 12, 13 and 39 Neks, respectively. In general, ciliated or flagellar organisms possess more members of the Nek family (Quarmby and Mahjoub, 2005; Parker et al., 2007). The Giardia genome encodes 198 Neks, which is strikingly more than any other reported organism (Morrison et al., 2007; Manning et al., 2011). Proteomic analysis of giardial mitosomes and a basal body enriched fraction identified six and 11 Neks, respectively. Localization studies have confirmed two of the Neks (Gl50803_16279 and GL50803_92498) to be basal body-associated proteins (Lauwaet et al., 2011), while several other Neks are either primarily cytoplasmic (Gl50803_101534 and Gl50803_15409) or associate with the anterior and caudal axonemes (Gl50803_5375) (Manning et al., 2011). The increased number of giardial Neks may be needed for the complex processes of flagellar maturation, cytoskeletal rearrangements and faithful chromosome segregation during the cell and life cycles (Nohynkova et al., 2006; Dawson et al., 2007; Parker et al., 2007; Tumova et al., 2007).
In this study, we characterized the two giardial Neks that share highest sequence identity with the kinase domains of human Nek1 and Nek2 and studied their roles in the cell and life cycles. Our study shows that these kinases are involved in regulating cell division and excystation.
2. Materials and methods
2.1. HMMER search of genome
The dataset containing the predicted proteins of Giardia lamblia Assemblage A isolate WB was downloaded from http://giardiadb.org (release-2.5). The single sequence query JackHMMER program (Johnson et al., 2010) was used to query the protein dataset for the presence of human Nek1 (Swiss-Prot, Q96PY6) and human Nek2 (Swiss-Prot, P51955). The inclusion threshold was set at 0.001.
2.2. Cell culture, encystation and excystation
Giardia trophozoites were cultured in modified TYI-S-33 medium with bovine bile (Keister, 1983), with encystation (Davids et al., 2011) and excystation induced as previously described (Keister, 1983; Boucher and Gillin, 1990). At 21 h encystation, the numbers of ESV were counted in live trophozoites using differential interference contrast optics. Cysts were collected after 48 h encystation from the bottom of the culture flasks and remaining trophozoites were lysed by incubation in double-distilled water for 20 min at 4°C. Water-resistant cysts were washed three times, counted and stored overnight in double-distilled water at 4°C. In Stage 1 of excystation, cysts were exposed to an acidic-reducing solution (57 mM L-cysteine HCl, 32.5 mM reduced glutathione, 0.1 M NaHCO3, in Hank s balanced salt solution, pH 2.0) for 30 min at 37°C. In Stage 2, acid-treated cysts were washed and treated with trypsin in pH 8.0 bicarbonate-buffered Tyrode s solution for 1 h at 37°C. Finally, excysting cysts were transferred to regular growth medium for 60 min at 37°C. The numbers of excyzoites were counted in three independent experiments. Simple one-way ANOVA was used to determine significance among excyzoite counts.
2.3. Epitope tagging of GlNeks
The promoter and coding region of GlNek1 and GlNek2 were amplified from 200 ng of G. lamblia genomic DNA using primers “GlNek1-Apa1-for” (5′-GGGCCCCCGGATGCGCGTCTGTTG-3′), “GlNek1-EcoRV-rev” (5′-GATATCCCTGACAGTATTGAACCTGTCC -3′), “GlNek2-Apa1-for” (5′-taagggccccagcatctagctgaatgccga-3′), and “GlNek2-EcoRV-rev” (5′-taagatatccatcttatacttgtaagcgcc-3′). The PCR products and a vector encoding the C-terminal HA tag were digested with ApaI and EcoRV. The digested PCR products and vector were gel extracted using a QIAquick Gel Extraction Kit (Qiagen, USA) and ligated overnight at 16°C. The targeted mutations, GlNek1 K22M (KM) and GlNek2 K64M, were introduced using the QuikChange site-directed mutagenesis method (Stratagene, USA). All plasmids were sequenced to confirm the presence of the desired mutation (Etonbio, USA). GlNek1 and GlNek2 over-expression plasmids were created by subcloning the Nek coding sequences from the wild type (WT) and mutant HA tag plasmids described above into the vector with the ornithine carbamoyl transferase (OCT) promotor using the primers 5′-CTCGAGATGGAACGCTACAAGGAGCTTAAGG-3′ for GlNek1, 5′-CTC GAGATGGCCAACGCAGGCGGACGAC-3′ for GlNek2, and the universal reverse primer 5′-GGGCCCCCTAAGTCGGATCCCTATGCATAGTCTGG-3′ (to include the 3xHA tag from the HA vector). The PCR products and the OCT over-expression vector were digested with XhoI and ApaI, gel extracted (Qiagen) and ligated overnight at 16°C. All plasmids were transformed into E. cloni 10G competent cells (Lucigen USA). DNA was purified from overnight bacterial cultures using a Maxiprep kit (Qiagen) and sequenced (Etonbio). Trophozoites were electroporated with 50 μg of plasmid DNA and transfectants were maintained through puromycin selection.
2.4. Cellular localization of GlNek1 and GlNek2
Localization of GlNek1 and GlNek2 during mitosis was assessed by IFA. Parasites were grown on coverslips in anaerobic chambers to enrich for adherent mitotic cells. The cells were fixed for 10 min in cold methanol (−20°C), dried, permeabilized in 0.5% Triton-X 100 for 10 min, and blocked for 1 h in block solution (10% goat serum, 1% glycerol, 0.1% BSA, 0.1% fish gelatin and 0.04% sodium azide in PBS). The coverslips with fixed parasites were incubated with 1/500 mouse anti-phosphorylated Aurora Kinase A (pAK) antibody (Abcam, USA) for 1 h, washed with PBS, incubated with 1/100 anti-HA-FITC and 1/800 goat-anti-mouse-Alexa, and washed four times with PBS. All antibodies were diluted in block solution. Cells were post-fixed with 4% paraformaldehyde, rinsed and mounted with Prolong Gold with DAPI (Molecular Probes, USA). Fluorescence was observed and photographed on a Nikon Eclipse E800 microscope equipped with an X-Cite™ 120 fluorescence lamp and 1000x magnification (Nikon Instruments Inc., USA).
2.5. Expression and purification rGlNek1 and GlNek2
The genes encoding GlNek1 (GL50803_92498) and GlNek2 (GL50803_5375) were cloned into the bacterial expression vector pETite C-His (Lucigen) using the primers 5′-GAAGGAGATATACATATGGAACGCTACAAGGAGCTTAAGG-3′ and 5′-GTGATGGTGGTGATGATGCCTGACAGTATTGAACCTGTCC-3′ for GlNek1, and 5′-GAAGGAGATATACATATGGCCAACGCAGGCGGACGAC-3 and 5′-GTGATGGTGGTGATGATGCATCTTATACTTGTAAGCGCC-3′ for GlNek2. The targeted mutations, GlNek1 K22M and GlNek2 K64M, were introduced using the QuickChange site-directed mutagenesis method (Stratagene). All plasmids were sequenced to confirm the desired mutations (Etonbio). The four resulting vectors were individually transformed into Hi-Control BL21(DE3) cells and cells were induced with 1 mM IPTG to express WT or mutant His-tagged GlNek1 and GlNek2. WT and mutant GlNeks were purified from induced bacterial lysates using HisExpress columns (Claremont Biosolutions, USA) according to the manufacturer's recommendations.
2.6. Western blot analysis
Proteins from trophozoites and cysts were precipitated with trichoroacetic acid (TCA), separated on 4–20% Tris-glycine gels and transferred as previously described (Lauwaet et al., 2007). Filters were blocked for 1 h in 5% milk in PBS supplemented with 0.1% Tween-20 (PBST), incubated for 1 h in 5% milk in PBST with anti-His-HRP (horseradish peroxidase; Bethyl, USA) or anti-HA-HRP (Roche, USA). Filters were washed and the signal was developed with ECL-Plus (GE Healthcare, USA). As a protein loading control, filters were re-probed with an antibody against taglin (Ward et al., 1987).
2.7. Kinase assay
Kinase reactions (60 μl) were performed in kinase buffer (20 mM Tris-HCl pH 7, 20 mM MgCl2, 2 mM MnCl2, 165 μM ATP (Invitrogen), 300 nM recombinant WT or mutant GlNek1 or GlNek2, and 125 ng/μl recombinant human Histone H1 (New England BioLabs, USA), recombinant human Histone H3 (New England BioLabs), or BSA (Invitrogen). The reactions were incubated at 30°C for 45 min and stopped by adding 12 μl of 6X reducing Laemmli sample buffer and heating for 5 min at 100 °C. Kinase reactions (15 μl) were resolved on two 14% SDS-PAGE gels. The first set of gels was fixed, stained with ProQ Diamond (Invitrogen), destained and imaged as directed by the manufacturer s recommendations. The intensity of the observed bands was quantified with the ImageJ software. After imaging, these gels were stained with Coomassie Brilliant Blue to assess total protein content of the kinase reactions. The duplicate 14% SDS-PAGE gels were transferred to polyvinylidene fluoride (PVDF) membranes and processed for western blotting with the anti-His-HRP antibody.
3. Results
3.1. GlNek1 and GlNek2 are homologous to active NIMA-related kinases (Neks)
We used JackHMMER searches and the human HsNek1 and HsNek2 protein sequences to query the sequences in the G. lamblia genome. Our searches identified GL50803_92498 and GL50803_5375 as the closest hits with 57% and 43% identity to HsNek1 and HsNek2, respectively, within the kinase domains. We will refer to the proteins encoded by GL50803_92498 as GlNek1 and by GL50803_5375 as GlNek2. GlNek1 is 898 amino acids in length and is predicted to be ~102 kDa, while GlNek2 is 405 amino acids in length and is predicted to have a mass of ~45 kDa. Outside of the conserved kinase domain, GlNek1 has two coiled-coil domains and one PEST domain, while HsNek1 contains five coiled-coil domains and three PEST domains (Fig. 1A). GlNek2 has one coiled-coil domain, but no PEST domain. HsNek2 has a similar predicted mass (~51 kDa) to GlNek2, and also has one coiled-coil domain. GlNek1 and GlNek2 lack additional domains that are found in HsNek2: one KEN-box domain, one D-Box domain, a leucine zipper motif and a protein phosphatase 1 binding site (Fig. 1A). PEST, KEN-box and D-box domains are common features of proteins targeted for rapid degradation (King et al., 1996; Rechsteiner and Rogers, 1996; Barford, 2011). Both putative giardial kinases are predicted to be active based on the presence of all conserved serine/threonine kinase sub-domains, including the glycine loop that anchors the non-transferable ATP phosphates, the invariant lysine (K33 in HsNek1) that is required for maximal activity, and the DFG triplet that helps orient the ATP gamma-phosphate for transfer (Fig. 1B) (Hanks and Hunter, 1995).
Fig. 1.

Domain and sequence analyses of Giardia lamblia NIMA-related serine/threonine kinases, GlNek1 and GlNek2. (A) Domains of GlNek1 (Gl50803_92498), GlNek2 (Gl50803_5375), human HsNek1 (Q96PY6) and HsNek2 (P51955) proteins are color coded as shown in the box. (B) The kinase domains of GlNek1 and GlNek2 contain highly conserved kinase sub-domains. Amino acids conserved in more than 75% of sequences are highlighted in black and similar residues are shaded grey. Several critical sub-domains are indicated. HsNek1 (Q96PY6), HsNek2 (P51955), Trypanosoma brucei TbNrkC (DQ054526), Drosophila melanogaster DmNek2 (NM_132187), and Aspergillus nidulans AnNIMA (P11837) were compared in this alignment.
3.2. GlNek1 and GlNek2 are active kinases in vitro
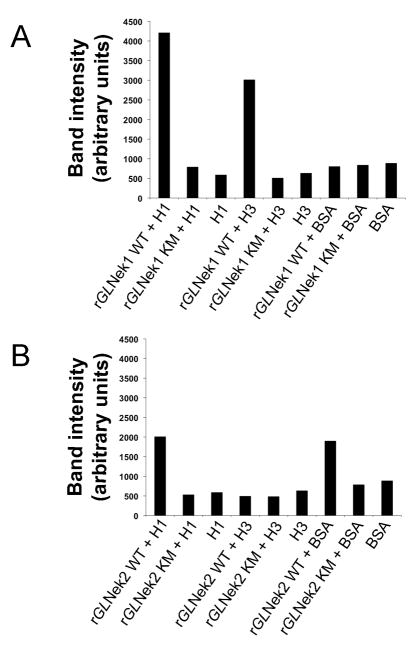
To determine whether GlNek1 and GlNek2 are active kinases, we expressed each in Escherichia coli as a C-terminal 6His-tagged protein and tested their activity with a standard kinase assay. Recombinant GlNek1 (rGlNek1-WT) and GlNek2 (rGlNek2-WT) were able to phosphorylate recombinant human histone H1 (H1), as indicated by the higher signal intensity compared with the background signal of the H1 substrate alone (Fig. 2A and B). rGlNek1-WT, but not rGlNek2-WT, phosphorylated recombinant human histone H3 (H3), while rGlNek2, but not rGlNek1, phosphorylated BSA. Mutated rGlNek1-KM and rGlNek2-KM, which have a methionine instead of the invariant lysine in sub-domain II of the kinase domain had no detectable kinase activity. This rules out the possibility that the kinase activity was due to bacterial contamination of the purified Neks. From these data we conclude that under these conditions, rGlNek1-WT and rGlNek2-WT are active kinases that display different preferences towards the artificial substrates tested. Moreover, the invariant lysine in subdomain II is necessary for kinase activity, as observed with human Nek2, trypanosome TbNRKC and plasmodial Nek2 (Fry et al., 1995; Pradel et al., 2006; Reininger et al., 2009).
Fig. 2.
Wild type recombinant Giardia lamblia NIMA-related serine/threonine kinases, GlNek1 and GlNek2, proteins are active in vitro. Bar graphs illustrate the band intensities of the substrates H1, H3 or BSA after incubation in the presence of wild type (WT) or mutant (KM) recombinant GlNek1 (rGlNek1) (A) or rGlNek2 (B). Reactions containing only H1, H3 or BSA substrates without wild type or mutant kinases were used as background controls.
3.3. GlNek1 and GlNek2 have dynamic localization during mitosis
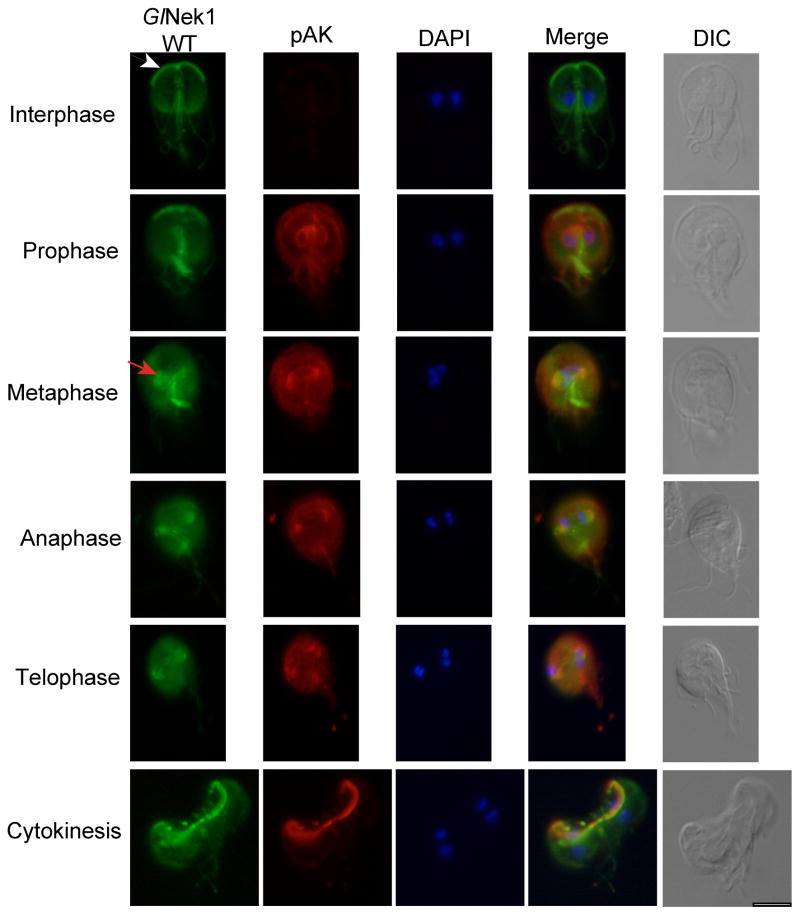
During interphase, GlNek1 localizes to the basal bodies, ventral disk, median body, anterior paraflagellar dense rods (PFR) and/or axonemes, and intracellular portions of the caudal and posterior-lateral axonemes (Fig. 3) (Lauwaet et al., 2011; Manning et al., 2011). During mitosis AK is activated by phosphorylation and is a valuable marker for identifying and characterizing cells in the progressive stages of mitosis (Davids et al., 2008). To identify Nek localization throughout mitosis, we double stained cells expressing HA-tagged GlNek1 or GlNek2 (GlNek1-WT and GlNek2-WT) under their own promoter with anti-HA and an antibody against the phosphorylated form of Aurora Kinase (anti-pAK). GlNek1-WT has a dynamic localization pattern throughout mitosis, similar, although not identical, to that observed for pAK (Fig. 3). The characteristic GlNek1-WT staining in the anterior PFR/axonemes (Fig. 3, white arrow) decreases from interphase to metaphase, where it is no longer detected. This is likely due to the internalization and re-localization of the anterior flagella during the cell cycle (Nohynkova et al., 2006). Like pAK, GlNek1-WT localizes to the re-organized basal bodies, some of which form the spindle poles, during metaphase and anaphase (Fig. 3, red arrow). GlNek1-WT and pAK both localize to the unfolding parental disk, which disassembles during cytokinesis. Overall, GlNek1-WT partially co-localizes with pAK during mitosis, with several areas of the cells being more strongly stained by either pAK (i.e. the area around the nuclei in prophase and spindle fibers in metaphase) or anti-HA (GlNek1-WT) (i.e. median bodies in prophase and metaphase) depending on the stage of mitosis.
Fig. 3.
Giardia lamblia NIMA-related serine/threonine kinase 1 (GlNek1) partially co-localizes with phosphorylated Aurora kinase during mitosis. Trophozoites expressing wild type GlNek1 under the control of its own promoter were co-stained with antibodies against the HA-tag and phosphorylated Aurora Kinase (pAK). DAPI illustrates the localization of the nuclei. The arrowhead indicates the anterior axonemes/paraflagellar rods. The arrow indicates the re-organized basal bodies. The corresponding differential interference contrast (DIC) image is shown. Bar = 10 μm.
GlNek2-WT localizes to the anterior and caudal flagellar axonemes during interphase (Manning et al., 2011) (Fig. 4, white arrow). Similar to GlNek1-WT, GlNek2-WT localization to the anterior PFR and axonemes is less intense in metaphase compared with interphase cells. During metaphase and anaphase, GlNek2-WT localizes to the spindle poles (Fig. 4, red arrow) and spindle microtubules, similar to pAK. During telophase and cytokinesis, GlNek2-WT is primarily observed in the developing daughter anterior PFR and axonemes (Fig. 4, white arrows). Unlike pAK and GlNek1-WT, GlNek2-WT is not readily detected in the disassembling parental disk.
Fig. 4.
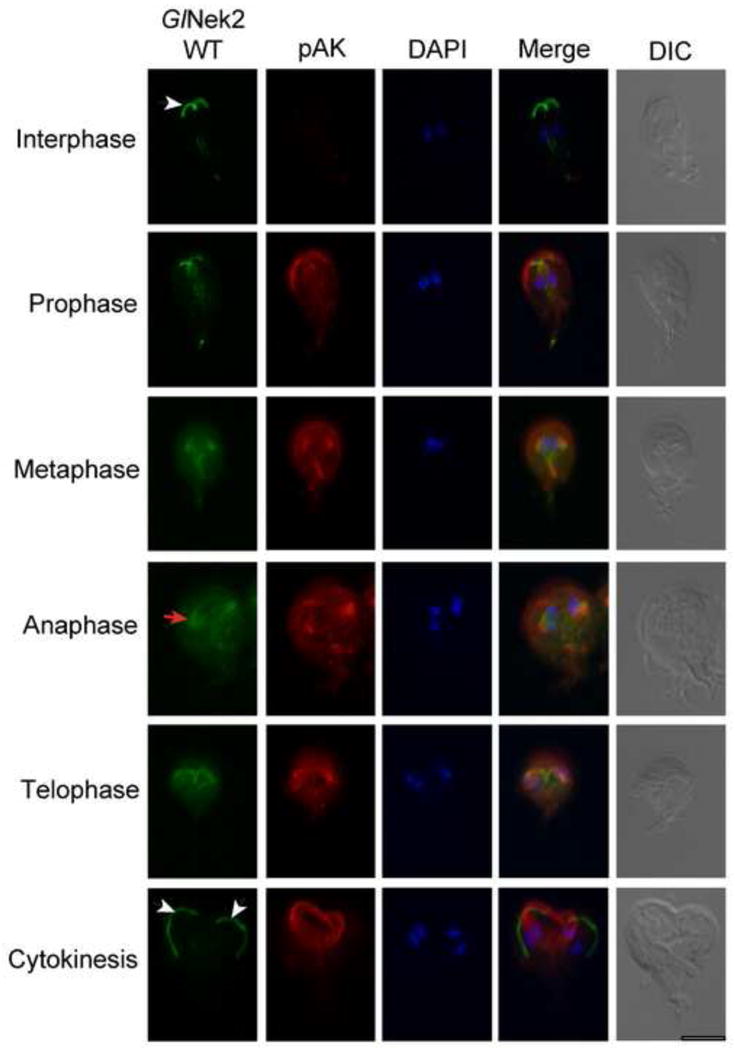
Giardia lamblia NIMA-related serine/threonine kinase 2 (GlNek2) localizes to the spindle poles and spindle apparatus during the cell cycle. Trophozoites expressing HA-tagged GlNek2-wild type (WT) under the control of its own promoter were co-stained with antibodies against the HA-tag and phosphorylated Aurora Kinase (pAK). DAPI illustrates the localization of the nuclei. The arrowheads indicate the anterior axonemes/paraflagellar rods. The arrow indicates the re-organized basal bodies. The corresponding differential interference contrast (DIC) image is shown. Bar = 10 μm.
3.4. Over-expression of kinase dead GlNek1 or GlNek2 leads to mitotic defects
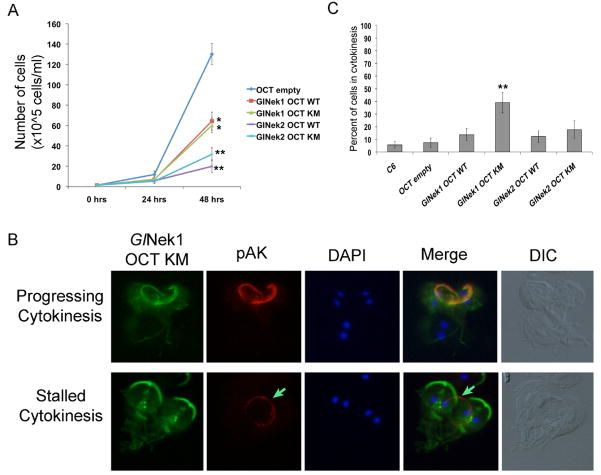
To assess whether GlNek1 and GlNek2 are involved in Giardia growth, we over-expressed both WT and KM GlNek1 and GlNek2 under the highly active OCT promoter and compared the growth rate of these cell lines with the growth of a control cell line with an empty expression vector (OCT-empty). Over-expression was confirmed by western blot (Supplementary Fig. S1). A slightly higher molecular weight band was observed in the GlNek1-OCT-KM sample, which may be the result of post-translational modification, such as phosphorylation. Cells expressing either GlNek1-OCT-WT or GlNek1-OCT-KM have significantly decreased growth rates, and cell lines expressing GlNek2-OCT-WT and GlNek2-OCT-KM have an even more pronounced growth defects (Fig. 5A). The localization of GlNek1-WT and KM, from interphase to telophase, in the over-expressing cell lines was the same as in the cell lines expressing endogenous GlNek1-WT levels (data not shown). However, approximately 50% of GlNek1-OCT-KM cells in cytokinesis appeared to be delayed in cytokinesis as evidenced by their intact parental disk outlined by pAK staining (Fig. 5B, “Stalled Cytokinesis”, green arrows). The remaining ~50% of GlNek1-OCT-KM cells in cytokinesis displayed the wild type localization of GlNek1 and pAK (Fig. 5B, “Progressing Cytokinesis”). In the “Stalled Cytokinesis” sub-population of cells, GlNek1-OCT-KM localized to the daughter disks, basal bodies, anterior PFR and axonemes, median body and intracellular portions of the caudal and posterior-lateral axonemes of the daughter cells. This indicated that the daughter cells are nearly fully formed. More dorsally focused images of the cells (Fig. 5B) did not reveal the presence of pAK labeled basal bodies or spindle microtubules, confirming that the cells are indeed in cytokinesis and not telophase (data not shown). Cell counts of live cultures revealed that ~39% of cells over-expressing the mutant form of GlNek1 were in cytokinesis compared with ~6 and ~7% in the C6 parental and OCT empty vector control cell lines, respectively (Fig. 5C). No significant differences were found among the cell lines expressing GlNek1-OCT-WT, GlNek2-OCT-WT, or GlNek2-OCT-KM. The significant increase in cells expressing GlNek1-OCT-KM in cytokinesis correlates with the phenotype observed by immunofluorescence, and further indicates that endogenous GlNek1 may have a role in regulating the disassembly of the parental disk. The growth defects of parasites over-expressing wild type GlNek1 or GlNek2 or mutant GlNek2 were not associated with any obvious morphological changes revealed by immunocytochemistry.
Fig. 5.
Effect of Giardia lamblia NIMA-related serine/threonine kinases, GlNek1 and GlNek2, on growth and cytokinesis. (A) Equal numbers of cells over-expressing wild type (WT) or mutant (KM) GlNek 1 or GlNek2 were inoculated at time 0 and cell growth was determined after 24 and 48 h. * P < 0.001, ** P < 0.0001. (B) Immunofluorescence analyses of GlNek-OCT-KM trophozoites with antibodies against the HA-tag and phosphorylated aurora kinase (pAK) show a large proportion of cells with an intact pAK labeled parental disk during cytokinesis (“Stalled Cytokinesis”), indicated by an arrow. Normally, cells in cytokinesis (“Progressing Cytokinesis”) display the typical pAK stained and pontoon shaped disassembling parental disk. The corresponding differential interference contrast (DIC) image is shown. (C) Bar graph represents the percentage of cells in cytokinesis per strain. ** P = 0.0001. Simple one-way ANOVA was used to determine statistical significances.
3.5. Over-expression of wild type GlNek1 or mutant GlNek2 inhibits excystation
To determine whether GlNek1 or GlNek2 have roles in regulating the giardial life cycle, we first tracked the endogenous protein expression levels of GlNek1-WT and GlNek2-WT in lysates of vegetative, encysting and excysting cells. Western blots show that the expression of GlNek1-WT dropped slightly during encystation and increased ~3.4 and ~2.4 fold in cysts and excystation stage 1 (S1), respectively, relative to the vegetative level of expression (Fig. 6A). The protein expression of GlNek2-WT increased ~1.7 fold during encystation through S1, relative to vegetative expression. Expression of both GlNeks decreased below vegetative levels during stage 2 of excystation. The protein expression of both GlNeks correlated well with the mRNA expression levels in the life cycle as shown by Serial Analysis of Gene Expression (SAGE) (www.giardiadb.org).
Fig. 6.
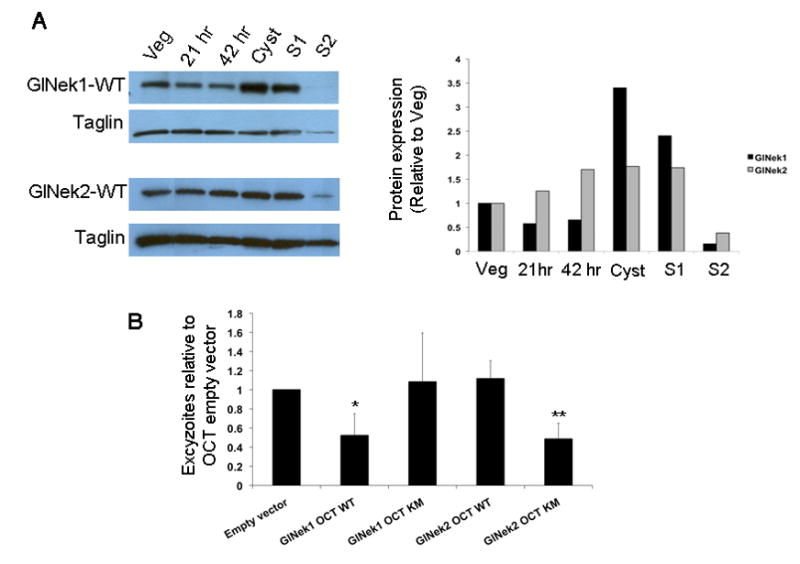
Giardia lamblia NIMA-related serine/threonine kinases, GlNek1 and GlNek2, in the Giardia life cycle. (A) Cell lines expressing GlNek1-HA or GlNek2-HA under their own promoter were encysted and excysted. Total protein levels of GlNek 1 and GlNek2 from vegetative trophozoites (Veg), 21 h or 42 h encysting trophozoites, cysts and Stage 1 (S1) and Stage 2 (S2) excysting cells were compared by Western blot with the anti-HA-HRP (horseradish peroxidase) antibody. Taglin was used as a protein loading control. The graph shows the band intensities of GlNek1 and GlNek2 relative to vegetative cells (Veg). (B) Equal numbers of cysts from cell lines over-expressing wild type (WT) or mutant (KM) GlNek1 or GlNek2 were excysted and excyzoites were counted. Bars represent the mean number of excyzoites from three independent experiments from each cell line relative to the empty vector control. * P < 0.05, ** P < 0.005. Simple one-way ANOVA was used to determine statistical significance.
We next assessed the ability and efficiency of cell lines over-expressing WT or KM GlNek1 and GlNek2 to encyst and excyst in vitro. There were no significant differences in the numbers of ESVs and cysts, relative to the parental and OCT-empty vector control cell lines (data not shown). However, water-resistant cysts from parasites expressing GlNek1-OCT-WT and GlNek2-OCT-KM both released ~ 50% fewer excyzoites than the cysts derived from the OCT-empty control (Fig. 6B). Cysts from GlNek1-OCT-KM and GlNek2-OCT-WT cell lines excysted equally as well as the OCT- empty-vector control cysts. These data indicate that excess WT GlNek1 can be deleterious to excystation and that the kinase activity of GlNek2 is necessary for efficient excystation.
4. Discussion
The giardial Nek kinase family is dramatically expanded (Morrison et al., 2007; Lauwaet et al., 2011; Manning et al., 2011), and there is a significant likelihood that several functional redundancies will be identified in future studies. However, the C- terminal domains of most of the 198 giardial Neks are highly divergent (Manning et al., 2011), indicating that these kinases can have unique protein-protein interactions, substrate specificities and cellular localizations, leading to a wide array of potential signaling functions. GlNek1 and GlNek2 share high sequence identity only within their kinase domains and significant overall domain architecture similarity to human HsNek1 and HsNek2, respectively. Both giardial Neks possess the conserved sub-domains required for serine/threonine kinase activity and our experiments validated this prediction. In interphase cells, both GlNek1 and GlNek2 localize to cytoskeletal structures, including the anterior and caudal axonemes, with GlNek1 additionally localizing to the basal bodies, median bodies, attachment disk and posterior-lateral axonemes. During the cell cycle, both GlNek1 and GlNek2 have dynamic localization patterns and target to the spindle poles (GlNek1 and GlNek2), the spindle microtubules (GlNek2) and the disassembling parental disk (GlNek1), suggesting they might have distinct functions during mitosis.
To assess the function of GlNek1 we over-expressed its active WT and inactive KM forms in Giardia. Both GlNek1-OCT-WT and GlNek1-OCT-KM over-expressing lines had growth deficiencies and a subset of GlNek1-OCT-KM cells stalled during cytokinesis. In contrast to GlNek1-OCT-WT cells, ~50% of the heart-shaped, dividing GlNek1-OCT-KM cells had parental disks that failed to disintegrate, likely inhibiting these cells from completing cytokinesis (Fig. 5B). This indicated that the kinase activity of the endogenous GlNek1 is likely involved in regulating the normal disassembly of the parental disk during cell division. Proper formation of the ventral disk is critical for trophozoites to complete cytokinesis and maintain infection (Tumova et al., 2007). Our data place GlNek1 in the correct location to influence ventral disk microtubule dynamics and indicate that GlNek1 may act as a positive regulator of parental disk microtubule disassembly. If this is the case, over-expression of active wild type GlNek1 would lead to premature disassembly of the parental disk or delayed assembly of the daughter disks during telophase, both of which could result in the decreased growth rate. In summary, our data suggest that an imbalance of both active and inactive GlNek1 affects growth and cytokinesis.
Of the 198 giardial Neks, the sequence of GlNek2 is most similar to that of HsNek2. Expression of either GlNek2-OCT-WT or GlNek2-OCT-KM led to greatly decreased growth rates. Similar to HsNek2, the over-expression of active or inactive GlNek2 could result in reduced growth due to inhibition of mitotic onset (active) or inaccurate chromosome segregation (inactive) (Fry, 2002; Liu et al., 2010).
We next assessed the potential functions of GlNek1 and GlNek2 during the life cycle. We did not observe any significant effects on encystation of cell lines over-expressing active or inactive GlNek1 or GlNek2. However, GlNek1-OCT-WT and GlNek2-OCT-KM cysts were significantly reduced in their capacity to excyst. Our growth and localization data suggest that GlNek1 helps to regulate disassembly of the parental disk. Hence, cells over-expressing active GlNek1 may be less able to assemble new daughter disks (Tumova et al., 2007), resulting in fewer excyzoites. Since disk disassembly is not necessary until the second round of excyzoite division, it is not surprising that GlNek1-OCT-KM cells defective in disk disassembly had no phenotype in early excystation. For GlNek2, we saw the opposite effect: cells over-expressing KM GlNek2 but not the WT GlNek2 showed reduced excystation. Similar to HsNEK2, GlNek2 may be involved in accurate chromosome segregation through regulation of kinetochore microtubules. During excystation, the 4 N content of each of the nuclei in the emerging excyzoite must be properly segregated twice to yield four trophozoites containing the correct DNA content (Bernander et al., 2001). Since GlNek2 localizes to the basal bodies, over-expression of inactive GlNek2-OCT-KM could inhibit activation of proteins necessary to properly connect the kinetochore microtubules to the chromosomes. This would adversely affect the accurate segregation of the chromosomes, resulting in non-viable excyzoites. Over-expression of HsNek2 adversely affects mitotic entry of 293T cells (Liu et al., 2010), however over-expression of GlNek2 did not inhibit the emergence and division of early excyzoites. Most likely, excyzoites that emerge from cysts are already past the mitotic commitment checkpoint, given their 16 N state. In this case, over-expressing GlNek2-OCT-WT would not inhibit the first round of cell division of excystation. Over-expression of the kinase-dead mutant GlNek1 and GlNek2 likely interferes with native GlNek signaling through sequestering upstream activating kinases and/or substrates in kinase inactive complexes.
We identified two members of the Giardia Nek family that are likely to have distinct functions during the giardial cell cycle and life cycle: GlNek1 in regulating the microtubule dynamics of the ventral disk and GlNek2 in regulating mitotic commitment and/or fidelity of chromosome distribution to daughter parasites. Our studies of these two giardial Neks begin to reveal the diversity of their expression, localization, kinase activity and function in growth and differentiation. Additional studies are needed to elucidate the complexity of the signaling pathways of these and other GlNeks and to help understand the striking expansion of these kinases in Giardia.
Supplementary Material
The GlNek-OCT-WT (wild type) and -KM (Lysine to Methionine mutant) cell lines over-express Giardia lamblia NIMA-related serine/threonine kinase GlNek1 or GlNek2. Total lysates from cell lines expressing HA-tagged GlNek1-WT or GlNek2-WT under their own promoters, or from cell lines over-expressing (OCT) WT or KM GlNek1 and GlNek2, were separated on SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes for western blot analysis. Membranes were probed with anti-HA-HRP (horseradish peroxidase) or with anti-taglin as a loading control. Bar graphs show the relative band intensities of the over-expressed GlNeks compared with the same GlNeks under control of their own promoters. The “empty” vector (OCT empty) was used as a negative control.
Highlights.
The first report of functional analysis of two Giardia lamblia Nek kinases.
Both GlNeks have dynamic re-localization during the cell cycle.
Over-expression of inactive Nek1 delays parental disk disassembly during the cell cycle.
Over-expression of wild type Nek1 or inactive Nek2 inhibits excystation.
Acknowledgments
We thank Nathan Low, Nele Nijs, Maya Millman-Gray and Cynthia Quindoza for excellent technical support and Dr. Heidi Elmendorf for additional support and guidance. This work was supported by National Institutes of Health (NIH), USA, grants AI42488 and AI75527. AJS was supported by the Gastroenterology Training Program, T32 DK007202.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel ES, Davids BJ, Robles LD, Loflin CE, Gillin FD, Chakrabarti R. Possible roles of protein kinase A in cell motility and excystation of the early diverging eukaryote Giardia lamblia. J Biol Chem. 2001;276:10320–10329. doi: 10.1074/jbc.M006589200. [DOI] [PubMed] [Google Scholar]
- Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado ME, Wasserman M. Analysis of phosphorylated proteins and inhibition of kinase activity during Giardia intestinalis excystation. Parasitol Int. 2010;59:54–61. doi: 10.1016/j.parint.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Barford D. Structural insights into anaphase-promoting complex function and mechanism. Philos Trans R Soc Lond B Biol Sci. 2011;366:3605–3624. doi: 10.1098/rstb.2011.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander R, Palm JE, Svard SG. Genome ploidy in different stages of the Giardia lamblia life cycle. Cell Microbiol. 2001;3:55–62. doi: 10.1046/j.1462-5822.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- Boucher SE, Gillin FD. Excystation of in vitro-derived Giardia lamblia cysts. Infect Immun. 1990;58:3516–3522. doi: 10.1128/iai.58.11.3516-3522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budu-Amoako E, Greenwood SJ, Dixon BR, Barkema HW, McClure JT. Foodborne illness associated with Cryptosporidium and Giardia from livestock. J Food Prot. 2011;74:1944–1955. doi: 10.4315/0362-028X.JFP-11-107. [DOI] [PubMed] [Google Scholar]
- Chen Y, Riley DJ, Zheng L, Chen PL, Lee WH. Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J Biol Chem. 2002;277:49408–49416. doi: 10.1074/jbc.M207069200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen CF, Chiang HC, Pena M, Polci R, Wei RL, Edwards RA, Hansel DE, Chen PL, Riley DJ. Mutation of NIMA-related kinase 1 (NEK1) leads to chromosome instability. Mol Cancer. 2011a;10:5. doi: 10.1186/1476-4598-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen CF, Riley DJ, Chen PL. Nek1 kinase functions in DNA damage response and checkpoint control through a pathway independent of ATM and ATR. Cell Cycle. 2011b;10:655–663. doi: 10.4161/cc.10.4.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids BJ, Williams S, Lauwaet T, Palanca T, Gillin FD. Giardia lamblia aurora kinase: a regulator of mitosis in a binucleate parasite. Int J Parasitol. 2008;38:353–369. doi: 10.1016/j.ijpara.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Davids BJ, Gilbert MA, Liu Q, Reiner DS, Smith AJ, Lauwaet T, Lee C, McArthur AG, Gillin FD. An atypical proprotein convertase in Giardia lamblia differentiation. Mol Biochem Parasitol. 2011;175:169–180. doi: 10.1016/j.molbiopara.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot Cell. 2007;6:2354–2364. doi: 10.1128/EC.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SC, House SA. Life with eight flagella: flagellar assembly and division in Giardia. Curr Opin Microbiol. 2010;13:480–490. doi: 10.1016/j.mib.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca JG, Howell BJ, Canman JC, Hickey JM, Fang G, Salmon ED. Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr Biol. 2003;13:2103–2109. doi: 10.1016/j.cub.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Du J, Cai X, Yao J, Ding X, Wu Q, Pei S, Jiang K, Zhang Y, Wang W, Shi Y, Lai Y, Shen J, Teng M, Huang H, Fei Q, Reddy ES, Zhu J, Jin C, Yao X. The mitotic checkpoint kinase NEK2A regulates kinetochore microtubule attachment stability. Oncogene. 2008;27:4107–4114. doi: 10.1038/onc.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol Biol Cell. 2003;14:2876–2889. doi: 10.1091/mbc.E03-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Schultz SJ, Bartek J, Nigg EA. Substrate specificity and cell cycle regulation of the Nek2 protein kinase, a potential human homolog of the mitotic regulator NIMA of Aspergillus nidulans. J Biol Chem. 1995;270:12899–12905. doi: 10.1074/jbc.270.21.12899. [DOI] [PubMed] [Google Scholar]
- Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene. 2002;21:6184–6194. doi: 10.1038/sj.onc.1205711. [DOI] [PubMed] [Google Scholar]
- Fu G, Ding X, Yuan K, Aikhionbare F, Yao J, Cai X, Jiang K, Yao X. Phosphorylation of human Sgo1 by NEK2A is essential for chromosome congression in mitosis. Cell Res. 2007;17:608–618. doi: 10.1038/cr.2007.55. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- Johnson LS, Eddy SR, Portugaly E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinformatics. 2010;11:431. doi: 10.1186/1471-2105-11-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- King RW, Glotzer M, Kirschner MW. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol Biol Cell. 1996;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MH, Herwaldt BL, Craun GF, Calderon RL, Juranek DD. Surveillance for waterborne-disease outbreaks--United States, 1993–1994. MMWR CDC Surveill Summ. 1996;45:1–33. [PubMed] [Google Scholar]
- Lauwaet T, Davids BJ, Torres-Escobar A, Birkeland SR, Cipriano MJ, Preheim SP, Palm D, Svard SG, McArthur AG, Gillin FD. Protein phosphatase 2A plays a crucial role in Giardia lamblia differentiation. Mol Biochem Parasitol. 2007;152:80–89. doi: 10.1016/j.molbiopara.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwaet T, Smith AJ, Reiner DS, Romijn EP, Wong CC, Davids BJ, Shah SA, Yates JR, 3rd, Gillin FD. Mining the Giardia genome and proteome for conserved and unique basal body proteins. Int J Parasitol. 2011;41:1079–1092. doi: 10.1016/j.ijpara.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Hirohashi Y, Du X, Greene MI, Wang Q. Nek2 targets the mitotic checkpoint proteins Mad2 and Cdc20: a mechanism for aneuploidy in cancer. Exp Mol Pathol. 2010;88:225–233. doi: 10.1016/j.yexmp.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- Lou Y, Yao J, Zereshki A, Dou Z, Ahmed K, Wang H, Hu J, Wang Y, Yao X. NEK2A interacts with MAD1 and possibly functions as a novel integrator of the spindle checkpoint signaling. J Biol Chem. 2004;279:20049–20057. doi: 10.1074/jbc.M314205200. [DOI] [PubMed] [Google Scholar]
- Mahjoub MR, Qasim Rasi M, Quarmby LM. A NIMA-related kinase, Fa2p, localizes to a novel site in the proximal cilia of Chlamydomonas and mouse kidney cells. Mol Biol Cell. 2004;15:5172–5186. doi: 10.1091/mbc.E04-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Manning G, Reiner DS, Lauwaet T, Dacre M, Smith A, Zhai Y, Svard S, Gillin FD. The minimal kinome of Giardia lamblia illuminates early kinase evolution and unique parasite biology. Genome Biol. 2011;12:R66. doi: 10.1186/gb-2011-12-7-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Regos A, Li Y, Schraner EM, Wild P, Muller N, Knopf LG, Hehl AB. An ancestral secretory apparatus in the protozoan parasite Giardia intestinalis. J Biol Chem. 2003;278:24837–24848. doi: 10.1074/jbc.M302082200. [DOI] [PubMed] [Google Scholar]
- Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, Best AA, Cande WZ, Chen F, Cipriano MJ, Davids BJ, Dawson SC, Elmendorf HG, Hehl AB, Holder ME, Huse SM, Kim UU, Lasek-Nesselquist E, Manning G, Nigam A, Nixon JE, Palm D, Passamaneck NE, Prabhu A, Reich CI, Reiner DS, Samuelson J, Svard SG, Sogin ML. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–1926. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- Nohynkova E, Tumova P, Kulda J. Cell division of Giardia intestinalis: flagellar developmental cycle involves transformation and exchange of flagella between mastigonts of a diplomonad cell. Eukaryot Cell. 2006;5:753–761. doi: 10.1128/EC.5.4.753-761.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan L, Blot J, Fry AM. Mitotic regulation by NIMA-related kinases. Cell Div. 2007;2:25. doi: 10.1186/1747-1028-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm D, Weiland M, McArthur AG, Winiecka-Krusnell J, Cipriano MJ, Birkeland SR, Pacocha SE, Davids B, Gillin F, Linder E, Svard S. Developmental changes in the adhesive disk during Giardia differentiation. Mol Biochem Parasitol. 2005;141:199–207. doi: 10.1016/j.molbiopara.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Parker JD, Bradley BA, Mooers AO, Quarmby LM. Phylogenetic analysis of the Neks reveals early diversification of ciliary-cell cycle kinases. PLoS One. 2007;2:e1076. doi: 10.1371/journal.pone.0001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel LC, Bonhivers M, Landrein N, Robinson DR. NIMA-related kinase TbNRKC is involved in basal body separation in Trypanosoma brucei. J Cell Sci. 2006;119:1852–1863. doi: 10.1242/jcs.02900. [DOI] [PubMed] [Google Scholar]
- Quarmby LM, Mahjoub MR. Caught Nek-ing: cilia and centrioles. J Cell Sci. 2005;118:5161–5169. doi: 10.1242/jcs.02681. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- Reiner DS, McCaffery M, Gillin FD. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur J Cell Biol. 1990;53:142–153. [PubMed] [Google Scholar]
- Reininger L, Tewari R, Fennell C, Holland Z, Goldring D, Ranford-Cartwright L, Billker O, Doerig C. An essential role for the Plasmodium Nek-2 Nima-related protein kinase in the sexual development of malaria parasites. J Biol Chem. 2009;284:20858–20868. doi: 10.1074/jbc.M109.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 2006;22:203–208. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Shalom O, Shalva N, Altschuler Y, Motro B. The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett. 2008;582:1465–1470. doi: 10.1016/j.febslet.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Tumova P, Kulda J, Nohynkova E. Cell division of Giardia intestinalis: assembly and disassembly of the adhesive disc, and the cytokinesis. Cell Motil Cytoskeleton. 2007;64:288–298. doi: 10.1002/cm.20183. [DOI] [PubMed] [Google Scholar]
- Ward HD, Lev BI, Kane AV, Keusch GT, Pereira ME. Identification and characterization of taglin, a mannose 6-phosphate binding, trypsin-activated lectin from Giardia lamblia. Biochemistry. 1987;26:8669–8675. doi: 10.1021/bi00400a027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The GlNek-OCT-WT (wild type) and -KM (Lysine to Methionine mutant) cell lines over-express Giardia lamblia NIMA-related serine/threonine kinase GlNek1 or GlNek2. Total lysates from cell lines expressing HA-tagged GlNek1-WT or GlNek2-WT under their own promoters, or from cell lines over-expressing (OCT) WT or KM GlNek1 and GlNek2, were separated on SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes for western blot analysis. Membranes were probed with anti-HA-HRP (horseradish peroxidase) or with anti-taglin as a loading control. Bar graphs show the relative band intensities of the over-expressed GlNeks compared with the same GlNeks under control of their own promoters. The “empty” vector (OCT empty) was used as a negative control.