Abstract
AIMS
This study aimed to evaluate the influence of hormonal receptor and Ki-67 proliferation marker in predicting MRI accuracy of measuring residual tumor size in HER2 negative breast cancer receiving neoadjuvant chemotherapy (NAC).
METHODS
Fifty-four women were studied. Patients received AC and/or taxane-based regimens. The accuracy of MR determined clinical complete response (CCR) was compared to pathological complete response (pCR). The size of detectable residual tumor on MRI was correlated with pathology-diagnosed tumor size using Pearson’s correlation.
RESULTS
MRI correctly diagnosed 16 of the 17 pCR patients. There were 8 false negative diagnoses, 7 hormonal receptor (HR) positive and one HR negative. The overall sensitivity, specificity, and accuracy of MRI were 78%, 94%, and 83%, respectively. The positive predictive value was 97% and the negative predictive value was 67%. For MRI-pathology tumor size correlation, HR negative cancers showed a higher correlation (R=0.79) than HR positive cancers (R= 0.58). A worse MRI-pathology size discrepancy was found in HR positive cancer than in HR negative cancer (1.6±2.8 cm vs. 0.56±0.9 cm, p=0.05). Tumors with a low Ki-67 proliferation (<40%) showed a larger size discrepancy than those with a high Ki-67 proliferation (≥40%) (1.2±2.0 cm vs. 0.4±0.8 cm p=0.05).
CONCLUSIONS
The results showed that the diagnostic performance of MRI for breast cancer undergoing NAC is associated with molecular biomarker profile. Among HER2 negative tumors, the accuracy of MRI was worse in HR positive than negative cancers, and also worse in low proliferative than high proliferative tumors. These findings may help in surgical planning.
INTRODUCTION
Breast cancer is heterogeneous, and each individual patient responds differently to neoadjuvant chemotherapy (NAC). NAC is traditionally used to down-stage inoperable cancers or to facilitate better outcomes in breast-conservation surgery.1-3 However, how to perform a successful breast-conservation surgery after NAC is challenging. It is difficult to determine how much tissue should be removed, especially in patients who responded well to the treatment. NAC-treated breast cancers having a pathologically complete remission (pCR) are associated with an overall higher survival benefit.5-8 The favorable prognosis resulting from pCR is consistent among all different histological types of breast cancer, although this relationship strength is varied, and is molecular subtype specific.8 The pCR rates are known to vary among different subtypes. For example, hormonal receptor (HR) negative tumors tend to respond better to chemotherapy than do hormonal receptor positive cancers, and HER2 positive tumors treated with the targeted therapy trastuzumab are more likely to achieve pCR than HER2 negative tumors treated with NAC that does not include trastuzumab.
Many studies have investigated the role of breast magnetic resonance imaging (MRI) as a diagnostic tool for evaluating the extent of residual disease after NAC.9-11 Despite superior accuracy when compared with other modalities, MRI can over- or under- estimate residual tumor extent. This inaccurate assessment may be influenced by tumor response (some tumors shrink concentrically down to a single focus of residual tumor cells, while others break apart into scattered tumor cells in patchy patterns4), chemotherapeutic agent, or NAC-induced reactive changes within the tumor.12 The accuracy of MRI in patients who undergo NAC is also affected by the molecular characteristics of cancer.13, 14
The traditional prognostic makers for breast cancer, such as tumor size, stage, lymph node status, hormonal receptors, and HER2 receptor, have been well studied. A newer classification method to separate luminal and basal types based on molecular characterization through high-throughput gene expression profiling is being investigated. Luminal-A, luminal-B, and basal types have different responses to chemotherapy and different clinical outcomes. In particular, one subtype, triple negative (HER2 negative, estrogen receptor negative, and progesterone receptor negative) cancers, do not receive targeted therapy or hormonal therapy to control the disease, and usually have a poor prognosis. The highly proliferative nature of this tumor, however, makes it highly susceptible to NAC, allowing for optimal chemotherapeutic treatments to possibly change the prognosis.5,8,15-17 For example, in neoadjuvant setting, higher pCR rates following chemotherapy lead to improved outcome. The other two major molecular subtypes of breast cancer, HER2 positive cancers, and HER2 negative but hormonal receptor positive cancers 18,19 have a better prognosis than triple negative tumors.
It has been suggested that breast tumors that have HER2 overexpression may be less sensitive to taxane therapy than are HER2 negative tumors.20 However, targeted therapy using trastuzumab has been shown to greatly improve patient outcome.8,19,21 HER2 negative and hormonal receptor positive cancer (i.e. the luminal type) has no targeted therapy, but since some forms of hormonal therapy can be offered, the patient can still achieve a favorable prognosis. Two subtypes of luminal cancer, luminal-A and luminal-B, show different Ki-67 expression, with luminal-A more likely to have negative or low Ki-67 and luminal-B with high Ki-67.5,6 Typically luminal-A cancer is less aggressive and the disease can be controlled very well by hormonal therapy alone.2 Improved knowledge about the detection accuracy of residual disease after NAC by imaging may help the planning of an optimal surgery to achieve a tumor free margin. This is important to decrease re-excision rate and minimize local recurrence. The basal, Luminal-A, luminal-B types are associated with tumors with different molecular biomarkers including HER2, hormonal receptor and Ki-67, and these biomarkers may have different influence on the accuracy of post-NAC size measurement made by MRI.
Previous studies 13, 22 have shown that the diagnostic accuracy of MRI was better in HER2 positive than in HER2 negative cancer. More specifically, a higher false negative rate and a larger size discrepancy between imaging and pathology are more frequently found in HER2 negative than in HER2 positive cancer. Factors affecting the inaccurate evaluation of HER2 negative cancer are still not well known, but the evidence in the literature suggest that the hormonal receptor and Ki-67 may play an import role. In this study, we measured the tumor size after NAC with MRI in HER2 negative cancer, and compared the imaging findings with final pathology between hormonal receptor positive and hormonal receptor negative subgroups, as well as between high and low Ki-67 subgroups, to understand their influence on the diagnostic accuracy of MRI.
MATERIALS AND METHODS
Subjects
Between May 2002 and February 2010, 77 female patients with biopsy proven HER2 negative breast cancer undergoing NAC were evaluated with MRI. Several MRI studies before, during, and after the NAC were usually acquired. Since the purpose of this study was to correlate the MR imaging findings with final pathology, only patients who had a final MRI scan after completing NAC infusion and before surgery were included for analysis. Based on these criteria, 23 patients were excluded: 18 did not have a final scan following NAC, and 5 did not have surgery. The remaining 54 patients (age range 31-82, mean age 51) were analyzed. The pre-treatment tumor size ranged from 0.5 cm to 11.8 cm (mean±STD 4.6cm±2.8cm). The histological types included invasive ductal carcinoma (N=44), invasive lobular carcinoma (N=7), and mixed ductal carcinoma with lobular features (N=3). The morphological types included N=43 mass lesions and N=11 non-mass like enhancement lesions.
Immunohistochemical (IHC) and Fluorescent in Situ Hybridization (FISH) Analysis
Biomarker status was determined by immunohistochemical (IHC) and/or FISH analysis from biopsied tissue prior to NAC treatment. The HER2 was measured either by IHC analysis and or by FISH. On IHC analysis, a score of 3+ was considered positive, and scores of 0 to 1+ negative. Patients with a score of 2+ were further examined using FISH for HER2 gene amplification. By FISH analysis, HER2 was considered positive when the HER2 to chromosome 17 centromere ratio was above 2.0. Tumors were classified as estrogen receptor or progesterone receptor positive if immunoperoxidase staining of tumor cell nuclei 10% or greater. Of these 54 patients, 36 had positive estrogen receptor expression (≥10% staining) and 32 had positive progesterone receptor expression (≥10% staining). If either estrogen or progesterone receptor was positive, the tumor was considered HR positive, and there was a total of 38 HR positive cases and 16 HR negative cases. Ki-67 staining was evaluated as percent of nuclei showing a positive reaction. Only 44 patients had Ki-67 staining. By using 40% or greater as the cut-off value for high Ki-67 expression, there were 20 patients in the high Ki-67 group and 24 patients in the low Ki-67 group.
Neoadjuvant Chemotherapy Protocol
Treatment protocols were chosen by oncologists who evaluated all available information (renal function, overall health, tumor type, etc) at the time. Thirty-six patients received a combination of doxorubicin and cyclophosphamide (AC) and taxane based regimens (cremophor or albumin-bound paclitaxel and carboplatin), while two patients received only AC and sixteen patients only a taxane-based regimen. The 36 patients undergoing a combination of both regiments, received 2 to 4 cycles of AC given every 2 weeks, followed by weekly doses of taxane based regimens. Eleven of these 36 patients receiving this combination also received bevacizumab with taxane. The remaining 16 patients receiving only a taxane-based regimen, all received a weekly infusion of albumin-bound paclitaxel and carboplatin with the addition of bevacizumab every other week.
MRI Acquisition
The patients received breast MRI scans in either a 3T (N=27) or a 1.5T (N=27) MR scanner. The MRI acquired at 3T was performed on a Philips Achieva scanner (Philips Medical Systems, Best, Netherlands) with a dedicated, SENSE-enabled, bilateral 4-channel breast coil. The bilateral axial DCE-MRI was acquired using a 3D gradient-echo, fat-suppressed sequence with FOV= 31-36cm, acquisition slice thickness= 2mm, reconstructed slice thickness= 1mm, slice overlap= 1mm, image-matrix 480×480, TR/TE= 6.2/1.26 ms, flip-angle= 12 degrees, NSA= 1, SENSE-factor= 2. Seven dynamic frames, including 2 pre-enhanced and 5 post-enhanced, were acquired. The imaging temporal resolution was 1 minute and 38 seconds for each frame. The MRI acquired at 1.5T was performed on a Philips Eclipse unit (Philips Medical Systems, Cleveland, Ohio). The body radiofrequency coil was used for transmission, and a dedicated four-channel phased-array breast coil was used for receiving. The bilateral DCE-MRI was acquired using a 3D spoiled gradient-recalled echo (SPGR) radio frequency Fourier-acquired steady-state (RF-FAST) pulse sequence. A total of 32 axial slices with 4-mm thickness were used to cover both breasts. The imaging parameters were as follows: TR= 8.1 msec, TE= 4.0 msec, flip angle= 20°, matrix size= 256 × 128, and FOV= 38 cm. The scan time was 42 seconds per acquisition. The sequence was repeated 16 times for dynamic acquisitions, using four pre-contrast sets and 12 post-contrast sets. The earlier MRI studies from 2002 to 2007 were done at 1.5T; and at that time the protocol was designed to have a high temporal resolution. When the study was moved from 1.5T to 3.0T in 2007, in order to meet the breast MRI guideline the protocol was changed to improve the spatial resolution by reducing the temporal resolution. The elapsed time between the last MRI and surgery was on average 36 days (range=1-93, median =34).
MRI Interpretation
The tumor response after completion of NAC in the final MRI was interpreted based on subtracting the pre-contrast images from the post-contrast images, and the maximum intensity projections (MIPs) generated from the subtraction images. Two radiologists (J.C. for 1.5T and S.B. for 3.0T), with 6 and 5 years of experience in interpreting breast MRI, performed the MR residual tumor size measurement using the same measurement standard. The longest dimension of the tumor measured in MRI was used to correlate with pathological size. MR-pathology tumor size discrepancy was defined as the difference of MR-determined and pathology-determined tumor size. The range referred to the lowest and the highest MRI-pathology tumor size difference. When measuring the tumor size in MRI, the radiologist was blinded to the pathology results. Complete clinical response (CCR) was diagnosed when no enhancement or faint enhancement equal to the background normal breast tissue was noted in the previous lesion site in MRI. MR determined CCR was used to evaluate the accuracy of MRI in prediction of pCR.
Pathological Determination of Residual Disease Extent
Surgical specimens were fixed with 10% neutral-buffered formalin and stained with H&E for evaluation. For tumors that were clearly visible, usually 2 cm or larger, only gross measurements were made. Small residual tumors that were not clearly visible were measured microscopically across slides of known thickness. If no invasive tumor was found within all examined slides from the region of the tumor, a final diagnosis of pathological complete response (pCR) was given. The largest dimension provided by the pathologist was used in the comparative study. Patients with no residual tumor were given a size of 0.
Statistical Analysis
The statistical analysis was performed by using the GraphPad Software (La Jolla, California, USA). A Pearson correlation was used for comparing MRI-determined residual tumor size and pathological size. An unpaired t-test with Welch’s correction was used to evaluate the presence of significance between high and low proliferation of Ki-67 tumors as well as between HR positive and HR negative tumors. An F-test compares 2 populations’ variances. In this study we used F-test to compare the range (or variances) of tumor size discrepancy between groups. P<0.05 was considered significant.
RESULTS
Tumor Subtypes and Biomarker Status
The HR status, histological types, and morphological types of mass and non-mass lesions, are listed in Table 1. Among the 54 analyzed patients, 38 (38/54, 70%) had HR positive tumors, while 16 (16/54, 30%) were HR negative. HR positive tumors included 29 mass (76%) and 9 non-mass lesions (24%); HR negative tumors had 14 mass (87.5%) and only 2 non-mass lesions (12.5%). For histological types, all 8 tumors with lobular features were in the HR positive group. Of the 44 patients with available Ki-67 information, 37 patients presented as mass lesion and 7 presented as non-mass like enhancement lesions. Six of the 7 non-mass lesions had lower Ki-67 expression and only one showed high Ki-67 expression (6/24, 25% vs. 1/20, 5%).
Table 1.
Comparison between HR positive and HR negative breast cancer
| HR positive | HR negative | P value | |
|---|---|---|---|
| pCR Rate | 9/38 | 8/16 | 0.10 |
| Lesion Type | |||
| Mass Lesion | 29/38 | 14/16 | 0.22 |
| Non-mass Lesion | 9/38 | 2/16 | 0.22 |
| Histological Type | |||
| Pure Ductal Cancer | 28/38 | 16/16 | 0.06 |
| Mixed Ductal and Lobular Cancer | 10/38 | 0/16 | 0.06 |
| Surgery | |||
| Mastectomy | 21/38 | 12/16 | 0.23 |
| Lumpectomy | 15/38 | 3/16 | 0.21 |
| Excision | 2/38 | 1/16 | 1.0 |
| Diagnosis | |||
| True Negative | 9/38 | 7/16 | 0.44 |
| True Positive | 22/38 | 7/16 | 0.22 |
| False Negative | 7/38 | 1/16 | 0.27 |
| False Positive | 0/38 | 1/16 | 0.55 |
| Δ (MRI – Path) Size Range (cm) | 0 ~ 14 | 0 ~ 3.2 | <0.0001 |
| Δ (MRI – Path) Size Mean (cm) | 1.6±2.8 | 0.6±0.9 | 0.05 |
Diagnostic Performance of MRI
Table 1 also shows the comparison of pCR rate, surgical treatment, performance of MR diagnosis, and size discrepancy between MRI and pathology for both HR positive and HR negative cancers. Overall, 17 of the 54 patients (31%) were diagnosed as pCR, that is, without any remaining invasive cancer cells in the pathological examination. In this cohort, 50% (8/16) of HR negative patients achieved pCR, which was much higher than 24% (9/38) in HR positive patients. The difference was, however, not statistically significant (p=0.10). MRI correctly diagnosed 16 of the 17 pCR patients (true negative) as clinical complete response, showing no suspicious enhanced lesions (Figure 1). MRI had one false positive diagnosis, for which MRI showed lesion enhancement but pathological examination only showed ductal carcinoma in situ and hyperplasia. For the 44 patients with available Ki-67 information, 2 of the 24 (2/24, 8%) patients with low Ki-67 showed pCR, while 12 of the 20 patients (12/20, 60%) with high Ki-67 showed pCR. The difference of treatment response was statistically significant (p=0.0003) between these two groups.
Figure 1.
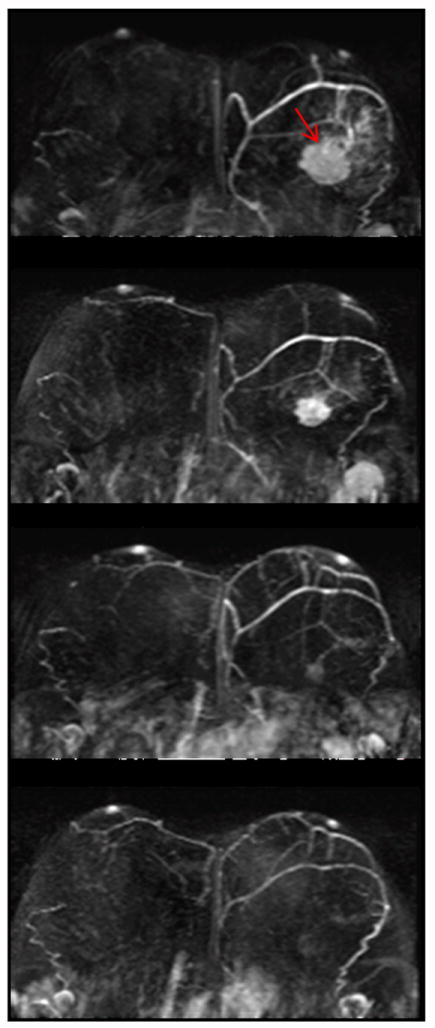
A triple negative tumor with high Ki67 proliferation in the left breast. The first image is baseline MRI prior to NAC. The second and the third images are follow-up MRI during NAC treatment. The fourth image is the last MRI after the completeness of NAC treatment. Following NAC, complete response was indicated by MRI, and confirmed by surgical pathology.
Pathological results showed residual invasive cancer in 37 patients (37/54, 69%). MRI detected enhanced lesions in 29 of these 37 patients (true positive). Among the remaining 8 patients with pathological-proven residual cancer, MRI found no suspicious enhancements (that is, false negative). Five of these 8 false negative patients were performed at 1.5T MR and three were performed at 3.0T MR. Seven of the 8 false negative diagnosis belonged to HR positive group, and only one patient was HR negative. Table 2 summarizes the MR tumor morphologies and final pathological findings of these eight patients falsely diagnosed as complete response by MRI.
Table 2.
The morphology of tumor, pathology diagnosis, and the elapsed time between MRI and surgery of patients who were incorrectly diagnosed as complete response by MRI.
| Patient | Morphology | Surgical pathology findings | Days between MRI and surgery |
|---|---|---|---|
| #1 | Mass | Three small scattered foci of infiltrating ductal carcinoma | 65 |
| #2 | NML | Infiltrating ductal carcinoma involving the skin | 43 |
| #3 | Mass | Infiltrating ductal carcinoma with a micropapillary pattern | 34 |
| #4 | Mass | Invasive ductal carcinoma with extensive fibrosis. | 4 |
| #5 | NML | Multiple scattered foci of invasive ductal carcinoma | 34 |
| #6 | NML | Invasive ductal carcinoma presented in an area of extensive fibrosis | 1 |
| #7 | Mass | Multiple scattered foci of invasive ductal carcinoma | 57 |
| #8 | NML | Infiltrating ductal carcinoma with ductal carcinoma in situ | 26 |
NML: Non-mass like enhancement lesion
The overall sensitivity, specificity, and accuracy of MRI diagnosis were found to be 78%, 94%, and 83%, respectively. The positive predictive value was 97% and the negative predictive value was 67%. In subgroup analysis, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of MRI diagnosis were 76%, 100%, 82%, 100%, and 56% for HR positive, and 88%, 88%, 88%, 88%, and 88% for HR negative cancers. It was obvious that the negative predictive value of MRI was higher in HR negative group than in HR positive group.
Accuracy of MRI in Diagnosing Residual Tumor Size
MRI and pathological tumor size discrepancy was analyzed for each case. The overall MRI-pathology correlation of residual tumor size for HER2 negative tumors showed Pearson R=0.59 (p<0.0001) (Figure 2). In subgroup analysis, HR negative (triple negative) cancers showed a higher correlation (R=0.79) than HR positive cancers (R=0.58). The average discrepancy between MRI and pathological tumor size for the whole cohort was 1.26 cm (median=0.3 cm, range=0-14 cm). A worse MRI-pathology tumor size discrepancy was found in HR positive cancer (Figures 3 and 4) than in HR negative cancer (Figure 5) (1.6±2.8 cm vs. 0.6±0.9 cm, p=0.05). The range of tumor size discrepancy was also significantly different between the two groups (0~14 cm vs. 0~3.2 cm, p<0.0001). In the HR positive group, four patients showed tumor size discrepancy larger than 5cm (5.8cm, 7cm, 7cm, and 14cm).
Figure 2.
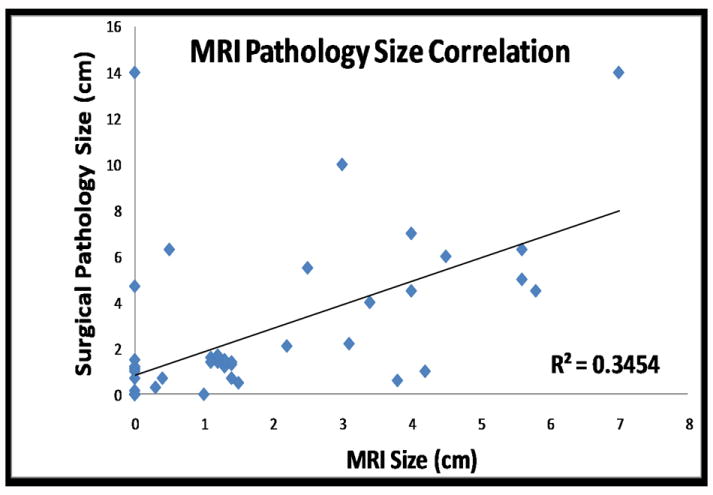
Correlation between residual tumor size post NAC determined by MRI and surgical pathology.
Figure 3.
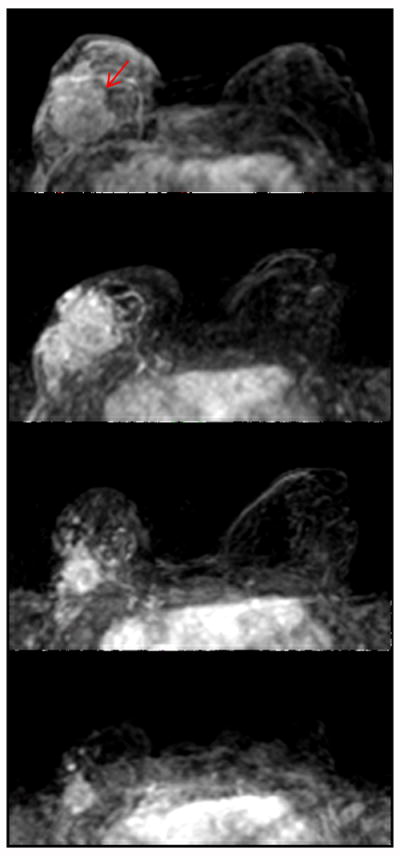
A hormonally positive, HER2 negative cancer with low Ki67 in the right breast. The first image is baseline MRI prior to NAC. The second and the third images are follow-up MRI during NAC treatment. The fourth image is the last MRI after the completeness of NAC treatment. MRI indicates a 2.5cm residual tumor after NAC, which differs markedly from a 5.5 cm tumor found in surgical pathology.
Figure 4.
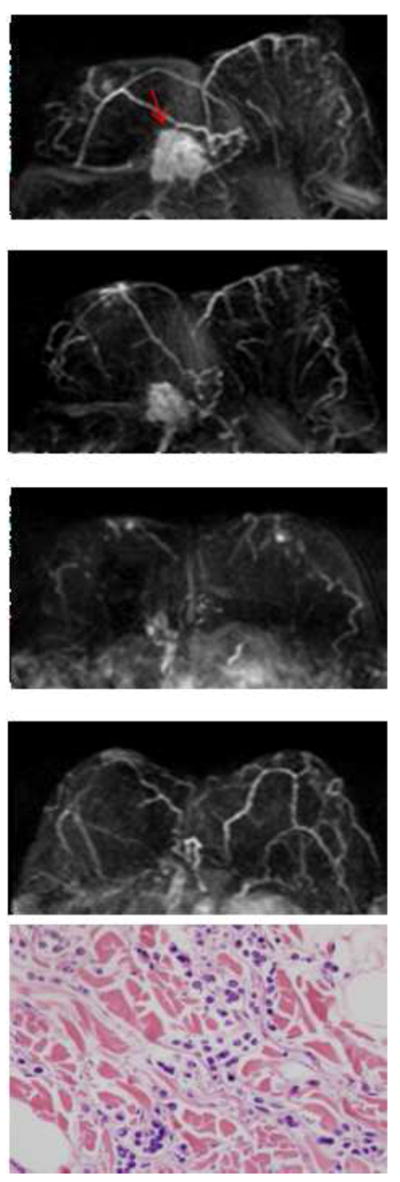
A hormonally positive, HER2 negative infiltrating lobular cancer in the right breast. The first image is baseline MRI prior to NAC. The second and the third images are follow-up MRI during NAC treatment. The fourth image is the last MRI after the completeness of NAC treatment. Following NAC, MRI showed a 0.5cm residual tumor, which differs markedly from a 6.3 cm tumor found in surgical pathology. In pathology, scattered residual tumor cells surrounded by chemotherapy associated changes, which include fibrosis and a lymphocyte infiltrate, are noted.
Figure 5.
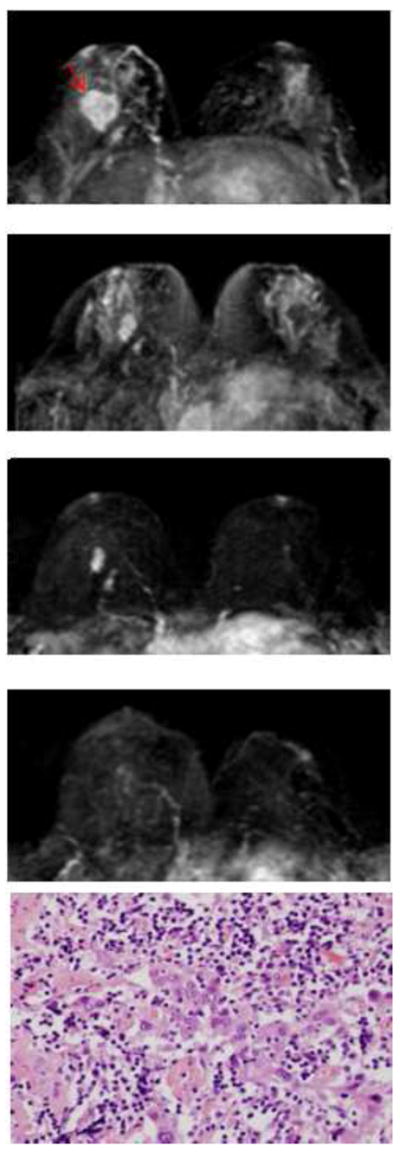
A triple negative tumor with high Ki67 proliferation in the right breast. The first image is baseline MRI prior to NAC. The second and the third images are follow-up MRI during NAC treatment. The fourth image is the last MRI after the completeness of NAC treatment. Following NAC, MRI indicated a 0.3cm residual tumor and surgical pathology found a 0.7cm tumor. Note that, compared to Figure 4, the residual tumor in pathology is more localized.
Tumors with a low Ki-67 proliferation (<40%) showed a higher size discrepancy than cancers with a high Ki-67 proliferation (≥40%) (1.2±2.0 cm vs. 0.4±0.8 cm, p=0.05). The range of tumor size discrepancy was also significantly different between the low and high Ki-67 proliferation groups (0~7 cm vs. 0~3.2 cm, p=0.0002).
Overall, the diagnostic performance of MRI for predicting residual tumor size is more accurate for triple negative cancers and cancers with high Ki-67 expression, which show a better NAC response (Table 1 and Table 3).
Table 3.
Tumor response and MRI performance between low and high Ki-67 tumor
| Low Ki-67 tumor (N=24) | High Ki-67 tumor (N=20) | P value | |
|---|---|---|---|
| Tumor response | 2/24 (8%) | 12/20 (60%) | 0.0003 |
| Accuracy of MRI | 1.2±2.0 cm | 0.4±0.8 cm | 0.05 |
| Range of size discrepancy | 0 - 7 cm | 0 - 3.2 cm | 0.0002 |
Tumor response was defined as pCR rate and accuracy of MRI was defined as MRI-pathology tumor size discrepancy
Comparison of MRI diagnostic accuracy between patients receiving different NAC regimens
Of the 38 HR positive patients, 26 sequentially received AC and taxane, 10 received only a taxane-based regimen, and 2 received only AC. In HR negative patients, 10 sequentially received AC and taxane, and 6 received only taxane-based regimen. Patients who received sequential AC and taxane-based regimen (N=36) had an average discrepancy between MRI and surgical pathology determined size of 1.1±2.5 cm. Patients who received only taxane-based regimen (N=16) were found to have average discrepancy of 1.8±2.4 cm, and the difference was not significant (p = 0.35). Two patients received AC only without taxane; due to the small case number this group was not compared with the other two treatment groups.
DISCUSSION
With the wide availability of increasingly effective chemo-regimens and targeted therapies, NAC can induce remarkable tumor shrinkage down to minimal residual tumor burden, or even to wipe out all invasive cancer and achieve pCR. Imaging assessment of NAC response may provide valuable information about the residual tumor and help the planning of an optimal surgery to achieve a tumor free margin. Compared with clinical examination, mammography, and ultrasound, MRI is considered to be the most accurate for evaluating the extent of residual tumor following NAC,11, 23-26, however, it may not detect small foci or scattered cancer cells/clusters that need little vascular supply to survive.13 It is, therefore, difficult to determine how much tissue should be removed, especially in patients who responded well to the treatment. Of the 607 patients studied in the German Preoperative Adriamycin Docetaxel trial, more than 70% were treated by breast conservation but 21.1% of these patients, who received breast conservation, required re-excision.27 It was found that for surgical planning, tumor characteristics and response to NAC should be taken into account.27
In our cohort of 54 patients with HER2 negative breast cancer, the diagnostic accuracy was different in subgroups with different hormonal receptor status and the proliferating activity measured by Ki-67. The post-NAC tumor size measured by MRI was more accurate in HR negative (that is, triple negative) than in HR positive cancers (p=0.05); and in tumors with high Ki-67 than low Ki-67 proliferating index (p=0.05). Triple negative cancers and high proliferating cancers are known to respond better to chemotherapy, and the result suggests that the diagnostic performance of MRI is more accurate when cancers show a better response.
The moderate overall correlation (Pearson R=0.59, p<0.0001) between tumor size determined by MRI and surgical pathology for HER2 negative tumors was consistent with previous studies.9,13,22 For HER2 positive cancer, which is very sensitive to the targeted therapy trastuzumab and chemotherapy, and generally highly proliferative, the treatment is more likely to eliminate the scattered residual cancer cells, leading to a higher negative predictive value of MRI.28 For HER2 negative cancer, there is no effective targeted regimen, and the residual disease is likely to present as scattered cells and difficult to diagnose by MRI.
Several studies have demonstrated a high accuracy of MRI for diagnosis of triple negative carcinoma.14, 22, 29 This high accuracy is probably, in part, due to its morphological characteristics. It is known that for non-mass lesions or lobular cancer, the residual disease is likely to present as the scattered pattern and is difficult to diagnose.13,28 Triple negative cancers are more likely to present as mass lesions, and have a lower prevalence of lobular features; these characteristics allow a higher diagnostic accuracy by MRI.22, 29-31 In our study, HR positive tumors were found more likely to be non-mass compared to triple negative tumors (9/38=24% vs. 2/16= 13%), and more likely to contain lobular features (8/38= 21% vs. 0/16= 0%). In Loo’s study on HER2 negative cancers, 38 of the 103 HR positive (37%) were non-mass like lesions, and this percentage was much lower in HR negative tumors (5 of 47, 11%). Similarly, they also showed that HR positive cancers were more likely to contain lobular carcinomas (18/103, 17.5%) than did the HR negative tumors (0%).22
Tumors with high Ki-67 proliferation achieved a higher pCR rate and had a smaller MRI-pathology size discrepancy when compared to tumors with low Ki-67 proliferation. As a cell proliferation marker, Ki-67 is associated with a higher expression of vascular endothelial growth factor (VEGF) in tumor cells,32 and the increased vascular supply and permeability may lead to a better delivery of chemo-regimens into the tumor tissue resulting in a better tumor response. Studies had shown that breast tumors with a low pre-treatment Ki-67 proliferation (< 15%) are less sensitive to chemotherapy.33 Therefore, the results of Ki-67 were in-line with that of hormonal receptor. A tumor with a high Ki-67 indicates it has a faster proliferation, thus a more aggressive tumor. An aggressive tumor is more likely to show a good response to chemotherapy, and that may lead to a more accurate diagnosis on MRI.
Literature regarding the cutoff value for defining high vs. low Ki-67 proliferation varied from study to study, ranging from 10% to 40%.34-39 In the analysis we have considered different values from 10% to 40% as the cut-off, and finally chose to separate the groups based on ≥40% (N=20) and <40% (N=24). Compared to using other numbers, choosing the 40% as the cutoff value showed the highest difference between the two groups with the p value reaching the significance level of p=0.05. Currently it is still not known how this cut-off value of 40% can be used for surgical planning following NAC. Further prospective studies are definitely needed to clarify its clinical role.
In this study, eight patients, four with mass lesions and four with non-mass like enhancement lesions, were falsely diagnosed by MRI as complete response. Following NAC, a breast cancer may be reduced to multiple tiny tumor foci distributed in an extensive fibrotic area,40-42 which may not be detected by MRI.13,28 This explains the high discrepancy in the tumor size measured by MRI and pathological examination. Chen et al have shown that size measured on MRI is highly accurate for mass lesions that shrink down to nodules; but is less accurate for non-mass lesions that reduce to scattered cells and clusters.13 In our current study, four patients had large size discrepancies greater than 5 cm between MRI and pathology. Three of the four patients presented with non-mass lesions. The limitation of MRI in detecting the minimal residual disease may affect surgical management. Residual tumor with minimal invasive cellularity may be associated with an increased risk of positive surgical margins.43 Since a positive margin is associated with an increased risk of local recurrence, breast-conserving surgery in these patients might be inadvisable.44
In our patient cohort, HR positive patients were more likely to receive a lumpectomy (15/38, 39%) than HR negative patients (3/16, 19%). This may be partly due to the fact that HR positive cancer can be controlled well by subsequent hormonal therapy, so that patients and their doctors are more willing to choose a less aggressive surgery. From the results of the present study and another study22, however, it was obvious that planning breast-conserving surgery for HR positive patients should be more conservative and cautious.
We also compared measurement accuracy of MRI in patients treated with different chemotherapy regimens, but did not find any significant association with over or underestimation of tumor size. Literature reports regarding the impact of different NAC regimens on the accuracy of MRI for estimating residual tumor size have been inconclusive. Denis et al. found underestimations larger than 15 mm more likely (p=0.02) among patients given taxane based NAC (11/28 of patients) than among those given anthracycline based treatment (0/12).45 Kwon et al. found consistent overestimation in tumor size among patients receiving doxorubicin and docetaxel for NAC.46 In patients receiving both doxorubicin and docetaxel treatments for NAC, Kim et al. found more likely occurrence of over estimation, than under estimation, of residual tumor size (13/50, 26% vs. 1/50, 2%).47 With the continuing evolution of NAC regimens for breast cancer, it would be difficult to compare results from different studies. Overall, the association of the diagnostic accuracy of MRI with different molecular biomarkers of breast cancer is better established than with different chemotherapy regimens. In this study, 27 patients also received Bevacizumab. Because Bevacizumab blocks angiogenesis, it may have impact on the accuracy of MRI in measuring tumor size after NAC. However, in a previous study of ours we have shown that the accuracy in patients with and without receiving Bevacizumab is comparable;28 therefore, in this study we did not perform the sub-group analysis based on Bevacizumab.
In conclusion, the diagnostic performance of MRI in HER2 negative breast cancer was studied, and the results in tumors with different hormonal receptor status and Ki-67 proliferation were compared. Overall, the size discrepancy between MRI and pathology was higher in HR positive than in HR negative cancers, and higher in low proliferating cancer (Ki-67 <40%) compared to high proliferating cancers (Ki-67 ≥40%). The results suggest that the diagnostic performance of MRI for HER2 negative breast cancer undergoing NAC is associated with the molecular biomarker profile of the tumor. When the diagnostic results of MRI are used for planning surgical procedures, the molecular biomarker status needs to be taken into consideration.
Clinical Practice Points.
Improved knowledge about the detection accuracy of residual disease after NAC by imaging may help the planning of an optimal surgery to achieve a tumor free margin. This is important to decrease re-excision rate and minimize local recurrence. Previous studies have shown that the diagnostic accuracy of MRI was better in HER2 positive than in HER2 negative cancer. A higher false negative rate and a larger size discrepancy between imaging and pathology are more frequently found in HER2 negative than in HER2 positive cancer. Factors affecting the inaccurate evaluation of HER2 negative cancer have not been well investigated. In this study we evaluated the influence of hormonal receptor and the Ki-67 proliferation marker in the accuracy of MRI-measured tumor size, by comparing to the pathological size analyzed from the post-NAC surgical specimen. Overall, the size discrepancy between MRI and pathology was worse in HR positive than in HR negative cancers, as also worse in low proliferating cancer (Ki-67 <40%) than in high proliferating cancers (Ki-67 ≥40%). The results suggest that the diagnostic performance of MRI for breast cancer undergoing NAC is associated with the molecular biomarker profile. When the diagnostic results of MRI are used for planning surgical procedures, the molecular biomarker status needs to be taken into consideration.
Acknowledgments
This study was supported in part by NIH/NCI R01 CA127927 and California Breast Cancer Research Program # 16GB-0056.
This study was conducted at the Center for Functional Onco-Imaging, Univ. of California Irvine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolmark N, Wang J, Mamounar E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project D-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Brown A, Mamounas E, et al. Effect of chemotherapy on local-regional disease in women with operable breast cancer: finding from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz TA, Lehman CD, Harris JR, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008;26:791–797. doi: 10.1200/JCO.2007.15.0326. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann M, Minckwitz GV, Rody A. Preoperative (neoadjuvant) systemic treatment of breast cancer. The Breast. 2005;14:576–581. doi: 10.1016/j.breast.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava R, Beriwal S, Badds DJ, et al. Immunohistochemical surrogate markers of breast cancer molecular classes predict response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. 2010;116(6):1431–1439. doi: 10.1002/cncr.24876. [DOI] [PubMed] [Google Scholar]
- 6.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 7.Straver ME, van Adrichem JC, Rutgers EJ. Neoadjuvant systemic therapy in patients with operable breast cancer: more benefits than breast conserving therapy. Ned Tijdschr Geneeskd. 2008;152(46):2519–2525. [PubMed] [Google Scholar]
- 8.Mersin H, Yildirim E, Berberogly U, Gulben K. The prognostic importance of triple negative breast carcinoma. Breast. 2008;17(4):341–346. doi: 10.1016/j.breast.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Moon HG, Han W, Lee JQ, et al. Age and HER2 expression status affect MRI accuracy in predicting residual tumor extent after neo-adjuvant systemic treatment. Ann Oncol. 2009;20(4):636–641. doi: 10.1093/annonc/mdn683. [DOI] [PubMed] [Google Scholar]
- 10.Partridge SC, Gibbs JE, Lu Y, et al. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002;179:1193–1199. doi: 10.2214/ajr.179.5.1791193. [DOI] [PubMed] [Google Scholar]
- 11.Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868–877. doi: 10.2214/ajr.184.3.01840868. [DOI] [PubMed] [Google Scholar]
- 12.Orel S. Who should have breast magnetic resonance imaging evaluation? J Clin Oncol. 2008;26:703–711. doi: 10.1200/JCO.2007.14.3594. [DOI] [PubMed] [Google Scholar]
- 13.Chen JH, Feig B, Agrawal G, et al. MRI evaluation of pathological complete response and residual tumors in breast cancer after neoadjubant chemotherapy. Cancer. 2008;112:17–26. doi: 10.1002/cncr.23130. [DOI] [PubMed] [Google Scholar]
- 14.Chen JH, Mehta RS, Carpenter PM, Nalcioglu O, Su MY. MRI in predicting pathological response of triple negative breast cancer following neoadjuvant chemotherapy. J Clin Oncol. 2007;25(35):5667–5669. doi: 10.1200/JCO.2007.14.6134. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Cho N, Cha JH, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008;9(1):10–18. doi: 10.3348/kjr.2008.9.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim E, Al-Gahmi AM, Zeenelin AA, et al. Basal vs. Luminal A breast cancer subtypes: a matched case-control study using estrogen receptor, progesterone receptor and HER2 as surrogate markers. Med Oncol. 2009;26(3):372–378. doi: 10.1007/s12032-008-9131-6. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RS. Dose-dense and/or metronomic schedules of specific chemotherapies consolidate the chemosensitivity of triple-negative breast cancer: a step toward reversing triple-negative paradox. J Clin Oncol. 2008;26(19):3286–8. doi: 10.1200/JCO.2008.17.1116. [DOI] [PubMed] [Google Scholar]
- 18.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 19.Sorlie T, Perous CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguishes tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;40:2667–2675. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 21.Dawood S, Broglio K, Gong Y, et al. Prognostic significance of HER2 status in women with Inflammatory breast cancer. Cancer. 2008;112(9):1905–1911. doi: 10.1002/cncr.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loo CE, Straver ME, Rodenhuis S, et al. Magnetic resonance imaging response monitoring breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. J Clin Onocol. 2011;28:1–7. doi: 10.1200/JCO.2010.31.1258. [DOI] [PubMed] [Google Scholar]
- 23.Londero V, Bazzocchi M, Del Frate C, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004;14:1371–1379. doi: 10.1007/s00330-004-2246-z. [DOI] [PubMed] [Google Scholar]
- 24.Wasser K, Klein SK, Junkermann H, et al. Neoadjuvant chemotherapy of breast carcinomas: what post-therapeutic (preoperative) information is provided by quantitative dynamic MRI? Radiologe. 2007;47:421–429. doi: 10.1007/s00117-005-1303-1. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya M, Ryan D, Carpenter R, Vinnicombe S, Gallagher CJ. Using MRI to plan breast-conserving surgery following neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2008;98:289–293. doi: 10.1038/sj.bjc.6604171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akazawa K, Tamaki Y, Taguchi T, et al. Preoperative evaluation of residual tumor extent by three-dimensional magnetic resonance imaging in breast cancer patients treated with neoadjuvant chemotherapy. Breast J. 2006;12:130–137. doi: 10.1111/j.1075-122X.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 27.Loibl S, von Minckwitz G, Raab G, et al. Surgical procedures after neoadjuvant chemotherapy in operable breast cancer: results of the GEPARDUO trial. Annals of Surgical Oncology. 2006;13(11):1434–1442. doi: 10.1245/s10434-006-9011-2. [DOI] [PubMed] [Google Scholar]
- 28.Bahri S, Chen JH, Mehta RS, et al. Residual breast cancer diagnosed by MRI in patients receiving neoadjuvant chemotherapy with and without bevacizumab. Annals of Surgical Oncology. 2009;16(6):1619–1628. doi: 10.1245/s10434-009-0441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009 Mar;250(3):638–647. doi: 10.1148/radiol.2503081054. [DOI] [PubMed] [Google Scholar]
- 30.Basu S, Chen W, Tchou J, et al. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for disease characterization. Cancer. 2008;112(5):995–1000. doi: 10.1002/cncr.23226. [DOI] [PubMed] [Google Scholar]
- 31.Chen JH, Agrawal G, Feig B, et al. Triple-negative breast cancer: MRI features in 29 patients. Ann Oncol. 2007;18(12):2042–2043. doi: 10.1093/annonc/mdm504. [DOI] [PubMed] [Google Scholar]
- 32.Szabo BK, Aspelin P, Kristoffersen Wiberg M, Tot T, Bone B. Invasive breast cancer: correlation of dynamic MR features with prognostic factors. Eur Radiol. 2003;13:2423–2435. doi: 10.1007/s00330-003-2000-y. [DOI] [PubMed] [Google Scholar]
- 33.Penault-Llorca F, Abrial C, Raoelfils I, et al. Changes and predictive and prognostic value of the mitotic index, Ki-67, cyclin D1, and cyclo-oxygenase-2 in 710 operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist. 2008;13:1235–1245. doi: 10.1634/theoncologist.2008-0073. [DOI] [PubMed] [Google Scholar]
- 34.Gadiyaram VK, Kurian S, Abraham J, et al. Correlation of Ki67 and p53 expression with obesity and pattern of metastatic spread in triple-negative and Her2/neu+ breast cancer. ASCO Breast Cancer Symposium. 2009 Abstract No. 111. [Google Scholar]
- 35.Ghebeh H, Tulbah A, Mohammed S, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121(4):751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 36.Wiesner FG, Magener A, Fasching PA, et al. Ki-67 as a prognostic molecular marker in routine clinical use in breast cancer patients. Breast. 2009;18(2):135–141. doi: 10.1016/j.breast.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura R, Osako T, Okumura Y, Hayashi M, Arima N. Clinical significance of Ki-67 in neoadjuvant chemotherapy for primary breast cancer as a predictor for chemosensitivity and for prognosis. Breast Cancer. 2010;17(4):269–275. doi: 10.1007/s12282-009-0161-5. [DOI] [PubMed] [Google Scholar]
- 38.Keam B, Im SA, Lee KH, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011 Mar 2;13(2):R22. doi: 10.1186/bcr2834. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeh SK, Kim SH, Kim HS, Kang BJ, Jeong SH, Yim HW, Song BJ. Correlation of the apparent diffusion coefficient value and dynamic magneitc resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging. 2011;33(1):102–109. doi: 10.1002/jmri.22400. [DOI] [PubMed] [Google Scholar]
- 40.Wasser K, Sinn HP, Fink C, et al. Accuracy of tumor size measurement in breast cancer using MRI is influenced by histological regression induced by neoadjuvant chemotherapy. Eur Radiol. 2003;13:1213–1223. doi: 10.1007/s00330-002-1730-6. [DOI] [PubMed] [Google Scholar]
- 41.Honkoop AH, Pinedo HM, De Jong JS, et al. Effects of chemotherapy on pathologic and biologic characteristics of locally advanced breast cancer. Am J Clin Pathol. 1997;107:211–218. doi: 10.1093/ajcp/107.2.211. [DOI] [PubMed] [Google Scholar]
- 42.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–695. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 43.Peintinger F, Kuerer HM, McGuire SE, Bassett R, Pusztai L, Symmans WF. Residual specimen cellularity after neoadjuvant chemotherapy for breast cancer. British Journal of Surgery. 2008;95:433–437. doi: 10.1002/bjs.6044. [DOI] [PubMed] [Google Scholar]
- 44.Buchholz TA, Hunt KK, Whitman GJ, Sahin AA, Hortobagyi GN. Neoadjuvant chemotherapy for breast carcinoma: multidisciplinary considerations of benefits and risks. Cancer. 2003;98:1150–1160. doi: 10.1002/cncr.11603. [DOI] [PubMed] [Google Scholar]
- 45.Denis F, Desbiez-Bourcier AV, Chapiron C, et al. Contrast enhanced magnetic resonance imaging underestimates residual disease following neoadjuvant docetaxel based chemotherapy for breast cancer. Eur J Surg Oncol. 2004;30:1069–1076. doi: 10.1016/j.ejso.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Kwong MS, Chung GG, Horwath LJ, et al. Postchemotherapy MRI overestimates residual disease compared with histopathology in responders to neoadjuvant therapy for locally advanced breast cancer. Cancer J. 2006;12:212–221. doi: 10.1097/00130404-200605000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Im YH, Han BK, et al. Accuracy of MRI for estimating residual tumor size after neoadjuvant chemotherapy in locally advanced breast cancer: relation to response patterns on MRI. Acta Oncol. 2007;46:996–1003. doi: 10.1080/02841860701373587. [DOI] [PubMed] [Google Scholar]


