Abstract
Background and Hypothesis
Klotho is a recently discovered anti-aging gene. The purpose of this study was to investigate if klotho gene transfer attenuates superoxide production and oxidative stress in rat aorta smooth muscle (RASM) cells.
Methods and Results
RASM cells were transfected with AAV plasmids carrying mouse klotho full-length cDNA (mKL) or LacZ as a control. Klotho gene transfer increased klotho expression in RASM cells. Notably, klotho gene expression decreased Nox2 NADPH oxidase protein expression but did not affect Nox2 mRNA expression, suggesting that the inhibition may occur at the post-transcriptional level. Klotho gene transfer decreased intracellular superoxide production and oxidative stress in RASM cells. Klotho gene expression also significantly attenuated the angiotensin II (AngII)-induced superoxide production, oxidative damage, and apoptosis. Interestingly, klotho gene delivery dose-dependently increased the intracellular cAMP level and PKA activity in RASM cells. Rp-cAMP, a competitive inhibitor of cAMP, abolished the klotho-induced increase in PKA activity, indicating that klotho activated PKA via cAMP. Notably, inhibition of cAMP-dependent PKA activity by RP-cAMP abolished klotho-induced inhibition of Nox2 protein expression, suggesting an important role of the cAMP-dependent PKA in this process.
Conclusions
The present finding revealed a previously unidentified role of klotho in regulating Nox2 protein expression in RASM cells. Klotho not only downregulated Nox2 protein expression and intracellular superoxide production but also attenuated AngII-induced superoxide production, oxidative damage, and apoptosis. The klotho-induced suppression of Nox2 protein expression may be mediated by the cAMP PKA pathway.
Keywords: klotho, Nox2, NADPH oxidase, superoxide, cAMP, PKA, smooth muscle cell
Introduction
Klotho is a recently discovered anti-aging gene (Kuro-o et al. 1997). Mutation of klotho gene expedites the aging process and shortens the life-span in mice (Kuro-o et al. 1997; Wang & Sun 2009a). Interestingly, over-expression of klotho slows the aging process and extends the life-span in mice (Kurosu et al. 2005). Klotho has two transcripts: a single-pass transmembrane protein and a secreted protein (Matsumura et al. 1998; Shiraki-Iida et al. 1998). The transmembrane klotho can be released into the circulation when its short transmembrane domain is removed by ectodomain shedding (Kuro-o 2008). Although klotho is mainly expressed in distal convoluted tubules in the kidneys and choroid plexus in the brain (Kuro-o et al. 1997), but it functions as a circulating hormone (Imura et al. 2004; Wang & Sun 2009a) and gets access to vascular smooth muscle cells (VSMCs).
The NAD(P)H oxidase was originally found in the plasma membrane of phagocytes which generates superoxide to participate in host defense by killing or destroying invading microbes. Recent studies indicated that the NAD(P)H oxidase is the primary source of reactive oxygen species (ROS) in vasculature (Griendling et al. 2000; Touyz & Schiffrin 2004; Tain & Baylis 2006). Membrane NADPH oxidases could generate superoxide (O2−) by transferring the electron to O2. Nox2 (gp91phox) and its homologs (Nox1, Nox4, and Nox5) are catalytic subunits of the NAD(P)H oxidase. Superoxide or ROS modulates the downstream signaling molecules by altering the intracellular redox state and by oxidative modification of proteins (Touyz 2004). In physiological condition, the NAD(P)H oxidase-derived superoxide is generated in a controlled manner at low concentrations and functions as a signaling molecule regulating VSMC contraction relaxation and VSMC growth (Rao & Berk 1992; Cosentino et al. 1994; Zafari et al. 1998; Touyz & Schiffrin 1999). But excess superoxide production could lead to increased contractility, VSMC growth and apoptosis, which have been implicated in hypertension, atherosclerosis, and diabetes (Rao & Berk 1992; Harrison 1997; Pawlak et al. 2004; San Martin et al. 2007). Nox-derived ROS are involved in a variety of oxidative stress damage and vascular diseases (Bedard & Krause 2007). It was found Nox2 deficiency reduces vascular inflammation, cellular proliferation, and neointimal thickening after experimental angioplasty (Chen et al. 2004; Carlstrom et al. 2009).
The prevalence of hypertension and vascular dysfunction is increased in the aged population (Burt et al. 1995; Ong et al. 2007) whereas the level of klotho is decreased in the aged population (Xiao et al. 2004). An increase in superoxide production or oxidative stress plays a role in vascular dysfunction and aging. Therefore, it is important to know if klotho has any effect on NAD(P)H oxidase expression and superoxide production. The purpose of this study was to test our hypothesis that klotho gene transfer decreases NAD(P)H oxidase expression and superoxide production in rat aorta smooth muscle (RASM) cells.
Method
Plasmid construction
The pAAV-MCS plasmid vector was purchased from Stratagene (Stratagene, La Jolla, CA, USA). The plasmid pAAV-mKlotho was constructed by inserting the mouse klotho full-length cDNA into the EcoRI and Xba I site of the pAAV-MCS expression vector. Plasmids were amplified in Escherichia coli DH5α cells, extracted by the alkaline lyses method, and purified using a Qiagen Endo-free Plasmid Maxi Kit (Qiagen Science, Maryland, USA). The quantity and quality of the purified plasmid DNA were assessed by determining the absorbance at 260 and 280 nm, and also by electrophoresis in agarose gels. The plasmids were dissolved in TE buffer before use.
Cell culture and transfection with pAAV-mKlotho
Rat aortic smooth muscle (RASM) cells (ATCC, Manassas, VA, USA) were cultured in DMEM (Cell Signaling, Danvers, MA, USA) supplemented with 10% fetal bovine serum (ATCC), 100 μg/ml of streptomycin (Sigma-Aldrich, Atlanta, GA, USA ) and 100 U/ml of penicillin (Sigma-Aldrich, Atlanta, GA, USA) at 37°C, 5% CO2. The cells were then transfected with either pAAV-lacZ or pAAV-mKlotho at 0, 2, 4, 8, 12 μg/well of using OptifectTM Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol, followed by 48–96 h of incubation in DMEM medium.
Quantitative Real-time RT-PCR (QRT-RT-PCR)
Mouse klotho mRNA and rat Nox2 mRNA expression were analyzed using QRT-RT-PCR as we described previously (Sun et al. 2003; Sun 2006). For details, refer to the Online Data Supplement.
Western blot
Western blot analysis was performed as described previously (Wang et al. 2005; de Oliveira 2006; Wang et al. 2006). For details, refer to the Online Data Supplement.
Measurement of intracellular superoxide production
Intracellular superoxide production was assessed by fluorescence microscopy using the oxidation sensitive dye dihydroethidium (DHE, Sigma-Aldrich, Atlanta, GA, USA) as we described recently (Dammanahalli & Sun 2008; Wang & Sun 2010b). For details, refer to the Online Data Supplement.
Immunocytochemical analysis of 4-HNE staining
The immunocytochemical procedure was described in our previous studies (Wang et al. 2005; Dammanahalli & Sun 2008). For details, refer to the Online Data Supplement.
Annexin V staining and cell counting
Apopotosis was assessed by an Annexin V staining kit (Invitrogen, Carlsbad, CA, USA), USA) after cells were transfected with the klotho plasmid for 24 h followed by incubation with or without angiotensin II (200 nM) for 72 hours. The cells were then harvested and resuspended with Annexin binding buffer at a concentration of 1.0×106 cells/mL and analyzed using an automated cell counter (TC 10™, Bio-RAD, CA, USA). One hundred microlitres of the suspension was mixed with 5 μL Annexin V conjugate and propidium iodide (PI) incubated for 15 min at room temperature in the dark. After incubation, 400 μL of binding buffer was added to each tube and fluorescence data for cells were acquired using flow cytometry(Sternfeld et al. 2009).
Intracellular cAMPimmunoassay
Intracellualr cAMP was measured using a kit from BioVision (BioVision, Mountain View, CA, USA) according to the manufacturer’s instruction. For details, refer to the Online Data Supplement.
Protein kinase assay
Protein kinase A (PKA) activity was measured using a PKA activity assay kit from EMD Biosciences (EMD Biosciences, Inc., Darmstadt, Germany) according to the manufacturer’s instruction. For details, refer to the Online Data Supplement.
Statistical analysis
Data were analyzed using a two-way ANOVA or an unpaired Student’s t-test, as appropriate probability values of < 0.05 were considered to be statistically significant. A regression analysis was used to analyze the dose-dependent response. A correlation analysis was used to analyze the correlation of klotho expression and Nox2 expression.
Results
Klotho gene transfer resulted in dose-dependent expression of mKL in the RASM cells
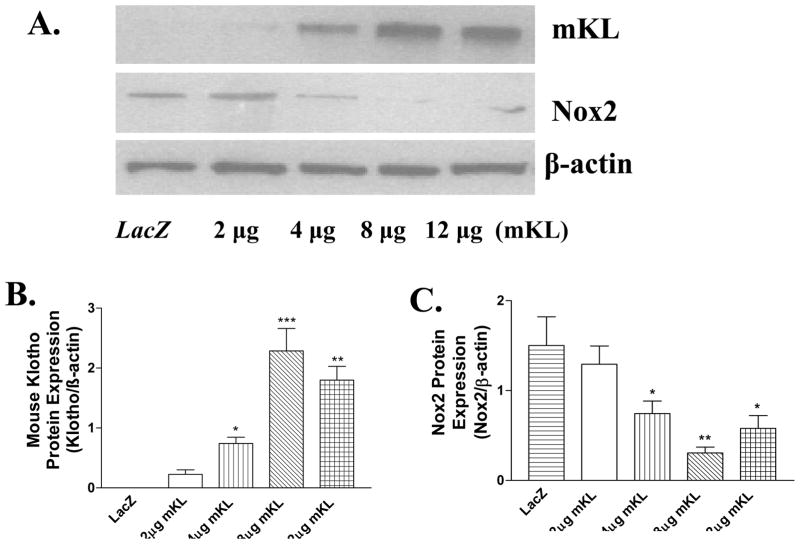
Transfer of mKL gene resulted in strong expression of transmembrane form klotho protein (130 kDa) and mRNA (710 bp) in RASM cells (Fig. 1A&B, Supplemental Figure S1 A&B, Fig. 2A). A quantitative and regression analysis showed that klotho protein was expressed in a dose-dependent manner following transfer of mKL plasmid (2–8 μg) (Figs. 1A&B). mKL at 8 μg resulted in a maximal expression of klotho protein while 12 μg of mKL did not further increase klotho expression. Klotho protein expression was not detectable in RASM cells transfected with pAAV-LacZ or in RASM cells without transfection (control) (Fig. 1A, S1A), indicating that the rat klotho protein may not be expressed in the RASM cells. The klotho antibody used can recognize both rat and mouse klotho protein (Wang & Sun 2009b).
Figure 1.
Mouse klotho (mKL) gene transfer decreased Nox2 protein expression in RASM cells. A, western blot bands of mouse klotho protein (transmembrane form) and Nox2 protein expression. B, Quantitative analysis of mouse klotho (mKL) protein expression. C, Quantitative analysis of Nox2 protein expression. Klotho protein and Nox2 protein were measured 48 hours following mKL klotho gene transfer. Values=means±SEM. *p<0.05, **p<0.01, ***p<0.001 vs the LacZ group. N=3 independent experiments with 3 measurements each.
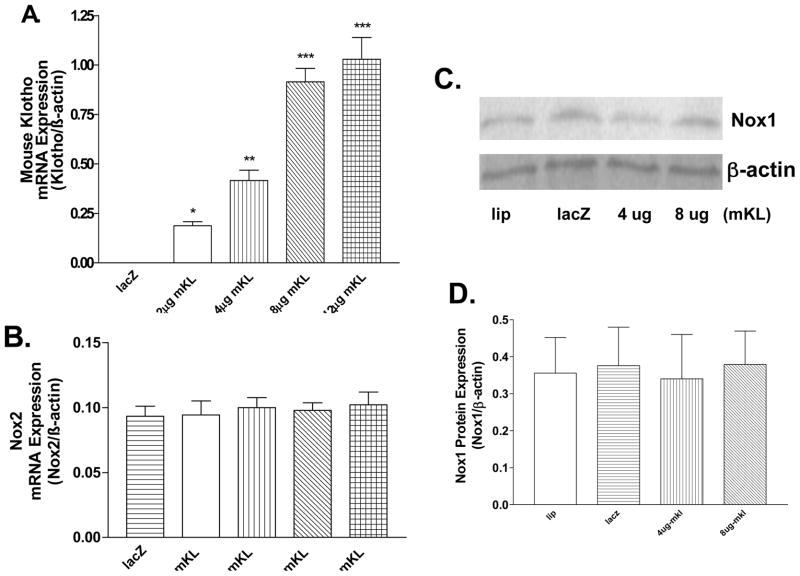
Figure 2.
mKL gene transfer did not alter Nox2 mRNA expression or Nox1 protein expression in RASM cells. A, Quantitative real-time RT-PCR (QRT-RT-PCR) analysis of mKL mRNA expression. B, QRT-RT-PCR analysis of rat Nox2 mRNA expression. mKL mRNA and rat Nox2 mRNA expression were measured 48 hours following mKL gene transfer. C, Nox1 protein expression. D, Quantification of Nox1 protein expression. Values=means±SEM. *p<0.05, **p<0.01, ***p<0.001 vs the LacZ group. N= 3 independent experiments with 3 measurements each.
Klotho gene transfer decreased Nox2 protein expression
To determine the effect of klotho expression on NADPH oxidases, we measured protein expression of Nox family members using western blot analysis. Klotho gene transfer selectively decreased Nox2 protein expression (Fig 1A&C, S2A), the major membrane-bound NADPH oxidase. A correlation analysis showed that klotho gene transfer decreased Nox2 protein expression in a dose-dependent manner. However, Nox2 mRNA expression was not altered significantly by klotho gene transfer (Fig. 2B), suggesting that klotho down-regulated Nox2 protein expression at the post-transcriptional level. In contrast, protein expression of Nox1 was not altered significantly by the klotho gene transfer (Fig. 2C&D). Nox4 protein expression was not detectable in RASM cells (S2B).
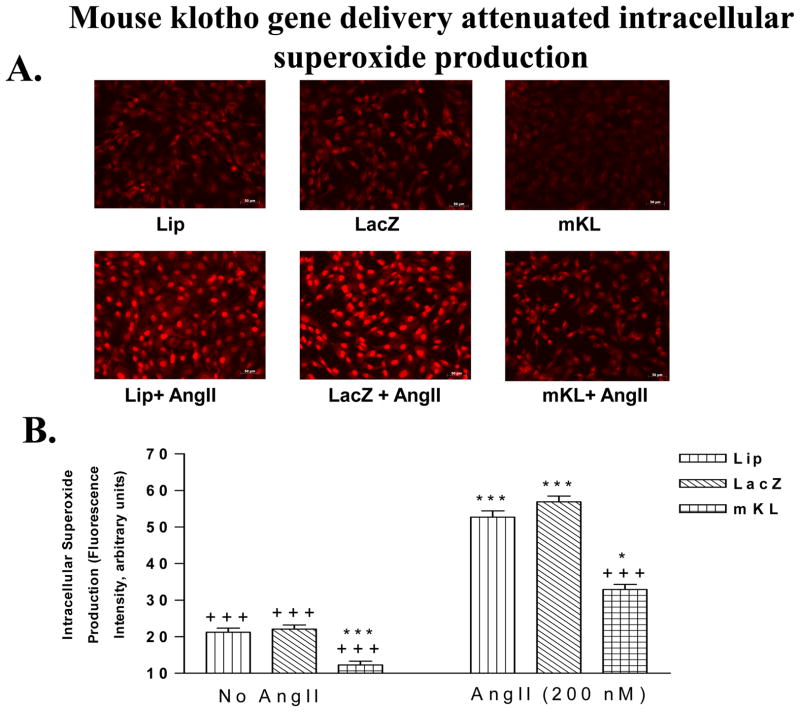
Klotho gene transfer attenuated intracellular superoxide production in RASM cells
The intracellular superoxide production in RASM cells were assessed by the oxidation of DHE. Klotho gene transfer significantly decreased the basal level of superoxide production (Fig. 3A&B). AngII increased superoxide production in RASM cells (Fig. 3A&B). Interestingly, klotho gene expression significantly attenuated AngII-induced superoxide production (Fig. 3A&B).
Figure 3.
mKL gene transfer attenuated the basal intracellular superoxide level and angiotensin II-induced superoxide production in RASM cells. A, Photomicrograph of DHE staining of superoxide production in RASM cells transfected with klotho for 24 h followed by incubation in the presence or absence of angiotensin II (200 nM) for 24 h. B, Quantitative analysis of superoxide production. Lip, lipofectamine. Values=means±SEM. ***p<0.001 vs the LacZ group (No AngII); +p<0.05, +++p<0.001 vs the LacZ+AngII group. N=3 independent experiments with 3 measurements each.
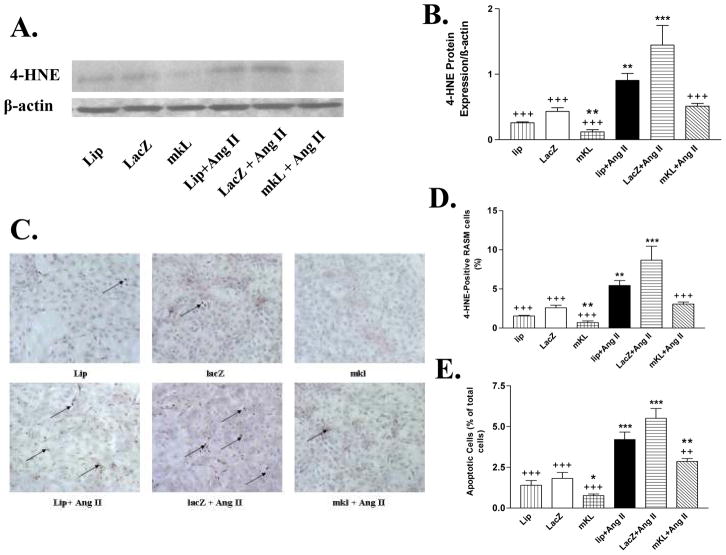
Klotho gene transfer decreased angiotensin II-induced oxidative stress and apoptosis
4 Hydroxynonenal (4-HNE) is a by-product of lipid peroxidation and serves as a marker of oxidativestress.(Wei et al. 2008) Klotho gene transfer decreased the basal level of 4-HNE protein level (Fig. 4A&B). AngII significantly increased the 4-HNE expression. Klotho gene transfer abolished the AngII-induced increase in 4-HNE expression (Fig. 4A&B), suggesting that klotho decreased oxidative stress-related cell damage. The immuncytochemical analysis showed that klotho gene transfer decreased the basal level of 4-HNE and prevented the AngII-induced oxidative damage (Fig. 4C&D).
Figure 4.
mKL gene transfer attenuated AngII-induced oxidative stress and apoptosis in RASM cells. A, Western blot bands of 4-HNE protein (lipid peroxidation product, oxidative stress marker) in RASM cells transfected with the klotho plasmid for 24 h followed by incubation with or without angiotensin II (200 nM) for 72 hours. B, Quantitative analysis of 4-HNE protein expression. C, Photomicrograph of 4-HNE staining in RASM cells. Arrows indicate 4-HNE-positive cells. D, Quantitative analysis of 4-HNE-positive cells. E, Quantitative analysis of apoptotic cells (Annexin V). Values=means±SEM. *p<0.05, **p<0.01, ***p<0.001 vs the LacZ group; ++p<0.01, +++p<0.001 vs the LacZ+AngII group. N=3 independent experiments with 3 measurements each.
Klotho gene transfer significantly decreased the number of apoptotic cells (Fig. 4E). AngII significantly increased apoptosis (Fig. 4E). Klotho gene transfer significantly attenuated AngII-induced apoptosis (Fig. 4E)
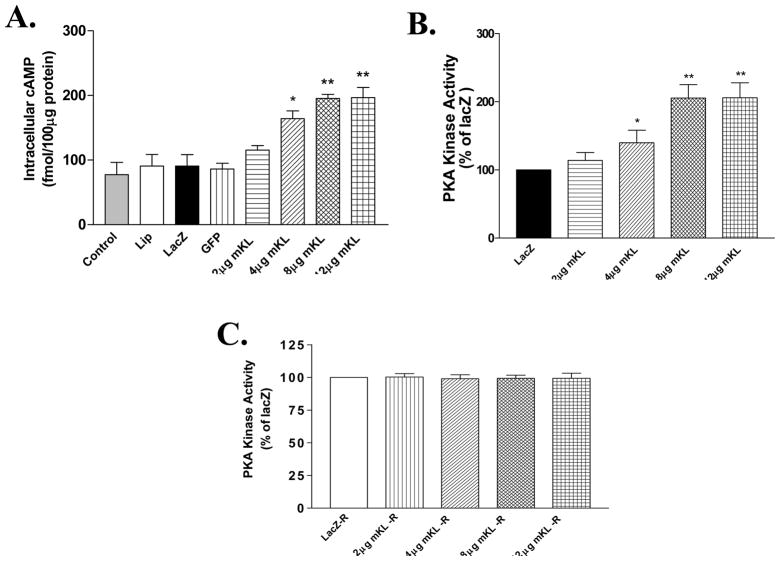
Klotho gene transfer increased intracellular cAMP production and PKA activity
To assess the intracellular mechanism that mediates the inhibitory effect of klotho on Nox2 protein expression, we measured cAMP and PKA activity in RASM cells. Klotho gene transfer dose-dependently increased cAMP production and PKA activity (Fig. 5A&B). The intracellular cAMP level and PKA activity were doubled by klotho gene transfer (8 μg mKL) (Fig. 5A&B). The increase in intracellular cAMP parallels with the increase in PKA activity. Interestingly, pretreatment with Rp-cAMP, a competitive inhibitor of cAMP, prevented the klotho-induced increase in PKA activity, suggesting that the increase in PKA activity is cAMP-dependent.
Figure 5.
mKL gene transfer increased intracellular cAMP and PKA activity in RASM cells. A, Intracellular cAMP level measured at 48 hours after mKL gene transfer. B, PKA activity. C, PKA activity measured in RASM cells pre-treated with Rp-cAMP (10−3 mol/L) followed by klotho gene transfer. Values=means±SEM. *p<0.05, **p<0.01 vs the LacZ group. N=3 independent experiments with 3 measurements each.
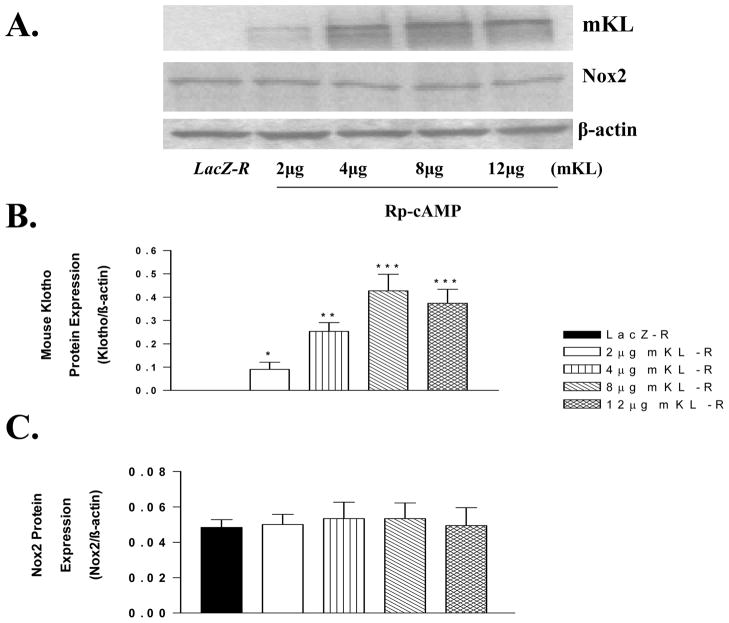
Rp-cAMP abolished klotho-induced inhibition of Nox2 protein expression
Klotho gene transfer dose-dependently increased klotho expression which, however, failed to decrease Nox2 protein expression in RASM cells pretreated with Rp-cAMP (Fig. 6A,B,C), an inhibitor of cAMP-dependent PKA. This result suggested that klotho-induced inhibition of Nox2 protein expression may be mediated by the cAMP-PKA pathway (Fig. 6).
Figure 6.
Inhibition of PKA activity by Rp-cAMP abolished mKL-induced inhibition of Nox2 expression. A, Western blot bands of mKL and Nox2 protein expression at 48 hours following treatment with Rp-cAMP (10 μM) and mKL gene transfer. B, Quantitative analysis of mKL protein expression. C, Quantitative analysis of Nox2 protein expression. Rp-cAMP (10 μM) is a common concentration used to inhibit protein kinase A. Values=means±SEM. *p<0.05, **p<0.01, ***p<0.001 vs the LacZ group. N=3 independent experiments with 3 measurements each.
Discussion
The present study reveals that mouse klotho gene transfer significantly decreased superoxide production in rat aortic smooth muscle (RASM) cells (Fig. 3). The suppressing effect of klotho gene transfer on superoxide production may be attributed, at least in part, to the downregulation of Nox2 protein expression. Indeed, klotho gene transfer dose-dependently decreased Nox2 protein expression in RASM cells (Fig. 1). The finding that klotho suppressed Nox2 protein expression in RASM cells is interesting, which suggests, for the first time, that klotho may regulate superoxide generation in vascular smooth muscle cells (SMCs). It is noted that klotho expression selectively downregulated Nox2 protein expression while Nox1 was not affected (Fig. 2C&D). Although the detailed molecular mechanism of the selective inhibition requires an additional study, it seems that klotho may downregulate Nox2 protein expression at the post-transcriptional level because klotho gene expression did not alter Nox2 mRNA expression (Fig. 2).
A further mechanistic investigation revealed that the klotho-induced suppression of Nox2 protein expression may be mediated by the cAMP-PKA pathway. This interesting observation is based on the following new findings: (1) Klotho gene transfer dose-dependently increased the intracellular cAMP level and the PKAactivity in RASM cells; (2) The activation of PKA is cAMP-dependent, i.e., cAMP-PKA; (3) Inhibition of the cAMP-PKA pathway by the Rp-cAMP abolished the klotho-induced decrease in Nox2 protein expression. Therefore, the present results suggest a new pathway that may mediate klotho-induced inhibition of Nox2 expression, i.e., klotho ↑ → cAMP ↑ → PKA ↑ → Nox2 protein expression ↓. PKA is normally inactive, but becomes activated upon binding to cAMP. The activated PKA phosphorylates a number of other proteins, many of which are also enzymes that are either activated or suppressed upon phosphorylation. It seems that the phosphorylated proteins may inhibit the translation of Nox2 or increase the degradation of Nox2 protein. This hypothesis, however, needs to be tested.
Nox2 is widely expressed in the vasculature (Bedard & Krause 2007). The Nox2 NAD(P)H oxidase is the major source of ROS in the vasculature (Dworakowski et al. 2008). Growing evidence suggests that AngII acts as one of the most important vasoactive factors in regulating vascular NAD(P)H oxidase (Berry et al. 2000; Griendling et al. 2000; Touyz et al. 2002). Nox2 can be activated by AngII and may play an important role in AngII-induced hypertension (Li & Shah 2003). Excess superoxide or ROS leads to oxidative stress damage which is involved in various cardiovascular diseases (Landmesser & Harrison 2001; Zalba et al. 2001). Our recent study indicates that the in vivo klotho gene delivery may attenuate the progression of spontaneous hypertension (Wang & Sun 2009b).
Klotho is mainly expressed in kidneys and brain choroid plexus (Wang & Sun 2009a; Wang & Sun 2010a). However, the kidney-derived klotho circulates and may regulate vascular function (Wang & Sun 2009a). Although klotho was reported to protect against endothelial function (Saito et al. 1998; Saito et al. 2000; Wang & Sun 2009a), this is the first study demonstrating that klotho may have beneficial effect in the SMCs. Interestingly, klotho not only decreased the basal superoxide level but also attenuated AngII-induced superoxide production in RASM cells (Fig. 3). Further, klotho gene transfer decreased oxidative damage as evidenced by a significant decrease in the level of 4-HNE (Fig. 4), a by-product of lipid peroxidation of cell membrane. Importantly, klotho gene expression prevented AngII-induced apoptosis (Fig. 4), suggesting that klotho may protect RASM cells from oxidative damage. The present finding points to a new direction for understanding how klotho may protect vascular SMCs. Klotho was discovered to have anti-aging effect (Kuro-o et al. 1997; Kurosu et al. 2005; Wang & Sun 2009a). In humans, the level of circulating klotho decreases after 40 years of age (Xiao et al. 2004). Aging is associated with increased prevalence of vascular disorders such as endothelial dysfunction, arterial stiffening, and hypertension (Burt et al. 1995; Ong et al. 2007). The suppressing action of klotho on oxidative stress in SMCs could contribute to its anti-aging effect.
Activation of the cAMP-PKA pathway by klotho was reported in HUVECs (Yang et al. 2003; Rakugi et al. 2007), which leads to up-regulation of angiotensin-I converting enzyme (ACE) and potentially increases AngII production (Yang et al. 2003). The ability of klotho to suppress AngII-induced oxidative stress in SMCs may counteract the increased AngII release from endothelial cells and contribute to prevention of vascular damage. Because klotho may increase the cAMP-dependent PKA activity in endothelial and smooth muscle cells, a further study is warranted to assess if this effect is due to activation of adenylate cyclase, a key enzyme for generating cAMP.
In summary, this present findings demonstrated a previously unidentified role of klotho in regulating Nox2 protein expression in RASM cells. This study further revealed that klotho decreased Nox2 protein expression through the cAMP-PKA-dependent pathway. In addition, klotho inhibited AngII-induced superoxide production and oxidative stress thereby attenuating apoptosis. Therefore, klotho gene transfer may offer a protection in smooth muscle cells.
Supplementary Material
Acknowledgments
This work was supported by the NIH HL105302 and HL102074.
Footnotes
Disclosures: Nothing to disclose.
The authors confirm that there are no conflicts of interest.
References
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJ, Dominiczak AF. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation. 2000;101:2206–2212. doi: 10.1161/01.cir.101.18.2206. [DOI] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- Carlstrom M, Lai EY, Ma Z, Patzak A, Brown RD, Persson AE. Role of NOX2 in the regulation of afferent arteriole responsiveness. Am J Physiol Regul Integr Comp Physiol. 2009;296:R72–79. doi: 10.1152/ajpregu.90718.2008. [DOI] [PubMed] [Google Scholar]
- Chen Z, Keaney JF, Jr, Schulz E, Levison B, Shan L, Sakuma M, Zhang X, Shi C, Hazen SL, Simon DI. Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proc Natl Acad Sci U S A. 2004;101:13014–13019. doi: 10.1073/pnas.0405389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino F, Sill JC, Katusic ZS. Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension. 1994;23:229–235. doi: 10.1161/01.hyp.23.2.229. [DOI] [PubMed] [Google Scholar]
- Dammanahalli JK, Sun Z. Endothelin (ET)-1 inhibits nicotinamide adenine dinucleotide phosphate oxidase activity in human abdominal aortic endothelial cells: a novel function of ETB1 receptors. Endocrinology. 2008;149:4979–4987. doi: 10.1210/en.2008-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580:5753–5758. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Dworakowski R, Alom-Ruiz SP, Shah AM. NADPH oxidase-derived reactive oxygen species in the regulation of endothelial phenotype. Pharmacol Rep. 2008;60:21–28. [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–241. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser U, Harrison DG. Oxidative stress and vascular damage in hypertension. Coron Artery Dis. 2001;12:455–461. doi: 10.1097/00019501-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem. 2003;278:12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- Pawlak K, Naumnik B, Brzosko S, Pawlak D, Mysliwiec M. Oxidative stress - a link between endothelial injury, coagulation activation, and atherosclerosis in haemodialysis patients. Am J Nephrol. 2004;24:154–161. doi: 10.1159/000076244. [DOI] [PubMed] [Google Scholar]
- Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, Maekawa Y, Kida I, Miyazaki J, Ogihara T. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82–87. doi: 10.1007/s12020-007-0016-9. [DOI] [PubMed] [Google Scholar]
- Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- Sternfeld T, Tischleder A, Schuster M, Bogner JR. Mitochondrial membrane potential and apoptosis of blood mononuclear cells in untreated HIV-1 infected patients. HIV Med. 2009;10:512–519. doi: 10.1111/j.1468-1293.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- Sun Z. Genetic AVP deficiency abolishes cold-induced diuresis but does not attenuate cold-induced hypertension. Am J Physiol Renal Physiol. 2006;290:F1472–1477. doi: 10.1152/ajprenal.00430.2005. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhang Z, Cade R. Renal responses to chronic cold exposure. Can J Physiol Pharmacol. 2003;81:22–27. doi: 10.1139/y03-002. [DOI] [PubMed] [Google Scholar]
- Tain YL, Baylis C. Dissecting the causes of oxidative stress in an in vivo model of hypertension. Hypertension. 2006;48:828–829. doi: 10.1161/01.HYP.0000242927.24428.25. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells -- implications in cardiovascular disease. Braz J Med Biol Res. 2004;37:1263–1273. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension. 1999;34:976–982. doi: 10.1161/01.hyp.34.4.976. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Cade R, Sun Z. Human eNOS gene delivery attenuates cold-induced elevation of blood pressure in rats. Am J Physiol Heart Circ Physiol. 2005;289:H1161–1168. doi: 10.1152/ajpheart.01306.2004. [DOI] [PubMed] [Google Scholar]
- Wang X, Skelley L, Cade R, Sun Z. AAV delivery of mineralocorticoid receptor shRNA prevents progression of cold-induced hypertension and attenuates renal damage. Gene Ther. 2006;13:1097–1103. doi: 10.1038/sj.gt.3302768. [DOI] [PubMed] [Google Scholar]
- Wang X, Sun Z. RNAi silencing of brain klotho potentiates cold-induced elevation of blood pressure via the endothelin pathway. Physiol Genomics. 2010a doi: 10.1152/physiolgenomics.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sun Z. Thyroid hormone induces artery smooth muscle cell proliferation: discovery of a new TRalpha1-Nox1 pathway. J Cell Mol Med. 2010b;14:368–380. doi: 10.1111/j.1582-4934.2008.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009a;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009b;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008;294:E345–351. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl) 2004;117:742–747. [PubMed] [Google Scholar]
- Yang J, Matsukawa N, Rakugi H, Imai M, Kida I, Nagai M, Ohta J, Fukuo K, Nabeshima Y, Ogihara T. Upregulation of cAMP is a new functional signal pathway of Klotho in endothelial cells. Biochem Biophys Res Commun. 2003;301:424–429. doi: 10.1016/s0006-291x(02)03056-5. [DOI] [PubMed] [Google Scholar]
- Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- Zalba G, San Jose G, Moreno MU, Fortuno MA, Fortuno A, Beaumont FJ, Diez J. Oxidative stress in arterial hypertension: role of NAD(P)H oxidase. Hypertension. 2001;38:1395–1399. doi: 10.1161/hy1201.099611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.