Abstract
Estrogen therapy used in combination with selective serotonin reuptake inhibitor (SSRI) treatment improves SSRI efficacy for the treatment of mood disorders. Desensitization of serotonin 1A (5-HT1A) receptors, which takes one to two weeks to develop in animals, is necessary for SSRI therapeutic efficacy. Estradiol modifies 5-HT1A receptor signaling and induces a partial desensitization in the paraventricular nucleus (PVN) of the rat within two days, but the mechanisms underlying this effect are currently unknown. The purpose of this study was to identify the estrogen receptor necessary for estradiol-induced 5-HT1A receptor desensitization. We previously showed that estrogen receptor β is not necessary for 5-HT1A receptor desensitization and that selective activation of estrogen receptor GPR30 mimics the effects of estradiol in rat PVN. Here, we used a recombinant adenovirus containing GPR30 siRNAs to decrease GPR30 expression in the PVN. Reduction of GPR30 prevented estradiol-induced desensitization of 5-HT1A receptor as measured by hormonal responses to the selective 5-HT1A receptor agonist, (+)8-OH-DPAT. To determine the possible mechanisms underlying these effects, we investigated protein and mRNA levels of 5-HT1A receptor signaling components including 5-HT1A receptor, Gαz, and RGSz1. We found that two days of estradiol increased protein and mRNA expression of RGSz1, and decreased 5-HT1A receptor protein but increased 5-HT1A mRNA; GPR30 knockdown prevented the estradiol-induced changes in 5-HT1A receptor protein in the PVN. Taken together, these data demonstrate that GPR30 is necessary for estradiol-induced changes in the 5-HT1A receptor signaling pathway and desensitization of 5-HT1A receptor signaling.
Introduction
Women in peri- to post-menopausal states experience a fluctuation, then decline, in estrogen levels (Banger, 2002). The greatest reduction of estrogen levels is observed during the late menopausal transition and the first year post-menopause (Archer et al., 1999; Santoro et al., 1996; Schmidt et al., 2004). Decreased levels of estrogens are associated with various neuropsychiatric disorders such as depression, anxiety, and panic disorders in women (Arpels, 1996). During peri-menopause, there is a higher incidence of first onset of mood disturbances (Freeman et al., 2004; Harlow et al., 2003). Change in serotonergic function, particularly serotonin 1A (5-HT1A) receptor function (Lemonde et al., 2003; Savitz et al., 2009; Shively and Bethea, 2004), is a hallmark of such disorders (Halbreich, 1990; Jimerson et al., 1997; Joffe and Cohen, 1998; Ressler and Nemeroff, 2000).
Selective serotonin reuptake inhibitors (SSRIs) are commonly used to treat mood disorders. Desensitization (attenuation) of both somatodendritic 5-HT1A autoreceptor signaling in the midbrain dorsal raphe nucleus (DRN) and postsynaptic 5-HT1A receptor signaling in the paraventricular nucleus (PVN) region of the hypothalamus are thought to contribute to the therapeutic efficacy of SSRIs (Lesch et al 1991, Sargent et al 1997, Bosker et al., 2001; Chaput et al., 1986; Czachura and Rasmussen, 2000; Lerer et al., 1999; Li et al., 1997; Gunther et al., 2008; Raap et al., 1999; Bleir and de Montigny, 1994), which can take three to 12 weeks to achieve (Rush et al., 2004). Multiple studies have provided direct evidence of a hyperactive hypothalamic-adreno-pituitary (HPA) axis response to the 5-HT1A partial agonist buspirone, as evidenced by elevated adrenocorticotropic hormone (ACTH) and cortisol levels, which can be prevented by SSRI treatment (Navines et al., 2007; Gomez-Gil et al., 2010; Nikisch et al., 2005). Desensitization of 5-HT1A receptor signaling in the PVN can be measured by neuroendocrine challenge tests that detect changes in oxytocin (OT) and ACTH levels in response to 5-HT1A receptor agonists (Li et al., 1997; Osei-Owusu et al., 2005). In humans, chronic treatment with SSRIs reduces the release of OT and ACTH in response to 5-HT1A receptor stimulation, thus demonstrating desensitization of the receptor signaling (Lerer et al., 1999). These studies suggest that functional adaptative changes in postsynaptic 5-HT1A receptor signaling underlie the effects of SSRIs.
Estrogens have been shown to enhance the efficacy of SSRIs for the treatment of mood disorders and hot flushes in women (Lomax and Schonbaum, 1993; Schneider et al., 1997). In rats, SSRIs produce full desensitization of 5-HT1A receptor signaling in the PVN in seven to 14 days (Li et al., 1996; Raap et al., 1999), while estradiol alone can produce a partial desensitization with two days of treatment (Raap et al., 2000). Therefore, it is hypothesized that estrogen treatment in combination with SSRIs may accelerate SSRI therapeutic effects.
In order to improve current therapies for mood disorders, it is important to understand which estrogen receptor is involved in the regulation of 5-HT1A receptors in the PVN. Of the two classical nuclear estrogen receptors (ERs) α and β, density of ERβ is higher than ERα in the PVN, especially in OT neurons (Alves et al., 1998; Hrabovszky et al., 2004; Laflamme et al., 1998; Simonian and Herbison, 1997). Recent work in our laboratory has shown that ERβ is not involved in 5-HT1A receptor desensitization (Rossi et al., 2010). This result, together with the low expression of ERα in the PVN, led us to investigate the non-classical, membrane estrogen receptor GPR30. GPR30 binds estrogen with high affinity (Revankar et al., 2005; Thomas et al., 2005), is expressed in the PVN, and colocalizes with 5-HT1A receptors, OT, and corticotrophin releasing factor (CRF) in the PVN (Brailoiu et al., 2007; Sakamoto et al., 2007; Xu et al., 2009). Previous work in our laboratory showed that treatment with the GPR30-selective agonist G-1 decreased 5-HT1A receptor signaling, similar to estradiol (Xu et al., 2009). These data suggest a role for GPR30 in the estradiol-mediated desensitization of 5-HT1A receptor signaling in the PVN.
The 5-HT1A receptor is known to couple to the pertussis-toxin-insensitive G protein subunit Gαz (Barr et al., 1997; Raymond et al., 1993). Stimulation of 5-HT1A receptors in the hypothalamus stimulates release of OT and ACTH via coupling to Gαz (Serres et al., 2000). Regulator of G protein signaling z1 (RGSz1), one of six splice variants of RGS20, is a highly selective GTP-activating protein (GAP) for Gαz. RGSz1 is exclusively found in the brain, where it accelerates hydrolysis of Gαz-bound GTP over 400-fold, with a Km of 2nM (Glick et al., 1998; Wang et al., 1998; Wang et al., 2002). Two day treatment with estradiol produces a dose-dependent upregulation of RGSz1 in the PVN, without changing Gαz levels, that parallels the decreased hormone response induced by estradiol (Carrasco et al., 2004), suggesting that RGSz1 is important in estradiol-induced desensitization of 5-HT1A signaling.
In this study, we investigated the hypothesis that GPR30 expression in the PVN is necessary for the estradiol-induced desensitization of 5-HT1A receptor signaling. To test this hypothesis, we injected a recombinant adenovirus containing a small interference RNA (siRNA) against GPR30 into the rat PVN and then evaluated the effects of estradiol on 5-HT1A receptor signaling, as well as changes in mRNA and proteins involved in 5-HT1A receptor signaling, including the 5-HT1A receptor, Gαz, and RGSz1.
Methods
Animals
Female Sprague-Dawley (SD) rats (225–250g) from Harlan (Haslett, MI) were housed two per cage in a temperature-, humidity-, and light-controlled room (12h light/dark cycle). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and as approved by the University of Kansas Institutional Animal Care and Use Committee. All efforts were made to minimize animal discomfort and to reduce the number of animals used.
Drugs
17β-Estradiol-3-benzoate (EB) was purchased from Sigma-Aldrich (St. Louis, MO). EB was first dissolved in 100% ethanol to a concentration of 1mg/ml and then diluted with sesame oil to a concentration of 25 μg/ml. The EB solution and sesame oil were administered at 0.4 ml/kg (EB dose 10 μg/kg, subcutaneous (s.c.)). (+)8-Hydroxy-2-dipropylaminotetralin ((+)8-OH-DPAT) was purchased from Tocris (Ellisville, MO). (+)8-OH-DPAT was dissolved in 0.85% NaCl (saline) at a concentration of 0.2mg/ml and administered at a dose of 0.2mg/kg, s.c. Solutions were made fresh before injection.
Procedures
Experiment 1: effect of estradiol on 5-HT1A receptor function
Prior to surgery, rats were anesthetized by an intraperitoneal (i.p.) injection of a cocktail of ketamine hydrochloride (100mg/kg) plus xylazine hydrochloride (7mg/kg). Rats were ovariectomized (OVX) by removing both ovaries via a single ventral midline incision. Five days after OVX, rats were given s.c. injections of either EB (10 μg/kg, 0.25ml/kg, s.c.) or vehicle (sesame oil) once a day for two days. In all experiments, plasma estradiol levels were reduced to below 10pg/ml by the seventh day after OVX, and two-day treatment with 10 μg/kg EB raised plasma estradiol levels to 100–140pg/ml. 18h following the last injection of EB or vehicle, rats were injected with the selective 5-HT1A receptor agonist, (+)8-OH-DPAT (0.2mg/kg, s.c.) or vehicle (saline). 15 minutes later, animals were sacrificed by decapitation. Trunk blood was collected in centrifuge tubes containing 0.5ml 0.3M EDTA (pH 7.4). Brains were removed and snap-frozen in dry-ice-cooled isopentane and then placed in dry ice. Plasma and brains were stored at −80°C until use.
Experiment 2: effect of recombinant adenovirus containing GPR30 siRNA on EB-induced desensitization of 5-HT1A receptors
Generation and evaluation of recombinant adenoviruses
Recombinant adenoviruses were generated as described previously (Luo et al., 2007; Rossi et al., 2010). Briefly, potential GPR30 siRNA sequences were designed using Block It™RNAi Designer program provided by Invitrogen. Four potential siRNA and wo mismatch sequences were converted to DNA sequences to further test and generate recombinant adenovirus. The sense sequences of the GPR30 siRNAs were GPR30-737 (sense: AGCCTGTGCTATTCCCTCATTTTT, antisense: AATGAGGGAATAGCACAGGCTTTT); GPR30-1135 (sense: AACGGAGCAGTCAGATGTCAAGTTCATTTT, antisense: ATGAACTTGACATCTGACTGCTCCGTTTTT); GPR30-402 (sense: AGGACGAGCAGTATTACGATTTTT, antisense: AATCGTAATACTGCTCGTCCTTTT); and GPR30-272 (sense: AGCAACATCCTCATCTTGGTGGTGAATTTT, antisense: ATTCACCACCAAGATGAGGATGTTGCTTTT). The mismatch sequences were GPR30-1135mis (sense: AACGGACGGACTTGTAGAACTAGTCATTTT, antisense: ATGACTAGTTCTACAAGTCCGTCCGTTTTT) and GPR30-402mis (sense: AGGAACGATATGCATGCGATTTTT, antisense: AATCGCATGCATATCGTTCCTTTT). The potential GPR30 siRNAs were evaluated using a pSOS-HUS vector, which contains an siRNA site and a target gene site that allows transfection of siRNAs and the target gene into the same cells. A sequence encoding green fluorescent protein (GFP) is adjacent to an internal ribosome entry site (IRES), followed by the target gene cloning site, so that observed GFP expression can be used as a marker for GPR30 expression. When an siRNA inhibits the transcription of GPR30, the expression of GFP is also reduced. Therefore, the expression of GFP observed can be used as a marker for GPR30 expression.
A full sequence of GPR30 (Accession No: U92802) was cloned into the target gene cloning site (SOS-GPR30-HUS). The siRNA or mismatch sequences were inserted into sfiI sites of SOS-GPR30-HUS (SOS-GPR30-siRNA-HUSs and SOS-GPR30-mis-HUSs, respectively) as described by Luo et al (2007), followed by NotI digestion after ligation. The clones containing siRNA sequences were identified by PCR with U6 forward and siRNA antisense primers. The SOS-GPR30-siRNA-HUSs or SOS-GPR30-mis-HUSs were transfected into HEK293 cells using lipofectamine reagent (Invitrogen, Carlsbad, CA) to evaluate the knockdown efficiency of the GPR30 siRNAs. The number and density of GFP-expressing cells were observed for five consecutive days after the transfection.
The sequences of siRNAs in the SOS-GPR30-siRNA-HUS that significantly reduced the number and brightness of GFP-containing cells as well as SOS-GPR30-mis-HUSs that had no effect on GFP were selected and inserted into pSES-HUS vector, which is a shuttle vector for adenovirus and contains a red fluorescent protein (RFP), as described above and Luo et al (2007). The SES-GPR30-siRNA-HUSs and SES-GPR30-mis-HUSs were further recombined into Ad-Easy-1 vector to generate high titer adenoviruses containing the GPR30 siRNA or mismatch sequences (GPR30-siRNA-Ads and GPR30-mis-Ads, respectively) (He et al., 1998). The high titer GPR30-siRNA-Ads and GPR30-mis-Ads (~1011–12 active viral particles/ml) were stored at −80°C. Before use, the high titer adenoviruses were dialyzed with saline for at least 40min at 4°C followed by 1:1 dilution with saline to reduce tissue damage caused by the high-salt storage solution.
To test the GPR30-siRNA-Ads in vivo, we conducted two studies. First, we tested the time-course of GPR30 knockdown by GPR30-siRNA-Ads. Then, we confirmed that knockdown of GPR30 was due to the GPR30-siRNA-Ads and not due to the viral injection or infection. Rats were anesthetized and OVX as described in experiment 1, then given unilateral intra-PVN injections of GPR30-siRNA-Ads (402, 737, and 1135 combined) using stereotaxic technique at a rate of 0.5 μl/min, 1 or 2 μl/side at the coordinates of AP= −1.8, ML= 0.5 and DV= −8.3 mm with respect to the bregma. The needle (31 gage) was left in the injection site for an additional 20min to reduce movement of the viral solution into the needle track. Three, five, or 10 days after injection, rats were sacrificed and the brains were removed. Brains were sectioned into 300 μm sections using a cryostat. Regions with viral injection as indicated by RFP and the contralateral regions (as controls) were punched out for immunoblot analysis of GPR30. The percent inhibition of GPR30-siRNA-Ads was calculated by comparing the ratio of GPR30:β-actin between the injected side (I) and the contralateral side (C) of each animal (% inhibition = (1−I/C) × 100%).
To confirm that observed knockdown of GPR30 protein was due to the GPR30-siRNA-Ads and not a result of the virus itself or tissue damage caused by the injection, OVX rats were given unilateral intra-PVN injections of saline, control-Ad (Adtrack, empty recombinant shuttle vector), GPR30-mis-Ads (737 and 1135 combined), or GPR30-siRNA-Ads (402, 737, and 1135 combined) using stereotaxic technique as described above. Five days after injection, rats were sacrificed and the brains were collected to evaluate the efficiency of knockdown of GPR30 expression by the siRNAs, compared to the various controls, as described above.
Effect of GPR30-siRNA-Ad on EB-induced desensitization of 5-HT1A receptors in the PVN
Rats were anesthetized and OVX as described above, then given bilateral intra-PVN injections of control-Ad or GPR30-siRNA-Ads (402, 737, 1135 combined) using stereotaxic technique at a rate of 0.5 μl/min, 1 μl/side at the coordinates of AP= −1.8, ML= ±0.5, and DV= −8.3 mm with respect to bregma. The needle was left in the injection site for an additional 20min to reduce movement of the viral solution into the needle track. Five days after OVX and viral injection, rats were treated with either 10 μg/kg/day EB or oil (s.c.) once a day for two days. 18h after the last EB or oil treatment, rats were injected with 0.2mg/kg (+)8-OH-DPAT or saline (s.c.) and sacrificed via decapitation 15 minutes later. Brains were removed and snap-frozen in dry-ice-cooled isopentane and then placed in dry ice. Trunk blood was collected in tubes containing 0.5ml 3M EDTA (pH 7.4). Brains and plasma were stored at −80°C until use.
Radioimmunoassay of plasma OT and ACTH
Plasma OT was determined by a radioimmunoassay as previously described with minor modifications (Li et al., 1997). Briefly, OT was extracted from 0.5 ml plasma with 1ml cold acetone followed by 2.5ml petroleum ether. The ether layer was aspirated and the samples were dried in a Centrivap vacuum concentrator at 4°C. The dried OT residue was resuspended in 1ml of cold assay buffer (0.05M phosphate buffer pH 7.4 containing 0.125% bovine serum albumin and 0.001M EDTA). The plasma extracts were used for the radioimmunoassay as previously described. The radioactive 125I oxytocin (specific activity: 2200 Ci/mmol) was obtained from Perkin Elmer (Waltham, MA). Several standard recovery samples containing 0.5ml pooled plasma and 8 and 16 pg OT were included throughout the extraction and assay procedure. The plasma OT concentrations were calculated based on the recovery and dilution factors. Plasma ACTH concentrations were determined by radioimmunoassay as previously described (Li et al., 1993). 125I ACTH (0.00102mCi) was obtained from DiaSorin (Stillwater, MN).
Quantitative Real-Time PCR
RNA was isolated from the PVN using TRI-Reagent according to the manufacturer’s instruction (Sigma-Aldrich, St. Louis, MO). Briefly, 5 μg RNA was treated with 5 μl DNase I in DNase I buffer (total reaction volume 50 μl) for 15min at room temperature; the reaction was stopped with 5 μl 25mM EDTA followed by incubation at 65°C for 10min. 10 μl of the DNase-treated RNA reaction mixture was incubated with 1 μl 0.3M oligo(dT)20 and 1 μl 10mM dNTPs at 65°C for 5 minutes. A master mix containing 5x reverse transcriptase buffer, 0.1M DTT, RNase Out, and 200 units Superscript II enzyme (Invitrogen, Carlsbad, CA) was added to each sample according to the manufacturer’s protocol. The reverse transcription was performed by incubation at 42°C for 50min and then reaction was stopped by incubation at 70°C for 15min. To cleave RNA templates, 1 μl RNase H was then added to the reaction which was then incubated at 37°C for 20 min. The complimentary DNA (cDNA) generated was used for real-time PCR.
Real-time quantitative PCR (qPCR) was performed in 96-well plates using SYBR Green Plus master mix (Invitrogen, Carlsbad, CA) and the Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems, Carlsbad, CA). mRNA levels of GPR30 (forward primer: CCACGCTCAAGGCAGTCATA; reverse primer: GCACTGCTGAACTTGACATCTGA), 5-HT1A receptor (forward primer: GATCTCGCTCACTTGGCTCAT; reverse primer: GCGCCAGCCCAGCAT), and RGSz1 (forward primer: AGACATTCCAGCGTGTGAAGAA; reverse primer: GGGCCCAGGCACAGACTT) were examined. The mRNA levels were normalized to TATA-box binding protein (TBP) mRNA (forward primer: CAGGAGCCAAGAGTGAAGAACA; reverse primer: GCTTCTGCACAACTCTAGCGTATT). Each qPCR reaction was conducted in a total volume of 20 μl, containing 2 μl cDNA, 0.2 μM of each primer, and 10 μl SYBR Green Plus master mix. PCR was performed at 50°C for 2min, 95°C for 10min, then 40 cycles of 95°C for 15s and 60°C for 1min per cycle. All samples were run in triplicate. ΔCt was calculated as the target gene – TBP mRNA for each sample; ΔΔCt was calculated as ΔCt for the experimental condition − ΔCt for the control condition, for each target gene.
Immunoblot assays
The PVN was punched out from 300 μm-thick sections prepared using a cryostat microtome. PVN tissue was homogenized in 100 μl homogenization buffer (10mM Tris pH 7.6, 100mM NaCl, 1mM EDTA, 1% sodium cholate, 1% phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO), 0.1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO)) by brief sonication followed by shaking for 1hr at 4°C. Samples were then centrifuged at 25,000×g for 1hr; the supernatant was collected and stored at −80°C. Protein concentration was measured using the Pierce BCA protein assay (Thermo Scientific, Rockford, IL). Protein (10 μg/lane) was resolved on a 12% SDS-PAGE gel followed by transfer to polyvinylidene fluoride (PVDF) membrane. The membranes were incubated in blocking buffer (5% milk in Tris-buffered saline with 0.1% Tween-20) to reduce non-specific binding and probed overnight using the following primary antibodies: rabbit anti-GPR30 (1:1000, Novus Biologicals, Littleton, CO); rabbit anti-5-HT1A receptor (1:1000, Abcam Cat.#85615, San Francisco, CA); rabbit anti-Gαz (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-RGSz1 (1:5000); rabbit anti-ERβ (1:1000, Alexis Biochemicals/Enzo Life Sciences, Plymouth Meeting, PA). After washing, membranes were incubated with the appropriate secondary antibody conjugated with horse radish peroxidase. Bands were detected with ECL substrate solution (GE Healthcare Biosciences, Piscataway, NJ) using BioRad ChemiDoc XRS+ molecular imager (BioRad, Hercules, CA). Due to the limited amount of PVN protein, all primary antibodies were used on the same blots, with washing in between uses. Monoclonal mouse actin antibody (1:10 000, MP Biomedicals, Solon, OH) was used as a loading control. Bands were analyzed densitometrically (integrated optical density, IOD) using ImageLab software (BioRad, Hercules, CA). Each band was normalized to actin and calculated as percent of the control group in each blot. All samples were run in duplicate or triplicate.
Characterization of anti-RGSz1
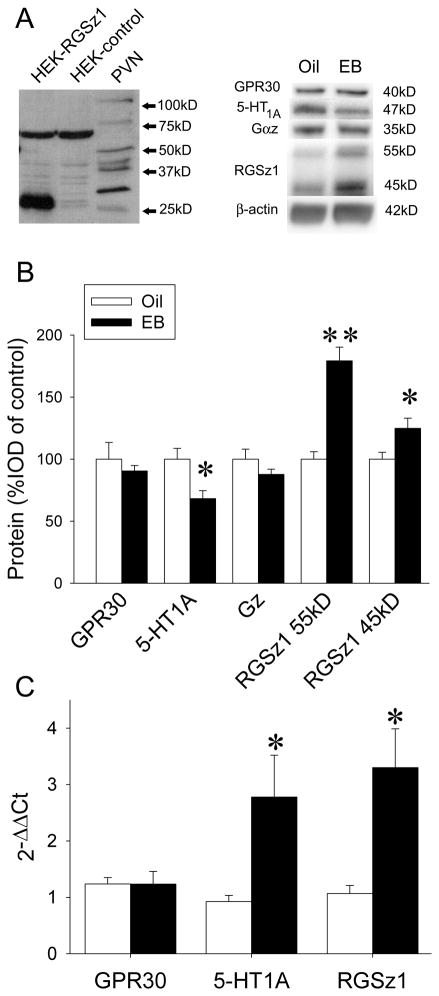
RGSz1 antiserum was raised in rabbits against the last 15 amino acids of the C-terminal of RGSz1 (YKDLLTSLAEKTVEA) and affinity purified by Biosynthesis (Lewisville, TX). To characterize the antibody, the full sequence of RGSz1 was inserted into a pcDNA vector (Invitrogen, Carlsbad, CA) which was then transfected into HEK293 cells. Cell lysates were collected, protein was resolved by SDS-PAGE, and Western blotting was performed as described above. Representative Western blot is shown in Figure 2A (left).
Figure 2.
EB-induced changes in 5-HT1A signaling pathway. A) Left, characterization of RGSz1 antibody. RGSz1 protein at approximately 27kD was detected in HEK293 cells transfected with a vector expressing RGSz1 protein but not empty-vector transfected cells. In rat PVN, higher molecular weight proteins were also detected most likely due to post-translational modifications. Lane 1, HEK293 cells transfected with RGSz1. Lane 2, HEK293 cells transfected with empty vector (pcDNA). Lane 3, PVN tissue lysate. Right, Western blot of PVN protein after oil or EB treatment with antibodies against GPR30, 5-HT1A, Gαz, and RGSz1, with β-actin as a loading control. B) Quantitation of protein levels after EB pretreatment. Bands were analyzed densitometrically (integrated optical density, IOD). Each band was normalized to β-actin and expressed as percent of control (oil). Data are expressed as mean ± SEM (n=6), *p<0.05, **p<0.001 by Student-Newman-Keuls post hoc test. C) qPCR of mRNA isolated from PVN tissue, normalized to control (oil). ΔCt was calculated as the target gene – TBP mRNA for each sample; ΔΔCt was calculated as ΔCt for the experimental condition − ΔCt for the control condition, for each target gene. Changes in mRNA levels are expressed as mean 2−ΔΔCt ± SEM (n=4–6), *p<0.05 by Student-Newman-Keuls post hoc test.
Microscopy
18 μm-Thick tissue sections were collected from the beginning, middle, and end of each 300 μl PVN section as described above to evaluate RFP expression. Images were captured using a Nikon Eclipse Ti2000 and Metamorph Software (Molecular Devices, Sunnyvale, CA) at 4x magnification.
Statistical analysis
All data are expressed as means ± SEM. Two- or three-way analysis of variance (ANOVA) and Student-Newman-Keuls post hoc tests were conducted using a statistical program (Statview version 5.0 software, SAS Institute Inc., Cary, NC).
Results
Experiment 1: Effects of EB on 5-HT1A receptor signaling in the PVN
EB induces desensitization of 5-HT1A receptor signaling
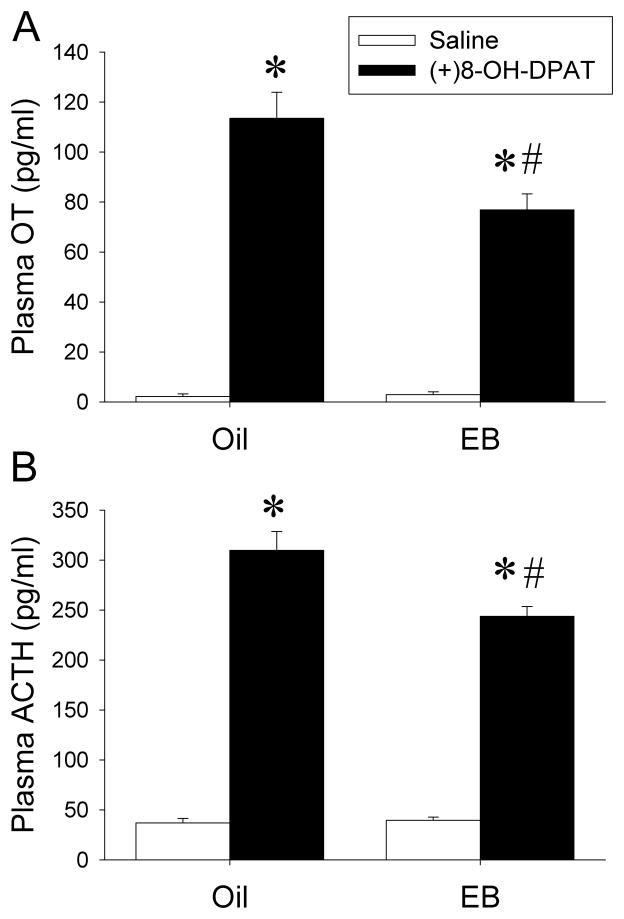
In our first experiment, female SD rats were OVX and treated with EB (10 μg/kg/day) for two days. To confirm that two-day EB treatment resulted in desensitization of 5-HT1A receptor signaling in our rat model, 18 hours after the second EB injection, the rats were challenged with the selective 5-HT1A receptor agonist (+)8-OH-DPAT (0.2mg/kg) and plasma hormone levels were examined. EB treatment did not alter the baseline levels of plasma OT in comparison to vehicle treatment (Figure 1A). Activation of 5-HT1A receptors by (+)8-OH-DPAT increased plasma OT levels in oil-treated rats as expected. The magnitude of the OT response to (+)8-OH-DPAT was significantly reduced in EB-treated rats (two-way ANOVA: main effect of (+)8-OH-DPAT: F(1,25)=299, p<0.0001; main effect of EB: F(1,25)=11.2, p<0.01; interaction between EB and (+)8-OH-DPAT: F(1,25)=12.2, p<0.01).
Figure 1.
EB treatment induces 5-HT1A desensitization. Plasma OT (A) and ACTH (B) levels in response to saline or (+)8-OH-DPAT challenge in rats treated with oil or EB. Data are presented as mean ±SEM (n=6–8/group). *Significantly different from saline-challenged animals with same treatment, p<0.0001; #significantly different from oil/(+)8-OH-DPAT-treated animals, p<0.005 by Student-Newman-Keuls post hoc test.
Baseline levels of plasma ACTH were unchanged by two-day EB treatment (Figure 1B), and stimulation of 5-HT1A receptors by (+)8-OH-DPAT increased plasma ACTH levels significantly. The ACTH response to (+)8-OH-DPAT was significantly reduced in EB-treated animals compared to oil treatment (two-way ANOVA: main effect of (+)8-OH-DPAT: F(1,27)=533, p<0.0001; main effect of EB: (F(1,27)=9.47, p<0.01; interaction between EB and (+)8-OH-DPAT: F(1,27)=11.0, p<0.01). Thus, both oxytocin and ACTH plasma concentration results suggest the desensitization of 5-HT1A receptor signaling in the PVN.
EB treatment alters expression of components of the 5-HT1A receptor signaling pathway To determine the cellular mechanisms mediating desensitization of 5-HT1A receptor signaling, we examined effects of EB treatment on the 5-HT1A receptor system in the PVN at the molecular level. EB reduced the protein levels of 5-HT1A receptors by 32% as measured on Western blots (Figure 2A, right) (F(1,5)=9.08, p<0.05). Two RGSz1 bands (55kD, 45kD) were measured. The protein levels in both bands were significantly increased by 79% (F(1,5)=21.8, p<0.01) and 25% (F(1,5)=6.75, p<0.05), respectively, in the EB group compared to the oil-treated group. There were no significant differences in the protein levels of either Gαz protein (F(1,5)=2.26, p=0.21) or GPR30 protein (F(1,5)=0.32, p=0.59) (Figure 2B).
Quantitative real-time PCR was conducted to further examine the expression of components of the 5HT1A receptor signaling pathway. Our results showed that EB treatment increased mRNA levels of 5-HT1A receptor by 299% (EB vs. oil, F(1,6)=6.13, p<0.05). RGSz1 mRNA levels increased by 308% (EB vs. oil, F(1,6)=10.2, p<0.05), while GPR30 mRNA did not change significantly (F(1,6)=0.0001, p=0.99) (Figure 2C).
Experiment 2: effects of reduction in the GPR30 expression on EB-induced desensitization of 5-HT1A receptor in the PVN
Generation and evaluation of GPR30-siRNA-Ads
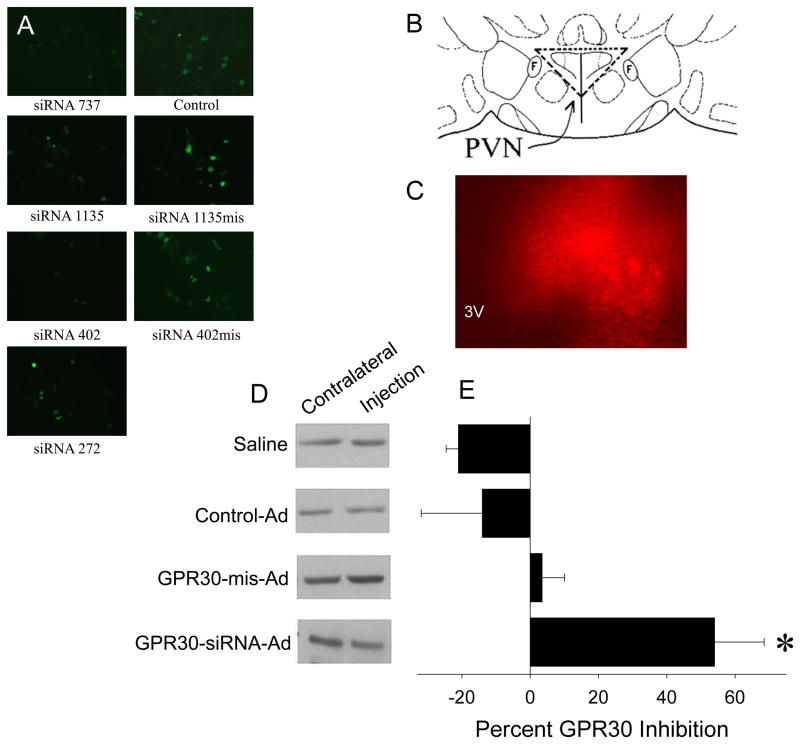
To select efficient GPR30 siRNA sequences, SOS-GPR30-siRNA-HUSs and SOS-GPR30-mis-HUSs were transfected into HEK293 cells. The cells transfected with GPR30-siRNA-HUS 402, 737 and 1135, but not 272 (numbers refer to first base pairs of each siRNA sequence), displayed reductions in the number of GFP-containing cells and the intensity of GFP compared to SOS-GPR30-mis-HUS and SOS-GPR30-HUS plasmids, beginning two days after transfection. Therefore, we selected the 402, 737, and 1135 siRNA constructs for production of GPR30 siRNA recombinant adenovirus (GPR30-siRNA-Ad). Figure 3A shows examples from 3 days after transfection.
Figure 3.
Generation and evaluation of recombinant adenoviruses containing GPR30-siRNAs. A) Selection of GPR30-siRNAs: SOS-GPR30-siRNA-HUS (left panels), SOS-GPR30-mis-siRNA- HUS (right panels), and SOS-GPR30-HUS (control) (right panels) constructs were transfected into HEK293 cells. The number and brightness of GFP-containing cells were observed three days after the transfection. B) Diagram of PVN injection site. F = fornix. Vertical line represents the 3rd ventricle. PVN is within the dashed triangle. C) An example of RFP expression in the PVN five days after unilateral injection of GPR30-siRNA-Ads. 3V = 3rd ventricle. D) Examples of Western blot for GPR30 protein from PVN tissue with unilateral injection of saline, control-Ad, GPR30-mis-Ads, and GPR30-siRNA-Ads five days after infection. E) Quantitation of GPR30 knockdown. Bands were measured densitometrically and normalized to β-actin. The percent inhibition of GPR30-siRNA-Ads was calculated by comparing the ratio of GPR30:β-actin between the injected side (I) and the contralateral side (C) of each animal (% inhibition = (1−I/C) × 100%). Data are presented as mean percent inhibition. ± SEM (n=4–5). *p<0.05 by Student-Newman-Keuls post hoc test.
To test the time course of GPR30 knockdown by GPR30-siRNA-Ads, we unilaterally injected 1 or 2 μl GPR30-siRNA-Ads into the PVN of rats (Figure 3B) and collected the brains 3, 5 and 10 days after the injection. By comparing GPR30 expression in the viral infected sides to their contralateral sides, we observed that the GPR30-siRNA-Ads significantly inhibited the expression of GPR30 in a dose-dependent manner (% inhibition: 59 ± 8 (n=3) for 1 μl vs. 78± 5 (n=6) for 2 μl viral injection). This inhibition of GPR30 expression was observed 3 days after the injections. There was no difference in inhibition between 3 and 5 days, but there was a slight, non-significant reduction in inhibition 10 days after the injection (89% inhibition for 3 days, 86% for 5 days and 66% for 10 days after 2 μl viral injection). These data demonstrated that the GPR30-siRNA-Ads are able to efficiently knock down GPR30 expression 3 days after injection. Although no visible tissue damage was observed, to avoid possible toxicity and to restrict the knockdown of GPR30 to the PVN, we used 1 μl of GPR30-siRNA-Ads and the 5 day time point in further studies.
To compare the effects of the GPR30-siRNA-Ads with the GPR30-mis-Ads in vivo, we unilaterally injected 1 μl of GPR30-siRNA-Ads into the PVN, with saline, empty viral vector (Adtrack, control-Ad), or GPR30-mis-Ads as controls. In the GPR30-siRNA-Ads construct, red fluorescent protein (RFP) is expressed independently of the siRNAs, making the presence of RFP useful for tracking the virus infection (Figure 3C). Five days after the injection of the virus, GPR30 protein level in the viral-infected side was measured and percent inhibition was calculated relative to the contralateral (non-injected) side of each animal (representative Western blots shown in Figure 3D). The GPR30-siRNA-Ads were successful in reducing GPR30 protein expression by about 42%, compared to the contralateral side (F(3,10)=6.23, p<0.01). None of the three controls had a significant effect on GPR30 protein levels (Figure 3E). However, in a separate experiment (not shown), GPR30-mis-Ads interfered with the neuroendocrine response. Therefore, we used control-Ad as the control for the following experiment.
Effect of GPR30-siRNA-Ads on GPR30 in the PVN
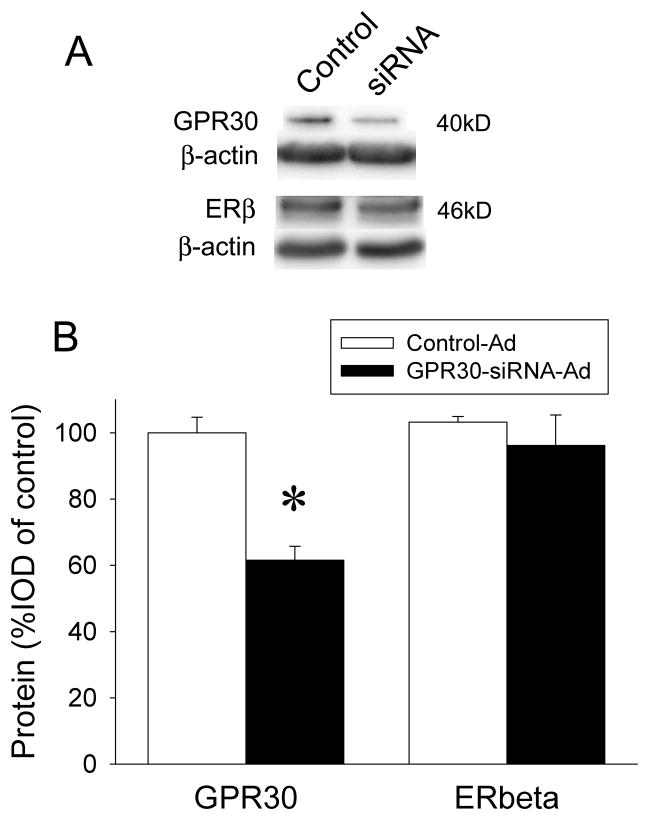
To determine the role of GPR30 in the EB-induced desensitization of 5-HT1A receptor signaling in the PVN, we performed bilateral injections of control-Ad or GPR30-siRNA-Ad constructs in the PVN of OVX rats. Five days after viral injection, rats were treated with 10 μg/kg/day EB or oil (s.c.) for two days and then challenged with 0.2mg/kg (+)8-OH-DPAT (s.c.) before being sacrificed, as described above. Knockdown of GPR30 was verified by Western blotting (Figure 4A). We used both reduction in GPR30 levels and the location of RFP expression as exclusion criteria for incorrect injections of GPR30-siRNA-Ads; rats with no RFP or RFP that was not in the PVN were excluded from subsequent analyses. GPR30 protein was successfully reduced to about 68% of control (F(1,12)=7.89, p<0.05) (Figure 4B). This 32% inhibition is a little lower than the reduction in the previous test. This could be due to the way tissue was collected and GPR30 levels compared: we measured GPR30 in the entire PVN if there was viral infection in at least part of the PVN, whereas previously we only injected one side of the PVN and only used tissue that had RFP expression. ERβ expression can be influenced by treatment with estradiol (Rossi et al., 2010); to ensure that effects of EB on 5-HT1A receptor signaling in the neuroendocrine challenge tests were due to the reduction of GPR30 and not a change in ERβ levels, we measured ERβ protein in the PVN following infection with GPR30-siRNA-Ads and found no significant change (F(1,15)=1.91, p=0.18) (Figure 4B).
Figure 4.
Confirmation of GPR30 knockdown. A) Examples of Western blots of GPR30 and ERβ with β-actin as loading control in GPR30-siRNA-Ad- and control-Ad-injected PVN. B) Quantitation of GPR30 knockdown and ERβ protein levels in the PVN of GPR30-siRNA-Ad- and control-Ad-injected rats. Bands were measured densitometrically and normalized to β-actin. Data are expressed as mean percent of control-Ad ± SEM (n=4–7), *p<0.01 by Student-Newman-Keuls post hoc test.
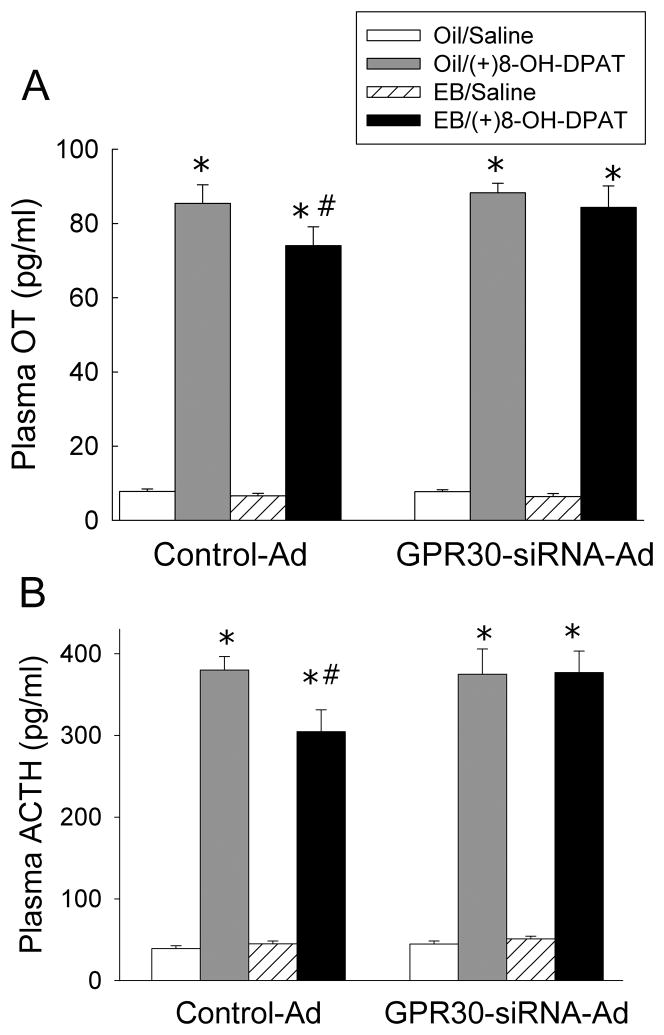
Effect of GPR30-siRNA-Ads on EB-induced desensitization of 5-HT1A receptor signaling
Desensitization of 5-HT1A receptor signaling was examined by measuring the plasma levels of OT (Figure 5A) and ACTH (Figure 5B). The baseline level of plasma OT was not altered by EB treatment or GPR30-siRNA-injection. In animals injected with the control-Ad, activation of 5-HT1A receptors by (+)8-OH-DPAT increased plasma OT levels. In the control-Ad-infected group, there was a significant reduction in OT response to (+)8-OH-DPAT after EB treatment compared to oil treatment, demonstrating desensitization of 5-HT1A receptor signaling. In the GPR30-siRNA-Ads-injected group, OT levels in response to (+)8-OH-DPAT were not significantly different from the control-Ad-injected, oil-treated group, and EB treatment did not induce 5-HT1A receptor signaling desensitization (three-way ANOVA: main effect of viral injection, F(1,56)=2.19, p=0.14; main effect of (+)8-OH-DPAT, F(1,56)=1219, p<0.0001; main effect of EB, F(1,56)=4.21, p<0.05; interaction between EB and (+)8-OH-DPAT, F(1,56)=2.16, p=0.15; interaction between EB and viral injection, F(1,56)=0.72, p<0.5; interaction between (+)8-OH-DPAT and viral injection, F(1,56)=2.39, p=0.13; interaction between EB, (+)8-OH-DPAT, F(1,56)=0.39, p=0.76).
Figure 5.
Effects of GPR30-siRNA-Ads on plasma OT (A) and ACTH (B) responses to (+)8-OH-DPAT in EB-treated rats. Data are expressed as mean ± SEM, (n=7–10). *Indicates significantly different from saline-challenged animals with same treatment, p<0.0001, #indicates significantly different from control-Ad/oil-treated rats with same challenge, p<0.05 by Student-Newman-Keuls post hoc test.
Plasma ACTH levels in response to (+)8-OH-DPAT showed a similar pattern. Neither EB treatment nor GPR30-siRNA-Ad injection had an effect on baseline levels of plasma ACTH. In animals injected with the control-Ad, activation of 5-HT1A receptors by (+)8-OH-DPAT increased plasma ACTH levels, while EB treatment produced a significant reduction in ACTH response to (+)8-OH-DPAT, demonstrating desensitization of 5-HT1A receptor signaling. In the GPR30-siRNA-Ad-injected group, ACTH levels in response to (+)8-OH-DPAT were not significantly different from the control-Ad-injected, oil-treated group, and EB treatment did not have an effect on plasma ACTH levels (three-way ANOVA: main effect of viral injection, F(1,62)=1.94, p=0.17; main effect of (+)8-OH-DPAT, F(1,62)=802, p<0.0001; main effect of EB, F(1,62)=0.69, p=0.41; interaction between (+)8-OH-DPAT and EB, F(1,62)=1.93, p=0.17; interaction between (+)8-OH-DPAT and viral injection, F(1,62)=0.38, p=0.77; interaction between EB and viral injection, F(1,62)=5.12, p=0.27; interaction between (+)8-OH-DPAT, EB, and viral injection, F(1,62)=5.04, p<0.05).
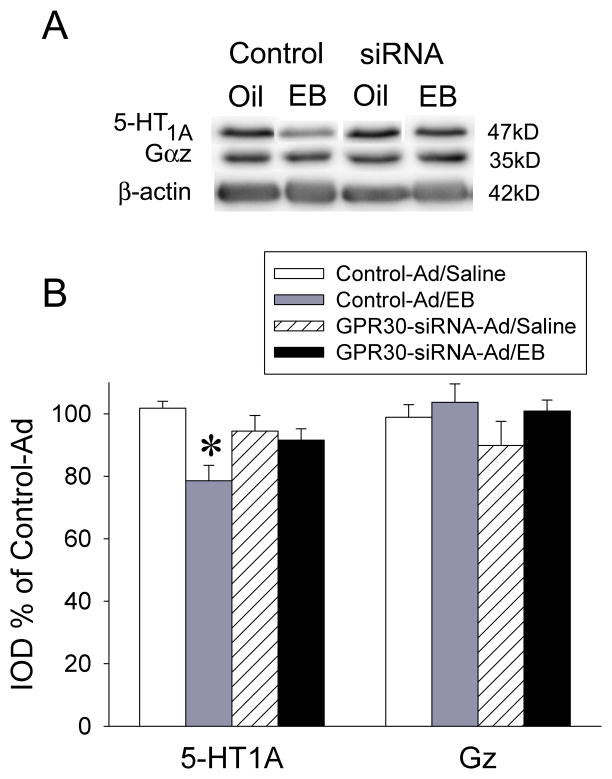
To further investigate the effects of EB treatment on 5-HT1A receptor signaling, we next examined 5-HT1A receptor protein and the G protein mediating the hormone responses, Gαz, using Western blotting (Figure 6A). EB pretreatment resulted in reduced levels of 5-HT1A receptor protein in the control-Ad-injected group (F(1,35)=6.69, p<0.001); this effect was not seen in the GPR30-siRNA-Ad-injected group, further suggesting that GPR30 may be involved in the reduction of 5-HT1A receptor protein. The GPR30-siRNA-Ad injection itself had no effect on 5-HT1A receptor protein levels. Levels of Gαz protein were unchanged, regardless of treatment (F(1,35)=1.05, p=0.39) (Figure 6B).
Figure 6.
GPR30-siRNA-Ads prevented EB-induced reductions of 5-HT1A receptor protein in the PVN. A) Representative Western blots of 5-HT1A receptor and Gαz protein levels with β-actin loading control in the PVN of rats injected with control-Ad or GPR30-siRNA-Ad followed by treatment with oil or EB. B) Quantitation of Western blots. Bands were measured densitometrically and normalized to β-actin. Data are expressed as mean percent of control-Ad/oil, ± SEM (n=7–12). *p<0.001 by Student-Newman-Keuls post hoc test.
Discussion
We previously reported that 2-day peripheral administration of EB resulted in the desensitization of 5-HT1A receptor signaling in the rat PVN (D’Souza et al., 2004; Raap et al., 2000). Our previous study demonstrated that this desensitization is not mediated by ERβ, as treatment with a selective ERβ agonist, DPN, did not mimic the results of EB treatment. Furthermore, siRNA knockdown of ERβ in the PVN did not prevent EB-induced desensitization of 5-HT1A receptor signaling (Rossi et al., 2010). However, 2-day intra-PVN injections of the selective GPR30 agonist, G-1, did result in desensitization of 5-HT1A receptor signaling (Xu et al., 2009). Therefore, in this study we chose to focus on the role of GPR30. We show for the first time that GPR30 expression is necessary for the EB-induced desensitization of 5-HT1A receptor signaling, and that this mechanism involves a decrease in 5-HT1A receptor protein expression and possibly an increase in the rate of Gαz-GTP hydrolysis, as suggested by an increase in RGSz1 protein expression.
To identify the role of GPR30 in EB-induced changes in 5-HT1A receptor signaling, we injected an adenovirus containing siRNA sequences against GPR30 directly into the PVN. Treatment with GPR30-siRNA-Ads produced a modest reduction of GPR30 protein, by about 32%. Despite such a modest reduction in GPR30 protein, GPR30-siRNA-Ads infection was successful in completely preventing the reduction of both the OT and ACTH hormone responses to (+)8-OH-DPAT produced by EB treatment, reinforcing the hypothesis that GPR30 is necessary for estradiol-induced 5-HT1A receptor desensitization.
To further elucidate the GPR30-mediated mechanism through which EB impacts 5-HT1A receptor desensitization, we examined changes in the 5-HT1A receptor signaling pathway. Two-day treatment with EB resulted in a decrease in 5-HT1A receptor protein in the PVN, which is consistent with previous studies that have reported a decrease in 5-HT1A receptor binding sites in the hypothalamus and DRN of nonhuman primates (Lu and Bethea, 2002) and reduced 5-HT1A receptor protein levels in the DRN (Henderson and Bethea, 2008) after chronic estradiol treatment. In contrast, we found that 2-day EB treatment increased 5-HT1A receptor mRNA. This upregulation of 5-HT1A receptor mRNA in the PVN may be a feedback response to the decrease in protein levels induced by estradiol, or estradiol signaling may be affecting 5-HT1A receptor translational control. Other studies have demonstrated that acute estradiol administration decreased 5-HT1A receptor gene expression in the rat limbic system (Osterlund and Hurd, 1998), while chronic estradiol decreased 5-HT1A receptor mRNA in the rat DRN (Birzniece et al., 2001) but increased 5-HT1A receptor mRNA in the DRN in nonhuman primates (Pecins-Thompson and Bethea, 1999). These discrepancies could be due to tissue-specific regulatory mechanisms, differences in chronic versus short-term estradiol administration, or species differences. Notably, the 32% reduction in GPR30 induced by infection with the GPR30-siRNA-Ads prevented the EB-induced changes in 5-HT1A receptor protein, thus reinforcing an important role for GPR30 in this mechanism.
There are some studies linking changes in 5-HT1A receptor expression to depression in human female patients (Szewczyk et al., 2009), which would indicate a role for estradiol in mediating 5-HT1A receptor expression as well as function. Pet-1, which is necessary for development of the serotonin system (Hendricks et al., 1999; Hendricks et al., 2003; Iyo et al., 2005; Maurer et al., 2004), directly binds to Pet-1 elements in the human 5-HT1A receptor promoter region, and is critical for 5-HT1A receptor expression in the midbrain (Jacobsen et al., 2011). In macaques, long-term OVX reduced expression of Pet-1 in the DRN (Bethea et al., 2011), while in rats, two-day administration of estradiol after OVX increased Pet-1 mRNA levels in the DRN (Rivera et al., 2009), indicating a clear role for regulation of midbrain 5-HT1A receptor expression by estradiol. A reduction in inhibitory DRN 5-HT1A autoreceptors, induced by estradiol via Pet-1, may play a role in desensitizing post-synaptic 5-HT1A receptors in the PVN by increasing serotonergic tone, similar to chronic SSRI treatment.
Though GPR30 is a membrane receptor that signals through heterotrimeric G proteins, studies have demonstrated that signaling through GPR30 has effects on gene transcription independent of classical estrogen response elements (Albanito et al., 2007; Maggiolini et al., 2004). GPR30 has been shown to act independently of ERα and ERβ to stimulate the MAPK/ERK1/2 signaling system via a Bordetella-pertussis-toxin-sensitive (PTX), Gβγ pathway (Filardo, 2002; Filardo et al., 2000). We showed previously that treatment with PTX inhibits modulation of 5-HT1A receptor signaling by estradiol (Xu et al., 2009), which again suggests that GPR30 signaling plays a role in this mechanism. In the current study, we saw an estradiol-induced decrease in 5-HT1A receptor expression that was precluded by GPR30 protein reduction. This suggests that estradiol could alter 5-HT1A receptor expression levels through the GPR30-Gβγ, MAPK/ERK1/2 pathway by transiently decreasing 5-HT1A receptor mRNA in the PVN. The subsequent decrease in 5-HT1A receptor protein levels could then feedback to lead to upregulation of mRNA expression, though the mechanisms remain unclear
5-HT1A receptors in the PVN are coupled to the G protein Gαz to stimulate OT and ACTH (via CRF) release (Barr et al., 1997; Raymond et al., 1993; Serres et al., 2000). Previous studies have shown that chronic estradiol treatment results in decreased DRN protein levels of Gαi3, but not Gαi1, Gαi/o, or Gαz in nonhuman primates (Lu and Bethea, 2002). Similarly, in the present study we found that 2-day EB administration had no effect on Gαz protein or mRNA levels in the PVN. These results are consistent with previous reports showing that estradiol treatment does not affect levels of Gαz protein in the rat PVN (Carrasco et al., 2004; D’Souza et al., 2004). Knockdown of GPR30 by GPR30-siRNA-Ads also had no effect on Gαz levels, indicating that GPR30 signaling does not regulate Gαz protein levels, and that alteration of Gαz levels is not responsible for estradiol-mediated 5-HT1A receptor desensitization.
While a decrease in 5-HT1A receptor protein expression may play a role in EB-induced desensitization, it is interesting that estradiol treatment also reduces 5-HT1A receptor function (D’Souza et al., 2004; Jackson and Uphouse, 1996, 1998; Raap et al., 2000) and coupling to G proteins (Mize and Alper, 2000), though we show that Gαz protein expression is not affected by EB. We therefore examined protein and mRNA expression of RGSz1, the GAP that is highly selective for Gαz (Glick et al., 1998; Wang et al., 1998; Wang et al., 2002). Consistent with a previous report (Carrasco et al., 2004), we found that 2-day EB treatment increased RGSz1 protein and now have found an increase in RGSz1 mRNA in the PVN. By increasing the hydrolysis of Gαz-GTP by over 400-fold (Wang et al., 1998), high levels of RGSz1 could effectively reduce the ability of Gαz to activate downstream effectors and hormone release upon 5-HT1A receptor stimulation, thus contributing to the desensitized response. Work is currently being done in our laboratory to investigate the potential interactions between RGSz1, Gαz, and 5-HT1A receptors.
Since OT-containing neurons express very little ERα (Simonian and Herbison, 1997), we have not yet directly addressed ERα as a potential candidate for desensitization of 5-HT1A receptor signaling. However, some studies have shown that hypothalamic neurons, as well as other cell types, have a number of ERα splice variants that are located in the plasma membrane and could mediate estradiol signaling (Acconcia et al., 2005; Bollig and Miksicek, 2000; Dominguez et al., 2009; Dominguez and Micevych, 2010; Gorosito et al., 2008; Ishunina and Swaab, 2008; Li et al., 2003). Recent studies have found a novel, membrane-targeted, 36kD splice variant (ERα36) with a unique C-terminal sequence in human tissues and human cell lines (Wang et al., 2005; Zou et al., 2009), and suggest that ERα36 may be involved in responses heretofore believed to be mediated by GPR30. In ER-negative breast cancer cell lines that express ERα36 (e.g. MDA-MB-231 and SK-BR-3), the selective estrogen receptor modulator tamoxifen and ER antagonist ICI 182, 780 were unable to block nongenomic estrogen signaling (Lee et al., 2008; Wang et al., 2006), similar to effects seen when GPR30 is expressed (Filardo et al., 2006). A recent study by Kang et al. (2010) demonstrated that GPR30 expression and signaling induced ERα36 expression, that G-1 specifically binds and activates ERα36, and that ERα36 mediates nongenomic estrogen signaling independent of GPR30 in full-length-ERα-negative but ERα36-positive breast cancer cells. They suggest that GPR30 signals through the Src/MAPK/AP-1 pathway to activate ERα36 promoter activity and induce ERα36 expression, and that G-1 may be an agonist for ERα36, not GPR30. Other studies have reported ERα36 effects or immunoreactivity in rodent tissues; however, they failed to directly demonstrate expression of this splice variant (Jia et al., 2011; Patkar et al., 2011). Indeed, we performed immunohistochemical staining of rat brain tissue using the antibody kindly provided by Wang’s group (Wang et al., 2006), raised against the unique C-terminal sequence of ERα36, and found strong immunoreactivity in neurons throughout the rat brain. However, we conducted a BLAST search for the unique 88 base pair sequence of human ERα36 in the rat genome and no comparable sequence was detected. These data suggest that while it is possible that the rat brain may contain an ERα splice variant that plays a role in estradiol-GPR30 signaling, this putative splice variant is not the same as the ERα36 described in humans.
The results of this study demonstrate that GPR30 expression is necessary for estradiol-induced desensitization of 5-HT1A receptor signaling in the PVN, and that the mechanism likely involves a decrease in 5-HT1A receptor protein levels and an increase in RGSz1 expression. Further studies are needed to clarify whether ERα or any of its splice variants play a role in this mechanism. Because the effects of SSRIs occur slowly and are thought to involve desensitization of pre-synaptic and post-synaptic 5-HT1A receptors (Bosker et al., 2001; Chaput et al., 1986; Czachura and Rasmussen, 2000; Rush et al., 2004; Li et al., 1997; Gunther et al., 2008; Raap et al., 1999, Bleir and de Montigny, 1994), elucidating the estrogen-receptor-mediated mechanisms that result in rapid desensitization of 5-HT1A signaling will be important in the design of novel treatments that activate specific estrogen receptors in conjunction with SSRI therapy, for the improved treatment of depression and other mood disorders.
Acknowledgments
We would like to thank Dr. He at the University of Chicago for providing the recombinant adenoviral vectors for preparation of the siRNA constructs and technical advice. We would also like to thank Dr. Ying Dai, Dr. Laura J. Miller, Dr. Dania Rossi, J. Matthew Sturgeon, and Cynthia Gouvion for their assistance in the animal experiments. Funding for this study was provided by NIMH.
Funding for this study was provided by NIMH RO1MH058448; the NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
None of the authors have any actual or potential financial and other conflicts of interest related to the submitted manuscript. This includes disclosure of all financial considerations (ownership, equity position, stock options, consulting fees, patent rights, employee status and corporate affiliations) associated with any drug, product, process, or commercial laboratory mentioned in the submitted material.
Contributors
Drs. Qian Li and Nancy Muma designed the study and the protocol and made major contributions to the writing of the manuscript. Dr. Qian Li designed and generated the recombinant adenovirus. Carrie McAllister designed and performed the assays, data analysis and prepared the first drafts of the manuscript including the literature searches. Renea Creech and Paul Kimball played a major role in the animal experiments. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Bio Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. P Natl Acad Sci USA. 1998;95:3281–3286. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DF, Furst K, Tipping D, Dain MP, Vandepol C. A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch Study Group. Obstet Gynecol. 1999;94:498–503. doi: 10.1016/s0029-7844(99)00359-2. [DOI] [PubMed] [Google Scholar]
- Arpels JC. The female brain hypoestrogenic continuum from the premenstrual syndrome to menopause. A hypothesis and review of supporting data. J Reprod Med. 1996;41:633–639. [PubMed] [Google Scholar]
- Banger M. Affective syndrome during perimenopause. Maturitas. 2002;41(Suppl 1):S13–18. doi: 10.1016/s0378-5122(02)00011-7. [DOI] [PubMed] [Google Scholar]
- Barr AJ, Brass LF, Manning DR. Reconstitution of receptors and GTP-binding regulatory proteins (G proteins) in Sf9 cells. A direct evaluation of selectivity in receptor G protein coupling. J Biol Chem. 1997;272:2223–2229. doi: 10.1074/jbc.272.4.2223. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Smith AW, Centeno ML, Reddy AP. Long-term ovariectomy decreases serotonin neuron number and gene expression in free ranging macaques. Neuroscience. 2011;192:675–688. doi: 10.1016/j.neuroscience.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birzniece V, Johansson IM, Wang MD, Seckl JR, Backstrom T, Olsson T. Serotonin 5-HT(1A) receptor mRNA expression in dorsal hippocampus and raphe nuclei after gonadal hormone manipulation in female rats. Neuroendocrinology. 2001;74:135–142. doi: 10.1159/000054679. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;7:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Bollig A, Miksicek RJ. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Mol Endocrinol. 2000;14:634–649. doi: 10.1210/mend.14.5.0460. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Cremers TI, Jongsma ME, Westerink BH, Wikstrom HV, den Boer JA. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem. 2001;76:1645–1653. doi: 10.1046/j.1471-4159.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Barker SA, Zhang Y, Damjanoska KJ, Sullivan NR, Garcia F, D’Souza DN, Muma NA, van De Kar LD. Estrogen treatment increases the levels of regulator of G protein signaling-Z1 in the hypothalamic paraventricular nucleus: possible role in desensitization of 5-hydroxytryptamine1A receptors. Neuroscience. 2004;127:261–267. doi: 10.1016/j.neuroscience.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. N-S Arch Pharmakol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Czachura JF, Rasmussen K. Effects of acute and chronic administration of fluoxetine on the activity of serotonergic neurons in the dorsal raphe nucleus of the rat. N-S Arch Pharmakol. 2000;362:266–275. doi: 10.1007/s002100000290. [DOI] [PubMed] [Google Scholar]
- D’Souza DN, Zhang Y, Damjanoska KJ, Carrasco GA, Sullivan NR, Garcia F, Battaglia G, Doncarlos LL, Muma NA, Van de Kar LD. Estrogen reduces serotonin-1A receptor-mediated oxytocin release and Galpha(i/o/z) proteins in the hypothalamus of ovariectomized rats. Neuroendocrinology. 2004;80:31–41. doi: 10.1159/000080795. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29:4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. J Neurosci. 2010;30:12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006:6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiat. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- Glick JL, Meigs TE, Miron A, Casey PJ. RGSZ1, a Gz-selective regulator of G protein signaling whose action is sensitive to the phosphorylation state of Gzalpha. J Biol Chem. 1998;273:26008–26013. doi: 10.1074/jbc.273.40.26008. [DOI] [PubMed] [Google Scholar]
- Gomez-Gil E, Navines R, Martinez De Osaba MJ, Diaz-Ricart Ml, Escolar G, Salamero M, Martin-Santos R, Galan A, Gasto C. Hormonal responses to the 5-HT1A agonist buspirone in remitted endogenous depressive patients after long-term imipramine treatment. Psychoneuroendocrinol. 2010;35:481–489. doi: 10.1016/j.psyneuen.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Gunther L, Liebscher S, Jahkel M, Oehler J. Effects of chronic citalopram treatment on 5-HT1A and 5-HT2A receptors in group- and isolation-housed mice. Eur J Pharmacol. 2008;593:49–61. doi: 10.1016/j.ejphar.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Halbreich U. Gonadal hormones and antihormones, serotonin and mood. Psychopharmacol Bull. 1990;26:291–295. [PubMed] [Google Scholar]
- Harlow BL, Wise LA, Otto MW, Soares CN, Cohen LS. Depression and its influence on reproductive endocrine and menstrual cycle markers associated with perimenopause: the Harvard Study of Moods and Cycles. Arch Gen Psychiat. 2003;60:29–36. doi: 10.1001/archpsyc.60.1.29. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. P Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JA, Bethea CL. Differential effects of ovarian steroids and raloxifene on serotonin 1A and 2C receptor protein expression in macaques. Endocrine. 2008;33:285–293. doi: 10.1007/s12020-008-9087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Steinhauser A, Merchenthaler I, Coen CW, Petersen SL, Liposits Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. J Comp Neurol. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Estrogen receptor-alpha splice variants in the human brain. Gynecol Endocrinol. 2008;24:93–98. doi: 10.1080/09513590701705148. [DOI] [PubMed] [Google Scholar]
- Iyo AH, Porter B, Deneris ES, Austin MC. Regional distribution and cellular localization of the ETS-domain transcription factor, FEV, mRNA in the human postmortem brain. Synapse. 2005;57:223–228. doi: 10.1002/syn.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Uphouse L. Prior treatment with estrogen attenuates the effects of the 5-HT1A agonist, 8-OH-DPAT, on lordosis behavior. Hormones and behavior. 1996;30:145–152. doi: 10.1006/hbeh.1996.0018. [DOI] [PubMed] [Google Scholar]
- Jackson A, Uphouse L. Dose-dependent effects of estradiol benzoate on 5-HT1A receptor agonist action. Brain Res. 1998;796:299–302. doi: 10.1016/s0006-8993(98)00238-8. [DOI] [PubMed] [Google Scholar]
- Jacobsen KX, Czesak M, Deria M, Le Francois B, Albert PR. Region-specific regulation of 5-HT1A receptor expression by Pet-1-dependent mechanisms in vivo. J Neurochem. 2011;116:1066–1076. doi: 10.1111/j.1471-4159.2010.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Zhang X, He DZ, Segal M, Berro A, Gerson TG, Wang Z, Casale TB. Expression and Function of a Novel Variant of Estrogen Receptor-ER-{alpha}36 in Mouse Airway. Am J Resp Cell Mol. 2011 Jun 3; doi: 10.1165/rcmb.2010-0268OC. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimerson DC, Wolfe BE, Metzger ED, Finkelstein DM, Cooper TB, Levine JM. Decreased serotonin function in bulimia nervosa. Arch Gen Psychiat. 1997;54:529–534. doi: 10.1001/archpsyc.1997.01830180043005. [DOI] [PubMed] [Google Scholar]
- Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiat. 1998;44:798–811. doi: 10.1016/s0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lee LM, Cao J, Deng H, Chen P, Gatalica Z, Wang ZY. ER-alpha36, a novel variant of ER-alpha, is expressed in ER-positive and -negative human breast carcinomas. Anticancer Res. 2008;28:479–483. [PMC free article] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer B, Gelfin Y, Gorfine M, Allolio B, Lesch KP, Newman ME. 5-HT1A receptor function in normal subjects on clinical doses of fluoxetine: blunted temperature and hormone responses to ipsapirone challenge. Neuropsychopharmacol. 1999;20:628–639. doi: 10.1016/S0893-133X(98)00106-7. [DOI] [PubMed] [Google Scholar]
- Lesch KP. 5-HT1A receptor responsivity in anxiety disorders and depression. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:723–733. doi: 10.1016/0278-5846(91)90001-h. [DOI] [PubMed] [Google Scholar]
- Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. P Natl Acad Sci USA. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Levy AD, Cabrera TM, Brownfield MS, Battaglia G, Van de Kar LD. Long-term fluoxetine, but not desipramine, inhibits the ACTH and oxytocin responses to the 5-HT1A agonist, 8-OH-DPAT, in male rats. Brain Res. 1993;630:148–156. doi: 10.1016/0006-8993(93)90652-4. [DOI] [PubMed] [Google Scholar]
- Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of G(i) and G(o) proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharm Exp Ther. 1997;282:1581–1590. [PubMed] [Google Scholar]
- Li Q, Muma NA, van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharm Exp Ther. 1996;279:1035–1042. [PubMed] [Google Scholar]
- Lomax P, Schonbaum E. Postmenopausal hot flushes and their management. Pharmacol Therapeut. 1993;57:347–358. doi: 10.1016/0163-7258(93)90060-q. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacol. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He TC. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nature Prot. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- Maurer P, Rorive S, de Kerchove d’Exaerde A, Schiffmann SN, Salmon I, de Launoit Y. The Ets transcription factor Fev is specifically expressed in the human central serotonergic neurons. Neurosci Lett. 2004;357:215–218. doi: 10.1016/j.neulet.2003.12.086. [DOI] [PubMed] [Google Scholar]
- Mize AL, Alper RH. Acute and long-term effects of 17beta-estradiol on G(i/o) coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPgammaS binding. Brain Res. 2000;859:326–333. doi: 10.1016/s0006-8993(00)01998-3. [DOI] [PubMed] [Google Scholar]
- Navines R, Martin-Santos R, Gomez-Gil E, Martinez de Osaba MJ, Imaz ML, Gasto C. Effects of citalopram treatment on hypothermic and hormonal responses to the 5-HT1A receptor agonist buspirone in patients with major depression and therapeutic response. Psychoneuroendocrinol. 2007;32:411–416. doi: 10.1016/j.psyneuen.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Mathe AA, Czernik A, Thiele J, Bohner J, Eap CB, Agren H, Baumann P. Long-term citalopram administration reduces responsiveness of HPA axis in patients with major depression: relationship with S-citalopram concentrations in plasma and cerebrospinal fluid (CSF) and clinical response. Psychopharmacol. 2005;181:751–760. doi: 10.1007/s00213-005-0034-3. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P, James A, Crane J, Scrogin KE. 5-Hydroxytryptamine 1A receptors in the paraventricular nucleus of the hypothalamus mediate oxytocin and adrenocorticotropin hormone release and some behavioral components of the serotonin syndrome. J Pharm Exp Ther. 2005;313:1324–1330. doi: 10.1124/jpet.104.082073. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Hurd YL. Acute 17 beta-estradiol treatment down-regulates serotonin 5HT1A receptor mRNA expression in the limbic system of female rats. Brain research Mol Brain Res. 1998;55:169–172. doi: 10.1016/s0169-328x(98)00018-7. [DOI] [PubMed] [Google Scholar]
- Patkar S, Farr TD, Cooper E, Dowell FJ, Carswell HV. Differential vasoactive effects of oestrogen, oestrogen receptor agonists and selective oestrogen receptor modulators in rat middle cerebral artery. Neurosci Res. 2011;71:78–84. doi: 10.1016/j.neures.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Raap DK, DonCarlos L, Garcia F, Muma NA, Wolf WA, Battaglia G, Van de Kar LD. Estrogen desensitizes 5-HT(1A) receptors and reduces levels of G(z), G(i1) and G(i3) proteins in the hypothalamus. Neuropharmacol. 2000;39:1823–1832. doi: 10.1016/s0028-3908(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Raap DK, Evans S, Garcia F, Li Q, Muma NA, Wolf WA, Battaglia G, Van De Kar LD. Daily injections of fluoxetine induce dose-dependent desensitization of hypothalamic 5-HT1A receptors: reductions in neuroendocrine responses to 8-OH-DPAT and in levels of Gz and Gi proteins. J Pharm Exp Ther. 1999;288:98–106. [PubMed] [Google Scholar]
- Raymond JR, Olsen CL, Gettys TW. Cell-specific physical and functional coupling of human 5-HT1A receptors to inhibitory G protein alpha-subunits and lack of coupling to Gs alpha. Biochem. 1993;32:11064–11073. doi: 10.1021/bi00092a016. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depression and anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rivera HM, Oberbeck DR, Kwon B, Houpt TA, Eckel LA. Estradiol increases Pet-1 and serotonin transporter mRNA in the midbrain raphe nuclei of ovariectomized rats. Brain Res. 2009;1259:51–58. doi: 10.1016/j.brainres.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DV, Dai Y, Thomas P, Carrasco GA, DonCarlos LL, Muma NA, Li Q. Estradiol-induced desensitization of 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus is independent of estrogen receptor-beta. Psychoneuroendocrinol. 2010;35:1023–1033. doi: 10.1016/j.psyneuen.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinol. 2007;148:5842–5850. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocr Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- Sargent P, Williamson DJ, Pearson G, Odontiadis J, Cowen PJ. Effect of paroxetine and nefazodone on 5-HT1A receptor sensitivity. Psychopharmacol. 1997;132:296–302. doi: 10.1007/s002130050348. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiat. 2004;161:2238–2244. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Small GW, Hamilton SH, Bystritsky A, Nemeroff CB, Meyers BS. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiat. 1997;5:97–106. [PubMed] [Google Scholar]
- Serres F, Li Q, Garcia F, Raap DK, Battaglia G, Muma NA, Van de Kar LD. Evidence that G(z)-proteins couple to hypothalamic 5-HT(1A) receptors in vivo. J Neurosci. 2000;20:3095–3103. doi: 10.1523/JNEUROSCI.20-09-03095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Bethea CL. Cognition, mood disorders, and sex hormones. ILAR J. 2004;45:189–199. doi: 10.1093/ilar.45.2.189. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Herbison AE. Differential expression of estrogen receptor alpha and beta immunoreactivity by oxytocin neurons of rat paraventricular nucleus. J Neuroendocrinol. 1997;9:803–806. doi: 10.1046/j.1365-2826.1997.00659.x. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, Meltzer HY, Konick LC, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA, Austin MC. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009;12:155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinol. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Wang J, Ducret A, Tu Y, Kozasa T, Aebersold R, Ross EM. RGSZ1, a Gz-selective RGS protein in brain. Structure, membrane association, regulation by Galphaz phosphorylation, and relationship to a Gz gtpase-activating protein subfamily. J Biol Chem. 1998;273:26014–26025. doi: 10.1074/jbc.273.40.26014. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ho G, Zhang JJ, Nieuwenhuijsen B, Edris W, Chanda PK, Young KH. Regulator of G protein signaling Z1 (RGSZ1) interacts with Galpha i subunits and regulates Galpha i-mediated cell signaling. J Biol Chem. 2002;277:48325–48332. doi: 10.1074/jbc.M206116200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Comm. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. P Natl Acad Sci USA. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Ding L, Coleman M, Wang Z. Estrogen receptor-alpha (ER-alpha) suppresses expression of its variant ER-alpha 36. FEBS Lett. 2009;583:1368–1374. doi: 10.1016/j.febslet.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]