Abstract
The docking protein FRS2 is a major downstream effector that links fibroblast growth factor (FGF) and nerve growth factor receptors with the Ras/mitogen-activated protein kinase signaling cascade. In this report, we demonstrate that FRS2 also plays a pivotal role in FGF-induced recruitment and activation of phosphatidylinositol 3-kinase (PI3-kinase). We demonstrate that tyrosine phosphorylation of FRS2α leads to Grb2-mediated complex formation with the docking protein Gab1 and its tyrosine phosphorylation, resulting in the recruitment and activation of PI3-kinase. Furthermore, Grb2 bound to tyrosine-phosphorylated FRS2 through its SH2 domain interacts primarily via its carboxyl-terminal SH3 domain with a proline-rich region in Gab1 and via its amino-terminal SH3 domain with the nucleotide exchange factor Sos1. Assembly of FRS2α:Grb2:Gab1 complex induced by FGF stimulation results in activation of PI3-kinase and downstream effector proteins such as the S/T kinase Akt, whose cellular localization and activity are regulated by products of PI3-kinase. These experiments reveal a unique mechanism for generation of signal diversity by growth factor-induced coordinated assembly of a multidocking protein complex that can activate the Ras/mitogen-activated protein kinase cascade to induce cell proliferation and differentiation, and PI3-kinase to activate a mediator of a cell survival pathway.
Fibroblast growth factors (FGFs) play key roles in diverse cellular processes including mitogenesis, differentiation, migration, and survival (reviewed in refs. 1 and 2). We have recently identified a major downstream mediator of signaling through the activated FGF receptors (FGFRs), termed FRS2, that was redesignated FRS2α when a highly homologous isoform, FRS2β, was identified (3, 4). The FRS2 proteins are targeted to the plasma membrane by myristylation at the N terminus and contain a phosphotyrosine-binding domain that mediates direct interaction with FGF or nerve growth factor (NGF) receptors (4–8). The C-terminal region of FRS2 contains multiple tyrosine residues that are phosphorylated by activated FGF or NGF receptors, serving as recognition motifs for the SH2 domains of Grb2 and Shp2 (3, 9). Recruitment of both Grb2 and Shp2 are required for FGF-mediated mitogen-activated protein kinase (MAPK) activation, proliferation of fibroblasts, and neuronal differentiation of PC12 cells (3, 9).
Although FGFs and neurotrophins are strongly implicated in cell survival, the intracellular signaling machinery and mechanisms involved are not clear (1, 10, 11). Numerous studies have provided evidence that the PI3-kinase/Akt (or PKB) signaling cascade activated by multiple growth factors and cytokines results primarily in cell survival (12–14), as opposed to the Ras/MAPK pathway, which mainly signals to control cell proliferation and differentiation (15, 16). Although some receptors such as the platelet-derived growth factor (PDGF) and ErbB3 recruit PI3-kinase directly (17, 18), other receptors such as the insulin and epidermal growth factor (EGF) receptors use specific docking proteins such as IRS1 and Gab1, respectively (19–22). In EGF signaling, PI3-kinase is recruited by hetero-oligomerization with ErbB3 and by tyrosine phosphorylation of Gab1; a docking protein that upon tyrosine phosphorylation recruits and activates PI3-kinase (18, 21, 23). Moreover, PI3-kinase activity recruited through Gab1 was shown to be essential for NGF-induced protection from apoptosis induced by serum deprivation, as well as for neurite outgrowth in PC12 cells (24–26). Gab1 was originally cloned as a Grb2-associated protein that is tyrosine phosphorylated in cells upon stimulation with EGF or insulin (20). Gab1 possesses an N-terminal pleckstrin homology (PH) domain, a Met-binding domain (MBD) that mediates interactions with the Met receptor and other receptor tyrosine kinases, and a large C-terminal portion containing multiple tyrosine residues (20, 27). Tyrosine-phosphorylated Gab1 is capable of recruiting several SH2 domain-containing signaling molecules including Grb2, Shp2, PLCγ, Crk1, PI3-kinase, and Nck (20, 24, 28, 29). Recently, a highly homologous isoform of Gab1, termed Gab2, was isolated (30, 31). Unlike the broad expression pattern of Gab1, Gab2 is predominantly expressed in hematopoietic cells (30). Several reports have shown that the Gab family of docking proteins are involved in the activation of PI3-kinase in response to growth factors or cytokines stimulation and by activation of the B cell antigen receptor (20, 21, 24, 25, 27, 28, 30, 32–35).
In this report, we show that in response to FGF stimulation, FRS2α and Gab1 associate indirectly via Grb2 resulting in tyrosine phosphorylation of Gab1 and activation of the PI3-kinase/Akt survival pathway. These experiments reveal a mechanism for activation of multiple signaling pathways by coordinated assembly of docking proteins.
Materials and Methods
Antibodies.
Antibodies for FGFR1, FRS2, Grb2, Shc, Sos1, and pTyr were previously described (3, 4, 36). Anti-Shp2 antibodies were from Transduction Laboratories (Lexington, KY). Anti-Gab1 antibodies were from Upstate Biotechnology (Lake Placid, NY). Anti-Akt and anti-pS473-Akt antibodies were from New England Biolabs. Anti-FLAG (M2) antibodies were from Sigma.
Cell Lines.
Swiss 3T3, NIH 3T3, 293, COS-1, SH-SY5Y, and L6-FGFR1 cells were cultured in DMEM supplemented with 10% FBS, 10 mM l-glutamine, and 100 μg each of penicillin and streptomycin/ml, all from GIBCO/BRL. PC12 cells were grown in DMEM supplemented with 10% FCS and 10% heat-inactivated horse serum (GIBCO/BRL).
Expression Constructs.
The FGFR1 expression vectors for wild-type (wt) or kinase-inactive (KD) mutant were previously described (36). FRS2α vectors for wt or the 6F-FRS2α mutant were described in refs. 3 and 4. Mouse Gab1 cDNA was kindly provided by W. Birchmeier (Max Delbrück Center for Molecular Medicine, Berlin, Germany). Gab1 mutants consisting of the PH domain or the MBD or point mutations were generated by standard PCR and subcloning procedures. The Akt plasmid was from P. Cohen (University of Dundee, Dundee, U.K.). Transient transfection of cells was performed by using Lipofectamine (GIBCO/BRL). Glutathione S-transferase (GST) fusion proteins of Grb2 (amino acids 1–217), Grb2-SH2 (amino acid 54–164), Grb2-N-SH3 (amino acid 1–68), Grb2-C-SH3 (amino acid 156–199), Gab1 (amino acids 1–695), and Gab1-MBD (amino acids 450–532) were expressed in Escherichia coli and purified with glutathione-conjugated agarose beads (Sigma) according to established procedures.
Immunoprecipitations, Protein Binding Studies, and PI3-Kinase Assays.
The protocols for immunoprecipitations, binding studies with GST fusion proteins and in vitro PI3-kinase assays were as described previously (3, 4, 17).
Results
In a survey of tyrosine phosphoproteins involved in FGF signaling, we have identified proteins that coimmunoprecipitate with Grb2 upon FGF stimulation. Lysates from FGF-stimulated Swiss 3T3 fibroblasts were subjected to immunoprecipitation with anti-Grb2 antibodies followed by SDS–PAGE and immunoblotting with anti-pTyr antibodies. FGF induced the association of multiple tyrosine-phosphorylated proteins with Grb2 (Fig. 1A). Four prominent tyrosine-phosphorylated proteins that were detected include FRS2α, Shp2, Shc, and a previously undescribed broad band with apparent molecular mass of 120 kDa, termed here p120.
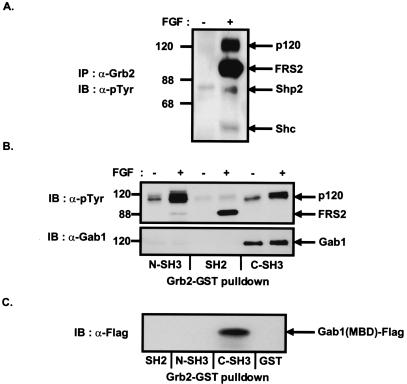
Figure 1.
Complex formation between Grb2 and Gab1 in FGF-stimulated cells. (A) Quiescent Swiss 3T3 cells were unstimulated or stimulated with FGF1 and heparin (100 ng/ml and 5 μg/ml, respectively) for 10 min. Lysates were immunoprecipitated with anti-Grb2 antibodies and followed by SDS–PAGE and immunoblotting with anti-pTyr antibodies. (B) Swiss 3T3 cells were unstimulated or stimulated with FGF1 and heparin (100 ng/ml and 5 μg/ml, respectively) for 10 min. Cell lysates were incubated with immobilized GST fusion proteins of the N-terminal SH3, SH2, or C-terminal SH3 domains of Grb2. Bound proteins were eluted and resolved by SDS–PAGE and then followed by immunoblotting with anti-pTyr (Upper) or anti-Gab1 antibodies (Lower). (C) Gab1 binds to the C-terminal SH3 domain of Grb2 through the MBD. 293 cells were transfected with the expression vector for Flag-tagged MBD of Gab1. The lysates were incubated with immobilized GST fusion proteins of the SH2, N-SH3, or C-SH3 domains of Grb2. Bound proteins were eluted and resolved by SDS–PAGE and followed by immunoblotting with anti-Flag antibodies.
To delineate the nature of interaction between p120 and Grb2, GST fusion proteins of the SH2 or SH3 domains of Grb2 were used to precipitate p120 from lysates of quiescent or FGF-stimulated Swiss 3T3 fibroblasts. Bound proteins were eluted, resolved by SDS–PAGE, and probed by immunoblotting with specific antibodies. Although the SH2 domain of Grb2 predominantly precipitated tyrosine-phosphorylated FRS2, the N- and C-terminal SH3 domains of Grb2 apparently precipitated the p120 species (Fig. 1B Upper). Immunoblotting with specific antibodies revealed that distinct components of the p120 species interacted specifically with the N- and C-terminal SH3 domains of Grb2. The p120 component that precipitated with the C-terminal SH3 domain of Grb2 was identified by immunoblotting with anti-Gab1 antibodies (Fig. 1B Lower), whereas the p120 component bound to the N-terminal SH3 domain of Grb2 remained unidentified.
Having established that the C-terminal SH3 domain of Grb2 associated with Gab1, we next determined the region on Gab1 that mediates this interaction. A likely candidate was the proline-rich region that resides in the MBD of Gab1 as proline-rich sequences had been shown to bind to SH3 domains (27). 293 cells were transfected with Gab1-MBD tagged at the N terminus with the FLAG-epitope, termed Gab1(MBD)-FLAG. The lysate was incubated with immobilized GST fusion proteins of the SH2 or SH3 domains of Grb2, or with GST alone. Bound proteins were eluted, resolved by SDS–PAGE, and probed with anti-FLAG antibodies (Fig. 1C). This experiment showed that the C-terminal SH3 domain of Grb2 binds specifically to the MBD of Gab1. To further characterize the proteins associating with Gab1 in FGFR signaling, 293 cells were transfected with expression vector for wt FGFR1 resulting in autoactivation, or with expression vector for kinase-inactive mutant of FGFR1 (KD) as a control, together with expression vectors for FRS2α, Shc, or Shp2 as indicated (Fig. 2 A–C). Lysates from the transfected cells were subjected to pull-down experiments with GST fusion protein of full-length Gab1 or the MBD. FRS2α, Shc, and Shp2 were precipitated by both full-length Gab1 or the MBD when coexpressed with wt FGFR1 but not when coexpressed with FGFR1 (KD). When lysates from serum-starved or FGF-stimulated NIH 3T3 cells were used for a similar binding assay, tyrosine-phosphorylated FRS2, Shc and Shp2 were similarly found to be precipitated by Gab1 or the MBD alone (data not shown). We have shown in previous reports that these three proteins bind directly to the SH2 domain of Grb2 in FGF-stimulated cells (3, 9). By contrast, FGFR1 was not readily detected in a same complex with Gab1 or the MBD (data not shown). Taken together, these data indicate that Grb2 serves as an intermediary between Gab1 and FRS2, Shc and Shp2, by binding to Gab1 directly through its C-SH3 domain and to phosphorylated FRS2, Shc, and Shp2 through its SH2 domain. Indeed, in an immunoprecipitate of Gab1 from PC12 cells stimulated with FGF, Gab1, Grb2, FRS2, and Shp2 were all detectable in a same complex (Fig. 2D). Therefore, the interaction of docking proteins with the SH2 domain of Grb2 may be a general mechanism to target Grb2:Gab1 complexes to the plasma membrane for phosphorylation in response to activated growth factor or cytokine receptors.
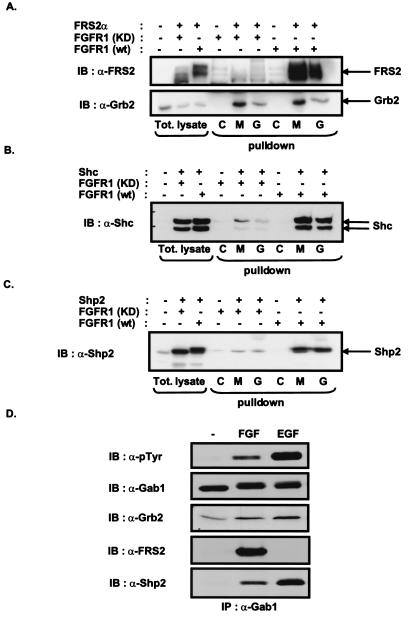
Figure 2.
Gab1 and the MBD of Gab1 form a complex with FRS2α, Shc, or Shp2 in cells expressing activated FGF receptor. 293 cells were transfected with expression vectors for kinase-inactive FGFR1 (KD) mutant, wt FGFR1, and FRS2α, Shc, or Shp2 (A–C) as indicated. Cell lysates were incubated with immobilized GST fusion proteins of full-length Gab1 (G) or the MBD of Gab1 (M). GST alone was used as a control (C). Bound proteins were eluted and analyzed by SDS–PAGE and immunoblotting with FRS2, Grb2, Shc, Shp2 antibodies (A–C). (D) Quiescent PC12 cells were unstimulated or stimulated with FGF1 and heparin (100 ng/ml and 5 μg/ml, respectively) or EGF (50 ng/ml) for 10 min. The lysates were immunoprecipitated with anti-Gab1 antibodies, resolved by SDS–PAGE, and followed by immunoblotting with anti-pTyr, Gab1, Grb2, FRS2, or Shp2 antibodies.
Grb2 Mediates Complex Formation Between Gab1 and FRS2.
In response to FGF stimulation, FRS2 forms a complex with Grb2 directly and indirectly via Shp2 (9, 40). Because Gab1 interacts with FGFR by an indirect mechanism, we examined the possibility of whether the binding of Grb2 and Shp2 via their SH2 domains to tyrosine-phosphorylated FRS2 enables the recruitment of Grb2:Gab1 complex in the vicinity of FGFR, resulting in the tyrosine phosphorylation of Gab1. We have previously reported that FGF stimulation results in the phosphorylation of Y196, Y306, Y349, and Y392 of FRS2α, residues that function as binding sites for the SH2 domain of Grb2, Y436 and Y471, residues that function as binding sites for the SH2 domains of Shp2 (3, 9). The substitution of these six tyrosine residues by phenylalanine resulted in a mutant FRS2α, termed 6F-FRS2α, that is not detectably tyrosine phosphorylated in cells upon FGF stimulation (9). Because 6F-FRS2α is deficient in recruiting Grb2 and Shp2 in response to FGF stimulation, this mutant is potentially incapable of recruiting Gab1. To test this hypothesis, FGFR1 (wt or kinase-inactive mutant) and FRS2α (wt or 6F-FRS2α) were transiently expressed in 293 cells. Cell lysates were resolved by SDS–PAGE and immunoblotted with anti-FGFR1 or anti-FRS2 antibodies to verify the expression levels of FGFR1 and FRS2α, or incubated with immobilized GST fusion protein of the MBD of Gab1. Bound proteins were eluted, resolved by SDS–PAGE, and probed for the presence of FRS2α or the 6F-FRS2α mutant by immunoblotting with anti-FRS2α antibodies (Fig. 3A). Although wt FRS2α, when cotransfected with wt FGFR1, coprecipitated with GST-Gab1-MBD, the 6F-FRS2α mutant did not. Similar results were obtained with full-length GST-Gab1 whereas GST alone showed no binding (data not shown). This experiment demonstrated that the recruitment of Grb2 to tyrosine-phosphorylated FRS2 mediates complex formation with Gab1, allowing the formation of a ternary FRS2:Grb2:Gab1 complex thus enabling tyrosine phosphorylation of Gab1 by FGFR.
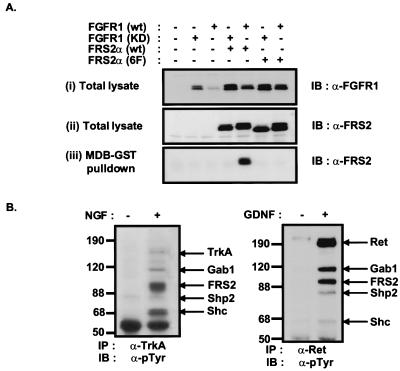
Figure 3.
Gab1 and the MBD of Gab1 form a complex with tyrosine-phosphorylated FRS2α. (A) 293 cells were transfected with expression vectors for wt FGFR1 or kinase-inactive FGFR1 (KD) mutant and wt FRS2α or an FRS2α mutant in which six tyrosine phosphorylation sites were replaced by phenylalanines (6F) as indicated. Equivalent amounts of the total cell lysates were resolved and immunoblotted with anti-FGFR1 (i) or anti-FRS2 antibodies (ii). Alternatively, cell lysates were incubated with immobilized GST fusion proteins of the MBD of Gab1 (iii). Bound proteins were eluted and resolved by SDS–PAGE and then followed by immunoblotting with anti-FRS2 antibodies. (B) FRS2 and Gab1 form a complex with tyrosine-phosphorylated NGF or GDNF receptors. Quiescent PC12 cells were unstimulated or stimulated with NGF (100 ng/ml, 10 min). Lysates were immunoprecipitated with anti-TrkA antibodies (Left). Similarly, lysates from unstimulated or GDNF-stimulated (50 ng/ml, 10 min) SH-SY5Y neuroblastoma cells were immunoprecipitated with anti-Ret antibodies (Right). In both cases the immunoprecipitates were resolved by SDS–PAGE and followed by immunoblotting with anti-pTyr antibodies.
Activation of NGF and glial-derived neurotrophic factor (GDNF) receptors also induce tyrosine phosphorylation of FRS2α, resulting in the recruitment of Gab1 by means of association to FRS2:Grb2 complex. As shown in Fig. 3B, activated TrkA immunoprecipitated from NGF-stimulated PC12 cells (Left), and activated Ret immunoprecipitated from GDNF-stimulated SH-SY5Y neuroblastoma cells (Right), form a complex with tyrosine-phosphorylated Gab1, FRS2, Shc, and Shp2. Therefore it appears that NGF, GDNF, and FGF use a common mechanism for activation of these effector proteins through their interaction with the Grb2:FRS2 complex.
Gab1 Mediates FGF Stimulation of PI3-Kinase Activity.
Tyrosine phosphorylation of Gab1 induced by EGF, insulin, or NGF stimulation has been reported to result in the recruitment of PI3-kinase, via the binding of the SH2 domains of PI3-kinase to specific pTyr sites on Gab1 (20, 21, 24, 25). To explore the possibility that FGF-stimulation of PI3-kinase is mediated by Gab1, lysates from FGF, insulin, or PDGF-stimulated L6 cells were immunoprecipitated with anti-Gab1 antibodies followed by analysis of PI3-kinase activity in the immunocomplexes. As shown in Fig. 4A (Upper), FGF and PDGF stimulations result in enhancement of PI3-kinase activity in Gab1 immunocomplexes. By contrast, insulin stimulation did not result in stimulation of tyrosine phosphorylation of Gab1 and in enhancement of PI3-kinase activity in Gab1 immunocomplexes (Fig. 4A Lower).
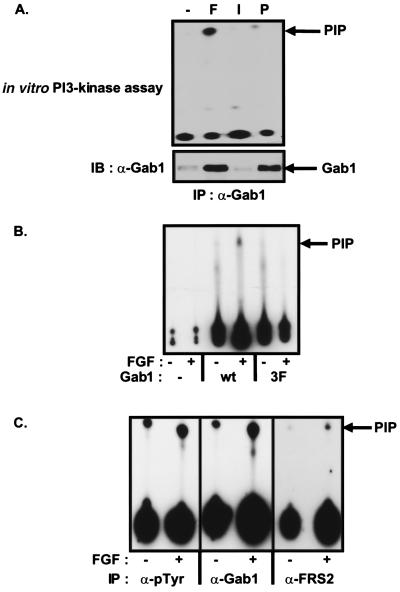
Figure 4.
FGF induces PI3-kinase activity associated with Gab1. (A) Quiescent L6-myoblasts stably expressing FGFR1 were unstimulated or stimulated with FGF1 and heparin (100 ng/ml and 5 μg/ml, respectively), insulin (100 ng/ml), or PDGF (100 ng/ml) for 10 min. The lysates were immunoprecipitated with anti-Gab1 antibodies. One-half of the immunocomplex was subjected to an in vitro PI3-kinase assay (Upper), and the second half was resolved by SDS–PAGE and immunoblotted with anti-pTyr antibodies (Lower). (B) COS-1 cells were transfected with expression vectors for Gab1-Flag (wt) or for 3F mutant of Gab1-Flag, (3F). Lysates from unstimulated or FGF1 and heparin- (100 ng/ml and 5 μg/ml, respectively, 10 min) stimulated cells were immunoprecipitated with anti-Flag antibodies, and the immunocomplexes were assayed for associated PI3-kinase activity. (C) Quiescent PC12 cells were unstimulated or stimulated with FGF1 and heparin (100 ng/ml and 5 μg/ml, respectively). The lysates were immunoprecipitated with anti-pTyr, anti-Gab1, or anti-FRS2 antibodies and the immunocomplexes assayed for associated PI3-kinase activity.
Gab1 contains three potential tyrosine residues (Y448, Y473, and Y590) with the consensus YXXM sequences that function as binding sites for the SH2 domain of the p85 subunit of PI3-kinase. The mutation of these tyrosine residues to phenylalanine, generating the 3F-Gab1 mutant was shown to abolish NGF- or EGF-induced recruitment of PI3-kinase by Gab1 (21, 24). To investigate whether 3F-Gab1 was also defective in recruiting PI3-kinase in FGF signaling, COS-1 cells transfected with either wt Gab1 or 3F-Gab1, were stimulated with FGF or left unstimulated. Gab1 was immunoprecipitated from the lysates with anti-FLAG antibodies and assayed for association of PI3-kinase activity. As shown in Fig. 4B, FGF induced the association of PI3-kinase activity with wt Gab1, but not with the 3F-Gab1 mutant, indicating that the YXXM motifs in Gab1 are indeed responsible for recruitment of PI3-kinase in response to FGF-stimulation. We further confirm that FRS2 is involved in the activation of PI3-kinase by showing that immunoprecipitates of FRS2 from PC12 cells stimulated with FGF contained significant levels of PI3-kinase activity above the control (Fig. 4C).
Gab1 and Sos1 Associate in a Same Complex with Grb2.
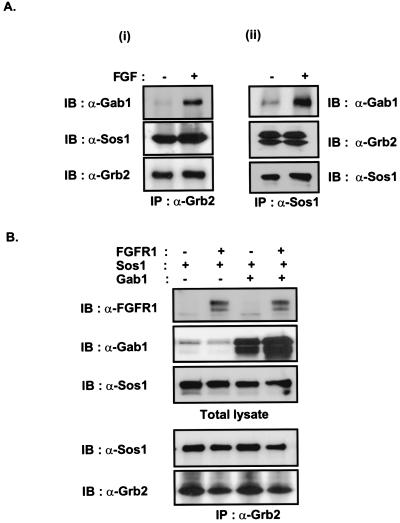
We have demonstrated that Gab1 is bound to Grb2 via its C-SH3 domain, as was also shown by others recently (28, 41). This is in contrast to the nucleotide exchange factor Sos1, which binds predominantly to the N-terminal SH3 of Grb2 (42–44). It is therefore possible that a single Grb2 molecule may bind both Gab1 and Sos1 through its C- and N-SH3 domains, respectively. The formation of such a tertiary complex in cells upon growth factor stimulation may enable both Sos1 and Gab1 to be recruited simultaneously to the membrane through association with Grb2/FRS2 complexes, to activate both the Ras/MAPK and the PI3-kinase pathways. Alternatively, Sos1 and Gab1 may bind to different pools of Grb2 molecules, resulting in competition between the two molecules for the binding to Grb2. We proceeded to examine the nature of the interaction among Grb2, Sos1, and Gab1. Lysates from unstimulated or FGF-stimulated NIH 3T3 cells were immunoprecipitated with anti-Grb2 or anti-Sos1 antibodies and followed by SDS–PAGE and immunoblotting with anti-Gab1, anti-Sos1, or anti-Grb2 antibodies (Fig. 5A). Although similar amounts of Sos1 were coprecipitated with Grb2 from lysates of unstimulated or FGF-stimulated cells, the level of Gab1 that coprecipitated with Grb2 or Sos1 was enhanced upon FGF stimulation. Furthermore, the overexpression of Gab1 did not affect the association of Sos1 with Grb2 (Fig. 5B). Similarly, the overexpression of Sos1 did not affect the association of Gab1 with Grb2 (data not shown). We interpret these results as an indication that Sos1 and Gab1 associate with Grb2 simultaneously via the N- and C-SH3 of Grb2, respectively, allowing the coexistence of Sos1 and Gab1 with Grb2 in a same complex. The increased association of Gab1 with Grb2 upon FGF stimulation, could be caused by additional molecules of Gab1 that are recruited through other proteins such as Shp2, which binds avidly to phosphorylated Gab1 through its SH2 domains (20, 28, 29), and is itself phosphorylated and capable of binding another population of Grb2/Gab1 complexes. Alternatively, it could be that Gab1 is more highly concentrated in the vicinity of the cell membrane because of additional interactions with membrane components such as inositol phospholipids via its PH domain.
Figure 5.
Gab1 and Sos1 associate with Grb2 in a same complex. (A) Quiescent NIH 3T3 cells were unstimulated or stimulated with FGF1 and heparin (100 ng/ml and 5 μg/ml, respectively) for 5 min. The lysates were immunoprecipitated with Grb2 (i) or Sos1 (ii) antibodies and followed by SDS–PAGE and immunoblotting with Gab1, Sos1, or Grb2 antibodies. (B) The 293 cells were transfected with expression vectors for FGFR1, Sos1, and Gab1 as indicated. Equivalent amounts of total cell lysates were resolved by SDS–PAGE and immunoblotted with anti-FGFR1, Gab1, or Sos1 antibodies. Alternatively, the lysates were immunoprecipitated with anti-Grb2 and followed by SDS–PAGE and immunoblotting with anti-Sos1 or Grb2 antibodies.
Positive and Negative Regulation of Akt Activity by Overexpression of wt or Mutant Gab1 and FRS2α.
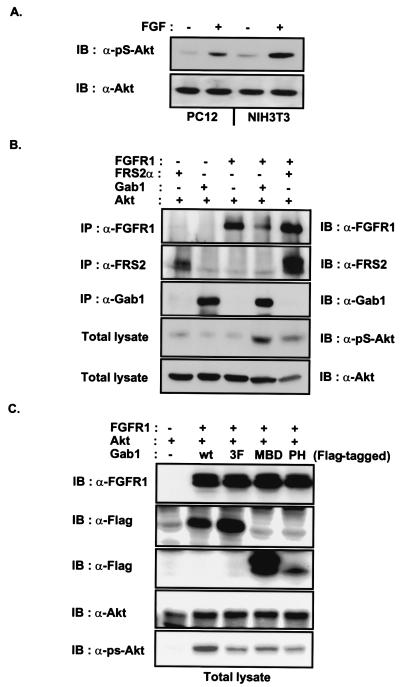
The products of PI3-kinase have been shown to play an important role in the activation of Akt/PKB (45–47). We therefore tested whether FGF stimulation of cells would result in the activation of Akt as determined by immunoblotting with anti-pS-Akt, antibodies that recognize specifically activated Akt. Lysates from quiescent PC12 or NIH 3T3 cells that were unstimulated or stimulated with FGF were resolved by SDS–PAGE and then followed by immunoblotting with anti-Akt or anti-pS-Akt antibodies (Fig. 6A). We observed that FGF treatment induced significant activation of Akt, consistent with the activation of PI3-kinase described earlier (Fig. 4). To investigate whether FRS2 and Gab1 play a role in Akt activation, 293 cells were cotransfected with expression vectors for FGFR1, FRS2α, Gab1, and Akt. The expression of FGFR1, FRS2α, and Gab1 was confirmed by immunoprecipitation and immunoblotting with antibodies against these proteins (Fig. 6B). Equivalent amounts of total cell lysates were resolved by SDS–PAGE and then followed by immunoblotting with anti-Akt or anti-pS-Akt antibodies. This experiment demonstrated that the overexpression of FRS2α or Gab1 potentiates FGF-induced Akt stimulation. We next examined whether overexpression of Gab1-MBD, the region mediating the interaction of Gab1 with Grb2, would exert a dominant inhibitory effect on FGF-induced Akt activation. 293 cells were transfected with Akt alone or together with FGFR1 and wt Gab1, 3F-Gab1, Gab1-MBD, or the PH domain of Gab1. FGFR1 was transfected at levels that induce autoactivation, resulting in tyrosine phosphorylation of FRS2α and Gab1 and stimulation of PI3-kinase and Akt activities. Equivalent amounts of lysates from the transfected cells were resolved by SDS–PAGE and then followed by immunoblotting with anti-FGFR1, anti-FLAG, or anti-pS-Akt antibodies (Fig. 6C). Although overexpression of wt Gab1 results in enhancement of Akt activity, overexpression of 3F-Gab1, Gab1-MBD, or the PH domain decreased FGFR-induced stimulation of Akt. We propose that Gab1-MBD interferes with FGF-induced stimulation of Akt by preventing complex formation between Grb2 and endogenous Gab1; 3F-Gab1 interferes with recruitment of PI3-kinases by wt Gab1. The overexpression of the PH domain is likely to interfere with membrane translocation by competing with Gab1 for binding to phosphatidylinositol 3,4,5-triphosphate.
Figure 6.
FRS2 and Gab1 potentiate FGF-induced activation of Akt. (A) Quiescent PC12 or NIH 3T3 cells were unstimulated or stimulated with FGF1 and heparin (100 ng/ml and 5 μg/ml, respectively) for 5 min. Equivalent amounts of total cell lysates were resolved by SDS–PAGE and then followed by immunoblotting with anti-pS-Akt or anti-Akt antibodies. (B) The 293 cells were transfected with expression vectors for FGFR1, FRS2α, Gab1 and Akt as indicated. The expression of FGFR1, FRS2α, and Gab1 was verified by immunoprecipitation with antibodies specific to these proteins and followed by SDS–PAGE and immunoblotting with the same antibodies. Equivalent amounts of total cell lysates were resolved by SDS–PAGE and immunoblotted with anti-pS-Akt or anti-Akt antibodies. (C) The MBD of Gab1 inhibits Gab1-potentiated FGFR-induced activation of Akt. 293 cells were transfected with the expression vectors for FGFR1, Akt and Gab1-Flag, Gab1(3F)-Flag, Gab1-MBD-Flag, or Gab1-PH-Flag as indicated. Equivalent amounts of total cell lysate were resolved by SDS–PAGE and followed by immunoblotting with anti-FGFR1, anti-Flag, anti-Akt, or anti-pS-Akt antibodies.
Discussion
Numerous studies have implicated the PI3-kinase/Akt-signaling pathway as a component of a cell survival pathway that is activated by growth factors, neurotrophic factors, and cytokines (reviewed in refs. 14 and 26). Certain growth factor receptors (i.e., PDGF receptor and ErbB3) possess binding sites for direct recruitment of PI3-kinase. Others, such as the insulin receptor, lack these sites and recruit PI3-kinase indirectly through association with IRS proteins; a family of docking proteins containing multiple tyrosine residues that are phosphorylated by activated insulin or insulin-like growth factor I receptors to recruit SH2 domain-containing molecules, such as PI3-kinase and Grb2 (reviewed in ref. 19). Gab1 and Gab2 represent another family of docking proteins capable of recruiting PI3-kinase in response to growth factor or cytokine stimulation. It has been shown that activation of NGF, hepatocyte growth factor, or EGF receptors results in tyrosine phosphorylation of Gab1 and its association with PI3-kinase (20, 21, 24, 25, 27, 28). Similarly, IL-2 or IL-3 stimulation of hematopoietic cells leads to tyrosine phosphorylation of Gab2 and activation of PI3-kinase (30, 32).
Although FGF stimulation has been shown to play a role in cell survival (2, 10, 11), it has not been resolved whether the PI3-kinase/Akt pathway is involved in this process. Furthermore, the activated FGF receptor does not appear to recruit PI3-kinase directly. In this report, we show that activation of FGF receptor induces PI3-kinase and Akt activities through the recruitment and tyrosine phosphorylation of the docking protein Gab1. Most interestingly, unlike the IRS-docking proteins that bind to the insulin receptor directly, the recruitment of Gab1 to the FGF receptor occurs indirectly, mediated by the FRS2:Grb2 complex. FGF stimulation results in tyrosine phosphorylation of Gab1 and activation of PI3-kinase. Activation of PI3-kinase is mediated by binding of the SH2 domain of Grb2 to tyrosine-phosphorylated FRS2 and by binding of the C-SH3 domain of Grb2 to a proline-rich region in the MBD of Gab1. The assembly of Gab1 in a complex with Grb2:FRS2 enables tyrosine phosphorylation of Gab1 by FGF receptor to generate binding sites for the SH2 domain of p85, the regulatory subunit of PI3-kinase, thus resulting in recruitment and activation of PI3-kinase.
Of interest, both the guanine nucleotide releasing factor Sos1 and Gab1, which bind predominantly to the N- and C- terminal SH3 domains of Grb2, respectively, coexist in a same complex with Grb2. The formation of such a complex allows the concurrent recruitment of both Sos1 and Gab1 to the membrane by the binding of Grb2 to the membrane linked FRS2, eliciting bifurcating signals to activate the Ras/MAPK and PI3-kinase/Akt pathways simultaneously.
The ability of tyrosine-phosphorylated FRS2 to mediate FGF-induced PI3-kinase activation via indirect recruitment of Gab1 enables FRS2 to activate many additional effector proteins whose cellular localization and activity are regulated by the reaction product of PI3-kinase. These will increase the repertoire of signaling pathways that are recruited by FGF, NGF, and other growth factors that induce the tyrosine phosphorylation of FRS2α. Because Shc and Shp2 are also tyrosine phosphorylated in cells upon FGF stimulation, leading to their association with the SH2 domain of Grb2, we propose that Shc and Shp2 may also contribute toward the recruitment of the Grb2/Gab1 complex thus indirectly activating PI3-kinase and its downstream effector proteins. Indeed, it has been recently reported that, in response to IL-2 or IL-3 stimulation of hematopoeitic cells, tyrosine-phosphorylated Shc forms a ternary complex with Grb2 and Gab2, resulting in tyrosine phosphorylation of Gab2 and activation of PI3-kinase (32). These results together with the experiments described in this report demonstrate that growth factor or cytokine receptors that do not recruit PI3-kinase directly may use docking proteins such as FRS2 as a platform for recruitment of additional adaptors and docking proteins, which do not interact with the receptors directly. The assembly of such large multidocking/adaptor protein complexes and the effectors that are bound to them may provide a mechanism for generation of signal diversity.
Acknowledgments
This work was partially supported by the National Science and Technology Board of Singapore and fellowships from the Human Frontier Science Program Organization.
Abbreviations
- PI3-kinase
phosphatidylinositol 3-kinase
- FGFR
fibroblast growth factor receptor
- NGF
nerve growth factor
- wt
wild type
- MBD
Met-binding domain
- PH
Pleckstrin homology
- KD
kinase inactive mutant
- MAPK
mitogen-activated protein kinase
- PDGF
platelet-derived growth factor
- EGF
epidermal growth factor
- GST
glutathione S-transferase
- SH2 and 3
Src homology 2 and 3
- GDNF
glial-derived neurotrophic factor
References
- 1.Barde Y A. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 2.Goldfarb M. Cytokine Growth Factor Rev. 1996;7:311–325. doi: 10.1016/s1359-6101(96)00039-1. [DOI] [PubMed] [Google Scholar]
- 3.Kouhara H, Hadari Y R, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 4.Ong S H, Guy G R, Hadari Y R, Laks S, Gotoh N, Schlessinger J, Lax I. Mol Cell Biol. 2000;20:979–989. doi: 10.1128/mcb.20.3.979-989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhalluin C, Yan S K, Plotnikova O, Lee W K, Zeng L, Kuti M, Mujtaba S, Goldfarb P M, Zhou M M. Mol Cell. 2000;6:921–929. doi: 10.1016/s1097-2765(05)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Lee K W, Goldfarb M. J Biol Chem. 1998;273:17987–17990. doi: 10.1074/jbc.273.29.17987. [DOI] [PubMed] [Google Scholar]
- 7.Meakin S O, MacDonald J I, Gryz E A, Kubu C J, Verdi J M. J Biol Chem. 1999;274:9861–9870. doi: 10.1074/jbc.274.14.9861. [DOI] [PubMed] [Google Scholar]
- 8.Easton J B, Moody N M, Zhu X, Middlemas D S. J Biol Chem. 1999;274:11321–11327. doi: 10.1074/jbc.274.16.11321. [DOI] [PubMed] [Google Scholar]
- 9.Hadari Y R, Kouhara H, Lax I, Schlessinger J. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow R L, Roux G D, Roghani M, Palmer M A, Rifkin D B, Moscatelli D A, Lang R A. Development (Cambridge, UK) 1995;121:4383–4393. doi: 10.1242/dev.121.12.4383. [DOI] [PubMed] [Google Scholar]
- 11.Desire L, Courtois Y, Jeanny J C. J Neurochem. 2000;75:151–163. doi: 10.1046/j.1471-4159.2000.0750151.x. [DOI] [PubMed] [Google Scholar]
- 12.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 13.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.Datta S R, Brunet A, Greenberg M E. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 15.Cowley S, Paterson H, Kemp P, Marshall C J. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 16.Marshall C J. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 17.Hu P, Margolis B, Skolnik E Y, Lammers R, Ullrich A, Schlessinger J. Mol Cell Biol. 1992;12:981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H H, Sierke S L, Koland J G. J Biol Chem. 1994;269:24747–24755. [PubMed] [Google Scholar]
- 19.Yenush L, White M F. BioEssays. 1997;19:491–500. doi: 10.1002/bies.950190608. [DOI] [PubMed] [Google Scholar]
- 20.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. Nature (London) 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues G A, Falasca M, Zhang Z, Ong S H, Schlessinger J. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunnick J M, Dorsey J F, Munoz-Antonia T, Mei L, Wu J. J Biol Chem. 2000;275:13842–13848. doi: 10.1074/jbc.275.18.13842. [DOI] [PubMed] [Google Scholar]
- 23.Kim H H, Vijapurkar U, Hellyer N J, Bravo D, Koland J G. Biochem J. 1998;334:189–195. doi: 10.1042/bj3340189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holgado-Madruga M, Moscatello D K, Emlet D R, Dieterich R, Wong A J. Proc Natl Acad Sci USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korhonen J M, Said F A, Wong A J, Kaplan D R. J Biol Chem. 1999;274:37307–37314. doi: 10.1074/jbc.274.52.37307. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan D R, Miller F D. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 27.Weidner K M, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Nature (London) 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 28.Schaeper U, Gehring N H, Fuchs K P, Sachs M, Kempkes B, Birchmeier W. J Cell Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maroun C R, Naujokas M A, Holgado-Madruga M, Wong A J, Park M. Mol Cell Biol. 2000;20:8513–8525. doi: 10.1128/mcb.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu H, Pratt J C, Burakoff S J, Neel B G. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhao C, Yu D H, Shen R, Feng G S. J Biol Chem. 1999;274:19649–19654. doi: 10.1074/jbc.274.28.19649. [DOI] [PubMed] [Google Scholar]
- 32.Gu H, Maeda H, Moon J J, Lord J D, Yoakim M, Nelson B H, Neel B G. Mol Cell Biol. 2000;20:7109–7120. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingham R J, Holgado-Madruga M, Siu C, Wong A J, Gold M R. J Biol Chem. 1998;273:30630–30637. doi: 10.1074/jbc.273.46.30630. [DOI] [PubMed] [Google Scholar]
- 34.Sachs M, Brohmann H, Zechner D, Muller T, Hulsken J, Walther I, Schaeper U, Birchmeier C, Birchmeier W. J Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hibi M, Hirano T. Leuk Lymphoma. 2000;37:299–307. doi: 10.3109/10428190009089430. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadi M, Dionne C A, Li W, Li N, Spivak T, Honegger A M, Jaye M, Schlessinger J. Nature (London) 1992;358:681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J, Cooper J A, Schlessinger J. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bazenet C E, Gelderloos J A, Kazlauskas A. Mol Cell Biol. 1996;16:6926–6936. doi: 10.1128/mcb.16.12.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamin C W, Jones D A. J Biol Chem. 1994;269:30911–30916. [PubMed] [Google Scholar]
- 40.Ong S H, Lim Y P, Low B C, Guy G R. Biochem Biophys Res Commun. 1997;238:261–266. doi: 10.1006/bbrc.1997.7272. [DOI] [PubMed] [Google Scholar]
- 41.Lock L S, Royal I, Naujokas M A, Park M. J Biol Chem. 2000;275:31536–31545. doi: 10.1074/jbc.M003597200. [DOI] [PubMed] [Google Scholar]
- 42.Vidal M, Montiel J L, Cussac D, Cornille F, Duchesne M, Parker F, Tocque B, Roques B P, Garbay C. J Biol Chem. 1998;273:5343–5348. doi: 10.1074/jbc.273.9.5343. [DOI] [PubMed] [Google Scholar]
- 43.Wittekind M, Mapelli C, Lee V, Goldfarb V, Friedrichs M S, Meyers C A, Mueller L. J Mol Biol. 1997;267:933–952. doi: 10.1006/jmbi.1996.0886. [DOI] [PubMed] [Google Scholar]
- 44.Goudreau N, Cornille F, Duchesne M, Parker F, Tocque B, Garbay C, Roques B P. Nat Struct Biol. 1994;1:898–907. doi: 10.1038/nsb1294-898. [DOI] [PubMed] [Google Scholar]
- 45.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 46.Franke T F, Kaplan D R, Cantley L C, Toker A. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 47.Frech M, Andjelkovic M, Ingley E, Reddy K K, Falck J R, Hemmings B A. J Biol Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]