Abstract
5,6-dihydroxy-5,6-dihydrothymidine (thymidine glycol) is a major product of the reaction of thymidine with reactive oxygen species, including those generated by ionizing radiation. Thymidine glycol exists as 2 diastereomeric pairs by virtue of the chirality of the C(5) and C(6) atoms. A simple procedure is described for synthesizing and purifying each of the diastereomeric pairs separately. After brominating thymidine, the two trans 5-bromo-6-hydroxy-5,6-dihydrothymidine (thymidine bromohydrin) C(5) diastereomers were easily separated by High Performance Liquid Chromatography. Each thymidine bromohydrin was quantitatively converted to the corresponding diastereomeric thymidine glycol pair by reflux in aqueous solution. The concentrations at equilibrium of the cis (5S,6R),(5R,6S) and trans (5S,6S),(5R,6R) forms of the thymidine glycol diastereomers were determined and were 80% cis and 20% trans for the 5S pair and 87% cis and 13% trans for the 5R pair. At equilibrium, the rate of cis-trans epimerization of the two sets of diastereomers was essentially identical. The 5S diastereomeric pair was significantly more alkali labile than the 5R pair due to the higher concentration of the 5S trans epimer at equilibrium. This differential alkali lability was also manifest when the thymine glycol moiety was present in chemically oxidized poly(dA-dT).poly(dA-dT) indicating that the chemical differences between the diastereomeric pairs are preserved in DNA. These chemical differences may affect the biological properties of this important oxidative derivative of thymine in DNA.
Full text
PDF

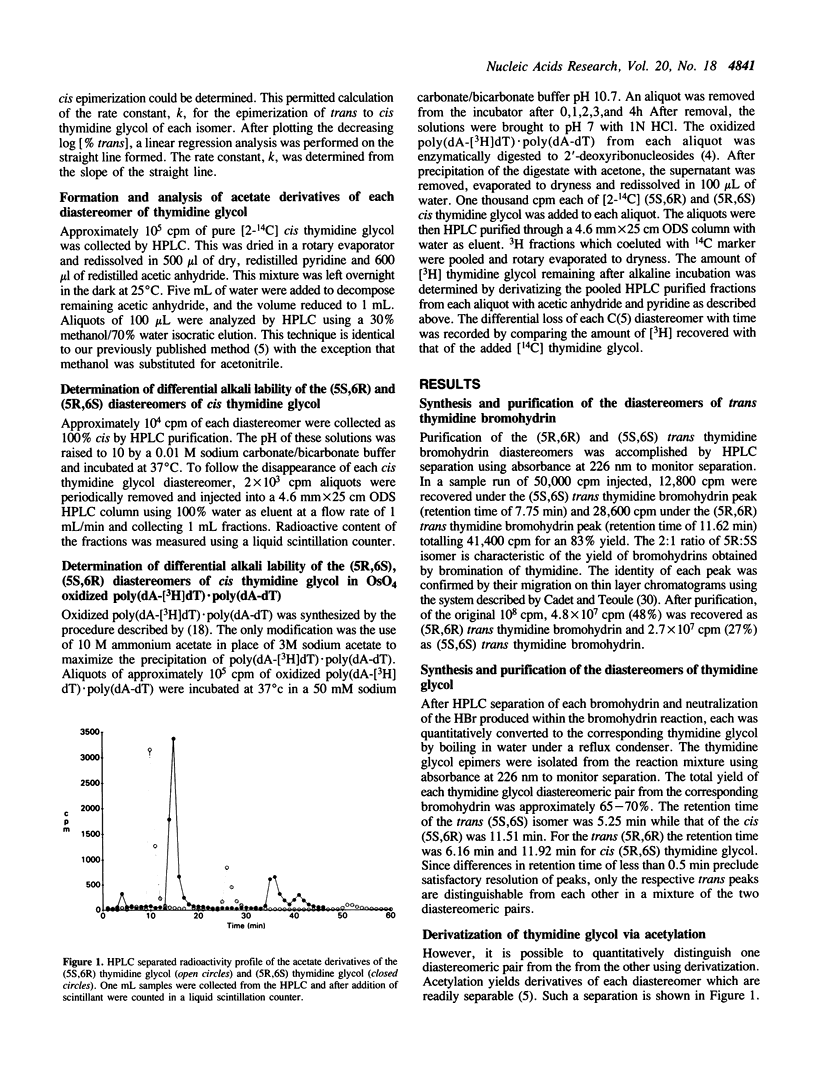
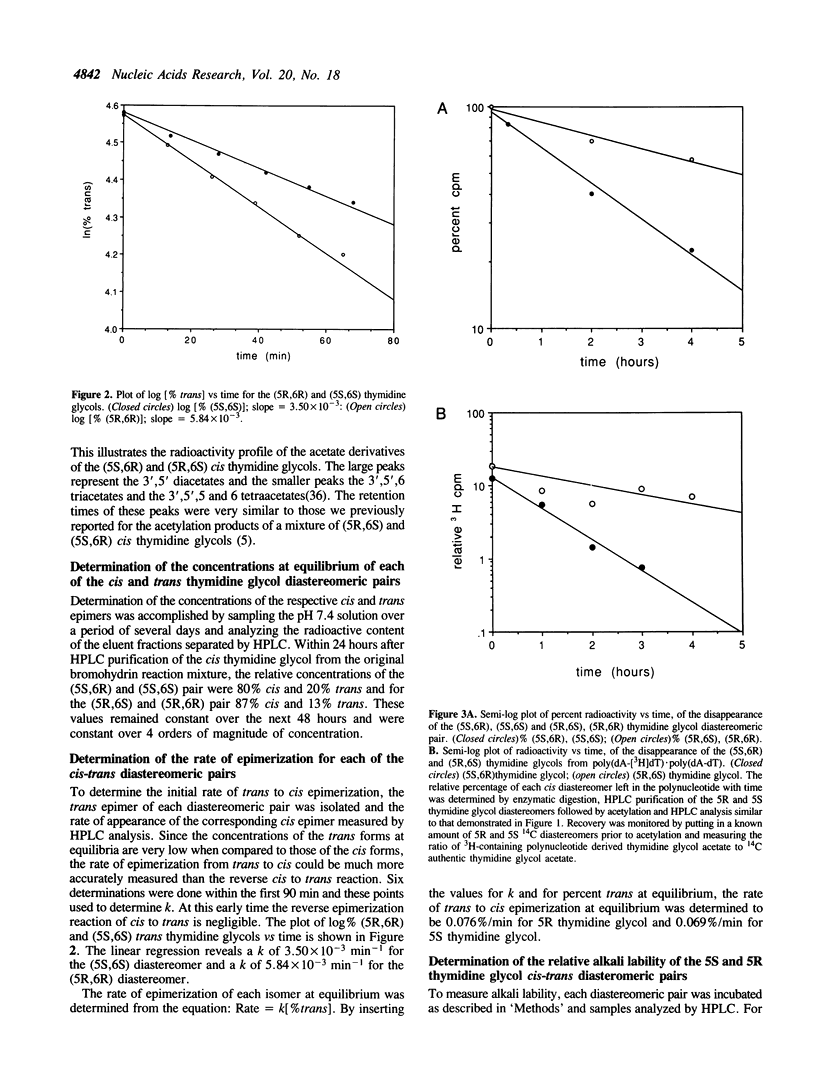
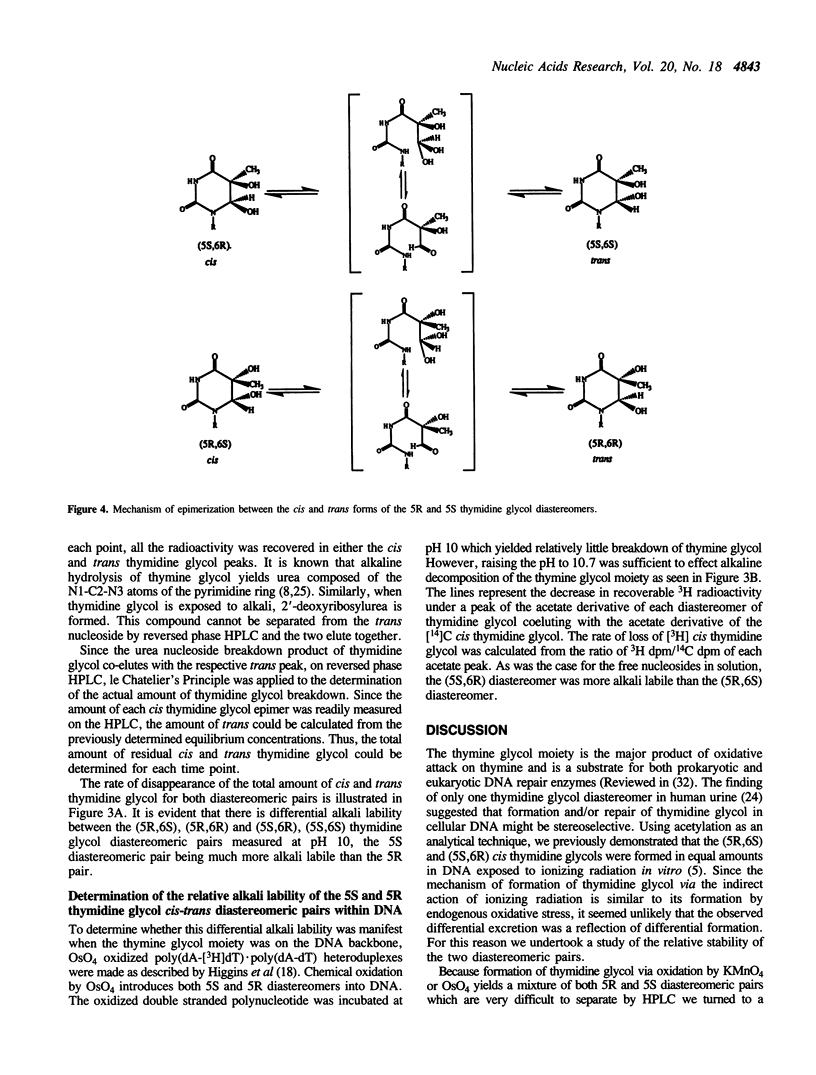


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu A. K., Loechler E. L., Leadon S. A., Essigmann J. M. Genetic effects of thymine glycol: site-specific mutagenesis and molecular modeling studies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7677–7681. doi: 10.1073/pnas.86.20.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. DNA glycosylase activities for thymine residues damaged by ring saturation, fragmentation, or ring contraction are functions of endonuclease III in Escherichia coli. J Biol Chem. 1984 May 10;259(9):5543–5548. [PubMed] [Google Scholar]
- Breimer L. H. Urea--DNA glycosylase in mammalian cells. Biochemistry. 1983 Aug 30;22(18):4192–4197. doi: 10.1021/bi00287a005. [DOI] [PubMed] [Google Scholar]
- Cadet J., Ducolomb R., Hruska F. E. Proton magnetic resonance studies of 5,6-saturated thymidine derivatives produced by ionizing radiation. Conformational analysis of 6-hydroxylated diastereoisomers. Biochim Biophys Acta. 1979 Jun 20;563(1):206–215. doi: 10.1016/0005-2787(79)90021-2. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Saul R. L., Ames B. N. Thymine glycol and thymidine glycol in human and rat urine: a possible assay for oxidative DNA damage. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5633–5637. doi: 10.1073/pnas.81.18.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Functional effects of cis-thymine glycol lesions on DNA synthesis in vitro. Biochemistry. 1987 Aug 25;26(17):5398–5403. doi: 10.1021/bi00391a027. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Template length, sequence context, and 3'-5' exonuclease activity modulate replicative bypass of thymine glycol lesions in vitro. Biochemistry. 1989 Jan 24;28(2):775–779. doi: 10.1021/bi00428a054. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Thymine glycol lesions terminate chain elongation by DNA polymerase I in vitro. Nucleic Acids Res. 1986 Jan 24;14(2):737–749. doi: 10.1093/nar/14.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Helland D. E., Haseltine W. A. Mechanism of action of a mammalian DNA repair endonuclease. Biochemistry. 1986 Apr 22;25(8):2212–2220. doi: 10.1021/bi00356a054. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Henner W. D., Cunningham R. P., Toney J. H., Helland D. E. A highly conserved endonuclease activity present in Escherichia coli, bovine, and human cells recognizes oxidative DNA damage at sites of pyrimidines. Mol Cell Biol. 1987 Jan;7(1):26–32. doi: 10.1128/mcb.7.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel K., Chrzan K., Troll W., Teebor G. W., Steinberg J. J. Radiation-like modification of bases in DNA exposed to tumor promoter-activated polymorphonuclear leukocytes. Cancer Res. 1986 Nov;46(11):5533–5540. [PubMed] [Google Scholar]
- Frenkel K., Goldstein M. S., Teebor G. W. Identification of the cis-thymine glycol moiety in chemically oxidized and gamma-irradiated deoxyribonucleic acid by high-pressure liquid chromatography analysis. Biochemistry. 1981 Dec 22;20(26):7566–7571. doi: 10.1021/bi00529a035. [DOI] [PubMed] [Google Scholar]
- Frenkel K., Zhong Z. J., Wei H. C., Karkoszka J., Patel U., Rashid K., Georgescu M., Solomon J. J. Quantitative high-performance liquid chromatography analysis of DNA oxidized in vitro and in vivo. Anal Biochem. 1991 Jul;196(1):126–136. doi: 10.1016/0003-2697(91)90128-g. [DOI] [PubMed] [Google Scholar]
- Hayes R. C., LeClerc J. E. Sequence dependence for bypass of thymine glycols in DNA by DNA polymerase I. Nucleic Acids Res. 1986 Jan 24;14(2):1045–1061. doi: 10.1093/nar/14.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. A., Frenkel K., Cummings A., Teebor G. W. Definitive characterization of human thymine glycol N-glycosylase activity. Biochemistry. 1987 Mar 24;26(6):1683–1688. doi: 10.1021/bi00380a029. [DOI] [PubMed] [Google Scholar]
- Ide H., Kow Y. W., Wallace S. S. Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res. 1985 Nov 25;13(22):8035–8052. doi: 10.1093/nar/13.22.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Wallace S. S. Mechanism of action of Escherichia coli endonuclease III. Biochemistry. 1987 Dec 15;26(25):8200–8206. doi: 10.1021/bi00399a027. [DOI] [PubMed] [Google Scholar]
- Kow Y. W., Wallace S. S., Van Houten B. UvrABC nuclease complex repairs thymine glycol, an oxidative DNA base damage. Mutat Res. 1990 Mar;235(2):147–156. doi: 10.1016/0921-8777(90)90068-g. [DOI] [PubMed] [Google Scholar]
- Leadon S. A. Production of thymine glycols in DNA by radiation and chemical carcinogens as detected by a monoclonal antibody. Br J Cancer Suppl. 1987 Jun;8:113–117. [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Sancar A. A new mechanism for repairing oxidative damage to DNA: (A)BC excinuclease removes AP sites and thymine glycols from DNA. Biochemistry. 1989 Oct 3;28(20):7979–7984. doi: 10.1021/bi00446a002. [DOI] [PubMed] [Google Scholar]
- Rouet P., Essigmann J. M. Possible role for thymine glycol in the selective inhibition of DNA synthesis on oxidized DNA templates. Cancer Res. 1985 Dec;45(12 Pt 1):6113–6118. [PubMed] [Google Scholar]
- Saul R. L., Ames B. N. Background levels of DNA damage in the population. Basic Life Sci. 1986;38:529–535. doi: 10.1007/978-1-4615-9462-8_55. [DOI] [PubMed] [Google Scholar]
- Sharma M., Box H. C., Kelman D. J. Fluorescence postlabeling assay of cis-thymidine glycol monophosphate in X-irradiated calf-thymus DNA. Chem Biol Interact. 1990;74(1-2):107–117. doi: 10.1016/0009-2797(90)90062-r. [DOI] [PubMed] [Google Scholar]
- Teebor G. W., Boorstein R. J., Cadet J. The repairability of oxidative free radical mediated damage to DNA: a review. Int J Radiat Biol. 1988 Aug;54(2):131–150. doi: 10.1080/09553008814551591. [DOI] [PubMed] [Google Scholar]
- Teebor G., Cummings A., Frenkel K., Shaw A., Voituriez L., Cadet J. Quantitative measurement of the diastereoisomers of cis thymidine glycol in gamma-irradiated DNA. Free Radic Res Commun. 1987;2(4-6):303–309. doi: 10.3109/10715768709065296. [DOI] [PubMed] [Google Scholar]



