Abstract
Müller cells, the primary glial cells are a crucial component of the retinal tissue performing a wide range of functions including maintaining the blood-retinal barrier. Several studies suggest that diabetes leads to Müller cell dysfunction and loss. The pathophysiology of hyperglycemia-induced cellular injury of Müller cells remains only poorly understood. Recently, the concept that translocation of the predominantly cytosolic glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to the nucleus and its accumulation in this cellular compartment alters transcriptional events associated with cell death induction has gained major interest. High glucose conditions induce nuclear translocation and accumulation of GAPDH in the nucleus of Müller cells in vivo and in vitro. With regards to Müller cell dysfunction, the effects of nuclear accumulation of GAPDH are multifaceted. Considering the functional versatility of GAPDH including gene regulation, DNA repair, telomere protection, etc., it is of immense importance to explore possible GAPDH actions to unravel the mysteries around the role of GAPDH in hyperglycemia-induced cellular changes in order to develop novel therapeutic strategies. Therefore, this review focuses on the molecular events associated with the nuclear translocation of GAPDH and how it affects the fate of Müller cells in diabetes.
Keywords: Diabetic retinopathy, Müller cells, Nuclear GAPDH, Inflammation
Introduction
Diabetes is the leading cause of new cases of blindness in adults aged 20–70 years [1] and an estimated 92.6 million adults worldwide are affected by diabetic retinopathy [2]. In spite of improved life expectancy, with longer duration of diabetes, most diabetics still develop retinopathy to some degree. Often the condition progresses to the proliferative stage leading to blindness. Various pathways including accumulation of sorbitol and advanced glycation end products, protein kinase C, inflammation, oxidative damage, upregulation of renin–angiotensin system, and VEGF etc. (reviewed in [3]) have been implicated in the pathogenesis of diabetic retinopathy and its progression to the proliferative stage.
Retinal cells undergo accelerated death in diabetic retinopathy and it has been speculated that loss of cells in the retina enhances the progression of diabetic retinopathy. One cell type affected by hyperglycemia is the Müller cells. Müller cells, which span the entire retina, are the primary glial cells maintaining the integrity and the microvasculature of the retina [4–11]. Müller cells perform a number of functions including glycolytic, providing metabolic support to neighboring energy-demanding neurons by releasing lactate [12, 13] and recycle excessive glutamine from the extracellular spaces [14–16]. Müller cells take up excess K+ in the extracellular retinal space and participate in the regulation of retinal water and ion homeostasis [7, 17]. They also maintain the blood-retinal barrier through physical interaction and secretion of factors that induce formation of tight junctions [4, 11]. Selective elimination of Müller cells in vivo compromises the tissue integrity and increases retinal degeneration [18]. High glucose causes defects in the glutamate transporter resulting in excessive extracellular glutamate [19, 20] which may further worsen the oxidative damage in the diabetic retina [19]. Hyperglycemia also induces the production of pro-inflammatory cytokines by Müller cells. Thus, Müller cells are a potent source for pro-inflammatory cytokines and might play a crucial role in retinal inflammation associated with the development of diabetic retinopathy [21–26]. Cytokine secretion by Müller cells influences function and viability of surrounding retinal cells, such as the retinal endothelial cells, in a paracrine fashion and their own function and viability in an autocrine manner [22]. How hyperglycemia alters Müller cell function and how it affects Müller cell survival is only poorly understood. Recently, the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been implicated in the detrimental effects of hyperglycemia. Nuclear translocation and accumulation of GAPDH has strongly been associated with cellular dysfunction and cell death in the Müller cells [27]. The idea that a well-known glycolytic enzyme can challenge the fate of Müller cells in diabetes is an intriguing and novel concept and the major focus of this review.
Diverse function of glyceraldehyde-3-phosphate dehydrogenase
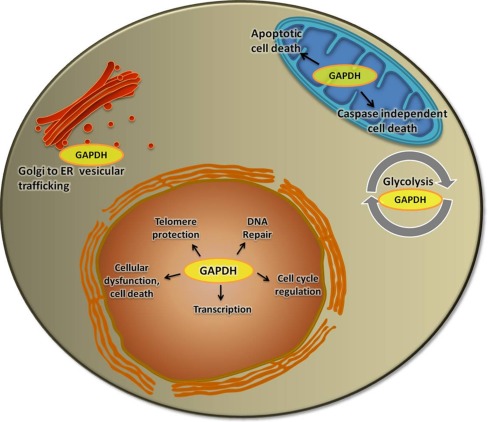
Glyceraldehyde-3-phosphate dehydrogenase is a tetramer protein composed of 37 kDa subunits. The enzyme plays a major role in glycolysis in catalyzing the conversion of glyceraldehyde-3-phosphate to d-glycerate 1, 3-biphosphate in the presence of NAD+, inorganic phosphate facilitating the formation of NADH, and ATP. Although traditionally considered as a house-keeping gene, GAPDH expression seems to vary among different tissues, with highest in energy demanding tissues [28]. Studies in recent years have revealed the multifacets of GAPDH. Besides its active role in glycolysis, GAPDH performs a variety of other functions (Fig. 1).
Fig. 1.
Diverse functions of GAPDH
GAPDH plays an important role in the intracellular membrane trafficking by modulating the cytoskeletal architecture [29–31] and via its association with by Rab2, the Ras-related small GTPase required for intracellular membrane trafficking mediating the endoplasmic reticulum to Golgi transport of cargo [32].
GAPDH also acts as a transcriptional regulator like trans-activating the androgen receptor [33]. In addition, GAPDH recognizes the sequence and the structure of RNA to accomplish tRNA transport [34]. Studies have identified GAPDH as a 3′UTR binding protein and as well as a 5′ UTR binding protein at the AU-rich elements in the respective mRNA. Through such interaction, it may stabilize or destabilize the mRNA it is associated with. While GAPDH destabilizes the mRNA of endothelial vasoconstrictor ET-1 via its interaction and eventual mRNA degradation [35], GAPDH stabilizes the mRNA of the hematopoietic cytokine colony stimulating factor 1 [36, 37]. GAPDH also interacts with the angiotensin II type 1 receptor (AT1R) mRNA [38] and decreases the AT1R translation but does not degrade the AT1R mRNA.
GAPDH regulates cyclin B-cdk1 activity and thereby the cell cycle progression [39]. It also plays an indispensable role during the S phase-dependent transcription of histone H2B by directly interacting with the DNA binding transcription factor OCT1 while serving a redox sensing role in the H2B gene transcription [40]. A strong correlation between cell cycle-regulated expression of GAPDH with increased uracil DNA glycosylase activity has been observed suggesting the role of GAPDH in DNA repair [41, 42]. GAPDH directly interacts with the apurinic/apyrimidinic endonuclease enzyme APE1 to reducing it from an oxidized state, thereby making it available for the DNA base excision repair pathway [43]. GAPDH protects the integrity of telomeric DNA and protects it from shortening [44, 45]. The telomeric DNA competes with the NAD+ binding site of GAPDH when the latter associates with it. GAPDH directly binds to the single stranded telomeric DNA to protect it from shortening and to maintain the structural integrity [45]. The GAPDH-telomeric DNA binding requires the Asp32 and Cys149 of GAPDH.
GAPDH regulates the intracellular Ca+ signaling by binding to inositol 1,4,5-trisphosphate receptor and releasing NADH near the channels [46]. Under stress conditions, GAPDH undergoes an oxidative inhibition, enabling the cells to redirect their carbohydrate flux from glycolysis to the pentose phosphate pathway, generating the reducing NADPH and protecting the cells [47]. Oxidative stress-induced DNA strand breaks activate the DNA repair enzyme poly(ADP-ribose) polymerase-1, which inactivates GAPDH by ADP-ribosylation and creating energy deficits and driving the cells towards accelerated cell death [48, 49]. Thus, GAPDH relays the stress signals and regulates the ATP production to control the viable population of cells [49]. During apoptosis, GAPDH associates with the voltage dependent anion channel 1 and induces mitochondrial membrane permeabilization leading to the release of cytochrome c and apoptosis-inducing factor [50]. GAPDH also protects cells from caspase-independent cell death (CICD) [51]. GAPDH helps the cells to recover from mitochondrial outer membrane potential (MOMP), provides ATP to maintain the mitochondrial membrane potential via the F0F1 ATPase activity and thereby compensating the energy deficit because of the loss of mitochondrial function. Under the same conditions that induce MOMP, GAPDH enhances the expression of Atg12 which is required for autophagy, towards the protection from CICD.
Nuclear GAPDH and cell death
Studies in different systems show that GAPDH translocates to the nucleus under certain stress conditions and that this event is associated with cell death [52–57]. GAPDH interacts with the E3 ubiquitin ligase SIAH1 (seven in absentia homolog 1) to translocate to the nucleus [58]. A stress-like NO S-nitrosylates the GAPDH at Cys 150 and facilitates its interaction with SIAH1. The interaction with SIAH1 enables GAPDH to translocate to the nucleus to bring about cellular dysfunction and or cell death. On the other hand, GOSPEL [59], a cytosolic protein, is S-nitrolsylated by nitric oxide stress and competes with SIAH1 to bind to GAPDH and in that way preventing its nuclear translocation via SIAH1. Thus, the GOSPEL–GAPDH interaction is in a way a protective mechanism against the nitric oxide-induced stress. However, when the level of stress exceeds the limit, GAPDH–SIAH1 interaction prevails.
Studies on neuronal and non-neuronal cells identified nuclear translocation of GAPDH to be associated with apoptosis and oxidative stress [57]. GAPDH however, lacks a nuclear translocation signal (NLS), which is essential for the translocation into the nucleus. The molecular basis for the translocation thus remained a mystery until researchers found that the stressors activate nitric oxide synthase, which in turn induces the S-nitrosyaltion of GAPDH, a post-translational modification [58]. The S-nitrosylation facilitates the binding of GAPDH with the E3 ubiquitin ligase protein SIAH1, which has the NLS. SIAH1 proteins are human homologs of the Drosophila E3 ubiquitin ligase sina (seven in absentia) protein, that degrades selected target proteins [60, 61]. SIAH1contains an N-terminal RING domain that facilitates the ligase function, two cysteine-rich zinc finger domains, and a substrate-binding domain, which is localized on the C-terminal of the protein and is also the NLS containing domain [62–64]. The last 12 amino acids on the C-terminal in the substrate-binding domain are necessary for GAPDH–SIAH1 interaction. While SIAH1 shares the NLS with GAPDH helping it to translocate to the nucleus, GAPDH stabilizes the otherwise rapid turn-over protein SIAH1 enhancing its activity [58]. SIAH1 is an ubiquitin ligase that catalyses the proteosome-dependent protein degradation and is known to play a role in apoptosis. Thus, by associating with SIAH1 for nuclear translocation and stabilizing it, GAPDH facilitates target protein degradation and cell death. The S-nitrosylated GAPDH, that translocated to the nucleus is acetylated at Lys 160 by the nuclear protein acetyltransferase p300/CREB binding protein by direct interaction [65]. The interaction requires the Lys160 to be intact. The studies on iNOS deleted, LPS/IFNγ-treated macrophages with a fused GAPDH-NLS showed that although the NO stress does not affect the GAPDH-p300/CBP interaction directly, it governs the nuclear translocation of GAPDH and, thereby, the downstream events. The study also revealed that GAPDH-p300/CBP acetylates p53 and that in the absence of p300, GAPDH cannot bind to p53. Studies on U2OS cells revealed that GAPDH-p300-p53 induces the downstream target gene of p53—the PUMA and that the cell death signaling associated with the nuclear translocation of GAPDH is induced by the p300-p53–PUMA pathway. Later studies also showed that the S-nitrosylated GAPDH physiologically transnitrosylates other nuclear proteins like the sirtuin-1 (SIRT1), histone deacetylase-2, and DNA-activated protein kinase [66]. Transnitrosylation of SIRT1 by GAPDH inhibits the activity of SIRT1 leading to decreased PGC1α transcription.
Hyperglycemia-induced GAPDH nuclear translocation and accumulation in Müller cells
Hyperglycemia causes GAPDH nuclear accumulation in Müller cells in vitro and in vivo. In the rat retinal Müller cells rmc-1 and in cultured human primary cells incubated in high glucose conditions, GAPDH translocates to and accumulates in the nucleus. Similar nuclear accumulation of GAPDH in Müller cells was observed in retinal sections from diabetic rats at 4 months of diabetes.
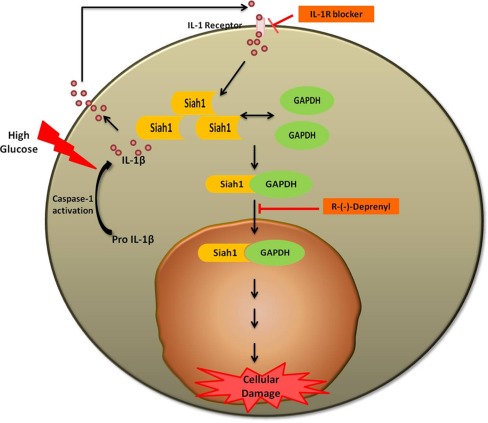
As stated above, nuclear accumulation of GAPDH requires a carrier protein for GAPDH to move from the cytosol into the nucleus. In vitro studies have shown that similar to stress conditions, SIAH1 acts as the carrier protein to help the nuclear translocation of GAPDH in Müller cells under hyperglycemic conditions [67]. Under high glucose conditions, SIAH1 expression is increased at both mRNA and protein levels [67]. As seen for GAPDH, nuclear localization of SIAH1 also increases significantly under high-glucose conditions. Si-RNA-mediated knockdown of SIAH1 abolished GAPDH nuclear accumulation indicating the essential role of SIAH1 as a carrier in the process. The nuclear accumulation of GAPDH in high glucose conditions not only requires the presence of SIAH1 but also requires the interaction of GAPDH with SIAH1. Co-immunoprecipitation assays have shown that GAPDH and SIAH1 form a tight complex that is stable and detectable in the nucleus of Müller cells treated with elevated glucose levels. Transfection of Müller cells with truncated form of SIAH1 which is unable to bind to GAPDH did not facilitate nuclear translocation and accumulation of GAPDH [67].
Nuclear translocation of GAPDH in Müller cells by the caspase-1/interleukin-1beta pathway
It has been speculated that cytokine production and retinal cell death are associated with diabetic retinopathy. High glucose induces the production of various acute-phase cytokines [21–26]. In the Müller cells, high glucose activates caspase-1 which catalyzes the production of mature, active IL-1β from pro IL-1β and IL-18 [68]. Caspase-1 activation and IL-1β production strongly associated with the nuclear accumulation of GAPDH implicating the involvement of the caspase-1 activation, IL-1β signaling pathway in the nuclear translocation and accumulation of GAPDH. Inhibition of caspase-1 activation and IL-1β receptor action prevents high glucose-induced nuclear accumulation of GAPDH in Müller cells. Nuclear accumulation of GAPDH is significantly reduced in cells treated with an IL-1β receptor blocker. Similarly, the caspase-1 inhibitor YVAD-fmk reduces hyperglycemia-induced nuclear accumulation of GAPDH in Müller cells. Treatment of cells with exogenous IL-1β also induced nuclear accumulation of GAPDH in a concentration-dependent manner starting at 2 ng/ml of exogenous IL-1β. In the rat retinal Müller cells, IL-1β-induced nuclear accumulation of GAPDH is significantly elevated at 12 h and remains the same through 24 h [27].
The process of pro-inflammatory cytokine-mediated GAPDH nuclear accumulation seems to be tightly regulated by the ratio of pro-inflammatory cytokines. IL-6, which can act as a pro-inflammatory or as an anti-inflammatory cytokine, prevents nuclear accumulation of GAPDH in Müller cells exposed to hyperglycemia [26]. Of note, TNFα, a pro-inflammatory cytokine commonly associated with diabetes and the development of diabetic complications, does neither induce nor prevent nuclear accumulation of GAPDH in Müller cells under hyperglycemia conditions. These studies indicate specificity for the caspase-1/IL-1β signaling pathway as initiator of hyperglycemia-induced GAPDH nuclear accumulation (Fig. 2).
Fig. 2.
Hyperglycemia-mediated nuclear translocation of GAPDH in Müller cells
Nuclear GAPDH and Müller cell death
Our studies have shown that hyperglycemia-induced nuclear translocation and accumulation of GAPDH in the Müller cells is associated with cellular dysfunction and cell death [27]. High glucose induced an increase in p53 phosphorylation and the expression of its target gene Bax. The Si-RNA knockdown of SIAH1 reversed this effect by reducing the phosphorylated p53 levels and the expression of Bax. Si-RNA-mediated knockdown of SIAH1 also reduced the high glucose induced cell death associated with the nuclear accumulation of GAPDH. Nuclear accumulation seems to be an early event in the hyperglycemia associated cell death of Müller cells preceding common downstream markers of cell death, such as the activation of caspase-6 and 3. Although high glucose induces death in Müller cells, the process involved is not apoptosis but rather pyroptosis, a type of death that requires strong caspase-1 activation, IL-1β production, and the production of reactive oxygen species but does not lead to classical DNA cleavage seen in apoptosis or leakage as defined for necrosis. Execution of this type of cell death is only vaguely defined and includes potential autophagic pathways.
Experimental evidences show that loss of Müller cells in the diabetic retina of rats is observed as early as 4 months after induction of diabetes. The Müller cell loss is accompanied by capillary basement thickening, sacking of the vasculature and aneurysms [62, 63]. Nuclear accumulation of GAPDH is also observed around the same time [27]. Immunohistochemistry staining showed that the Müller cells are regularly aligned in the middle of the inner nuclear layer in a normal retina. However, in the diabetic rat retina, the regular order was disrupted, indicating a loss of Müller cells. Since nuclear translocation and accumulation of GAPDH is strongly associated with loss of Müller cells in the retinal cells and tissue, it might also serve as a potential marker for cells undergoing cell death in general. Although no specific cell type was determined, studies have shown increased levels of nuclear GAPDH in nuclear fraction of retinas from diabetic rats [69] confirming the association of GAPDH nuclear accumulation in the nuclei of retinal cells and diabetic retinopathy.
Conclusion and outlook
High glucose conditions induce nuclear translocation and accumulation of GAPDH in the nucleus. The events underlying this nuclear accumulation and the cellular dysfunction and cell death associated with that in the Müller cells still need more studies. Overall, however, the current studies indicate that targeting GAPDH nuclear accumulation might serve as a new strategy to prevent cellular injury caused by diabetes. The involvement of cytokines in regulating the nuclear accumulation of GAPDH emphasizes the need to study the inflammatory signaling in the diabetic retina and its role in retinal tissue damage. However, anti-inflammatory drugs that specifically target the caspase-1 pathway, such as minocycline [70], might represent a new treatment strategy. Treatment with R-(−)-Deprenyl, a monoamine oxidase-B inhibitor, prevents hyperglycemia-induced nuclear translocation and accumulation of GAPDH in the Müller cells [55] and classes of these drugs might be beneficial of treating diabetic retinopathy although more studies are needed.
Although high glucose induces cell death, there is always a population of cells that are not undergoing cell death. The fate of that remaining population of cells and how they contribute to the transfer of stress signals remains to be investigated. Gene, protein expression, post-translational modifications and thereby modified signaling pathways initiated by the nuclear accumulation of GAPDH is unexplored. Multiple independent studies on glyceraldehyde-3-phosphate dehydrogenase discussed earlier have highlighted its functional versatility. The high glucose-induced post-translational modifications in GAPDH and the resulting nuclear accumulation could alter its crucial role like protecting the telomeres. Studies indicate that telomere shortening, a marker of senescence is more pronounced in type 1 diabetes [71]. Similarly, reduced proliferative capacity has been reported in monocytes of type 1 diabetes subjects [72]. Another study has indicated that the decreased proliferative efficiency is linked to the shortening of telomeres and that both controlled and uncontrolled group of type 1 diabetes subjects show telomere shortening with heightened shortening in uncontrolled group [73]. The study also reports that although glycemic control attenuated telomere shortening, it failed to abolish the same. Therefore, diabetes-induced cellular damage could be viewed as “cellular aging”. In such a case, the cellular aging and the inflammation caused by the cytokines under the influence of nuclear accumulation of GAPDH may together form a vicious cycle, kicking in a phenomenon recently labeled “inflamm-aging”. The involvement of GAPDH in diabetic retinopathy has opened new doors for diabetes researchers. It has given a new perspective in identifying the signaling pathways in the development and progression of diabetic retinopathy. This also means identifying newer targets and novel therapeutic strategies.
Acknowledgments
This manuscript was supported by NIH/NEI EY-017268 (SM).
References
- 1.National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.Yau J, Rogers S, Kawasaki R, Lamoureux E, Kowalski J, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 4.Distler C, Dreher Z. Glia cells of the monkey retina–II. Müller cells. Vis Res. 1996;36(16):2381–2394. doi: 10.1016/0042-6989(96)00005-3. [DOI] [PubMed] [Google Scholar]
- 5.Kannan R, Bao Y, Wang Y, Sarthy V, Kaplowitz N. Protection from oxidant injury by sodium-dependent GSH uptake in retinal Müller cells. Exp Eye Res. 1999;68(5):609–616. doi: 10.1006/exer.1998.0639. [DOI] [PubMed] [Google Scholar]
- 6.Miller R, Dowling J. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol. 1970;33(3):323–341. doi: 10.1152/jn.1970.33.3.323. [DOI] [PubMed] [Google Scholar]
- 7.Newman E, Frambach D, Odette L. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science. 1984;225(4667):1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichenbach A, Stolzenburg J, Eberhardt W, Chao T, Dettmer D, Hertz L. What do retinal Müller (glial) cells do for their neuronal ‘small siblings’? J Chem Neuroanat. 1993;66(4):201–213. doi: 10.1016/0891-0618(93)90042-3. [DOI] [PubMed] [Google Scholar]
- 9.Sarthy V. Müller cells in retinal health and disease. Arch Soc Esp Oftalmol. 2000;75(6):367–368. [PubMed] [Google Scholar]
- 10.Schütte M, Werner P. Redistribution of glutathione in the ischemic rat retina. Neurosci Lett. 1998;246(1):53–56. doi: 10.1016/S0304-3940(98)00229-8. [DOI] [PubMed] [Google Scholar]
- 11.Tout S, Chan-Ling T, Holländer H, Stone J. The role of Müller cells in the formation of the blood–retinal barrier. Neuroscience. 1993;55(1):291–301. doi: 10.1016/0306-4522(93)90473-S. [DOI] [PubMed] [Google Scholar]
- 12.Poitry-Yamate C, Poitry S, Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci Off J Soc Neurosci. 1995;15(7):5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsacopoulos M, Magistretti P. Metabolic coupling between glia and neurons. J Neurosci Off J Soc Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui K, Hosoi N, Tachibana M. Active role of glutamate uptake in the synaptic transmission from retinal nonspiking neurons. J Neurosci Off J Soc Neurosci. 1999;19(16):6755–6766. doi: 10.1523/JNEUROSCI.19-16-06755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riepe R, Norenburg M. Müller cell localisation of glutamine synthetase in rat retina. Nature. 1977;268:654–655. doi: 10.1038/268654a0. [DOI] [PubMed] [Google Scholar]
- 16.White R, Neal M. The uptake of l-glutamate by the retina. Brain Res. 1976;111(1):79–93. doi: 10.1016/0006-8993(76)91050-7. [DOI] [PubMed] [Google Scholar]
- 17.Newman E, Reichenbach A. The Müller cell: a functional element of the retina. Trends Neurosci. 1996;19(8):307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- 18.Dubois-Dauphin M, Poitry-Yamate C, Bilbao F, Julliard A, Jourdan F, Donati G. Early postnatal Müller cell death leads to retinal but not optic nerve degeneration in NSE-Hu-Bcl-2 transgenic mice. Neuroscience. 2000;95(1):9–21. doi: 10.1016/S0306-4522(99)00313-9. [DOI] [PubMed] [Google Scholar]
- 19.Puro D, Mano T. Modulation of calcium channels in human retinal glial cells by basic fibroblast growth factor: a possible role in retinal pathobiology. J Neurosci Off J Soc Neurosci. 1991;11(6):1873–1880. doi: 10.1523/JNEUROSCI.11-06-01873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward M, Jobling A, Kalloniatis M, Fletcher E. Glutamate uptake in retinal glial cells during diabetes. Diabetologia. 2005;48(2):351–360. doi: 10.1007/s00125-004-1639-5. [DOI] [PubMed] [Google Scholar]
- 21.Abu el Asrar A, Maimone D, Morse P, Gregory S, Reder A. Cytokines in the vitreous of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 1992;114(6):731–736. doi: 10.1016/s0002-9394(14)74052-8. [DOI] [PubMed] [Google Scholar]
- 22.Busik J, Mohr S, Grant M. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57(7):1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joussen A, Poulaki V, Le M, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J Off Publ Fed Am Soc Exp Biol. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 24.Mohr S, Xi X, Tang J, Kern T. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51(4):1172–1179. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]
- 25.Vincent J, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56(1):224–230. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 26.Yego E, Vincent J, Sarthy V, Busik J, Mohr S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Müller cells by IL-1beta and IL-6. Invest Ophthalmol Vis Sci. 2009;50(4):1920–1928. doi: 10.1167/iovs.08-2082. [DOI] [PubMed] [Google Scholar]
- 27.Kusner L, Sarthy V, Mohr S. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase: a role in high glucose-induced apoptosis in retinal Müller cells. Invest Ophthalmol Vis Sci. 2004;45(5):1553–1561. [PubMed] [Google Scholar]
- 28.Barber R, Harmer D, Coleman R, Clark B. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–395. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- 29.Tisdale E. Glyceraldehyde-3-phosphate dehydrogenase is phosphorylated by protein kinase Ciota/lambda and plays a role in microtubule dynamics in the early secretory pathway. J Biol Chem. 2002;277(5):3334–3341. doi: 10.1074/jbc.M109744200. [DOI] [PubMed] [Google Scholar]
- 30.Tisdale E, Azizi F, Artalejo C. Rab2 utilizes glyceraldehyde-3-phosphate dehydrogenase and protein kinase C{iota} to associate with microtubules and to recruit dynein. J Biol Chem. 2009;284(9):5876–5884. doi: 10.1074/jbc.M807756200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrade J, Pearce S, Zhao H, Barroso M. Interactions among p22, glyceraldehyde-3-phosphate dehydrogenase and microtubules. Biochem J. 2004;384(Pt 2):327–336. doi: 10.1042/BJ20040622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tisdale E, Kelly C, Artalejo C. Glyceraldehyde-3-phosphate dehydrogenase interacts with Rab2 and plays an essential role in endoplasmic reticulum to Golgi transport exclusive of its glycolytic activity. J Biol Chem. 2004;279(52):54046–54052. doi: 10.1074/jbc.M409472200. [DOI] [PubMed] [Google Scholar]
- 33.Harada N, Yasunaga R, Higashimura Y, Yamaji R, Fujimoto K, Moss J, et al. Glyceraldehyde-3-phosphate dehydrogenase enhances transcriptional activity of androgen receptor in prostate cancer cells. J Biol Chem. 2007;282(31):22651–22661. doi: 10.1074/jbc.M610724200. [DOI] [PubMed] [Google Scholar]
- 34.Singh R, Green M. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993;259(5093):365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Pascual F, Redondo-Horcajo M, Magán-Marchal N, Lagares D, Martínez-Ruiz A, Kleinert H, et al. Glyceraldehyde-3-phosphate dehydrogenase regulates endothelin-1 expression by a novel, redox-sensitive mechanism involving mRNA stability. Mol Cell Biol. 2008;28(23):7139–7155. doi: 10.1128/MCB.01145-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonafé N, Gilmore-Hebert M, Folk N, Azodi M, Zhou Y, Chambers S. Glyceraldehyde-3-phosphate dehydrogenase binds to the AU-Rich 3′ untranslated region of colony-stimulating factor-1 (CSF-1) messenger RNA in human ovarian cancer cells: possible role in CSF-1 posttranscriptional regulation and tumor phenotype. Cancer Res. 2005;65(9):3762–3771. doi: 10.1158/0008-5472.CAN-04-3954. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Yi X, Jn S, Bonafe N, Gilmore-Hebert M, McAlpine J, et al. The multifunctional protein glyceraldehyde-3-phosphate dehydrogenase is both regulated and controls colony-stimulating factor-1 messenger RNA stability in ovarian cancer. Mol Cancer Res: MCR. 2008;6(8):1375–1384. doi: 10.1158/1541-7786.MCR-07-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Backlund M, Paukku K, Daviet L, Boer R, Valo E, Hautaniemi S, et al. Posttranscriptional regulation of angiotensin II type 1 receptor expression by glyceraldehyde 3-phosphate dehydrogenase. Nucleic Acids Res. 2009;37(7):2346–2358. doi: 10.1093/nar/gkp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carujo S, Estanyol J, Ejarque A, Agell N, Bachs O, Pujol M. Glyceraldehyde 3-phosphate dehydrogenase is a SET-binding protein and regulates cyclin B-cdk1 activity. Oncogene. 2006;25:4033–4042. doi: 10.1038/sj.onc.1209433. [DOI] [PubMed] [Google Scholar]
- 40.Zheng L, Roeder R, Luo Y. S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell. 2003;114(2):255–266. doi: 10.1016/S0092-8674(03)00552-X. [DOI] [PubMed] [Google Scholar]
- 41.Mansur N, Meyer-Siegler K, Wurzer J, Sirover M. Cell cycle regulation of the glyceraldehyde-3-phosphate dehydrogenase/uracil DNA glycosylase gene in normal human cells. Nucleic Acids Res. 1993;21(4):993–998. doi: 10.1093/nar/21.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronai Z. Glycolytic enzymes as DNA binding proteins. Int J Biochem. 1993;25(7):1073–1076. doi: 10.1016/0020-711X(93)90123-V. [DOI] [PubMed] [Google Scholar]
- 43.Azam S, Jouvet N, Jilani A, Vongsamphanh R, Yang X, Yang S, et al. Human glyceraldehyde-3-phosphate dehydrogenase plays a direct role in reactivating oxidized forms of the DNA repair enzyme APE1. J Biol Chem. 2008;283(45):30632–30641. doi: 10.1074/jbc.M801401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundararaj K, Wood R, Ponnusamy S, Salas A, Szulc Z, Bielawska A, et al. Rapid shortening of telomere length in response to ceramide involves the inhibition of telomere binding activity of nuclear glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 2004;279(7):6152–6162. doi: 10.1074/jbc.M310549200. [DOI] [PubMed] [Google Scholar]
- 45.Demarse N, Ponnusamy S, Spicer E, Apohan E, Baatz J, Ogretmen B, et al. Direct binding of glyceraldehyde 3-phosphate dehydrogenase to telomeric DNA protects telomeres against chemotherapy-induced rapid degradation. J Mol Biol. 2009;394(4):789–803. doi: 10.1016/j.jmb.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patterson R, Rossum D, Kaplin A, Barrow R, Snyder S. Inositol 1,4,5-trisphosphate receptor/GAPDH complex augments Ca2+ release via locally derived NADH. Proc Natl Acad Sci USA. 2005;102(5):1357–1359. doi: 10.1073/pnas.0409657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravichandran V, Seres T, Moriguchi T, Thomas J, Johnston R. S-thiolation of glyceraldehyde-3-phosphate dehydrogenase induced by the phagocytosis-associated respiratory burst in blood monocytes. J Biol Chem. 1994;269(40):25010–25015. [PubMed] [Google Scholar]
- 48.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112(7):1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devalaraja-Narashimha K, Padanilam B. PARP-1 inhibits glycolysis in ischemic kidneys. J Am Soc Nephrol: JASN. 2009;20(1):95–103. doi: 10.1681/ASN.2008030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarze A, Deniaud A, Bras M, Maillier E, Molle D, Larochette N, et al. GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene. 2007;26(18):2606–2620. doi: 10.1038/sj.onc.1210074. [DOI] [PubMed] [Google Scholar]
- 51.Colell A, Ricci J-E, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129(5):983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 52.Saunders P, Chalecka-Franaszek E, Chuang D. Subcellular distribution of glyceraldehyde-3-phosphate dehydrogenase in cerebellar granule cells undergoing cytosine arabinoside-induced apoptosis. J Neurochem. 1997;69(5):1820–1828. doi: 10.1046/j.1471-4159.1997.69051820.x. [DOI] [PubMed] [Google Scholar]
- 53.Sawa A, Khan A, Hester L, Snyder S. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci USA. 1997;94(21):11669–11674. doi: 10.1073/pnas.94.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishitani R, Tanaka M, Sunaga K, Katsube N, Chuang D. Nuclear localization of overexpressed glyceraldehyde-3-phosphate dehydrogenase in cultured cerebellar neurons undergoing apoptosis. Mol Pharmacol. 1998;53(4):701–707. doi: 10.1124/mol.53.4.701. [DOI] [PubMed] [Google Scholar]
- 55.Kragten E, Lalande I, Zimmermann K, Roggo S, Schindler P, Muller D, et al. Glyceraldehyde-3-phosphate dehydrogenase, the putative target of the antiapoptotic compounds CGP 3466 and R-(−)-deprenyl. J Biol Chem. 1998;273(10):5821–5828. doi: 10.1074/jbc.273.10.5821. [DOI] [PubMed] [Google Scholar]
- 56.Carlile G, Chalmers-Redman R, Tatton N, Pong A, Borden K, Tatton W. Reduced apoptosis after nerve growth factor and serum withdrawal: conversion of tetrameric glyceraldehyde-3-phosphate dehydrogenase to a dimer. Mol Pharmacol. 2000;57(2):2–12. [PubMed] [Google Scholar]
- 57.Dastoor Z, Dreyer J. Potential role of nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase in apoptosis and oxidative stress. J Cell Sci. 2001;114:1643–1653. doi: 10.1242/jcs.114.9.1643. [DOI] [PubMed] [Google Scholar]
- 58.Hara M, Agrawal N, Kim S, Cascio M, Fujimuro M, Ozeki Y, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following SIAH1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 59.Sen N, Hara M, Ahmad A, Cascio M, Kamiya A, Ehmsen J, et al. GOSPEL: a neuroprotective protein that binds to GAPDH upon S-nitrosylation. Neuron. 2009;63(1):81–91. doi: 10.1016/j.neuron.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sourisseau T, Desbois C, Debure L, Bowtell D, Cato A, Schneikert J, et al. Alteration of the stability of Bag-1 protein in the control of olfactory neuronal apoptosis. J Cell Sci. 2001;114(Pt 7):1409–1416. doi: 10.1242/jcs.114.7.1409. [DOI] [PubMed] [Google Scholar]
- 61.Fiucci G, Beaucourt S, Duflaut D, Lespagnol A, Stumptner-Cuvelette P, Géant A, et al. SIAH-1b is a direct transcriptional target of p53: identification of the functional p53 responsive element in the siah-1b promoter. Proc Natl Acad Sci USA. 2004;101(10):3510–3515. doi: 10.1073/pnas.0400177101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polekhina G, House C, Traficante N, Mackay J, Relaix F, Sassoon D, et al. SIAH ubiquitin ligase is structurally related to TRAF and modulates TNF-alpha signaling. Nat Struct Biol. 2002;9(1):68–75. doi: 10.1038/nsb743. [DOI] [PubMed] [Google Scholar]
- 63.House C, Frew I, Huang H-L, Wiche G, Traficante N, Nice E, et al. A binding motif for SIAH ubiquitin ligase. Proc Natl Acad Sci USA. 2003;100(6):3101–3106. doi: 10.1073/pnas.0534783100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.House C, Hancock N, Möller A, Cromer B, Fedorov V, Bowtell D, et al. Elucidation of the substrate binding site of SIAH ubiquitin ligase. Structure (London, England: 1993) 2006;14(4):695–701. doi: 10.1016/j.str.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 65.Sen N, Hara M, Kornberg M, Cascio M, Bae B-I, Shahani N, et al. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat Cell Biol. 2008;10:866–873. doi: 10.1038/ncb1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kornberg M, Sen N, Hara M, Juluri K, Nguyen J, Snowman A, et al. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12(11):1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yego E, Mohr S. SIAH-1 protein is necessary for high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation and cell death in Müller cells. J Biol Chem. 2010;285:3181–3190. doi: 10.1074/jbc.M109.083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Creagh E, Conroy H, Martin S. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003;193:10–21. doi: 10.1034/j.1600-065X.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- 69.Madsen-Bouterse S, Mohammad G, Kowluru R. Glyceraldehyde-3-phosphate dehydrogenase in retinal microvasculature: implications for the development and progression of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(3):1765–1772. doi: 10.1167/iovs.09-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krady J, Basu A, Allen C, Xu Y, LaNoue K, Gardner T, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54(5):1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- 71.Jeanclos E, Krolewski A, Skurnick J, Kimura M, Aviv H, Warram J, et al. Shortened telomere length in white blood cells of patients with IDDM. Diabetes. 1998;47(3):482–486. doi: 10.2337/diabetes.47.3.482. [DOI] [PubMed] [Google Scholar]
- 72.González M, Sanz I, Silva V, Asenjo S, Gleisner A, Bustamante M. Differential modulation by native and glycated low density lipoproteins of peripheral blood mononuclear cells proliferation induced by phytohemagglutinin in insulin-dependent diabetes mellitus patients. Clin Chim Acta Int J Clin Chem. 2000;293(1–2):223–228. doi: 10.1016/S0009-8981(99)00223-5. [DOI] [PubMed] [Google Scholar]
- 73.Uziel O, Singer J, Danicek V, Sahar G, Berkov E, Luchansky M, et al. Telomere dynamics in arteries and mononuclear cells of diabetic patients: effect of diabetes and of glycemic control. Exp Gerontol. 2007;42(10):971–978. doi: 10.1016/j.exger.2007.07.005. [DOI] [PubMed] [Google Scholar]