Abstract
In diabetic retinopathy (DR), abnormalities in vascular and neuronal function are closely related to the local production of inflammatory mediators whose potential source is microglia. Adenosine and its receptors have been shown to possess anti-inflammatory properties that have only recently been studied in DR. Here, we review recent studies that determined the roles of adenosine and its associated proteins, including equilibrative nucleoside transporters, adenosine receptors, and underlying signaling pathways in retinal complications associated with diabetes.
Keywords: Diabetic retinopathy, Adenosine, Microglia, Cannabinoids, Adenosine receptors
Introduction
Diabetic retinopathy (DR) is a leading cause of blindness among working-age adults [1]. Despite many years of research, treatment options for DR, including photocoagulation, vitrectomy, and repeated intraocular injections of steroids and anti-VEGF, remain limited and with adverse effects. Discovery of new molecular entities with adequate clinical activity for DR remains one of the key research priorities in ophthalmology.
Activation of retinal microglial cells in early diabetes is critical in causing the major complications in DR, including losses of blood–retinal barrier (BRB) function and retinal neurons [2, 3]. Although these losses may be a major vision-threatening complication in diabetes, by the time they become easily demonstrable, the progress of DR is already irreversible. The preceding microglial activation and other changes that cause the development of vascular and neuronal changes are highly significant to the understanding and treatment of DR.
Activation of retinal microglial cells is most likely associated with oxidative stress and inflammation. Tissue inflammation is modulated by extracellular adenosine via adenosine receptors. Our research in DR has focused on delineating the inflammatory processes involved. We have identified new noninvasive receptor-based therapies for mitigating microglial activation associated with diabetes. This review is focused on the therapeutic effects of cannabidiol (which are linked with adenosine) and adenosine receptor agonists on animal models of DR. Special emphasis is placed on novel mechanisms described in recent studies of retinal models which help to explain some of the pharmacological effects observed with these therapies.
Diabetic retinopathy
DR is a chronic ocular disorder that will lead to blindness if untreated. In the USA, over 20 million, or 10% of the total population, currently have diabetes. Of this group, over 12,000 patients will be diagnosed with new-onset blindness annually, making it one of the leading causes of legal blindness in Americans within the age group of 20–74 [4]. Type 1 diabetics usually have high incidence of DR, and it occurs in almost all patients with diabetes for 20 years or more [1]. The earliest detectable signs of DR are categorized as nonproliferative diabetic retinopathy (NPDR). NPDR is clinically subdivided into mild, moderate, and severe categories. Loss of retinal pericytes and alterations in retinal blood flow are preclinical changes that are often non-detectable by physical exam [5, 6]. Retinal venous dilation and microaneurysms are the first alterations detectable by ophthalmoscopy. Following these alterations, intraretinal hemorrhage and exudation may occur. These may then lead to macular edema, which may lead to blindness if untreated. As hyperglycemia persists, the disease progresses which presents with hemorrhages and venous beading, suggesting decreased retinal circulation and dilated capillaries [7]. Proliferative diabetic retinopathy (PDR) is the next stage when proliferation of new blood vessels begins. Approximately 50% of patients with severe NPDR progress to PDR within 1 year [8]. This stage is characterized by the onset of ischemia-induced new vessel proliferation from the optic nerve head as well as in the retina. These new vessels are fragile and tend to bleed easily resulting in vitreous hemorrhage. If untreated, the neovascularization will undergo fibrosis and contraction leading to traction retinal detachments.
The early signs of DR in experimental diabetic models include vascular inflammatory reactions due to glycated albumin, oxidative stress, pro-inflammatory cytokines, and the consequent binding of leukocyte adhesion molecules CD18 and intercellular adhesion molecule 1 (ICAM-1) [9]. These reactions lead to breakdown of the BRB function, vascular occlusion, and tissue ischemia, which in turn leads to neuronal cell death. However, diabetes could also directly affect metabolism within the neural retina leading to neuronal cell death [9–14]. Whether diabetes affects vascular or neural retina first, both microglial and macroglial cells are activated [15]. The function of activated macroglia in transporting [16] and metabolizing glutamate may be impaired [16, 17]. This leads to glutamate accumulation [18–21]. Glutamate excitotoxicity occurs via activation of N-methyl-d-aspartic acid (NMDA) and non-NMDA receptors, to directly or indirectly induce calcium influx and the release of superoxides, leading to neuronal cell death [21]. This is followed by neuroinflammation, during which activated microglial cells migrate toward dying neurons and release inflammatory cytokines to further exacerbate the damage [22]. These findings suggest that pharmacological interventions that reduce oxidative stress and inflammation might be effective neuroprotectants for DR [20, 23].
Microglia in DR
Microglia are very sensitive to small changes in their environment, and they can be activated by a variety of factors including: pro-inflammatory cytokines, lipopolysaccharide, damaged cells, or any immune-stimulatory agents [24]. Activated microglia have phagocytic and cytotoxic ability to destroy foreign materials by secreting cytokines and other signaling molecules. However, if microglia remain in a sustained activated state, the secreted cytokines can affect other cell types in the proximity, particularly neuronal and vascular cells [25]. Recently, overwhelming evidence has sculpted the concept of activated microglia as an important player in the pathogenesis of DR. This input has originated partly from histopathologic studies that showed clustering of apparently activated microglia in the diabetic rat retina [2, 3]. These initial observations have been supported in postmortem human retinas [26] and reinforced by additional histopathological studies showing that many inflammatory molecules, such as tumor necrosis factor alpha (TNF-α), can be detected in the diabetic retina, often in association with microglia [27–29]. The retinal expression of TNF-α has been reported to be associated with neuronal and endothelial cell death, hallmark features of the disease [12, 30], and inhibition of TNF-α has demonstrated beneficial effects in the prevention of early DR [31]. Moreover, the in vitro studies on co-cultured retinal neurons R28 with activated microglia have shown that microglia produce cytotoxins that kill retinal neuronal cells [32]. It remains unclear why diabetes would incite microglia activation in the retina to release inflammatory cytokines. However, recent studies from our group have recognized Amadori-glycated albumin (AGA)/inflammation cascade as a potential culprit mechanism contributing to microglia activation and their secretion of inflammatory cytokines [33]. AGA is present in the retinal capillaries of patients with DR [34] and in the retina of STZ-induced diabetic rats [33, 35] in regions of microglial distribution. Treatment of diabetic rats with A717, a specific AGA-neutralizing antibody, significantly attenuated overexpression of both Iba1, a microglial marker, and TNF-α mRNAs. These observations, together with the finding that intravitreal injection of AGA per se in normal rats induced Iba-1 expression as well as TNF-α release, have strengthened the notion that increased levels of AGA in the diabetic retina is an important contributor to microglial activation and thereby inflammation [33]. Accordingly, the direct relationship between microglia and AGA has been explored through in vitro study. The results showed that formation of reactive oxygen species (ROS) with subsequent activation of extracellular signal-regulated kinase (ERK) and P38, but not JNK, are molecular events underpinning retinal microglial TNF-α release during AGA treatment [33]. Therefore, treatment of cultured microglia with glycated proteins has been used as an in vitro model to simulate inflammation during diabetes [33, 36–38].
Roles of adenosine receptors in DR
After having shown the contribution of microglia to retinal inflammation and DR, the next question is how the protective, anti-inflammatory actions are being harnessed to develop new drug targets for DR control. Adenosine has shown a non-redundant role in the attenuation of inflammation in other tissues through interaction with its receptors. Extracellular adenosine can activate transmembrane adenosine receptors (ARs), which are classified as A1, A2A, A2B, and A3 subtypes [39]. These receptors are classified based on their mechanism of signal transduction. A1 and A3 receptors interact with G proteins of the Gi and Go family to inhibit adenylate cyclase and stimulate phospholipases. The A2A receptor stimulates adenylate cyclase through Gs coupling [40]. In addition to interaction with Gs, A2B receptor also stimulates phospholipase C activity through Gq [41]. The increased adenosine at inflamed sites exhibits anti-inflammatory effects to protect against excessive cellular damage through A2AAR [42, 43]. Subthreshold doses of an inflammatory stimulus that caused minimal tissue damage in wild-type mice were sufficient to induce extensive tissue damage and more prolonged and higher levels of pro-inflammatory cytokines in A2AAR−/− mice [44]. Moreover, A2AAR agonist treatment blocks the inflammation, functional and histological changes associated with diabetic nephropathy in wild-type diabetic mice but not in the A2AAR−/− diabetic mice [45]. Activation of the A2AAR in the LPS-stressed retinal microglial cells is the most efficient in mediating TNF-α inhibition [46]. In the DR context, A2AAR−/− mice had significantly more retinal terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells, TNF-α release, and ICAM-1 expression compared with diabetic wild type [47]. Furthermore, knockout of A2AAR altered microglia phenotype in unison with TUNEL and cytokine expression profiles during diabetes. This was manifested by the finding that when microglia encountered diabetic milieu, they transformed from their ramified resting state into an amoeboid shape, the activated and cytokine-releasing state, and this phenotypic configuration became more obvious in A2AAR−/− diabetic mice than in diabetic wild-type mouse retina. Furthermore, treatment with the A2AAR agonist resulted in marked decreases in diabetes-induced retinal cell death and TNF-α release [47]. Following this further, we have addressed an interesting feature, acquisition of reactive microglial phenotype, that could be an important determinant for understanding the mechanisms by which A2AAR agonist affects TNF-α release. In this regard, we have noted that treatment of diabetic mice with A2AAR agonist attenuated the morphological transformation of ramified microglia into an activated ameboid microglia. Taken together, these results suggest that A2AAR plays a crucial role in limiting retinal inflammation, microglial activation, and neuronal cell injury associated with diabetes. Little, however, is known about how these receptors regulate inflammation in DR. Additional studies from our group have shown that activation of A2AAR inhibits Raf activation and TNF-α release in AGA-treated retinal microglial cells. These data suggest an important crosstalk regulation between adenosine and inflammation signaling (Ras/Raf/MEK/MAPK) through A2AAR-cAMP regulator. However, this remarkable regulator appears to both activate and inhibit MAPK activity in different cell line. The opposite effects of cAMP on MAPK activity in different cell line could be explained by dissimilar involvement of RAF isoform (C-Raf or B-Raf). Originally, it was assumed that cAMP-triggered inhibition of MAPK seemed to be mediated by C-RAf, while induction of MAPK by cAMP in another cell type involved B-Raf. In AGA-treated microglia, activation of A2AAR inhibits C-Raf activation without affecting B-Raf phosphorylation, suggesting that the mechanism of anti-inflammation involving negative crosstalk between A2AAR-cAMP and Ras/Raf/MEK/MAPK is operative. Furthermore, the observed specificity in the cAMP signaling that is PKA-independent and EPAC-dependent suggests a novel mode in controlling the inflammatory events associated with microglia activation [47].
Adenosine reuptake and degradation
The therapeutic application of adenosine and its agonists is limited by systemic side effects, such as hypotension, bradycardia, and sedation [48]. Adenosine disappears rapidly in physiological or inflammatory conditions due to rapid reuptake via nucleoside transporters (NTs) and subsequent intracellular metabolism [49]. Since endogenous adenosine levels are increased at inflamed sites, prevention of adenosine reuptake into the cells and its subsequent metabolism can selectively enhance extracellular levels of adenosine at the inflamed sites, resulting in a site-specific anti-inflammatory effect. There are two subtypes of NTs: concentrative NTs, which are dependent on the presence of extracellular sodium, and equilibrative NT (ENTs). In the microglial cells, the majority of adenosine transport is not affected by sodium removal, suggesting ENTs are the primary transporters functioning in these cells [50]. ENTs are classified into two subtypes on the basis of their sensitivities to inhibition by the drug S-(4-nitrobenzyl)-6-thioinosine (nitrobenzylmercaptopurine riboside, NBMPR). NBMPR-sensitive ENTs bind NBMPR with high affinity and have the functional designation equilibrative sensitive (ENT1). NBMPR-insensitive transporters are designated ENT2. Dipyridamole, an inhibitor for both ENT1 and ENT2 [51], is used clinically as a coronary vasodilator and a platelet aggregation inhibitor [52, 53]. Dipyridamole plus aspirin improves retinal vasculature patterns in experimental diabetes [54].
Role of ENT1 in adenosine function in diabetes
ENT1 plays an integral role in adenosine function in diabetes by regulating adenosine levels in the vicinity of adenosine receptors [55]. In this study, Vmax of adenosine transport in high glucose (HG)-treated human aortic smooth muscle cells (HASMCs) was increased by 40% without affecting Km. Similarly, Bmax of high-affinity [3H]NBMPR binding was increased without affecting Kd. Consistent with these observations, HG increased mRNA and protein expression of ENT1. Treatment of cells with the selective inhibitors of ERK, PD98059, and U0126 abolished the effect of HG on ENT1. These results suggest that HG upregulates the expression and functional activity of ENT1 in HASMCs via ERK-dependent pathways. Pathologically, the increase in ENT1 activity in diabetes may affect the availability of adenosine in the vicinity of adenosine receptors and thus alter vascular functions in diabetes. Because diabetes-induced changes in ENT1 expression vary depending on cell types [56], how diabetes alters retinal ENT1 is relevant for its role in the regulation of retinal inflammation.
Cannabinoids as neuroprotectant therapeutics
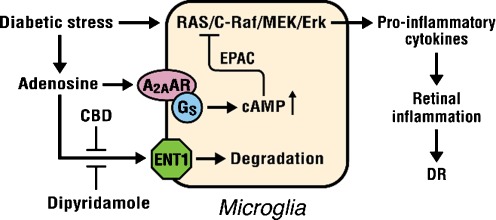
The marijuana-derived cannabinoids (−)-Δ9–tetrahydrocannabinol (THC) and (−)-cannabidiol (CBD) each has anti-oxidative and immunosuppressive effects [57]. THC is neuroprotective in a rat model of glaucoma [58]. The psychotropic and anti-inflammatory effects of THC are, at least in part, mediated by CB1 and CB2 cannabinoid receptors, respectively. CBD, though, does not bind well to these receptors, resulting in the inability of CBD to produce the subjective “high” and cognitive effects [59]. The anti-oxidative effect of CBD (≧1 μM) [60] is due to its ability to scavenge ROS. CBD decreases inflammation in arthritis [61] and uveitis [62], prevents cerebral damage in cerebral ischemia [63] and cerebral infarction [64], reduces hyperglycemia-induced endothelial cell inflammation and barrier disruption [65], decreases the incidence of diabetes in non-obese diabetic mice [66], and is neuroprotective and BRB-preserving in diabetic rats [12]. CBD is well tolerated when chronically administered to humans and has been approved for the treatment of inflammation and spasticity associated with multiple sclerosis in humans [67]. Nanomolar concentrations of CBD inhibit uptake of adenosine by ENT1 in microglia and macrophages [50]. In vivo treatment with a low dose of CBD decreases TNF-α production in serum in LPS-treated mice; this effect is reversed with an A2AAR antagonist and abolished in A2AAR−/− mice [50]. Similar results were obtained in the retinas of LPS-treated rats and retinal microglial cells [46]. These studies suggest that CBD has the ability to enhance adenosine signaling through inhibition of reuptake via ENT1, thus using a mechanism that is not cannabinoid receptor-mediated. The above results are summarized in a diagram (Fig. 1).
Fig. 1.
Regulation of inflammation by adenosine in diabetic retinopathy
Conclusion
This study is important for the development of adenosine receptor agonists or adenosine reuptake inhibitors as a potentially novel and effective therapy for DR. The effect of these therapies is based on their ability to attenuate microglial activation, which precedes the irreversible vascular and neuronal losses in DR. However, the therapeutic values of these agents should be confirmed by clinical trials. Furthermore, depending on the difference in the genetic makeups for the metabolism and pharmacological target of the A2AAR agonists, CBD, or dipyridamole, it may be important to consider these agents as a personalized medicine, i.e., adjusted dosages according to individual’s genetic makeups, to offer significant advantages over traditional clinical approaches [68].
Footnotes
This work has been supported by Vision Discovery Institute (GIL), Department of Defense (GIL), and Egyptian Cultural and Educational Bureau (ASI).
References
- 1.Klein R, et al. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 2.Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41(7):1971–1980. [PubMed] [Google Scholar]
- 3.Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000;17(3):463–471. doi: 10.1017/S0952523800173122. [DOI] [PubMed] [Google Scholar]
- 4.Ammary-Risch NJ, Huang SS. The primary care physician's role in preventing vision loss and blindness in patients with diabetes. J Natl Med Assoc. 2011;103(3):281–283. doi: 10.1016/s0027-9684(15)30288-1. [DOI] [PubMed] [Google Scholar]
- 5.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- 6.Bursell SE, et al. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37(5):886–897. [PubMed] [Google Scholar]
- 7.Benson WE, et al. Current popularity of pneumatic retinopexy. Retina. 1999;19(3):238–241. [PubMed] [Google Scholar]
- 8.Palmberg PF. Diabetic retinopathy. Diabetes. 1977;26(7):703–709. doi: 10.2337/diab.26.7.703. [DOI] [PubMed] [Google Scholar]
- 9.Joussen AM, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 10.Barber AJ, et al. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102(4):783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Remessy AB, et al. Experimental diabetes causes breakdown of the blood–retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol. 2003;162(6):1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Remessy AB, et al. Neuroprotective and blood–retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168(1):235–244. doi: 10.2353/ajpath.2006.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali TK, et al. Peroxynitrite mediates retinal neurodegeneration by inhibiting nerve growth factor survival signaling in experimental and human diabetes. Diabetes. 2008;57(4):889–898. doi: 10.2337/db07-1669. [DOI] [PubMed] [Google Scholar]
- 15.Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81(6):1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 16.Lieth E, et al. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. The Penn State Retina Research Group. Exp Eye Res. 2000;70(6):723–730. doi: 10.1006/exer.2000.0840. [DOI] [PubMed] [Google Scholar]
- 17.El-Remessy AB, et al. Cannabidiol protects retinal neurons by preserving glutamine synthetase activity in diabetes. Mol Vis. 2010;16:1487–1495. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Ambati J, et al. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1997;115(9):1161–1166. doi: 10.1001/archopht.1997.01100160331011. [DOI] [PubMed] [Google Scholar]
- 19.Lieth E, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47(5):815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 20.Kowluru RA, et al. Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int. 2001;38(5):385–390. doi: 10.1016/S0197-0186(00)00112-1. [DOI] [PubMed] [Google Scholar]
- 21.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 22.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 23.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9(4):315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 24.Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 25.Wood PL. Neuroinflammation: mechanisms and management. Totowa New York, NY: Humana Press; 2003. [Google Scholar]
- 26.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126(2):227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 27.Yang LP, et al. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50(5):2319–2327. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, et al. Expression of macrophage colony-stimulating factor (M-CSF) and its receptor in streptozotocin-induced diabetic rats. Curr Eye Res. 2009;34(2):123–133. doi: 10.1080/02713680802650369. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim AS, et al. Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Mol Vis. 2010;16:2033–2042. [PMC free article] [PubMed] [Google Scholar]
- 30.Joussen AM, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009;15:1418–1428. [PMC free article] [PubMed] [Google Scholar]
- 31.Joussen AM, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16(3):438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 32.Krady JK, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005. doi:10.2337/db(54.5.1559. [DOI] [PubMed]
- 33.Ibrahim AS, et al. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes. 2011;60(4):1122–1133. doi: 10.2337/db10-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schalkwijk CG, et al. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999;42(3):351–357. doi: 10.1007/s001250051162. [DOI] [PubMed] [Google Scholar]
- 35.Tang J, et al. Retina accumulates more glucose than does the embryologically similar cerebral cortex in diabetic rats. Diabetologia. 2000;43(11):1417–1423. doi: 10.1007/s001250051548. [DOI] [PubMed] [Google Scholar]
- 36.Wang AL, et al. AGEs mediated expression and secretion of TNF alpha in rat retinal microglia. Exp Eye Res. 2007;84(5):905–913. doi: 10.1016/j.exer.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Schalkwijk CG. Comment on “AGEs mediated expression and secretion of TNF alpha in rat retinal microglia” by Dr Wang et al. Exp Eye Res. 2007;85:572–573. doi: 10.1016/j.exer.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Quan Y, Du J, Wang X. High glucose stimulates GRO secretion from rat microglia via ROS, PKC, and NF-kappaB pathways. J Neurosci Res. 2007;85(14):3150–3159. doi: 10.1002/jnr.21421. [DOI] [PubMed] [Google Scholar]
- 39.Collis MG, Hourani SM. Adenosine receptor subtypes. Trends Pharmacol Sci. 1993;14(10):360–366. doi: 10.1016/0165-6147(93)90094-Z. [DOI] [PubMed] [Google Scholar]
- 40.Fredholm BB, et al. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- 41.Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999;55(4):726–734. [PubMed] [Google Scholar]
- 42.Bong GW, Rosengren S, Firestein GS. Spinal cord adenosine receptor stimulation in rats inhibits peripheral neutrophil accumulation. The role of N-methyl-d-aspartate receptors. J Clin Invest. 1996;98(12):2779–2785. doi: 10.1172/JCI119104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 44.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414(6866):916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 45.Awad AS, et al. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290(4):F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 46.Liou GI, et al. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci. 2008;49(12):5526–5531. doi: 10.1167/iovs.08-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ibrahim AS, et al. A(2A) adenosine receptor (A(2A)AR) as a therapeutic target in diabetic retinopathy. Am J Pathol. 2011;178(5):2136–2145. doi: 10.1016/j.ajpath.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams M. Challenges in developing P2 purinoceptor-based therapeutics. CIBA Found Symp. 1996;198:309–321. doi: 10.1002/9780470514900.ch18. [DOI] [PubMed] [Google Scholar]
- 49.Moser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol. 1989;256(4 Pt 1):C799–C806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- 50.Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103(20):7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunwiddie TV, Diao L. Regulation of extracellular adenosine in rat hippocampal slices is temperature dependent: role of adenosine transporters. Neuroscience. 2000;95(1):81–88. doi: 10.1016/S0306-4522(99)00404-2. [DOI] [PubMed] [Google Scholar]
- 52.Picano E, Michelassi C. Chronic oral dipyridamole as a ‘novel’ antianginal drug: the collateral hypothesis. Cardiovasc Res. 1997;33(3):666–670. doi: 10.1016/S0008-6363(96)00262-3. [DOI] [PubMed] [Google Scholar]
- 53.Schryver EL. Dipyridamole in stroke prevention: effect of dipyridamole on blood pressure. Stroke. 2003;34(10):2339–2342. doi: 10.1161/01.STR.0000090346.45784.C3. [DOI] [PubMed] [Google Scholar]
- 54.Cruz JP, et al. Effect of aspirin plus dipyridamole on the retinal vascular pattern in experimental diabetes mellitus. J Pharmacol Exp Ther. 1997;280(1):454–459. [PubMed] [Google Scholar]
- 55.Leung GP, Man RY, Tse CM. D-Glucose upregulates adenosine transport in cultured human aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;288(6):H2756–H2762. doi: 10.1152/ajpheart.00921.2004. [DOI] [PubMed] [Google Scholar]
- 56.Pawelczyk T, Podgorska M, Sakowicz M. The effect of insulin on expression level of nucleoside transporters in diabetic rats. Mol Pharmacol. 2003;63(1):81–88. doi: 10.1124/mol.63.1.81. [DOI] [PubMed] [Google Scholar]
- 57.Buckley NE, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396(2–3):141–149. doi: 10.1016/S0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 58.Crandall J, et al. Neuroprotective and intraocular pressure-lowering effects of (-)Delta9-tetrahydrocannabinol in a rat model of glaucoma. Ophthalmic Res. 2007;39(2):69–75. doi: 10.1159/000099240. [DOI] [PubMed] [Google Scholar]
- 59.Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42(11 Suppl):11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- 60.Hampson AJ, et al. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95(14):8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malfait AM, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97(17):9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El-Remessy AB, et al. Neuroprotective effects of cannabidiol in endotoxin-induced uveitis: critical role of p38 MAPK activation. Mol Vis. 2008;14:2190–2203. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Braida D, et al. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci Lett. 2003;346(1–2):61–64. doi: 10.1016/S0304-3940(03)00569-X. [DOI] [PubMed] [Google Scholar]
- 64.Mishima K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke. 2005;36(5):1077–1082. doi: 10.1161/01.STR.0000163083.59201.34. [DOI] [PubMed] [Google Scholar]
- 65.Rajesh M, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. 2007;293(1):H610–H619. doi: 10.1152/ajpheart.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss L, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39(2):143–151. doi: 10.1080/08916930500356674. [DOI] [PubMed] [Google Scholar]
- 67.Barnes MP. Sativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin Pharmacother. 2006;7(5):607–615. doi: 10.1517/14656566.7.5.607. [DOI] [PubMed] [Google Scholar]
- 68.Liou G, et al. Cannabidiol as a putative novel therapy for diabetic retinopathy: a postulated mechanism of action as an entry point for biomarker-guided clinical development. Curr Pharmacogenomics Person Med. 2009;7(3):215–222. doi: 10.2174/1875692110907030215. [DOI] [PMC free article] [PubMed] [Google Scholar]