Abstract
Diabetic retinopathy (DR) is a major complication of diabetes and a leading cause of blindness in working-age Americans. DR is traditionally regarded as a disorder of blood–retina barriers, and the leakage of blood content is a major pathological characteristic of the disease. While the breakdown of the endothelial barrier in DR has been investigated extensively, the vascular leakage through the retinal pigment epithelium (RPE) barrier in the disease has not been widely acknowledged. As the blood content leaked through the RPE barrier causes excessive water influx to the retina, the breakdown of the RPE barrier is likely to play a causative role in the development of some forms of diabetic macular edema, a major cause of vision loss in DR. In this article, we will discuss the clinical evidences of the diabetes-induced RPE barrier breakdown, the alteration of the RPE in diabetes, the molecular and cellular mechanism of RPE barrier breakdown, and the research tools for the analysis of RPE barrier leakage. Finally, we will discuss the methodology and potential applications of our recently developed fluorescent microscopic imaging for the diabetes- or ischemia-induced RPE barrier breakdown in rodents.
Keywords: Diabetic retinopathy, Macular edema, RPE, Barrier, Leakage, Fluorescent microscopy
Introduction
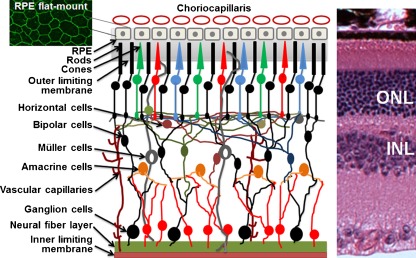
The retina is the most active tissue metabolically, and its activity and metabolism are supported by choroidal and retinal vascular circulations, a unique arrangement of blood supplies in mammals (Fig. 1). Approximately 20% of blood flow is supplied to the retina from the central retinal artery through the microvascular capillaries, which is utilized to support neuronal activities in the inner retina [1]. Tight junctions between adjacent retinal endothelial cells form the inner blood–retina barrier or endothelial barrier that separates the inner retina from the blood. Choroidal vasculature provides approximately 80% of the blood circulation to the retina via the choriocapillaris for activities of outer retinal neurons (mainly photoreceptors). The retinal pigmented epithelium (RPE) is a monolayer of cells separating the fenestrated choriocapillaris (leaking capillaries) and the neuronal retina (Fig. 1). The tight junctions between the RPE cells form the outer blood–retina barrier or the RPE barrier. Tight junctions, adherent junctions, and facilitated and active transporters perform important barrier functions in the physiological processes of the retina, by transporting nutrients, water, and ions, and removing metabolic wastes. The RPE is the site of phagocytosis, a process that renews and removes approximately 10% of lipid-rich photoreceptor outer segment discs daily [2]. As docosahexaenoic acid is a major component of photoreceptor outer segment membrane and is required for important retinal functions [3], the RPE barrier plays a crucial role in the transport and recycle of fatty acids [4]. To support the energy needs for neuronal activities, the RPE barrier also plays an important role in transporting glucose and lactose through glucose and monocarboxylate transporters [5, 6]. Retinoids are essential components of visual cycle and are replenished from the blood by the RPE [7]. The removal of excessive water from the subretinal space to the choriocapillaris, which is critical to the health of the retina, is also regulated by the active transport systems in the RPE [8–10].
Fig. 1.
Simplified illustration of the mammalian retina. ONL outer nuclear layer. INL inner nuclear layer. Top right immunohistochemical staining for occludin in mouse RPE flat mount. Left hematoxylin and eosin-stained mouse retinal section
Diabetic retinopathy (DR) is a major complication of diabetes and a leading cause of blindness in working-age Americans. DR is traditionally regarded as a disorder of blood–retina barriers, and the leakage of blood content to the retina is a major pathological characteristic of the disease. While the breakdown of the endothelial barrier in DR has been investigated extensively, the concept of RPE barrier breakdown in the disease has not been widely acknowledged. As the blood content leaked through the RPE barrier causes excessive water influx to the retina, the breakdown of the RPE barrier is likely to play a causative role in the development of some forms of diabetic macular edema (DME), a major cause of vision loss in DR. As two detailed reviews about the RPE barrier and tight junctions were published early this year [11, 12], the focus of this article will be on the regulation, function, and analysis of the RPE barrier in diabetes, particularly those related to tight junctions. In this article, we will discuss the clinical evidences of the diabetes-induced RPE barrier breakdown, the alteration of the RPE in diabetes, the molecular and cellular mechanisms of RPE barrier breakdown, and the research tools for the analysis of RPE barrier leakage. Finally, we will discuss the methodology and potential applications of our recently developed fluorescent microscopic imaging for the diabetes- or ischemia-induced RPE barrier breakdown in rodents.
Overview of the RPE barrier
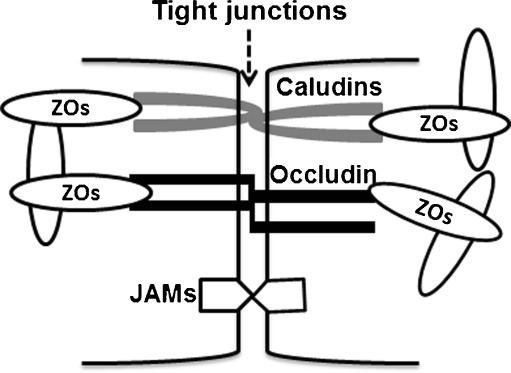
The RPE barrier comprises facilitated and active transporters, adherent junctions, and tight junctions. Tight junctions are elaborated network of transmembrane and cytosolic proteins that forms a selective semipermeable barrier between adjacent RPE cells. Figure 2 is a simplified illustration of the published core components of tight junctions in the RPE. Occludin, claudins, and junction adhesion molecules are major tight junction backbones [13–18] that are associated with zonula occludens-1 (ZO-1) and ZO-2 [16]. As the polarity of epithelial junctions is vital to their function [19], tight junctions are localized to the apical side of the RPE. The composition of tight junction proteins varies significantly among different species or at different developmental stages of a single species [11].
Fig. 2.
Simplified illustration of tight junctions in the RPE. ZOs zonula occludens, JAMs junction adhesion molecules
Cadherins and nectins, major adherent junction proteins in the RPE [20–22], are positioned basal to the tight junctions. They are anchored to the myosin through actin filaments that provide mechanical strength for their binding. E-cadherin plays a role in modulating the proper localization of other proteins, such as Na+ and K+-ATPase, in the RPE [22]. Nectin-1 has been shown to serve as the receptor for an invading virus [20, 21]. However, it is proposed that the major function of adherent junctions is to coordinate with tight junctions for modulating cell shape, polarity, and proliferation [11]. Likewise, gap junctions, located within the tight junctions in the RPE, may also assist tight junctions to exert their functions [11].
Clinical evidences of RPE barrier breakdown in diabetes
The presence of excessive albumin in the retina is a major characteristic of vascular leakage in DR and can be readily detected in diabetic human specimens and experimental animals by immunohistochemistry [23]. In a screening of 1,850 nonproliferative DR patients, 14 patients (19 eyes) were selected for a 4-year follow-up angiographic survey based on the following criteria: minimal microaneurysms and without clinically significant macular edema [24]. At the end of the survey, all 19 eyes appeared to have fluorescein leakage diffused from the RPE near the macular area, with no apparent cystic changes or cystoid macular edema, suggesting that the main lesions in these patients were in the RPE and the alteration of the RPE was responsible for the leakage [24]. These patients may have what is classified as DME without alterations in the morphology or thickness of the retinal layers [25]. Optical coherence tomography (OCT) images indicate that a significant portion of DME cases is exudative retinal detachment [26, 27]. In a survey of 78 eyes from 58 patients with DME, 24 (31%) demonstrated serous retinal detachment [28]. The largest group of DME patients is classified as diffusible DME as they show diffuse or localized thickening of the photoreceptor outer nuclear layer [25]. Diffusible DME patients with major pathological defects near the RPE area are likely to have an altered RPE barrier [25]. While the etiology for DME remains largely elusive, the inability for the retina to clear the leaked blood content and for the RPE to remove excessive water is likely a major cause of the disease [29]. It is understandable that the pathological changes in macular edema patients are not simply the consequence of water and protein leakage; however, it is safe to conclude that the RPE barrier dysfunction is involved in the development of the edema, such as exudative retinal detachment. Indeed, in our experimental modeling of retinal detachment with endotoxin-induced uveitis, the subretinal gap is filled with the macromolecules leaked through the RPE barrier (Fig. 3e). In summary, a significant amount of clinical evidences suggests that the RPE barrier dysfunction is a part of vascular complications in DR.
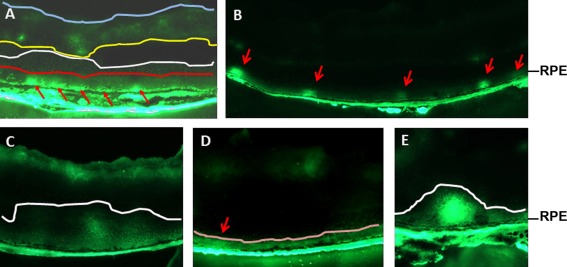
Fig. 3.
Imaging of the breakdown of the RPE barrier in retinal sections from mice injected with high molecular weight FITC–dextran intravenously. a One-year-old diabetic mice injected with 40 kDa FITC–dextran. b–d P17 ischemic mice injected with 10 kDa FITC–dextran. e Leakage of 10 kDa FITC–dextran in a mouse model of endotoxin-induced uveitis. Arrows severe break points, red line the outer limiting membrane, white line the boundary of the RPE barrier leakage, yellow line the boundary of the endothelial barrier leakage, blue line the inner limiting membrane, pink line the boundary of the evenly distributed RPE barrier leakage
Alterations of the RPE in diabetes
The tight junctions in both the endothelial and RPE barriers are compromised in DR [30–32]. Diabetes and hypoxia (occurred in late stage of DR) induce the breakdown of the RPE barrier [24, 32]. The ultrastructural changes in the RPE in early stage of diabetes can be readily detected in experimental animals by electron microscopy [33–35]. As a result of a diabetes-induced relaxation of the RPE barrier, an increased leakage of blood content can be observed in human diabetes and in diabetic animals [23, 30, 36]. An alteration of the RPE barrier in DR also affects the c wave of electroretinography, a signature of RPE integrity [37–39]. In a proteomic study comparing human donors of pre-DR and age-matched controls, 62% of the RPE proteins with significantly altered expression were those reported to have diabetes-induced change in nonretinal tissues, including proteins responsible for metabolism, chaperones in mitochondria, proteins in the cytosol and the endoplasmic reticulum (ER), and oxidative stress releasers [40]. The upregulation of proteins related to metabolism, ER stress, and oxidative stress indicates that the RPE may play a similar role as other tissues that respond to diabetic stress actively. Therefore, the RPE is an important tissue biochemically and mechanistically in DR.
Regulations of RPE barrier functions
Much of the work on the regulation of RPE barrier functions has been relied on the use of in vitro culture models, with a few exceptions. In this approach, the RPE barrier function is determined by the measurement of transepithelial resistance (TER). Hyperglycemia causes hypoxia, which in turn induces the upregulation of vascular endothelial growth factor (VEGF or VEGF-A) in the eye [31, 41]. VEGF is a major mediator of retinal vascular hyperpermeability in diabetes [42–44]. Overexpression of VEGF or its receptors is associated with DME [45–47]. Although the RPE is known to be important to the regulation of permeability for the RPE barrier [48], mechanistic studies on the function of the RPE barrier were carried out in RPE cultures supplemented with VEGF. However, in cultured ARPE-19 and RPE-51 cells, VEGF significantly upregulated ZO-1α+ and ZO-1α− mRNA and proteins, resulting in a significant increase in their TER [49]. In addition, the VEGF-treated cells exhibited an elevated ZO-1 membrane assembly [49]. This result suggests that VEGF may play a dual role in regulating the expression and posttranslational modification of tight junction proteins, as well as the assembly of tight junctions. Oxidative stress generated by tertiary butyl hydrogen peroxide stimulated VEGF-A and VEGF-C expression. The secretion of these cytokines to the apical side was higher than that to the basolateral side in polarized ARPE-19 cultures [50]. Co-cultures of bovine RPE cells with endothelial cells reduced the RPE barrier function significantly and led to a greater release of soluble VEGF into the conditioned media [51]. Neutralizing soluble VEGF with an antibody led to a partial recovery of barrier properties in the RPE and endothelial co-cultures [51]. In both ARPE-19 and primary porcine RPE cells, VEGF caused a significant reduction of TER, and this response was only observed following an apical VEGF administration [52]. The response to VEGF was blocked by a pretreatment with VEGF receptor 2 antagonists [52], suggesting that VEGF signaling through the apically oriented VEGF receptor 2, was responsible for the breakdown of the RPE barrier. In these RPE cultures, VEGF receptor 1 agonist, placental growth factor (PlGF), did not significantly alter the TER [52]. However, intravitreal injection of PlGF-1 relaxed the RPE tight junctions with subretinal fluid accumulation, retinal edema, and cytoplasmic translocation of junction proteins in rats [53]. This result suggests that PlGF-1 may play an opposite role in regulating tight junction assembly in the RPE barrier from that in endothelial barrier [54]. Pigment epithelium-derived factor, which was proposed to play an opposite role as that of VEGF in endothelial barrier breakdown, maintained the barrier function in ARPE-19 and primary porcine RPE cells by inhibiting VEGF receptor-2 signaling through gamma-secretase [55]. However, in claudin-rich fetal human RPE (fhRPE) cells, VEGF and anti-VEGF agents bevacizumab and ranibizumab demonstrated a minimal effect on the TER [56]. This result is supported by the observation that high glucose upregulated claudin mRNA expression in ARPE-19 cultures [57]. As the TER in fhRPE cells is eightfold of that in ARPE-19 cultures [58] and the roles of the RPE during fetal development may be substantially different from that in adulthood, the fhRPE probably needs a much tighter barrier. While high glucose did not alter the expression of occludin and ZO-1 mRNA significantly in ARPE-19 cultures [59], chronic oxidative stress relaxed the tight junctions and increased the permeability in the monolayer of ARPE-19 cells [60]. These seemingly conflicted results may be derived from the differences in experimental designs. It is also important to keep in mind that variations in the cells utilized in their studies, as demonstrated by the comparison of fhRPE and ARPE-19 cells recently [58], could also cause variation in experimental outcomes. Since the RPE can only be truly functional within its own cellular architecture, cautions must be taken in interpreting the data obtained in vitro. Therefore, we verified the tight junction integrity in ischemic and diabetic mice, which was shown to have a significant upregulation of their retinal VEGF [31, 41]. The loss of tight junction integrity in the RPE, as judged by immunohistochemical staining of occludin, was apparent in ischemic and diabetic mice [32].
Early studies showed that serum inhibited the formation of tight junctions and reduced the expression of ZO-1 in RPE cultures derived from the Royal College of Surgeons’ rats [61, 62]. In ARPE-19 cells grown in high glucose, erythropoietin was shown to be protective against the increased RPE barrier permeability through its downstream targets Janus kinase 2 and phosphoinositide 3-kinase [63]. Interleukin (IL) family cytokines, such as, IL6, IL-17A, and IL17F, significantly decreased the TER of the ARPE-19 monolayer and increased the rate of diffusion for fluorescein isothiocyanate (FITC)–dextran through the upregulation of chemokines [64, 65]. Neutrophils may also play a role in regulating the function of the RPE barrier. Basolateral incubation of bovine RPE–choroid explants with neutrophils decreased the expression of occludin and ZO-1 and significantly increased the permeability of the explants [66]. This effect was reversed by an antibody against matrix metallopeptidase 9 (MMP-9), suggesting that the neutrophil-derived MMP-9 may play an important role in disrupting the integrity of the RPE barrier [66].
Fenofibrate is a drug against cardiovascular risk in diabetes through the reduction of cholesterol by downregulating the levels of low-density lipoprotein, very low-density lipoprotein, and triglyceride and by upregulating the level of high-density lipoprotein level [67]. In ARPE-19 cells grown in high-glucose media, fenofibrate upregulated the expression of fibronectin and collagen IV, basement membrane components known to cause endothelial barrier breakdown [68]. Fenofibrate also reduced the IL-1β-induced RPE barrier permeability in ARPE-19 cultures grown in high-glucose media by suppressing adenosine monophosphate-activated protein kinase [68, 69]. Interestingly, fenofibrate may have a general beneficial effect on reducing high glucose- or hypoxia-induced oxidative and ER stresses in the RPE by reducing stress-mediated signaling and by inducing autophagy and survival pathways [70].
Methods for in vivo analysis of RPE barrier breakdown
Horseradish peroxidase, which can be detected by incubating with diaminobenzidine, has been used for ultrastructural tracing for the breakdown of the RPE barrier, the lesion in the outer retina and the RPE, and the alteration of tight junctions in diabetic rats [71], in pigs subjected to experimental blunt eye injury [72], and in rats with phototoxic retinopathy [73]. This qualitative method is particularly useful for identifying the lesions and for localizing the RPE barrier leakage. The leakage of the RPE barrier can also be measured by immunohistochemistry for albumin, a major parameter of vascular leakage in DR [23]. However, this method usually measures the cumulative effect of vascular leakage. As the blood content leakage through the RPE barrier migrates towards vitreous ([29], also see Fig. 3c in our model), the albumin leaked through the RPE barrier moves to the inner retina and vitreous and mixes with the blood content that leaked through the endothelial barrier. Therefore, it is difficult to assess the relative quantity of the RPE barrier-specific leakage with the method.
Fluorescent angiography-based technology is very useful for detecting the diabetes-induced vascular leakage in humans and experimental animals. As both the RPE and endothelial barriers are compromised in DR, it is challenging to distinguish the diabetes-induced RPE barrier leakage with this technology. However, this technology can be used to detect the leakage from the RPE barrier if there is no interference from the endothelial barrier, such as that in central serous (CSC). In CSC only the RPE is compromised and the RPE barrier leakage can be clearly detected by fluorescent angiography in humans and experimental animals [74, 75]. Therefore, fluorescent angiography can be used to identify the RPE barrier leakage in patients of nonproliferative DR, as discussed earlier [24]. This type of technology has also been used to detect the RPE barrier-specific leakage in short-term diabetic rats with no apparent leakage from the endothelial barrier at the time [71]. The development of OCT technology has largely enhanced our ability to perform diagnosis for DR patients, particularly for those with macular edema. A combination of OCT with fluorescent angiography is more advantageous for the classification of DR patients [76]. While OCT is a diagnostic tool for lesions, it is not ideal for the measurement of RPE barrier leakage. To our knowledge, fluorescent angiography and OCT has not been used extensively in analyzing the breakdown of the RPE barrier in experimental animals.
Fluorescent microscopic imaging of RPE barrier breakdown in rodents
To evaluate the significance of the diabetes-induced RPE barrier breakdown and to establish a quantitative assay for the RPE barrier-specific leakage, we recently developed a method to identify macromolecules leaked through the RPE barrier de novo in diabetic and ischemic rodents [32]. Immediately after injecting 40 kDa FITC–dextran intravenously to mice 12 months after the onset of diabetes, we detected strong fluorescent signals next to the RPE (Fig. 3a) [32], which was not present in the age-matched controls. The intense fluorescence suggests the presence of severe break points in the nearby RPE. Since the retina in late stage of diabetes is hypoxic, we took advantage of ischemic animals obtained with the oxygen-induced retinopathy (OIR) model [77], which allows us to generate our experimental animals in a very short time. By placing newborn mice in 75% oxygen from postnatal day 7 (P7) to P12 and in room air from P12 to P17, we generated ischemic mice. These ischemic mice demonstrated the breakdown of the RPE barrier similarly as that in diabetic animals (Fig. 3b). When we performed our imaging assay at P17 using sagitally cut serial cryosections (60 μm apart), we were able to demonstrate that the relative frequencies of severe break points in the RPE barrier were inversely proportional to the size of FITC–dextran (10, 20, 40, or 70 kDa) [32]. In both diabetic and ischemic mice, we were able to demonstrate the breakdown of tight junctions clearly by immunohistochemistry for occludin [32].
By using the fluorescent microscopic imaging assay, the contribution of the RPE barrier breakdown to overall vascular leakage in diabetic and ischemic rodents can also be evaluated [32]. As the FITC–dextran leaked through the RPE barrier was distinguishable from that of the endothelial barrier shortly after dissecting the eyeballs in the assay, the ratio of fluorescent intensity between the RPE barrier-specific leakage (area between the bottom white line and the RPE in Fig. 3a) and the endothelial barrier-specific leakage (area between the yellow line and the blue line in Fig. 3a) represented the severity of the RPE barrier-specific leakage. In our hands, the relative ratio of 10 kDa FITC–dextran leaked through the RPE barrier vs endothelial barrier was approximately 1:2.5 in diabetic mice. As the total fluorescence of the RPE barrier-specific leakage was contributed by fluorescence of severe break points, as well as by background fluorescence, sometimes a lower level of the RPE barrier-specific leakage occurs evenly across the whole retina (area between the pink line and the RPE in Fig. 3d). We are actively investigating the size limitation and cellular mechanisms of this background leakage. As our quantification did not exclude the bright fluorescent spots that were caused by intact vessels (Fig. 3a), the significance of endothelial barrier-specific leakage was somewhat inflated. However, the fluorescence in the vitreous might be contributed by the endothelial leakage, which could discount the extent of the endothelial barrier leakage. More importantly, the fluorescence in retinal sections represents the leakage between the time of injecting FITC–dextran and animal death. The actual rate may depend on the flow/pressure of endothelial capillaries and fenestrated choriocapillaris. Therefore, this quantification can only be considered as semiquantitative. Nevertheless, the ratio of the RPE leakage vs the endothelial leakage was comparable to that demonstrated in diabetic rats with fluorescein in a confocal fluorescence microscopy [78].
As predicted, the leaked FITC–dextran diffused towards vitreous if the retinal sections were obtained from the mice kept alive for additional 20 min after the injection of FITC–dextran (Fig. 3c). This result supports the notion that the blood content leaked through the RPE barrier is cleared through the vitreous [41, 79–81]. As both the RPE and endothelial barriers are interconnected [79–81] and the leakage from both barriers are mixed together in the fluidal retina, current technology for fluorescent fundus angiography may not be suitable for detecting the RPE barrier-specific leakage. This is, in our view, a major reason that the RPE barrier breakdown has been underappreciated, which results in a slow progress in the research in the biology and diseases of the RPE barrier breakdown.
Technical highlights of fluorescent microscopy for RPE barrier leakage
Our assay for the analysis of the RPE barrier-specific leakage is based on the imaging of fluorescent signals in cryosections. The detailed method is listed in our original publication [32]. While there are no major technical challenges in this assay, two cautionary measures must be taken to make it successful. First, the experimental eyes are not fixed and the eyeballs are embedded directly to the cryostat chuck with optimal cutting temperature media 10 min before sectioning. These procedures significantly reduce the diffusion of fluorescent signals. Second, maximal caution is needed to prevent the disturbance of fluorescent signals, including avoiding the overlap of eye slices during sectioning, keeping slide “dry” (no excessive moisture), and imaging immediately after sectioning. In summary, any actions that disturb/contaminate the actual fluorescence in the slides should be prevented. In our assay with 10 kDa FITC–dextran, we occasionally detected background fluorescence next to the RPE in the whole retina (fluorescence between the pink line and the RPE in Fig. 3d), which was likely resulted from many smaller break points. We are actively investigating this possibility. The presence of this background fluorescence (Fig. 3d) affects our ability to detect severe break points. To circumvent this problem, it is more advantageous to use 20 kDa FITC–dextran if the assay is intended for testing the effect of a drug on the RPE barrier.
We intentionally used 1-year-old diabetic mice and 9-month-old diabetic rats to establish our imaging assay [32], as we did not know the earliest time that severe break points of the RPE barrier can be observed in diabetic animals. We are currently optimizing our assay. The use of diabetic rats may shorten the time required for an experiment. However, the retina of late-stage DR is very similar to that in ischemic mice generated with the OIR [31, 41]. The use of OIR model is likely to reduce the time span for some experiments. In addition, supplying drugs/agents to OIR animals is considerably convenient, as it usually requires to supply a drug once, either intravitreally or intraperitoneally, whereas investigating drug effects in diabetes requires a much longer time, which is particularly challenging if ocular administration is the route. Finally, the time span required for a study with OIR model is shorter than that for most in vitro assays with RPE cells, which takes weeks of preparation for setting up the cultures.
Significance and potential applications of fluorescent microscopy for RPE barrier leakage
Alterations of the RPE barrier in diabetes and ischemia are not a new discovery [23, 24, 30, 33, 36–40, 82]. However, the extent and significance of the diabetes-induced RPE barrier breakdown have not been widely acknowledged. This situation is largely attributed to a lack of proper methodology for measuring the RPE barrier-specific leakage, due to the interference of the endothelial barrier leakage, as illustrated in our study ([32], also see Fig. 3c). The indirect measurement of albumin leakage and the direct assessment of leakage with low molecular weight compounds, such as fluorescein, are not sufficient to convince the field that the RPE barrier breakdown is a serious matter [23, 71]. As seeing is believing, we developed a semiquantitative assay based on fluorescent microscopic imaging of high molecular weight FITC–dextran leaked through the RPE barrier de novo, which is important to the understanding of the pathogenic mechanisms for DR. Our assay allows us to estimate the degree of the RPE barrier leakage semiquantitatively with the number of severely damaged break points in the RPE barrier in diabetic and ischemic rodents. The measurement of the relative ratio of RPE barrier-specific leakage in the retina demonstrates the contribution of RPE barrier leakage to the overall pathology in a particular disease model. In addition, if an assay can be performed using the ischemic model generated with OIR, one can address a question related to the RPE barrier function within a few days, which is faster than that from in vitro experiments. In short, this assay will allow us to measure the effect of manipulating the RPE barrier in preclinical investigations for mechanism, diagnosis, and treatment of the diabetes- or ischemia-induced RPE barrier breakdown. We are currently using this assay to identify agents that can be used to increase the RPE barrier permeability for mechanistic studies, as well as agents that can be used to treat the RPE barrier-specific leakage. Finally, this assay is a blueprint for investigating the RPE barrier-specific leakage in other mammals that are more closely related to humans.
The retina needs both the choroidal and endothelial circulations to keep a homeostatic microenvironment. Under pathological conditions, such as that in diabetes and ischemia, the balance is lost. The abnormalities in both endothelial and RPE barriers under these pathological conditions are a reflection of an internal regulatory mechanisms to maintain such a balance. The RPE barrier breakdown results in the influx of blood content, which is capable of causing the accumulation of fluid in the subretinal space (edema) under some pathological conditions. Eventually, it leads to exudative retinal detachment, as demonstrated in a model of endotoxin-induced uveitis (Fig. 3e). Our microscopic assay provides a method to estimate the severities of RPE barrier breakdown and establishes a cellular mechanism for some forms of DME, nonproliferative DR with RPE barrier leakage [24], uveitis, retinal detachment in preeclampsia, and soft drusen that causes wet age-related macular degeneration. Our method can be used for preclinical research related to these diseases. Since the subretinal fluids resulted from experimental retinal detachments in rabbits were reabsorbed very quickly [83], it is our understanding that the loss of the RPE junction integrity is unlikely the only reason that causes the pathological changes in these diseases. A major focus of our laboratory is to investigate the mechanisms of RPE barrier breakdown beyond the alterations of the RPE barrier.
Concluding remarks
The major message conveyed in this article is that the RPE barrier breakdown plays a causative role in DR, particularly for some forms of DME. We recently showed that the RPE barrier breakdown contributed to the overall vascular leakage substantially in diabetic rodents [32]. At this time, the extent and significance of the diabetes-induced RPE barrier breakdown in humans are not clear. However, treatment of the RPE barrier breakdown should be considered as an intervention in DR for the following reason. As the endothelial and RPE barrier are interconnected to the fluidal retina, the leakage through both barriers are additive to the overall insults. In Müller cell-specific VEGF knockout mice that reduced the overall retinal VEGF to approximately 50% of that in wild-type controls, the diabetes-induced endothelial barrier breakdown was reduced dramatically [31]. It is our view that reducing the overall insults under a “pathological threshold” is essential for keeping the disease under the control. In a preliminary study, genetic disruption of VEGF signaling in the mouse RPE caused a measurable reduction of overall diabetes-induced vascular leakage and inflammation (Le Y. Z. et al., unpublished observations). The beneficial effect of anti-VEGF therapies on the treatment of DME certainly supports our view [84].
Although many achievements have been made in the biology of the RPE barrier, a lack of progress in developing the methodology for clinical diagnosis and for research in the biology of the RPE barrier certainly makes it difficult to advance the field in a more significant way. As a result, not as many experiments related to the RPE barrier were carried out in in vivo settings. While our newly developed methodology for detecting the RPE barrier leakage is not ideal [32], it provides a way to assess in vivo function of the RPE barrier in a semiquantitative fashion. We, and certainly many other laboratories, should take advantage of the methodology and address important questions related to the RPE barrier function in vivo. As for the future of this fluorescent microscopic assay, it is our view that new technology is needed for imaging the RPE barrier-specific leakage in experimental animals, and perhaps in humans. We are actively working with bioengineers to achieve this goal. Recent development in tissue-specific gene expression tools for the RPE and animal models of RPE-specific gene knockout could potentially be used to manipulate the RPE barrier specifically [85–88], which is another avenue that greater progress can be made for in vivo analysis of the RPE barrier function. In conjunction with a combination of approaches in vivo and in vitro, we are certain that the significance of the RPE barrier breakdown in DR, as well as that in other retinal diseases, will be recognized appropriately in the near future.
Acknowledgments
We thank Dr. Gennadiy Moiseyev for the critical reading of this manuscript. Our research is supported by NIH grants R01EY20900, P20RR17703, P20RR024215, and P30EY21725, American Diabetes Association grant 1-10-BS-94, Beckman Initiative for Macular Research Grant 1003, and Oklahoma Center for the Advancement of Science and Technology contract HR09-058.
References
- 1.Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975;55:383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- 2.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benolken RM, Anderson RE, Wheeler TG. Membrane fatty acids associated with the electrical response in visual excitation. Science. 1973;182:1253–1254. doi: 10.1126/science.182.4118.1253. [DOI] [PubMed] [Google Scholar]
- 4.Gordon WC, Rodriguez de Turco EB, Bazan NG. Retinal pigment epithelial cells play a central role in the conservation of docosahexaenoic acid by photoreceptor cells after shedding and phagocytosis. Curr Eye Res. 1992;11:73–83. doi: 10.3109/02713689209069169. [DOI] [PubMed] [Google Scholar]
- 5.Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 6.Kumagai AK, Glasgow BJ, Pardridge WM. GLUT1 glucose transporter expression in the diabetic and nondiabetic human eye. Invest Ophthalmol Vis Sci. 1994;35:2887–2894. [PubMed] [Google Scholar]
- 7.Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 8.Bialek S, Miller SS. K+ and Cl− transport mechanisms in bovine pigment epithelium that could modulate subretinal space volume and composition. J Physiol. 1994;475:401–417. doi: 10.1113/jphysiol.1994.sp020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamann S. Molecular mechanisms of water transport in the eye. Int Rev Cytol. 2002;215:395–431. doi: 10.1016/S0074-7696(02)15016-9. [DOI] [PubMed] [Google Scholar]
- 10.Simo R, Villarroel M, Corraliza L, Hernandez C, Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier—implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzolo LJ, Peng S, Luo Y, Xiao W. Integration of tight junctions and claudins with the barrier functions of the retinal pigment epithelium. Prog Retin Eye Res. 2011;30:296–323. doi: 10.1016/j.preteyeres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Meth Mol Biol. 2011;686:133–148. doi: 10.1007/978-1-60761-938-3_5. [DOI] [PubMed] [Google Scholar]
- 13.Konari K, Sawada N, Zhong Y, Isomura H, Nakagawa T, Mori M. Development of the blood-retinal barrier in vitro: formation of tight junctions as revealed by occludin and ZO-1 correlates with the barrier function of chick retinal pigment epithelial cells. Exp Eye Res. 1995;61:99–108. doi: 10.1016/S0014-4835(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 14.Rizzolo LJ. Polarity and the development of the outer blood-retinal barrier. Histol Histopathol. 1997;12:1057–1067. [PubMed] [Google Scholar]
- 15.Kojima S, Rahner C, Peng S, Rizzolo LJ. Claudin 5 is transiently expressed during the development of the retinal pigment epithelium. J Membr Biol. 2002;186:81–88. doi: 10.1007/s00232-001-0137-7. [DOI] [PubMed] [Google Scholar]
- 16.Luo Y, Fukuhara M, Weitzman M, Rizzolo LJ. Expression of JAM-A, AF-6, PAR-3 and PAR-6 during the assembly and remodeling of RPE tight junctions. Brain Res. 2006;1110:55–63. doi: 10.1016/j.brainres.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 17.Daniele LL, Adams RH, Durante DE, Pugh EN, Jr, Philp NJ. Novel distribution of junctional adhesion molecule-C in the neural retina and retinal pigment epithelium. J Comp Neurol. 2007;505:166–176. doi: 10.1002/cne.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Economopoulou M, Hammer J, Wang F, Fariss R, Maminishkis A, Miller SS. Expression, localization, and function of junctional adhesion molecule-C (JAM-C) in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50:1454–1463. doi: 10.1167/iovs.08-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh SW. The chick retinal pigment epithelium grown on permeable support demonstrates functional polarity. Exp Cell Res. 1989;181:331–347. doi: 10.1016/0014-4827(89)90092-X. [DOI] [PubMed] [Google Scholar]
- 20.Shukla SY, Singh YK, Shukla D. Role of nectin-1, HVEM, and PILR-alpha in HSV-2 entry into human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:2878–2887. doi: 10.1167/iovs.08-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiwari V, Oh MJ, Kovacs M, Shukla SY, Valyi-Nagy T, Shukla D. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. FEBS J. 2008;275:5272–5285. doi: 10.1111/j.1742-4658.2008.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrs JA, Andersson-Fisone C, Jeong MC, Cohen-Gould L, Zurzolo C, Nabi IR, Rodriguez-Boulan E, Nelson WJ. Plasticity in epithelial cell phenotype: modulation by expression of different cadherin cell adhesion molecules. J Cell Biol. 1995;129:507–519. doi: 10.1083/jcb.129.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinores SA, Gadegbeku C, Campochiaro PA, Green WR. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am J Pathol. 1989;134:231–235. [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberger D, Fink-Cohen S, Gaton DD, Priel E, Yassur Y. Non-retinovascular leakage in diabetic maculopathy. Br J Ophthalmol. 1995;79:728–731. doi: 10.1136/bjo.79.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soliman W, Sander B, Jorgensen TM. Enhanced optical coherence patterns of diabetic macular oedema and their correlation with the pathophysiology. Acta Ophthalmol Scand. 2007;85:613–617. doi: 10.1111/j.1600-0420.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 26.Gaucher D, Sebah C, Erginay A, Haouchine B, Tadayoni R, Gaudric A, Massin P. Optical coherence tomography features during the evolution of serous retinal detachment in patients with diabetic macular edema. Am J Ophthalmol. 2008;145:289–296. doi: 10.1016/j.ajo.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Voo I, Mavrofrides EC, Puliafito CA. Clinical applications of optical coherence tomography for the diagnosis and management of macular diseases. Ophthalmol Clin North Am. 2004;17:21–31. doi: 10.1016/j.ohc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Ozdemir H, Karacorlu M, Karacorlu S. Serous macular detachment in diabetic cystoid macular oedema. Acta Ophthalmol Scand. 2005;83:63–66. doi: 10.1111/j.1600-0420.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 29.Marmor MF. Mechanisms of fluid accumulation in retinal edema. Doc Ophthalmol. 1999;97:239–249. doi: 10.1023/A:1002192829817. [DOI] [PubMed] [Google Scholar]
- 30.Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol. 1999;97:217–228. doi: 10.1023/A:1002136712070. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Xu X, Elliott MH, Zhu M, Le Y. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–3005. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52:2160–2164. doi: 10.1167/iovs.10-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aizu Y, Oyanagi K, Hu J, Nakagawa H. Degeneration of retinal neuronal processes and pigment epithelium in the early stage of the streptozotocin-diabetic rats. Neuropathology. 2002;22:161–170. doi: 10.1046/j.1440-1789.2002.00439.x. [DOI] [PubMed] [Google Scholar]
- 34.Vinores SA, Niel E, Swerdloff JL, Campochiaro PA. Electron microscopic immunocytochemical demonstration of blood-retinal barrier breakdown in human diabetics and its association with aldose reductase in retinal vascular endothelium and retinal pigment epithelium. Histochem J. 1993;25:648–663. doi: 10.1007/BF00157879. [DOI] [PubMed] [Google Scholar]
- 35.Vinores SA, Niel E, Swerdloff JL, Campochiaro PA. Electron microscopic immunocytochemical evidence for the mechanism of blood-retinal barrier breakdown in galactosemic rats and its association with aldose reductase expression and inhibition. Exp Eye Res. 1993;57:723–735. doi: 10.1006/exer.1993.1180. [DOI] [PubMed] [Google Scholar]
- 36.Kirber WM, Nichols CW, Grimes PA, Winegrad AI, Laties AM. A permeability defect of the retinal pigment epithelium. Occurrence in early streptozocin diabetes. Arch Ophthalmol. 1980;98:725–728. doi: 10.1001/archopht.1980.01020030719015. [DOI] [PubMed] [Google Scholar]
- 37.Pautler EL, Ennis SR. The effect of induced diabetes on the electroretinogram components of the pigmented rat. Invest Ophthalmol Vis Sci. 1980;19:702–705. [PubMed] [Google Scholar]
- 38.MacGregor LC, Matschinsky FM. Experimental diabetes mellitus impairs the function of the retinal pigmented epithelium. Metabolism. 1986;35:28–34. doi: 10.1016/0026-0495(86)90184-8. [DOI] [PubMed] [Google Scholar]
- 39.Rimmer T, Linsenmeier RA. Resistance of diabetic rat electroretinogram to hypoxemia. Invest Ophthalmol Vis Sci. 1993;34:3246–3252. [PubMed] [Google Scholar]
- 40.Decanini A, Karunadharma PR, Nordgaard CL, Feng X, Olsen TW, Ferrington DA. Human retinal pigment epithelium proteome changes in early diabetes. Diabetologia. 2008;51:1051–1061. doi: 10.1007/s00125-008-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y, Le YZ. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219:446–454. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 42.Aiello LP, Wong JS. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int Suppl. 2000;77:S113–S119. doi: 10.1046/j.1523-1755.2000.07718.x. [DOI] [PubMed] [Google Scholar]
- 43.Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest. 1996;74:819–825. [PubMed] [Google Scholar]
- 44.Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–406. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- 45.Mousa SA, Lorelli W, Campochiaro PA. Role of hypoxia and extracellular matrix-integrin binding in the modulation of angiogenic growth factors secretion by retinal pigmented epithelial cells. J Cell Biochem. 1999;74:135–143. doi: 10.1002/(SICI)1097-4644(19990701)74:1<135::AID-JCB15>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690–1696. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 47.Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- 48.Korte GE, Cushin S, Delman N. Permeability of regenerating and atrophic choriocapillaris in the rabbit. Acta Anat (Basel) 1989;134:144–150. doi: 10.1159/000146679. [DOI] [PubMed] [Google Scholar]
- 49.Ghassemifar R, Lai CM, Rakoczy PE. VEGF differentially regulates transcription and translation of ZO-1alpha+ and ZO-1alpha- and mediates trans-epithelial resistance in cultured endothelial and epithelial cells. Cell Tissue Res. 2006;323:117–125. doi: 10.1007/s00441-005-0046-7. [DOI] [PubMed] [Google Scholar]
- 50.Kannan R, Zhang N, Sreekumar PG, Spee CK, Rodriguez A, Barron E, Hinton DR. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol Vis. 2006;12:1649–1659. [PubMed] [Google Scholar]
- 51.Hartnett ME, Lappas A, Darland D, McColm JR, Lovejoy S, D'Amore PA. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 2003;77:593–599. doi: 10.1016/S0014-4835(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 52.Ablonczy Z, Crosson CE. VEGF modulation of retinal pigment epithelium resistance. Exp Eye Res. 2007;85:762–771. doi: 10.1016/j.exer.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyamoto N, Kozak Y, Jeanny JC, Glotin A, Mascarelli F, Massin P, BenEzra D, Behar-Cohen F. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50:461–470. doi: 10.1007/s00125-006-0539-2. [DOI] [PubMed] [Google Scholar]
- 54.Cai J, Wu L, Qi X, Shaw L, Li Calzi S, Caballero S, Jiang WG, Vinores SA, Antonetti D, Ahmed A, et al. Placenta growth factor-1 exerts time-dependent stabilization of adherens junctions following VEGF-induced vascular permeability. PLoS One. 2011;6:e18076. doi: 10.1371/journal.pone.0018076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Ablonczy Z, Prakasam A, Fant J, Fauq A, Crosson C, Sambamurti K. Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through gamma-secretase. J Biol Chem. 2009;284:30177–30186. doi: 10.1074/jbc.M109.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng S, Adelman RA, Rizzolo LJ. Minimal effects of VEGF and anti-VEGF drugs on the permeability or selectivity of RPE tight junctions. Invest Ophthalmol Vis Sci. 2010;51:3216–3225. doi: 10.1167/iovs.09-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villarroel M, Garcia-Ramirez M, Corraliza L, Hernandez C, Simo R. Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Exp Eye Res. 2009;89:913–920. doi: 10.1016/j.exer.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 58.Ablonczy Z, Dahrouj M, Tang PH, Liu Y, Sambamurti K, Marmorstein AD, Crosson CE. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci. 2011;52(12):8614–8620. doi: 10.1167/iovs.11-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villarroel M, Garcia-Ramirez M, Corraliza L, Hernandez C, Simo R. High glucose concentration leads to differential expression of tight junction proteins in human retinal pigment epithelial cells. Endocrinol Nutr. 2009;56:53–58. doi: 10.1016/S1575-0922(09)70552-2. [DOI] [PubMed] [Google Scholar]
- 60.Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:675–684. doi: 10.1167/iovs.03-0351. [DOI] [PubMed] [Google Scholar]
- 61.Chang C, Wang X, Caldwell RB. Serum opens tight junctions and reduces ZO-1 protein in retinal epithelial cells. J Neurochem. 1997;69:859–867. doi: 10.1046/j.1471-4159.1997.69020859.x. [DOI] [PubMed] [Google Scholar]
- 62.Chang CW, Ye L, Defoe DM, Caldwell RB. Serum inhibits tight junction formation in cultured pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997;38:1082–1093. [PubMed] [Google Scholar]
- 63.Garcia-Ramirez M, Hernandez C, Ruiz-Meana M, Villarroel M, Corraliza L, Garcia-Dorado D, Simo R. Erythropoietin protects retinal pigment epithelial cells against the increase of permeability induced by diabetic conditions: essential role of JAK2/PI3K signaling. Cell Signal. 2011;23:1596–1602. doi: 10.1016/j.cellsig.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y, Yang P, Li F, Kijlstra A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS One. 2011;6:e18139. doi: 10.1371/journal.pone.0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abe T, Sugano E, Saigo Y, Tamai M. Interleukin-1beta and barrier function of retinal pigment epithelial cells (ARPE-19): aberrant expression of junctional complex molecules. Invest Ophthalmol Vis Sci. 2003;44:4097–4104. doi: 10.1167/iovs.02-0867. [DOI] [PubMed] [Google Scholar]
- 66.Zhou J, He S, Zhang N, Spee C, Zhou P, Ryan SJ, Kannan R, Hinton DR. Neutrophils compromise retinal pigment epithelial barrier integrity. J Biomed Biotechnol. 2010;2010:289360. doi: 10.1155/2010/289360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hiukka A, Leinonen E, Jauhiainen M, Sundvall J, Ehnholm C, Keech AC, Taskinen MR. Long-term effects of fenofibrate on VLDL and HDL subspecies in participants with type 2 diabetes mellitus. Diabetologia. 2007;50:2067–2075. doi: 10.1007/s00125-007-0751-8. [DOI] [PubMed] [Google Scholar]
- 68.Trudeau K, Roy S, Guo W, Hernandez C, Villarroel M, Simo R, Roy S. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest Ophthalmol Vis Sci. 2011;52:6348–6354. doi: 10.1167/iovs.11-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villarroel M, Garcia-Ramirez M, Corraliza L, Hernandez C, Simo R. Fenofibric acid prevents retinal pigment epithelium disruption induced by interleukin-1beta by suppressing AMP-activated protein kinase (AMPK) activation. Diabetologia. 2011;54:1543–1553. doi: 10.1007/s00125-011-2089-5. [DOI] [PubMed] [Google Scholar]
- 70.Miranda S, Gonzalez-Rodriguez A, Garcia-Ramirez M, Revuelta-Cervantes J, Hernandez C, Simo R, Valverde AM. Beneficial effects of fenofibrate in retinal pigment epithelium by the modulation of stress and survival signaling under diabetic conditions. J Cell Physiol. 2011. doi:10.1002/jcp.22970. [DOI] [PubMed]
- 71.Tso MO, Cunha-Vaz JG, Shih CY, Jones CW. Clinicopathologic study of blood-retinal barrier in experimental diabetes mellitus. Arch Ophthalmol. 1980;98:2032–2040. doi: 10.1001/archopht.1980.01020040884020. [DOI] [PubMed] [Google Scholar]
- 72.Gregor Z, Ryan SJ. Blood-retinal barrier after blunt trauma to the eye. Graefe’s Arch Clinical Exp Ophthalmol. 1982;219:205–208. doi: 10.1007/BF00231236. [DOI] [PubMed] [Google Scholar]
- 73.Korte GE, Bellhorn RW, Burns MS. Ultrastructure of blood-retinal barrier permeability in rat phototoxic retinopathy. Invest Ophthalmol Vis Sci. 1983;24:962–971. [PubMed] [Google Scholar]
- 74.Uyama M, Matsunaga H, Matsubara T, Fukushima I, Takahashi K, Nishimura T. Indocyanine green angiography and pathophysiology of multifocal posterior pigment epitheliopathy. Retina. 1999;19:12–21. doi: 10.1097/00006982-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Yoshioka H, Katsume Y, Ishibashi R. Experimental central serous chorioretinopathy. II: further clinical findings. Kurume Med J. 1981;28:189–196. doi: 10.2739/kurumemedj.28.189. [DOI] [PubMed] [Google Scholar]
- 76.Soliman W, Sander B, Hasler PW, Larsen M. Correlation between intraretinal changes in diabetic macular oedema seen in fluorescein angiography and optical coherence tomography. Acta Ophthalmol. 2008;86:34–39. doi: 10.1111/j.1600-0420.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- 77.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 78.Do carmo A, Ramos P, Reis A, Proenca R, Cunha-vaz JG. Breakdown of the inner and outer blood retinal barrier in streptozotocin-induced diabetes. Exp Eye Res. 1998;67:569–575. doi: 10.1006/exer.1998.0546. [DOI] [PubMed] [Google Scholar]
- 79.Googe JM, Hirose T, Apple DJ, Melgen S. Vitreous hemorrhage secondary to age-related macular degeneration. Surv Ophthalmol. 1987;32:123–130. doi: 10.1016/0039-6257(87)90104-4. [DOI] [PubMed] [Google Scholar]
- 80.Baba F, Jarrett WH, 2nd, Harbin TS, Jr, Fine SL, Michels RG, Schachat AP, Green WR. Massive hemorrhage complicating age-related macular degeneration. Clinicopathologic correlation and role of anticoagulants. Ophthalmology. 1986;93:1581–1592. doi: 10.1016/s0161-6420(86)33540-1. [DOI] [PubMed] [Google Scholar]
- 81.Takeuchi A, Kricorian G, Marmor MF. Albumin movement out of the subretinal space after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:1298–1305. [PubMed] [Google Scholar]
- 82.Aizu Y, Katayama H, Takahama S, Hu J, Nakagawa H, Oyanagi K. Topical instillation of ciliary neurotrophic factor inhibits retinal degeneration in streptozotocin-induced diabetic rats. Neuroreport. 2003;14:2067–2071. doi: 10.1097/00001756-200311140-00012. [DOI] [PubMed] [Google Scholar]
- 83.Negi A, Marmor MF. Experimental serous retinal detachment and focal pigment epithelial damage. Arch Ophthalmol. 1984;102:445–449. doi: 10.1001/archopht.1984.01040030359038. [DOI] [PubMed] [Google Scholar]
- 84.Nicholson BP, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2011;248:915–930. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 85.Le YZ. Conditional gene targeting: dissecting the cellular mechanisms of retinal degenerations. J Ophthalmol. 2011;2011:806783. doi: 10.1155/2011/806783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Le YZ, Zheng W, Rao PC, Zheng L, Anderson RE, Esumi N, Zack DJ, Zhu M. Inducible expression of cre recombinase in the retinal pigmented epithelium. Invest Ophthalmol Vis Sci. 2008;49:1248–1253. doi: 10.1167/iovs.07-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le YZ, Bai Y, Zhu M, Zheng L. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J Neurochem. 2010;112:1584–1592. doi: 10.1111/j.1471-4159.2010.06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iacovelli J, Zhao C, Wolkow N, Veldman P, Gollomp K, Ojha P, Lukinova N, King A, Feiner L, Esumi N, et al. Generation of Cre transgenic mice with postnatal RPE-specific ocular expression. Invest Ophthalmol Vis Sci. 2011;52:1378–1383. doi: 10.1167/iovs.10-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]