Abstract
The endoplasmic reticulum (ER) is the primary cellular compartment where proteins are synthesized and modified before they can be transported to their destination. Dysfunction of the ER impairs protein homeostasis and leads to the accumulation of misfolded/unfolded proteins in the ER, or ER stress. While it has long been recognized that ER stress is a major cause of conformational disorders, such as Alzheimer's disease, Huntington's disease, certain types of cancer, and type 2 diabetes, recent evidence suggests that ER stress is also implicated in many chronic inflammatory diseases. These diseases include irritable bowel syndrome, atherosclerosis, diabetic complications, and many others. Diabetic retinopathy is a common microvascular complication of diabetes, characterized by chronic inflammation, progressive damage to retinal vascular and neuronal cells, vascular leakage, and abnormal blood vessel growth (neovascularization). In this review, we discuss the role and mechanisms of ER stress in retinal inflammation and vascular damage in diabetic retinopathy.
Keywords: ER stress, Unfolded protein response, Inflammation, Diabetic retinopathy
Introduction
Diabetes has been recognized as a major epidemic disease worldwide [1]. A recent study reveals that the global prevalence of diabetes has increased dramatically over the past three decades from 153 million in 1980 to 347 million in 2008 [2]. The rising prevalence of diabetes has caused a consequential increase of long-term diabetic complications such as retinopathy, nephropathy, and neuropathy. Diabetic retinopathy (DR) is one of the most common microvascular complications of diabetes, and it is the major cause of vision loss with higher incidence and more severe risk when compared to other diabetes-related ocular complications, such as fluctuation of visions, cataract, and glaucoma [3]. It has been shown that almost 100% of type 1 diabetic patients and more than 60% of type 2 diabetic patients develop DR during the first two decades of diabetes [3], and approximately 12,000–24,000 diabetic subjects lose their sight each year as a result of DR [4]. In the USA, DR is the most frequent cause of blindness in the working-age population. According to The Eye Diseases Prevalence Research Group, in adult diabetic patients, the estimated crude prevalence rates for retinopathy and vision-threatening retinopathy are 40.3% and 8.2%, respectively [5, 6], and 50% of all patients with untreated proliferative retinopathy will lose their sight within 5 years [7]. Furthermore, it is estimated that half of all Hispanic/Latino American with diabetes have DR [8]. As a consequence, DR is among the major visual disorders that cause substantial economic burden due to the significant loss of productivity. In 2006, the annual direct medical costs for Americans age 40 and older with DR was estimated to be $490 million [9]. It is clear that DR is a serious sight-threatening disease with considerable impact on both the patients and the society; however, an effective treatment for DR is currently not available. A potential cause is our lack of understanding of the pathogenic mechanisms by which diabetes induces damage to retinal cells. Recent studies demonstrate that dysfunction of the endoplasmic reticulum (ER), or ER stress, is implicated in the pathogenesis of diabetes and its complications [10–13]. In this review, we will summarize the recent progress in the research of ER stress and inflammation, with a focus on the association of ER stress and vascular damage in DR.
Diabetic retinopathy: rathophysiology and inflammation
DR is a major complication of diabetes that is characterized by damage to the retinal vasculature, retinal neuronal degeneration, and impaired distribution of blood supply to retinal tissue [14]. Diabetic macular edema (DME) resulting from the breakdown of the blood–retinal barrier (BRB) and uncontrolled retinal neovascularization (NV) are major causes of vision loss in DR. The BRB is composed of two spatially distinct barriers: (1) the inner barrier is located primarily at the tight junction between adjacent endothelial cells (EC); (2) the outer barrier is formed primarily by retinal pigment epithelial cells (RPE). Breaching the BRB in diabetes is thought to be an early event in the disease as leakage is found in numerous animal models by vastly different methods shortly after the onset of diabetes (reviewed by Gardner [15]). The increased permeability of retinal blood vessels results in the leakage of plasma macromolecules and fluid into the retina. In addition, damage to the outer BRB integrity, as observed in diabetic animals, may allow blood contents to diffuse from the outer BRB into the neural retina, contributing to increased retinal thickness [16]. The consequent edema of retinal tissue, which can be clearly visualized by optimal coherence tomography, if occurring in the central retina (macula), can cause significant impairment of the central vision in diabetic patients.
Aberrant blood vessel growth in the retina (retinal NV) is a hallmark of proliferative DR caused by retinal ischemia secondary to massive retinal capillary dropout. Studies demonstrate that apoptosis of retinal vascular cells occurs within 1 month of hyperglycemia in diabetes animal models [17–19], and preceded histological evidence of retinopathy in diabetic patients [18]. The loss of pericytes is one of the earliest changes in the diabetic retina resulting in dilation of capillaries and the formation of microaneurysms, which is observed as the first clinical sign of DR. The death of retinal EC results in the occlusion of capillaries and the ischemia of retinal tissue. This in turn induces the overexpression and overproduction of proangiogenic cytokines, such as vascular endothelial growth factor (VEGF), which stimulate retinal NV in an attempt to restore blood supply and to improve oxygenation in the retina. However, the abnormal structure of these new blood vessels often leads to hemorrhage and leakage of plasma proteins into the retina or vitreous consequently compromising the vision.
Besides vascular damage, recent studies suggest that loss of retinal neural cells is another critical component of DR, and it may occur independent of vascular injury [20]. Notably, apoptosis of ganglion cells and photoreceptors have been observed in early diabetes in animals [20]. Damage of retinal glial (Müller and astrocytes) cells by diabetes was also reported [21, 22]. In diabetic patients, the decrease in retinal nerve fiber layer thickness is detected even in those without retinopathy [23]. Furthermore, the structural damage of retinal neurons is associated with deficits in retinal functions such as abnormalities in the electroretinogram [24] and loss of contrast sensitivity [25]. Protection of retinal neurons is an important strategy to preserve and improve the visual function of diabetic patients with DR.
While hyperglycemia is the primary etiological factor for DR, compelling evidence shows that inflammation plays an important role in the BRB breakdown as well as retinal cell death caused by diabetes [26, 27]. In diabetic patients and animal models of diabetes, expressions of inflammatory mediators, such as VEGF, tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), interleukin-1β (IL-1β), interleukin 6 (IL-6), intercellular adhesion molecule-1 (ICAM-1), and other such mediators are increased in the retina or vitreous [26–28]. Significantly elevated VEGF levels correlated with the severity of vascular leakage in diabetic patients with DME [27]. The upregulation of VEGF is also responsible for retinal hyper-permeability in streptozotocin (STZ)-induced diabetic rats [29]. Inhibition of TNF-α or ICAM-1 expression or activity significantly reduced retinal leukostasis and vascular leakage in animal models of diabetes and uveitis [26, 29–31]. Pharmaceutical inhibition of TNF-α also effectively attenuated retinal cell apoptosis and reduced acellular capillary formation in diabetic rodents [32]. These findings strongly suggest a causal role of inflammation in BRB breakdown and vascular damage.
In addition to inflammation, several mechanisms have been identified to play a critical role in the development and progression of DR. These mechanisms include the polyol pathway, diacylglycerol-protein kinase C pathway, oxidative stress, and changes in macromolecule structure and function via the formation of advanced glycation end products [33]. It has been shown that hyperglycemia and the disturbed metabolism in diabetes increases production of reactive oxygen species resulting in oxidative damage to retinal cells [34]. Relative to other tissues, the retina has the highest metabolic rates, oxygen uptake, and glucose oxidation, and the retina is rendered highly susceptible to oxidative stress (reviewed by Kowlulu and Chan [34]). Administration of anti-oxidants or the NADPH oxidase inhibitor diphenyleneiodonium attenuates retinal inflammation and vascular leakage in rodents with diabetes; thus, this suggests that oxidative stress is a potential regulator of inflammatory response in DR [35]. In addition, increased oxidative stress also contributes to retinal basement membrane thickening and capillary cell apoptosis, both of which are central features of vascular pathology in DR [34].
ER stress and the unfolded protein response: cellular function and inflammation
The endoplasmic reticulum is the primary intracellular organelle responsible for protein folding, maturation, and trafficking [36, 37]. The ER consists of a network of folded membranes in which secretory and most membrane proteins are synthesized, post-translationally modified, and folded into their correct three-dimensional conformations. Only properly folded (mature) proteins can be transported to the Golgi apparatus for further processing. In addition, the ER also serves as a dynamic pool of calcium, governing the intracellular calcium homeostasis [38]. Other major functions of the ER include lipid and steroid hormone synthesis, carbohydrate metabolism, and drug detoxification. Importantly, compelling evidence indicates that the ER is one of the major machinery involved in sensing subtle environmental changes and cellular stresses, coordinating signaling pathways, and modulating cell function and cell survival. Various physiological and pathological circumstances such as excessive mutant proteins, viral infection, energy or nutrient deprivation, as well as alteration in the redox status can compromise the ER capacity in protein folding, resulting in the accumulation of unfolded or misfolded proteins in the ER lumen, or ER stress. In turn, misfolded proteins aggregate to form insoluble intracellular or extracellular deposit which is toxic to the cell. It has been demonstrated that a number of age-related diseases, such as Alzheimer’s diseases; inflammatory disorders, such as diabetes; and neurodegenerative diseases, such as Parkinson’s disease, are associated with the buildup of misfolded or unfolded protein aggregates [39–42]. To eliminate the toxic protein components, cells activate an adaptive mechanism that consists of a number of intracellular signaling pathways, collectively known as unfolded protein response (UPR). The UPR relieves ER stress and restores the protein homeostasis through three complementary strategies: (1) halt the generation of more unfolded proteins by suppression of protein translation; (2) induce ER-related molecular chaperones to promote refolding of the unfolded proteins; and (3) activate the ER-associated protein degradation (ERAD) system to remove the unfolded proteins.
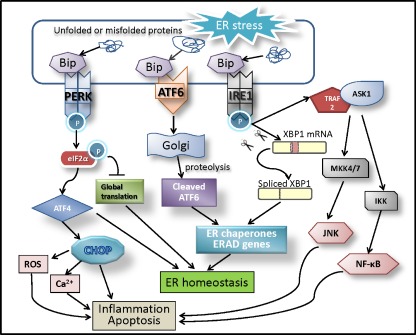
There are three branches of UPR that are initiated by distinct ER stress transducers located on the ER membrane: PKR-like endoplasmic reticulum kinase (PERK) [43], inositol-requiring enzyme 1 (IRE1) [44, 45], and activating transcription factor 6 (ATF6) [36]. In an unstressed condition, each of these three proteins is bound to a molecule called glucose-regulated protein 78 (GRP78), also known as immunoglobulin binding protein (Bip) [46, 47]. An accumulation of unfolded/misfolded proteins in the ER pulls GRP78, which is itself a chaperon protein, away from these three proteins triggering the activation of the UPR [48]. In eukaryotic cells, UPR is an adaptive cellular response to activate chaperon proteins to help correctly fold the misfolded proteins. It also attenuates translation of proteins to prevent further accumulation of misfolded proteins in the ER, and, as well, the UPR promotes the degradation of proteins through ERAD [49]. If ER stress becomes overwhelmed and the UPR fails to restore the normal function of the ER, it will signal the cell to enter apoptosis [50, 51] (Fig. 1).
Fig. 1.
ER stress-driven UPR signal pathways in inflammation and apoptosis. Accumulation of unfolded and misfolded proteins in the ER lumen (ER stress) is sensed by three ER membrane proteins (ER stress transducers)—IRE1, PERK, and ATF6. In response to ER stress, GRP78 dissociates from ER stress transducers and binds to unfolded and misfolded proteins, resulting in the activation of ER stress transducers. Upon activation, IRE1 splices the mRNA of XBP1 and produces an active transcription factor named spliced XBP1 (XBP1-S). The activation of PERK increases phosphorylation of eIF2α leading to a global attenuation of protein synthesis and a concomitant increase in ATF4 translation. ATF6 dissociated from GRP78 translocates to the Golgi apparatus where it is cleaved by proteolysis, and becomes an active transcription factor. XBP1 and ATF6 transcriptionally upregulate ER chaperones and proteins implicated in the ER-associated protein degradation (ERAD), which reduce ER stress and restore ER homeostasis. During prolonged ER stress, ATF4 induces CHOP expression, which increases reactive oxygen species (ROS) generation, promotes calcium influx from the ER to mitochondria, and activates inflammatory and proapoptotic cascades. In addition, IRE1 recruits TRAF2 and ASK1, resulting in JNK and NF-κB activation. These events contribute to pathological inflammation and apoptosis
The IRE1-UPR branch
There are two different IRE1 proteins in mammalian cells, IRE1α and IRE1β, both of which can participate in the ER stress response. Upon activation during ER stress, IRE1 acquires the activity of an endoribonuclease, and slices the mRNA of X-box binding protein 1 (XBP1) to generate a new transcription factor, spliced XBP1. XBP1 is widely held as a master regulator of the adaptive response to ER stress, and it is responsible for the activation and induction of many ER-related molecular chaperones like GRP78, facilitating protein folding and promoting cell recovery from ER stress. Importantly, our recent study indicates that XBP1 regulates inflammatory response in retinal EC [14]. We demonstrated that loss of XBP1 results in an increase in ICAM-1 and VCAM-1 expression. These adhesion molecules are key mediators of leukocyte adhesion, which disrupts the tight junctions in the retina leading to vascular leakage and endothelial apoptosis in DR. In addition, cells deficient in XBP1 are more susceptible to inflammation-induced cell death suggesting that XBP1 protects cells from apoptosis during ER stress [52]. Interestingly, in addition to its function as an endoribonuclease, IRE1 can form a complex with tumor necrosis factor-associated factor 2 (TRAF2) [53], which recruits apoptosis signal-regulating kinase 1 (ASKI) and activates the JNK pathway [54]. IRE1-dependent JNK activation is implicated in inflammatory response and signaling for apoptosis [55]. It has been shown that loss of XBP1 induces increased JNK activation through regulation of IRE1 phosphorylation, which may contribute, at least in part, to its protective effect on inhibition of inflammation and apoptosis.
The PERK-UPR branch
PERK is a serine/threonine protein kinase activated by ER stress via dimerization and autophosphorylation upon dissociation from GRP78. Activated PERK phosphorylates its downstream target protein, eIF2α, resulting in the inhibition of global protein translation [56]. However, some genes with upstream open reading frames (uORFs) in their 5′ untranslated region (UTR) could escape from the eIF2α-initiated translational attenuation. A representative example is activating transcription factor 4 (ATF4)–human ATF4 gene contains multiple uORFs in its 5′ UTR, whereas the murine mRNA has two uORFs [57]. These uORFs prevent the translation of ATF4 under normal conditions, but enhance its expression when eIF2α is phosphorylated [58, 59]. ATF4 in turn upregulates its downstream genes such as C/EBP homologous protein-10 (CHOP), which is responsible for signaling cells to enter apoptosis. One mechanism by which CHOP signals apoptosis is through the dephosphorylation of eIF2α which prevents the attenuation of translation resulting in the overwhelmed protein loading in the ER to continue [60]. As a transcription factor, CHOP binds directly to the promoter of the TRB3 gene which inhibits AKT activation resulting in apoptosis [14]. Alternatively, CHOP causes increased calcium influx from the ER to the mitochondria, resulting in the activation of the mitochondria-dependent apoptotic pathway [61]. In addition to regulating the apoptotic cascade, CHOP has also been shown to regulate expression of CD11b (Mac-1), a gene critical for macrophage infiltration and inflammation [62]. Deletion of CHOP attenuated the lipopolysaccharide (LPS)-induced inflammation in the lungs, including the IL-1beta activity in bronchoalveolar lavage fluid [63]. The induction of caspase-11 by LPS, which is needed for the activation of procaspase-1 and pro-IL-1beta, was also suppressed in CHOP knockout mice. In addition, it was found that circulating TNF-α and expression of acute-phase response proteins CRP and SAP were significantly lower in CHOP knockout mice compared to WT mice fed with a methionine and choline-deficient diet [64]. These findings suggest that CHOP is a critical mediator of inflammation, and its role in retinal inflammation and DR warrants future investigation.
The ATF6 branch
ATF6 consists of two closely related proteins ATF6α and ATF6β in mammalian cells, both of which are ER membrane-bound transcription factors activated during ER stress [65]. Unlike the IRE1 and PERK UPR branches, ATF6, the third UPR branch, is activated through transportation to the Golgi where it is cleaved by serine protease site 1 protease and metalloprotease site 2 protease [66]. Once ATF6 is cleaved, it is transported to the nucleus where it can play a role in ERAD and in apoptosis. ATF6 can also bind to XBP1 and increase expression of XBP1 and other molecular chaperons like GRP78 [67]. Deletion of ATF6α does not affect the basal expression of ER protein chaperones nor embryonic/postnatal development; however, ATF6α null mice showed compromised organ function and survival when challenged with chronic stress despite functional overlap between UPR sensors [68]. In addition, ATF6α null mice were more sensitive to ER stress and exhibited liver dysfunction and steatosis when given intraperitoneal injection of the ER stress-inducing reagent tunicamycin, whereas wild-type mice were able to recover from the insult [65]. In contrast, overexpressing ATF6 in the heart upregulated protective molecules like GRP78 and prevented damage to cardiac tissue after ischemia/reperfusion (I/R) in cardiac-specific ATF6 transgenic mice [69]. Although emerging evidence suggests that ATF6 may be involved in the development of type 2 diabetes [70], its role in the pathogenesis of diabetes and diabetic complications remains largely unknown.
Evidence of ER stress and UPR in DR
Although the role of ER stress has been extensively studied in the area of diabetes, in particular pancreatic beta cell death, its implication in diabetic complications was not investigated until recently. Using Akita mice, a model of type 1 diabetes, we, for the first time, demonstrated that multiple ER stress markers, including GRP78, phospho-IRE1α, and phospho-eIF2α were significantly upregulated in the diabetic retina [13]. These changes coincide with increased expression of inflammatory cytokines, such as TNF-α and VEGF. Using immunohistochemistry, we observed that increased GRP78 expression in the diabetic retina was mainly localized to the inner retina. In nondiabetic animals, GRP78 was constantly expressed at a modest level in the cytoplasm of cells in the inner nuclear layer, ganglion cell layer, and the inner segment of photoreceptors. GRP78 is a prominent ER chaperones that promotes the correct folding of proteins. In response to ER stress, GRP78 expression is upregulated rapidly, dissociates from the ER membrane proteins, and binds to unfolded/misfolded proteins for refolding. The dissociation of GRP78 from the ER membrane proteins (ER stress sensors) triggers the activation of the UPR. Indeed, we observed increased phosphorylation of IRE1α accompanied by enhanced splicing of XBP1 mRNA. In addition, increased phosphorylation of eIF2α and its downstream effects ATF4 and CHOP indicated the activation of the PERK UPR branch in the diabetic retina from Akita mice. Significantly increased expression of GRP78 and activation of PERK were also observed in 14-week-old db/db mice, a model of type 2 diabetes [71]. In addition, a recent study analyzed expression of ER stress-related genes in the retina from streptozotocin-induced diabetic rats, suggesting that some genes involved in ER stress response may be changed after short-term onset of hyperglycemia [72].
In our recent studies, we investigated the role of ER stress in diabetes-induced inflammation and vascular leakage. We showed that pharmacological induction of ER stress was sufficient to induce inflammatory gene expression in the retina [13]. Tunicamycin is a common ER stress inducer that inhibits N-linked glycosylation. Twenty-four hours after intravitreal injection of tunicamycin into the mouse eye, we found that retinal TNF-α and VEGF levels were significantly increased by 5.1- and 4.3-fold, respectively [13]. In parallel, ER stress was markedly increased in the eyes receiving tunicamycin treatment. In cultured retinal EC, tunicamycin upregulated TNF-α expression which also resulted in downregulation of claudin-5, a major tight junction protein, which may contribute to increased endothelial permeability in DR [73]. To further examine the role of ER stress in the development of retinal inflammation and vascular damage in DR, we administrated 4-phenyl butyrate (PBA), a small molecule chemical chaperone, to the Akita mice. We found that inhibition of ER stress by PBA significantly attenuated retinal VEGF expression in the diabetic animals. Moreover, we confirmed that ER stress is critical for inflammatory gene expression in retinal EC. Pretreatment of human retinal EC with PBA dose-dependently abolished hypoxia-induced TNF-α and VEGF expression [13]. Furthermore, we showed that enhancing the adaptive UPR by preconditioning with ER stress protects retinal EC against inflammatory injury [14]. We demonstrated that pretreatment of retinal EC with a very low dose of tunicamycin or thapsigargin, both of which are ER stress stimulators, for a short time, significantly blocked TNF-α-elicited NF-κB activation and adhesion molecule ICAM-1 and VCAM-1 expression in retinal EC. Preconditioning also successfully prevented TNF-α-induced retinal leukostasis and vascular leakage in mouse retinas [14]. These results collectively suggest a central role of ER stress in retinal inflammation and vascular damage in DR.
Oxygen-induced retinopathy (OIR) is a commonly used model for the study of retinal NV, a feature of advanced (proliferative) DR, as well as hypoxia-induced inflammation and vascular leakage [28]. To induce OIR, neonatal mice were exposed to 75% O2 from postnatal day 7 (P7) to P12 followed by returning the animals to room air to induce a relative hypoxia [74]. We demonstrated that inflammatory cytokines, including VEGF and TNF-α, are significantly upregulated in the retinas of OIR mice at P16, which contribute to subsequent retinal vascular leakage and neovascularization in the retina [28, 75]. These changes coincided with increase ER stress markers (GRP78 expression, phosphorylation of eIF2α, and expression of ATF4). Moreover, treatment of mice with intraperitoneal injections of PBA (40 mg/kg body weight/day) or PBS from P7 to P14 significantly reduced retinal VEGF levels in OIR [13]. These findings suggest that ER stress contributes to inflammation in ischemic retinal disease and may be implicated in retinal angiogenesis.
Pericyte injury and cell death are considered as a hallmark pathological change in DR. We have shown that pericytes activated by oxidized lipids secrete high levels of inflammatory cytokines, such as macrophage chemoattractant protein 1 (MCP-1) [76]. In a recent study, we evaluated the effects of constant and intermittent high glucose on inflammatory cytokine production in human retinal pericytes (HRP) and explored the role of ER stress in pericyte inflammation [77]. We found that exposure to constant high glucose did not alter ATF4 or CHOP expression, but intermittent high glucose induced a significant increase in nuclear levels of ATF4 and CHOP. Similarly, intermittent high glucose, but not constant high glucose, treatment for 8 days induced a significant increase in VEGF expression and MCP-1 secretion. Importantly, treatment with chemical chaperones PBA or tauroursodeoxycholic acid dose-dependently abolished intermittent high glucose-induced CHOP and VEGF expression and reduced MCP-1 secretion from HRP. These results suggest that ER stress is an important mediator of glucose fluctuation-induced inflammation in retinal pericytes. Intriguingly, we have shown that persistent high glucose for 8 days suppressed GRP78 expression, but it did not induce ATF4 and CHOP activation. In contrast, intermittent exposure to high glucose caused a more marked decrease in GRP78 expression accompanied by increased ATF4 and CHOP expression. Previously, Ikesugi and associates reported glucose deprivation, but not constant high glucose, elicits ER stress in rat retinal pericytes [78]. It is possible that the repetitive shift from high glucose to normal glucose during glucose fluctuation induces ER stress, while GRP78 suppression compromises the protein-folding capacity of the ER resulting in exaggerated ATF4/CHOP activation and inflammation in retinal pericytes. In addition, increased ATF4/CHOP activation may also contribute to pericyte apoptosis induced by glucose fluctuation. Future studies are warranted to investigate how ER stress-associated apoptotic pathways are implicated in retinal cell death in DR.
ER stress, UPR, and inflammatory signaling pathway
Perhaps of most interest, many recent reports have indicated that interaction between ER stress and inflammatory processes exists in many chronic diseases, such as DR. In general, inflammation is characterized by an increase in chemokines and cytokines. All three branches of the UPR have been demonstrated to be involved in the inflammatory response through the up-regulation of cytokines and chemokines by the activation of inflammatory pathways like VEGF, [79] c-Jun NH(2)-terminal kinase (JNK), [80] nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), [81], and toll-like receptor (TLR)-mediated pathways [82]. The implications of UPR in inflammatory response are complex and have been comprehensively reviewed in recent years [83–85]. In the following sections, we discuss the potential mechanisms of ER stress and UPR in regulation of retinal inflammatory signaling in DR.
VEGF
VEGF is a proangiogenic protein that signals for the growth of new blood vessels in order to restore proper oxygen supply to tissues where the levels of oxygen are inadequate. With an efficacy 5,000-fold higher than histamine in inducing vascular permeability [86], increased VEGF levels are thought to be the primary etiological factor in retinal vascular leakage and retinal edema in DR. VEGF also upregulates adhesion molecule expression, promoting leukocyte binding to the endothelial wall resulting in tight junction damage and endothelial cell injury [87]. Interestingly, ER stress and the UPR have been shown to play a critical role in angiogenesis by modulating VEGF expression. Our data show that reduction of ER stress attenuates VEGF production in cultured retinal cells, the diabetic retina, and the ischemic retina from OIR mice [13]. Furthermore, a study by Ghosh and associates demonstrated that all three UPR branches were necessary to induced VEGF expression to promote angiogenesis [79]. However, each of the branches is thought to be triggered by different stimuli, and activated at different times. Interestingly, a recent study by Droget and associates indicates that IRE1α is required for hypoxia-induced tumor angiogenesis [88]. Tumor cells expressing a dominant-negative IRE1α transgene as well as IRE1α-null mouse embryonic fibroblasts were unable to trigger VEGF upregulation upon either oxygen or glucose deprivation [88]. IRE1 null mice also exhibit low VEGF production and impaired angiogenesis in the placenta [89]. These findings suggest that IRE1 may play an important role in ER stress-mediated VEGF upregulation. In addition, a number of studies have shown that ATF4 is required for the upregulation of VEGF by various stimuli, including homocystine, arsenite, oxidized lipid, and growth factors in vascular cells and in RPE cells [90–92]. In human RPE cells, ATF4 binds to an amino acid response element in the first intron of the VEGF gene, which may be responsible for its regulatory effect on VEGF expression induced by arsenite [91].
JNK
JNK is a stress-activated protein kinase and belongs to the superfamily of mitogen-activated protein kinases. JNK has been implicated in cell proliferation, apoptosis, and cytokine production [93]. Activation of JNK has also been shown to be a novel mechanism in regulation of VEGF expression in retinal cells [94]. Guma and colleagues demonstrated that JNK is a critical mediator of VEGF upregulation and retinal NV in OIR [94]. Mice lacking JNK exhibit reduced pathological angiogenesis and lower levels of retinal VEGF production in OIR; furthermore, hypoxia induces JNK activation and regulates VEGF expression by enhancing the binding of phospho-c-Jun to the VEGF promoter [94]. Increased JNK activation was also observed in the diabetic retina; as well, inhibitors of JNK attenuated retinal VEGF expression, ICAM-1 levels, leukostasis, and BRB breakdown in STZ-diabetic rats, suggesting a pathogenic role of JNK activation in retinal inflammation and vascular leakage in DR. Interesting, as briefly discussed above, JNK has been considered as a key molecular link between ER stress and inflammatory response in intestinal epithelial cells and glial cells [95]. Induction of ER stress activates JNK through the IRE1 UPR branch, while inhibition of JNK reduces ATF4 expression [96], suggesting an underlying communication between the UPR pathways through this key molecule. In addition, JNK activation promotes ER stress in certain circumstances. For instance, IL-1β, a proinflammatory cytokine, stimulates JNK activation and enhances ER stress in pancreatic epithelial cells [97]. Pretreatment with JNK inhibitor abrogates IL-1β-induced ER stress, indicated by phosphorylation of eIF2α and increased expression of CHOP, GADD34, ATF4, and spliced XBP1; however, inhibition of ER stress does not affect JNK activation by IL-1β [97]. This suggests that JNK activation is essential for IL-1β-induced ER stress, which further increases JNK activity resulting in exacerbated inflammatory damage.
NF-κB
NF-κB, a key mediator of TNF-α-mediated signal transduction, is a major transcription factor in inflammatory signaling contributing to the pathogenesis of DR [76, 98–101]. Activation of NF-κB has been observed in the retinal vasculature of diabetic patients [102], in animal models [26, 101], and in retinal endothelial cells exposed to high glucose or TNF-α in vitro [99, 100]. Inhibiting NF-κB activation significantly reduced vascular leakage and cell death in the retina of diabetic animals [101, 103], suggesting that the NF-κB activation is necessary to elicit vascular damage caused by diabetes. Besides its role in regulating inflammatory response, NF-κB is also important for controlling cell fate. Moreover, it appears to have contradictory functions in apoptosis and cell survival. NF-κB mediates the survival response of many signals by inhibiting p53-dependent apoptosis and upregulating anti-apoptotic members of the Bcl-2 family, as well as, caspase inhibitors such as XIAP and FLIP. In contrast, NF-κB is also activated by apoptotic stimuli involved in DNA damage and mediates upregulation of proapoptotic genes such as TRAIL R2/DR5, Fas, and Fas ligand.
NF-κB is one of the first responders to harmful stimuli in a cell, and it can be activated by all three branches of the UPR. IRE1 is a well-known activator of the NF-κB pathway through interacting with TRAF2. Similar to the previously discussed complex of IRE1α-TRAF2 binding to ASK1, this complex binds with IκB kinase (IKK) to activate the NF-κB pathway and induce the transcription of inflammatory genes including TNF-α, IL-6, and IL-8. [54, 81] Our study demonstrates that enhancing the adaptive UPR by ER stress preconditioning or by overexpressing XBP1 suppresses NF-κB activation and inhibits adhesion molecule expression in retinal EC [14]. Furthermore, we identified that the inhibitory action of XBP1 on NF-κB is through inhibition of the IRE1/IKK pathway and upregulation of GRP78. Seen in another branch of the UPR, ATF6 activates NF-κB through phosphorylation of Akt, which, like IRE1 activation of NF-κB, induces transcription of inflammatory cytokines [104]. In addition, activation of the PERK-UPR induces phosphorylation of eIF2α, which reduces the translation of IκB, an inhibitor of NF-κB, resulting in NF-κB activation. Knockout PERK in mice alleviates free fatty acid-induced inflammation through inhibiting the activation of IKK and NF-κB [105].
Toll-like receptor signaling pathways
TLRs are part of the innate immune system and are responsible for recognizing microbes. Specifically, TLR4 recognizes the LPS from Gram-negative bacteria, while TLR2 is a key sensor implicated in recognizing Gram-positive bacteria. Upon activation, the TLRs initiate a complex signaling cascade through several adaptor molecules, including the common adaptor protein myeloid differentiation factor (MyD) 88, protein kinase, and other signaling intermediates [106]. All these signaling pathways culminate in activation of NF-κB resulting in upregulation of proinflammatory cytokines. Despite very few studies being published in the field of DR, expression of TLRs has been observed in the retina [106], isolated retinal astrocytes [107], and RPE cells [108]. Treatment of RPE cells with Poly I:C resulted in augmented protein expression of TLRs 2, 3, and 4, accompanied by increased production of IL-6, IL-8, MCP-1, and sICAM-1[108]. In cultured retinal astrocytes, PolyI:C increased the expression of MHC and induced the production of IL-6, IL-12, and IL-23. These cytokines were able to activate IRBP-specific T cells which produce IFN-γ and IL-17, and induce experimental autoimmune uveitis when injected into naive mice [107]. Deletion of TLR4 significantly attenuated NF-κB activation, reduced inflammatory gene expression and alleviated retinal damage after an ischemia–reperfusion injury [109]. It also abolished oxidative stress and the damage to mitochondria DNA in photoreceptors induced by complete Freund's adjuvant containing heat-killed Mycobacterium tuberculosis (CFA) [106]. The role of TLRs in retinal inflammatory response and vascular injury induced by diabetes remains to be investigated.
It has been suggested that TLR signaling can play a role in the severity of the ER stress response and activating UPR-associated transcription factors. Woo and associates reported that prior engagement of TLR3 or 4 suppressed ER stress-induced CHOP expression without affecting its upstream UPR pathway (phosphorylation of PERK or eIF-2α). Furthermore, they demonstrated that this protective effect is through the TRIF-mediated signaling pathways activated by TLRs, which selectively attenuate translational activation of ATF4 and its downstream target gene CHOP [110]. In a recent study, Mahedevan reported that TLR4 KO macrophages had reduced ER stress markers and less severe inflammation when exposed to conditioned medium from ER-stressed tumor cells [82]. Intriguingly, TLR4 has been shown to be essential for XBP1 activation in macrophages, which, instead of resulting in the normal activation of the UPR, triggered the transcription of proinflammatory genes [111]. These findings suggest that TLRs, in particular TLR4, may be important regulators of ER stress and inflammation in DR and other diabetic complications.
Perspective and future directions
DR is one of the most frequent microvascular complications of diabetes. Despite many advances in clinical management of diabetes, consistently effective treatment for diabetic complications is still lacking, and DR remains the leading cause of blindness in American adults. Understanding the cellular and molecular underpinning of the pathological changes in DR is important to identify therapeutic targets and develop better modalities, in conjunction with glycemic control, to protect the retina from diabetic damage. Cellular signaling pathways associated with disturbed protein homeostasis in stressed cells have been studied recently in many diseases. We hypothesize that during the early stage of diabetes, various stressors including hyperglycemia, mild oxidative stress, overnutrition or hypoxia, and others, induce a low-grade ER stress which stimulates the activation of the adaptive UPR. Through upregulating ER chaperones and promoting protein-degradation system (e.g., ERAD and autophagy) to remove harmful protein aggregation, the UPR is able to restore the ER homeostasis and maintain normal cell function. However, as the disease progresses, it accumulates chronic insults causing persistent and severe ER stress exceeding the capacity of the adaptive mechanism. When this occurs, the proapoptotic and proinflammatory components of the UPR will be activated, resulting in the development of detrimental inflammation and even cell death. Many elegant studies over the past decade, and our recent work, clearly demonstrate a critical role of ER stress and UPR in mediating and regulating the inflammatory response and damage to retinal cells by inflammatory cytokines. While as a nonspecific response of molecular mediators to the site of injury, inflammation can be beneficial when seen transiently; prolonged inflammation can have undesirable consequences inducing cell death. Although there is compelling evidence that ER stress is a possible link between inflammation and a diseased state, it remains poorly understood how ER stress and UPR pathways interacts with inflammatory signaling and how this interaction leads to tissue and cell injury in the disease. It is also noteworthy that many other mechanisms, in addition to ER stress, contribute to retinal inflammation and vascular and neuronal damage in DR. It is very likely that these major signaling pathways crosstalk with each other in the decision making process of cell death and cell survival. Nevertheless, early intervention that reduces ER stress and enhances the adaptive mechanism to restore ER homeostasis and promote cell survival may be important for protecting retinal vascular cells and neurons from diabetic damage. In this regard, it is plausible that ER stress inhibitors could be of therapeutic value in DR and other diabetic complications. A close look into the mechanisms by which ER stress initiates inflammatory processes and identification of key protective genes against ER stress may provide new insight into the pathogenesis of the disease and pave the way for new treatment in DR.
Acknowledgments
This work was supported by the National Institutes of Health grant EY019949 and research awards from American Diabetes Association, Juvenile Diabetes Research Foundation, Oklahoma Center for the Advancement of Science and Technology, American Health Assistance Foundation, and Harold Hamm Diabetes Center at University of Oklahoma (all to SXZ).
References
- 1.American Diabetes Association Implications of the diabetes control and complications trial. Diabetes Care. 2003;26(Suppl 1):25–27. doi: 10.2337/diacare.26.2007.s25. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Fong DS, Aiello L, Gardner TW, et al. Diabetic retinopathy. Diabetes Care. 2003;26(1):226–229. doi: 10.2337/diacare.26.1.226. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Diabetic nephropathy. Diabetes Care. 2000;23(Suppl 1):S69–S72. [PubMed] [Google Scholar]
- 5.The Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 6.The Eye Diseases Prevalence Research Group The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 7.Ferris FL., 3rd Results of 20 years of research on the treatment of diabetic retinopathy. Prev Med. 1994;23(5):740–742. doi: 10.1006/pmed.1994.1127. [DOI] [PubMed] [Google Scholar]
- 8.National Eye Institute (2004) Statement on the prevalence of diabetic retinopathy and age-related macular degeneration among Hispanic/Latino Americans. http://www.nei.nih.gov/news/statements/latinostudy.asp.
- 9.Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 10.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Sci. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Sci. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118(10):3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583:1521–1527. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Wang JJ, Zhang SX. Preconditioning with endoplasmic reticulum stress mitigates retinal endothelial inflammation via activation of X-box binding protein 1. J Biol Chem. 2011;286(6):4912–4921. doi: 10.1074/jbc.M110.199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner TW, Antonetti DA. Novel potential mechanisms for diabetic macular edema: leveraging new investigational approaches. Curr Diab Rep. 2008;8(4):263–269. doi: 10.1007/s11892-008-0047-5. [DOI] [PubMed] [Google Scholar]
- 16.Xu H-Z, Le Y-Z. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52(5):2160–2164. doi: 10.1167/iovs.10-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin PM, Roon P, Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45(9):3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 18.Joussen AM, Poulaki V, Tsujikawa A, et al. Suppression of diabetic retinopathy with angiopoietin-1 [comment] Am J Pathol. 2002;160(5):1683–1693. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998;47(12):1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- 20.Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 21.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusner LL, Sarthy VP, Mohr S. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase: a role in high glucose-induced apoptosis in retinal Muller cells. Invest Ophthalmol Vis Sci. 2004;45:1553–1561. [PubMed] [Google Scholar]
- 23.Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmol. 2005;219(6):379–385. doi: 10.1159/000088382. [DOI] [PubMed] [Google Scholar]
- 24.Yonemura D, Tsuzuki K. Electroretinogram in diabetic retinopathy. Arch Ophthalmol. 1962;68(1):19–24. doi: 10.1001/archopht.1962.00960030023005. [DOI] [PubMed] [Google Scholar]
- 25.Sokol S, Moskowitz A, Skarf B, Evans R, Molitch M, Senior B. Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol. 1985;103(1):51–54. doi: 10.1001/archopht.1985.01050010055018. [DOI] [PubMed] [Google Scholar]
- 26.Joussen AM, Poulaki V, Mitsiades N, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16(3):438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 27.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133(1):70–77. doi: 10.1016/S0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, J-x M. Pigment epithelium-derived factor (PEDF) is an endogenous anti-inflammatory factor. FASEB J. 2006;20(2):323–325. doi: 10.1096/fj.06-5668fje. [DOI] [PubMed] [Google Scholar]
- 29.Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42(10):2408–2413. [PubMed] [Google Scholar]
- 30.Joussen AM, Poulaki V, Qin W, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002;160(2):501–509. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koizumi K, Poulaki V, Doehmen S, et al. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44(5):2184–2191. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- 32.Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172(5):1411–1418. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz JP, Gonzalez-Correa JA, Guerrero A, De La Cuesta FS. Pharmacological approach to diabetic retinopathy. Diabetes Metabol Res Rev. 2004;20(2):91–113. doi: 10.1002/dmrr.432. [DOI] [PubMed] [Google Scholar]
- 34.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen P, Guo AM, Edwards PA, Trick G, Scicli AG. Role of NADPH oxidase and ANG II in diabetes-induced retinal leukostasis. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1619–R1629. doi: 10.1152/ajpregu.00290.2007. [DOI] [PubMed] [Google Scholar]
- 36.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 37.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8(9):663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 38.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8(9–10):1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 39.Bouman L, Schlierf A, Lutz A, et al. Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Diff. 2010;18:769–782. doi: 10.1038/cdd.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundar Rajan S, Srinivasan V, Balasubramanyam M, Tatu U. Endoplasmic reticulum (ER) stress & diabetes. Indian J Med Res. 2007;125(3):411–424. [PubMed] [Google Scholar]
- 41.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13(3):385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 42.Szegezdi E, Duffy A, O'Mahoney ME, et al. ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006;349(4):1406–1411. doi: 10.1016/j.bbrc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 44.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12(12):1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calfon M, Zeng H, Urano F, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 46.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2002;2(6):326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 47.Ng D, Watowich S, Lamb R. Analysis in vivo of GRP78-BiP/substrate interactions and their role in induction of the GRP78-BiP gene. Mol Biol Cell. 1992;3(2):143. doi: 10.1091/mbc.3.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haze K, Okada T, Yoshida H, et al. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J. 2001;355(Pt 1):19. doi: 10.1042/0264-6021:3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 50.Paschen W, Frandsen A. Endoplasmic reticulum dysfunction–a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem. 2001;79(4):719–725. doi: 10.1046/j.1471-4159.2001.00623.x. [DOI] [PubMed] [Google Scholar]
- 51.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11(4):372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 52.Jing G, Wang, J, Zhang, SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res 2012. doi:10.1155/2012/589589. [DOI] [PMC free article] [PubMed]
- 53.Mosbah B, Alfany-Fernandez I, Martel C, et al. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy undder ischemia-reperfusion. Cell Death and Dis. 2010;1:e52. doi: 10.1038/cddis.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gotoh T, Endo M, Oike Y. Endoplasmic reticulum stress-related inflammation and cardiovascular disease. Int J Inflamm. 2011;2011:259462. doi: 10.4061/2011/259462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Sci. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 56.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol cell. 2000;5(5):897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 57.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 58.Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 59.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101(31):11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marciniak SJYC, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giorgi C, Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41(10):1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Namba T, Tanaka K-I, Ito Y, et al. Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response in the development of colitis. Am J Pathol. 2009;174(5):1786–1798. doi: 10.2353/ajpath.2009.080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Endo M, Mori M, Akira S, Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol. 2006;176(10):6245–6253. doi: 10.4049/jimmunol.176.10.6245. [DOI] [PubMed] [Google Scholar]
- 64.Rahman SM, Schroeder-Gloeckler JM, et al. CCAAT/enhancing binding protein β deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45(5):1108–1117. doi: 10.1002/hep.21614. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto K, Takahara K, Oyadomari S, et al. Induction of liver steatosis and lipid droplet formation in ATF6α-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell. 2010;21(17):2975–2986. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X, Shen J, Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biochem. 2002;277:13045–13052. doi: 10.1074/jbc.M110636200. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto KST, Matsui T, Sato M, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 68.Wu J, Rutkowski DT, Dubois M, et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13(3):351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Martindale J, Fernandez R, Thuerauf D, et al. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98(9):1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 70.Thameem F, Farook VS, Bogardus C, Prochazka M. Association of amino acid variants in the activating transcription factor 6 gene (ATF6) on 1q21-q23 with type 2 diabetes in Pima Indians. Diabetes. 2006;55(3):839–842. doi: 10.2337/diabetes.55.03.06.db05-1002. [DOI] [PubMed] [Google Scholar]
- 71.Tang L, Zhang Y, Jiang Y, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med. 2011;236(9):1051–1063. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan S, Zheng C, Chen Z-q, et al. Expression of endoplasmic reticulum stress-related factors in the retinas of diabetic rats. Exp Dia Res, 2012; (in press) [DOI] [PMC free article] [PubMed]
- 73.Adachi T, Yasuda H, Nakamura S, et al. Endoplasmic reticulum stress induces retinal endothelial permeability of extracellular-superoxide dismutase. Free Radical Res. 2011;45(9):1083–1092. doi: 10.3109/10715762.2011.595408. [DOI] [PubMed] [Google Scholar]
- 74.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 75.Zhang SX, Ma J-X, Sima J, et al. Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol. 2005;166(1):313–321. doi: 10.1016/S0002-9440(10)62255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang SX, Wang JJ, Dashti A, et al. Pigment epithelium-derived factor (PEDF) mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized-LDL. J Mol Endocrinol. 2008;41(3):135–143. doi: 10.1677/JME-08-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhong Y, Wang JJ, Zhang SX. Intermittent but not constant high glucose induces ER stress and inflammation in human retinal pericytes Adv Exp Med Biol 2012;723:285–92. [DOI] [PMC free article] [PubMed]
- 78.Ikesugi K, Mulhern ML, Madson CJ, et al. Induction of endoplasmic reticulum stress in retinal pericytes by glucose deprivation. Curr Eye Res. 2006;31(11):947–953. doi: 10.1080/02713680600966785. [DOI] [PubMed] [Google Scholar]
- 79.Ghosh R, Ghosh R, Lipson KL, et al. Transcriptional regulation of VEGF-A by the unfolded protein reponse pathway. PLoS One. 2010;5(3). [DOI] [PMC free article] [PubMed]
- 80.Zhou Q, Zouh M, Lou A, Xie D, Hou F. Advanced oxidation protein products induce inflammatory response and insulin resistance in cultured adipocytes via induction of endoplasmic reticulum stress. Cell Physiol Biochem. 2010;26:775–786. doi: 10.1159/000322345. [DOI] [PubMed] [Google Scholar]
- 81.Fougeray S, et al. Metabolic stress promotes renal tubular inflammation by triggering the unfolded protein response. Cell Death Dis 2012; (in press) [DOI] [PMC free article] [PubMed]
- 82.Mahadevan N, et al. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A. 2011;108(16):6561–6566. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaser A, Blumberg RS. Endoplasmic reticulum stress in the intestinal epithelium and inflammatory bowel disease. Seminars Immunol. 2009;21(3):156–163. doi: 10.1016/j.smim.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hotamisligil GH. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Senger DRLS, Claffey KP, et al. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 87.Tang J, Kern TS. Inflammation and diabetic retinopathy. Progress in Retinal and Eye Research. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Drogat B, Auguste P, Nguyen DT, et al. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res. 2007;67(14):6700–6707. doi: 10.1158/0008-5472.CAN-06-3235. [DOI] [PubMed] [Google Scholar]
- 89.Iwawaki T, Akai R, Yamanaka S, Kohno K. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc Natl Acad Sci U S A. 2009;106(39):16657–16662. doi: 10.1073/pnas.0903775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roybal CN, Yang S, Sun CW, et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279(15):14844–14852. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- 91.Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005;280(21):20331–20339. doi: 10.1074/jbc.M411275200. [DOI] [PubMed] [Google Scholar]
- 92.Malabanan KP, Kanellakis P, Bobik A, Khachigian LM. Activation transcription factor-4 induced by fibroblast growth factor-2 regulates vascular endothelial growth factor-A transcription in vascular smooth muscle cells and mediates intimal thickening in rat arteries following balloon injury. Circ Res. 2008;103(4):378–387. doi: 10.1161/CIRCRESAHA.107.168682. [DOI] [PubMed] [Google Scholar]
- 93.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57(4–5):283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 94.Guma M, Rius J, Duong-Polk KX, Haddad GG, Lindsey JD, Karin M. Genetic and pharmacological inhibition of JNK ameliorates hypoxia-induced retinopathy through interference with VEGF expression. Proc Natl Acad Sci U S A. 2009;106(21):8760–8765. doi: 10.1073/pnas.0902659106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim H-T, Qiang W, Liu N, Scofield VL, Wong PK, Stoica G. Up-regulation of astrocyte cyclooxygenase-2, CCAAT/enhancer-binding protein, glucose-related protein 78, eukaryotic initiation factor 2α, and c-Jun N-terminal kinase by a neurovirulent murine retrovirus. J Neurovirol. 2005;11(2):166–179. doi: 10.1080/13550280590922810. [DOI] [PubMed] [Google Scholar]
- 96.Urano FWX, Bertolotti A, Zhang Y, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 97.Verma G, Datta M. IL-1¦Â induces ER stress in a JNK dependent manner that determines cell death in human pancreatic epithelial MIA PaCa-2 cells. Apoptosis. 2010;2010:1–13. doi: 10.1007/s10495-010-0498-4. [DOI] [PubMed] [Google Scholar]
- 98.Chen YM, Chiang WC, Lin SL, Wu KD, Tsai TJ, Hsieh BS. Dual regulation of tumor necrosis factor-alpha-induced CCL2/monocyte chemoattractant protein-1 expression in vascular smooth muscle cells by nuclear factor-kappaB and activator protein-1: modulation by type III phosphodiesterase inhibition. J Pharmacol Exp Ther. 2004;309(3):978–986. doi: 10.1124/jpet.103.062620. [DOI] [PubMed] [Google Scholar]
- 99.Chen W, Esselman WJ, Jump DB, Busik JV. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 2005;46(11):4342–4347. doi: 10.1167/iovs.05-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007;56(2):337–345. doi: 10.2337/db06-0789. [DOI] [PubMed] [Google Scholar]
- 102.Mitamura Y, Harada T, Harada C, et al. NF-kappaB in epiretinal membranes after human diabetic retinopathy. Diabetologia. 2003;46(5):699–703. doi: 10.1007/s00125-003-1084-x. [DOI] [PubMed] [Google Scholar]
- 103.Kaji Y, Usui T, Ishida S, et al. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007;48(2):858–865. doi: 10.1167/iovs.06-0495. [DOI] [PubMed] [Google Scholar]
- 104.Yamazaki HHN, Hayakawa K, Tagawa Y, et al. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiao P, Ma J, Feng B, et al. FFA-induced adipocyte inflammation and insulin resistance: involvement of ER stress and IKKβ pathways. Obesity. 2011;19:483–490. doi: 10.1038/oby.2010.200. [DOI] [PubMed] [Google Scholar]
- 106.Ko MK, Saraswathy S, Parikh JG, Rao NA. The role of TLR4 activation in photoreceptor mitochondrial oxidative stress. Invest Ophthalmol Vis Sci. 2011;52:5824–5835. doi: 10.1167/iovs.10-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang G, Ke Y, Sun D, Wang Y, Kaplan HJ, Shao H. Regulatory role of TLR ligands on the activation of autoreactive t cells by retinal astrocytes. Invest Ophthalmol Vis Sci. 2009;50:4769–4776. doi: 10.1167/iovs.08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov V. Toll-like receptor 4 contributes to retinal ischemia/reperfusion injury. Mol Vis. 2010;16:1907–1912. [PMC free article] [PubMed] [Google Scholar]
- 110.Woo CW, Cui D, Arellano J, et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martinon FC, Lee X, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune response in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]