Abstract
The pathogenesis of diabetic retinopathy is complex, reflecting the array of systemic and tissue-specific metabolic abnormalities. A range of pathogenic pathways are directly linked to hyperglycaemia and dyslipidaemia, and the retina appears to be exquisitely sensitive to damage. Establishing the biochemical and molecular basis for this pathology remains an important research focus. This review concentrates on the formation of a range of protein adducts that form after exposure to modifying intermediates known to be elevated during diabetes. These so-called advanced glycation end products (AGEs) and advanced lipoxidation end products (ALEs) are thought to play an important role in the initiation and progression of diabetic retinopathy, and mechanisms leading to dysfunction and death of various retinal cells are becoming understood. Perspective is provided on AGE/ALE formation in the retina and the impact that such adducts have on retinal cell function. There will be emphasis placed on the role of the receptor for AGEs and how this may modulate retinal pathology, especially in relation to oxidative stress and inflammation. The review will conclude by discussion of strategies to inhibit AGE/ALE formation or harmful receptor interactions in order to prevent disease progression from the point of diabetes diagnosis to sight-threatening proliferative diabetic retinopathy and diabetic macular oedema.
Keywords: Diabetic retinopathy, Advanced glycation, Lipoxidation
Diabetic retinopathy is a leading cause of blindness among people of working age in developed countries [2]. Despite its importance, there are currently few effective means to prevent or treat the disease. Beyond maintenance of tight glycaemic control, laser photocoagulation remains the principal therapy for sight-threatening diabetic retinopathy, but this is always at the expense of functional retina and visual performance [26]. With diabetes rapidly emerging as a global health care problem [78], there remains a genuine and urgent need to develop new, effective therapies for diabetic retinopathy that may be used to augment strict glucose management.
Most studies concerned with the pathogenesis of diabetic retinopathy have focused on alterations in the functional integrity of the intra-retinal blood vessels. The vasodegenerative phase of diabetic retinopathy is characterized by a breakdown of the blood–retinal barrier, a thickening of capillary basement membranes, pericyte and microvascular smooth muscle dropout, microaneurysms and capillary closure [26]. Persistent vascular leakage leads to macular oedema with or without cystoid degenerative changes, photoreceptor atrophy and an irreversible loss of central vision. In the proliferative phase of the disease, the ischaemic retina begins to secrete various growth factors, including vascular endothelial growth factor (VEGF), which stimulates neovascularisation. The new vessels that form during proliferative diabetic retinopathy are unable to replace the flow of necessary nutrients and instead are associated with an increased risk of severe visual loss through vitreal haemorrhage, retinal fibrogliosis and tractional retinal detachment.
While there is no argument that the retinal vasculature is central to the development of diabetic retinopathy, there is accumulating evidence that neuroretinal function is also compromised during this disease [4]. For instance, deficits in visual functioning, such as loss of colour vision [59], contrast sensitivity [63] and abnormalities in the electroretinogram [84] have been documented in patients shortly after the diagnosis of diabetes and before detection of clinically evident vascular retinopathy. Early neuronal changes are also apparent in the retinas of experimental rodent models of diabetes, including neurophysiological deficits similar to those described in human diabetes [57]. Because neuroretinal alterations occur at an early stage of the disease process, it has been proposed that they may a play a causative or contributory role in the initiation and progression of the vascular pathology associated with diabetic retinopathy [4].
Epidemiological signposts for AGE/ALE involvement in diabetic retinopathy
The Diabetes Control and Complications Trial (DCCT) was a major epidemiological study conducted from 1983 to 1993 in type 1 diabetic patients and established the relationship between hyperglycaemia and progression of retinopathy [19]. A similar long-term evaluation of type 2 diabetic patients called the UK Prospective Diabetes Study also highlighted hyperglycaemia as being critical for pathogenesis although it indicated that this occurs in unison with dyslipidaemia and hypertension [1]. Such population-based evidence and a wealth of follow-up clinical data provide a strong basis for current, ongoing research which is seeking to identify the cellular and molecular mechanisms that underpin diabetic retinopathy. Although the formation of advanced glycation end products (AGEs) and advanced lipoxidation end products (ALEs) and activation of receptors for AGEs in the diabetic retina is the focus for this review, hyperglycaemia can evoke many other important pathogenic pathways in the retina, so each should not necessarily be viewed as an independent phenomenon. Indeed, many of these share common biochemistry (such as free radical formation) and evoke common pathological events in various retinal cells. The so-called “unifying concept” whereby hyperglycaemia increases superoxide production (via the mitochondrial electron transport chain) links inter-related pathogenic responses [10]. This hypothesis suggests that enhanced flux through the hexosamine pathway, diacylglycerol-mediated activation of PKC-β and intra-cellular AGE formation can coalesce to cause cell damage. Evidence for this concept and its involvement in diabetic retinopathy has been provided through the use of the vitamin B1 thiamine derivative, benfotiamine, which stimulates transketolase activity and shunts excess triose phosphates toward the reductive pentose phosphate pathway which is impaired in high glucose/diabetes [70]. Importantly, benfotiamine can prevent AGE formation and retinal capillary degeneration in diabetic animals [35].
Biochemistry of AGE and ALE formation
Reactions between sugars and free amino groups on proteins, lipids and DNA are an inevitable consequence of aldehyde reactivity, and many molecules in our body manifest chemically attached carbohydrate. These reactions begin with the formation of Schiff bases and ε-amino groups that rearrange to Amadori adducts. These intermediates can undergo further oxidation and dehydration reactions to form irreversible protein-bound compounds collectively termed “AGEs”. During diabetes even modest hyperglycaemia can result in significant adduct accumulation on long-lived macromolecules [50].
Glucose is much less reactive than α-oxaloaldehydes which can form AGEs much more rapidly [72]. For example, glyoxal (GO) reacts with arginine residues to form carboxymethyl–arginine [28] while methylglyoxal (MGO) is a precursor for the AGEs Nε-(carboxyethyl)lysine and arginine-hydroimidazolone [71]. The concentrations of these reactive carbonyls rise in high glucose-exposed cells, occur at elevated levels in diabetic serum and are important precursors for AGEs in the body [40]. Other AGE adducts that form in the body are Nε-(carboxy-methyl) lysine (CML), crossline, pentosidine, furoyl-furanyl imidazole, hydroimidazolone, argpyrimidine, glyoxal lysine dimer and methylglyoxal lysine dimer [72].
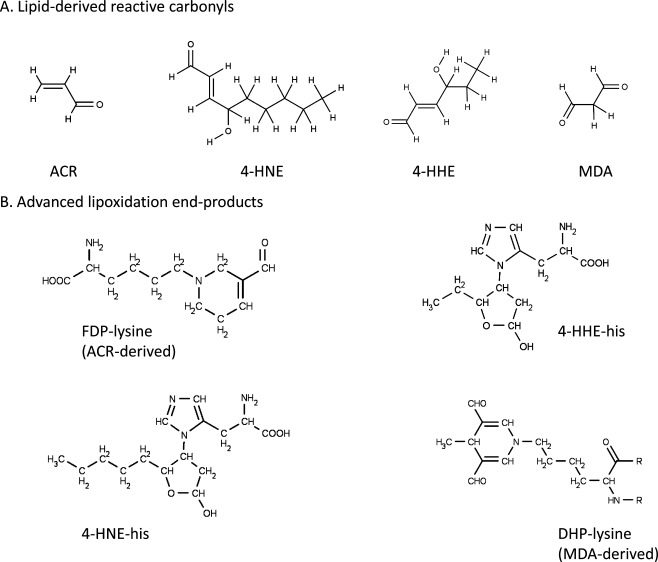
It is important to appreciate that proteins can be modified by lipids as well as carbohydrates. During oxidative stress, reactive oxygen species attack polyunsaturated fatty acids (PUFAs) either in the cell membrane or circulating lipoprotein molecules [3]. This oxidative decomposition of PUFAs initiates chain reactions that lead to the formation of a variety of reactive carbonyl species. Among them are 4-hydroxy-trans-2-nonenal (4-HNE), acrolein (ACR), malondialdehyde (MDA), 4-hydroxy-2-hexenal (4-HHE) and crotonaldehyde [75] (Fig. 1). These compounds subsequently react by the so-called Michael addition mechanism with cysteine, histidine and lysine residues in proteins, generating relatively stable adducts known as ALEs. ALEs chemically characterised to date include Nε-(2-propenal)lysine and dihydropyridine-type adducts (DHP-lysine, MDA-derived), hemiacetal and pyrrole adducts (HNE- and HHE-derived) and β-substituted propanal adducts and Nε-(3-formyl-3,4-dehydropiperidino)lysine (FDP-lysine; ACR-derived) [3] (Fig. 1). In addition to their formation through lipid peroxidation, ALEs can also be formed endogenously by carbohydrate or ascorbate autoxidation, amine-oxidases, cytochrome P450 enzymes and the myeloperoxidase system of neutrophils [54]. While ALEs are often grouped together with AGEs, they should be regarded as a distinct class of adducts due to their differing reaction chemistry. Lipid peroxidation is enhanced in diabetes, and diabetic patients have higher serum levels of MDA than non-diabetic subjects [30]. It has also been reported by our own group and others that FDP-lysine- and HNE-modified proteins accumulate in serum of patients with diabetes compared to control individuals [74, 88]. At present, however, our understanding of the role of ALEs in the pathogenesis of diabetic complications, particularly retinopathy, lags far behind that which is currently known about AGEs.
Fig. 1.
The structures of lipoxidation-derived reactive carbonyl species and the principal advanced lipoxidation adducts formed following their reaction with proteins
Most AGE/ALE adducts are highly stable at physiological pH, and their rate of accumulation in tissues depends on factors such as availability of metal ions, redox balances and longevity of the modified protein. Unlike other AGEs/ALEs, FDP-lysine is not a stable end product but a reactive intermediate that covalently binds to thiols, thereby exacerbating oxidative stress via depletion of the endogenous antioxidant glutathione [24]. Irrespective of their chemical derivation, AGE/ALE modifications can have a significant impact on protein structure and function by mediating protein–protein cross-linking reactions, changing tertiary structure and normal molecular function, conferring resistance to digestion or impairing receptor recognition.
Natural defence against AGE/ALEs
Cells have evolved natural defence mechanisms to protect against reactive carbonyl species. The capacity of cells to metabolise and detoxify carbonyls is dependent not only on the carbonyl but also on the cellular content of carbonyl-metabolising enzymes [54]. In general, aldehyde precursors of AGEs/ALEs are detoxified through oxidation reactions mediated by nicotinamide adenine dinucleotide (NAD+) and enzymes of the aldehyde dehydrogenase superfamily (ALDH). At least 19 ALDH isozymes have been identified localized to several cellular compartments and displaying varying affinities to a wide range of aldehyde substrates [90]. In addition to ALDH, other enzymes with high selectivity towards specific aldehydes have also been shown to play a detoxification role. For example, the glyoxalase complex (formed from glyoxalase I (GLO1) and glyoxalase II (GLO2) components) is an effective detoxification system for GO and MGO [42]. This has been shown in endothelial cells transfected to overexpress GLO1 which accumulate less MGO and subsequently contain less AGEs [62, 83]. Nematode worms engineered to overexpress GLO1 also contain fewer MGO-derived AGE adducts, and the worms demonstrate increased life-span [52, 61]. More recently it has been shown that overexpression of GLO1 in diabetic rats is significantly protective against key retinopathy lesions including Müller glia damage and formation of acellular capillaries [91].
AGES/ALES in diabetic retinopathy
Clinical correlates with diabetic retinopathy
In recent years, numerous studies have examined the correlation between serum levels of AGEs and the severity of retinopathy in patients with diabetes although the data have often been confusing and somewhat contradictory. For example, it has been reported that serum AGEs associate with progression of diabetic retinopathy in patients [55]. Conversely, other studies have demonstrated no significant correlation between AGE levels and retinopathy [69, 77]. Many of these reports are ELISA based using non-adduct-specific antibodies. Beyond this, investigation of defined AGEs such as CML, pentosidine or hydroimidazolone shows correlation of these adducts with retinopathy in diabetic patients [23, 69, 81].
From the ALE perspective, comparatively few studies have been undertaken to examine the association of these lipid-derived adducts and diabetic retinopathy. One study by Losado et al. reported that concentrations of the ALE precursor, MDA, are elevated in type 1 patients with retinopathy when compared to diabetics without retinopathy and healthy controls [45]. More recently, we have demonstrated that haemoglobin levels of the ACR-derived ALE, FDP-lysine, are associated with the severity of diabetic retinopathy in type 1 and type 2 diabetic patients [88]. Importantly, the relationship of FDP-lysine with retinopathy was unaltered after adjustment for HbA1c, or other clinical parameters. Thus, haemoglobin FDP-lysine could prove to be a very useful marker for stratifying patients at high risk of retinopathy not evident from simply measuring their long-term glycaemic control.
A number of studies have used skin collagen to investigate the correlations between AGEs and the severity of retinopathy in patient-based studies [49]. The most comprehensive work to date, conducted by the DCCT skin collagen ancillary study group, demonstrated a strong association between the glycooxidation product, CML, and the progression of diabetic retinopathy [48]. The Epidemiology of Diabetes Interventions and Complications trial further followed these patients and demonstrated that retinopathy was less frequent in the group initially maintained under “tight” glycaemic control. Importantly, these benefits extended far beyond the period of intensive insulin therapy [27]. The patients under “conventional” control for the first 10 years maintained a “hyperglycaemic memory” and showed considerable retinopathy progression. The Genuth and Monnier groups have shown that CML-modified skin collagen predicted the progression of retinopathy (and nephropathy) even after initiation of intensive insulin therapy [27]. Furthermore, they suggest that AGE accumulation on skin collagen is a robust biomarker for retinopathy risk and could offer a molecular-based explanation for hyperglycaemic memory.
AGEs/ALEs in the diabetic retina
AGEs have been extensively quantified in various ocular tissues and are often elevated during ageing and in diabetics when compared to non-diabetic controls [64]. This includes vitreous collagen [68], where the AGE levels correlate with diabetic retinopathy [22]. In the diabetic retina, AGEs and/or late Amadori products have been localized to vascular cells, neurons and glia [25, 33, 34, 53, 60, 66]. This would be expected to have pathogenic implications for the individual cells and retinal function. Although differential accumulation of AGEs exists in the retina over the course of life, diabetes significantly enhances the occurrence of these adducts in the vascular and neural tissue components [66].
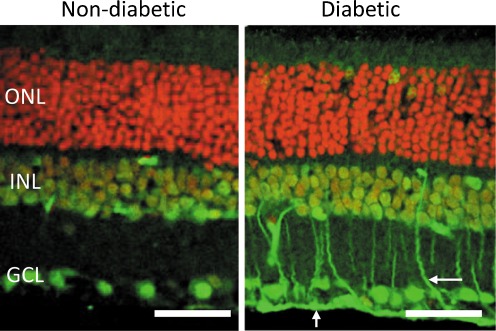
Recent studies by our group have focused on the distribution pattern of ALEs in the diabetic retina [85]. During early experimental diabetes, there is a selective accumulation of FDP-lysine in Müller glia (Fig. 2), whereas no significant differences in the levels of MDA-, 4-HNE- and 4-HHE-derived ALEs were apparent between control and diabetic retinas. Spatiotemporal studies revealed that FDP-lysine initially accumulated within the Müller glia end feet after only a few months of diabetes and thereafter spread distally throughout their inner radial processes. The observation that FDP-lysine selectively accumulates in Müller glia during diabetes is intriguing, and the underlying pathobiochemical mechanisms warrant further investigation. In this regard, since other ALE adducts do not appear to be elevated in diabetic Müller glia, it seems unlikely that the accumulation of FDP-lysine can simply be attributed to an increase in the levels of lipid peroxidation. A more likely explanation may relate to the fact that Müller glia are known to function as the main polyamine storage cells of the retina [7]. ACR, the precursor for FDP-lysine, can be formed from spermine by spermine oxidase and spermidine by spermidine/spermine N1-acetyltransferase and acetylpolyamine oxidase [73]. As such, the selective accumulation of FDP-lysine in Müller glia could reflect changes in polyamine catabolism in the diabetic retina, a possibility that is currently being addressed by ongoing studies within our laboratory.
Fig. 2.
FDP-lysine immunoreactivity (green channel) in retinal sections from non-diabetic and diabetic rats of 4-month disease duration. Cell nuclei are stained red by propidium iodide. In diabetes, prominent immunolabelling appeared in Müller glia end-feet and radial processes (arrows). GCL ganglion cell layer, INL inner nuclear layer, ONL outer nuclear layer. Scale bars = 50μm
AGEs/ALEs evoke retinal cell dysfunction and death
Retinal capillary degeneration remains a hallmark of retinopathy in diabetic animal models and patients [26]. Retinal capillaries appear to be important targets for AGE-induced toxicity. For example, AGEs induce toxic effects on retinal pericytes by inducing oxidative stress and subsequent apoptosis [16]. In addition, some studies have indicated that AGEs cause osteoblastic differentiation and calcification in retinal pericytes by the activation of alkaline phosphatases [80]. Pericytes growing on AGE-modified basement membrane show acute attenuation of endothelin-1 (ETA receptor mediated) contraction, suggesting that AGE-cross links in a surrounding matrix significantly influence pericyte physiology [36]. Indeed, longer exposure times to these substrate-AGEs induce loss of integrin signalling and apoptosis [44]. The GLO1 detoxification system is also critical for retinal pericyte survival, but this may be insufficient during diabetes since these cells undergo apoptosis as a direct result of MGO-derived AGE formation [47]. Strategies to enhance carbonyl detoxification in retinal cells could be an important future therapeutic strategy.
Retinal microvascular endothelial cells show pro-angiogenic responses to AGEs at lower concentrations by the involvement of MAPK, PKC and NF-κB signalling pathways [46], although at higher concentrations, these adducts are toxic to endothelial cells [17] and in vivo may eventually lead to enhanced microvascular closure [67]. Under hyperglycaemic conditions, retinal microvascular endothelial cells accumulate MGO and MGO-derived AGE adducts (such as hydroimidazolone and argpyrimidine) which in vivo contribute to premature closure of capillaries [56]. AGEs cause upregulation of ICAM which mediates retinal capillary leukocyte adherence and inner blood retinal barrier breakdown [51]. Independent of the complexities of the diabetic milieu, non-diabetic mice exposed to “diabetic-like” levels of injected AGE-albumin show increased retinal expression of VEGF concomitant with blood retinal barrier dysfunction [15]. Similar treatments may cause loss of pericytes [79], and taken together, this suggests that high serum levels of AGE-modified proteins (as particularly evident in diabetic patients with renal dysfunction) induce lesions that are comparable to those occurring during diabetic retinopathy.
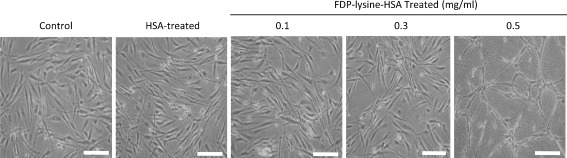
Whilst the detrimental effects of AGEs/ALEs on retinal vascular cells are well recognised, their potential role in mediating neuroretinal changes in diabetes has only recently begun to be explored. In retinal explants obtained from non-diabetic animals, incubation with AGE-modified albumin directly triggers neural cell apoptosis through a caspase-dependent pathway [43]. To better understand the possible pathogenic effects of FDP-lysine in Müller glia, we have recently developed an experimental in vitro model of FDP-lysine accumulation in human Müller cells [85]. Accumulation of FDP-lysine causes Müller glia dysfunction consistent with that observed in the diabetic retina, including the induction of oxidative stress, upregulation of pro-inflammatory cytokines and VEGF, and dysregulation of K+ channel protein expression [85]. Diabetes is one of the few pathological conditions where Müller cell apoptosis has been shown to occur [9]. Exposure of Müller cells to high concentrations of FDP-lysine causes extensive cell death (Fig. 3) through apoptosis induction [85], suggesting that FDP-lysine accumulation could be a major factor contributing to Müller cell death during long-term diabetes.
Fig. 3.
Phase-contrast micrographs of cultured human MIO-M1 Müller glia exposed to increasing concentrations of FDP-lysine-modified human serum albumin (FDP-lysine-HSA). High concentrations of FDP-lysine-HSA (0.5 mg/mL) induce cell death. Scale bars = 100μm
AGE/ALE inhibition and prevention of retinopathy
The first AGE inhibitor was aminoguanidine (Pimagedine) [11]. This small nucleophilic hydrazine drug was shown to prevent AGE formation and attenuate diabetic retinopathy in animal models [25, 32, 38]. In a subsequent clinical trial, aminoguanidine showed positive signs towards slowing the progression of retinopathy, but it ultimately failed [8]. This hydrazine is not a specific inhibitor of AGE formation and is also a potent iNOS inhibitor [58]. A class of drugs called “Amadorins” have an ability to scavenge reactive carbonyls and prevent conversion of Amadori intermediates to AGEs and ALEs [39]. The derivative of vitamin B6, pyridoxamine (Pyridorin™), is an efficacious and specific post-Amadori inhibitor [76] which reduces retinal AGE accumulation and also attenuates capillary acellularity in the diabetic rat retina [65]. Likewise, a drug called LR-90 has been developed as an effective multi-stage inhibitor of both AGE/ALE formation and prevents diabetic retinopathy [6].
It is noteworthy that drugs such as aminoguanidine and pyridoxamine which have been used widely to prevent advanced glycation reactions in diabetes are relatively poor scavengers of ACR [13]. Indeed, we have found that pyridoxamine is only moderately effective in preventing FDP-lysine accumulation in the diabetic retina, although it is still capable of attenuating Müller glial dysfunction and lesion formation [18]. On the other hand, hydrazino compounds such as hydralazine and dihydralazine react readily with ACR and have also been found to target protein-bound FDP-lysine adducts [12, 13]. Hydralazine and dihydralazine have been approved clinically as antihypertensive drugs for many years, but these cardiovascular actions complicate their potential use for the treatment of diabetic retinopathy. Since the hydrazine groups on hydralazine and dihydralazine are known to be responsible for their ACR scavenging abilities [31], but not their anti-hypertensive effects [21], it is evident that other compounds containing hydrazino groups could be highly suitable as therapeutic agents for diabetic retinopathy. Indeed, the non-vasoactive hydralazine analogue, 1-hydrazinoisoquinolone, has recently been proposed to protect against tobacco smoke-related lung toxicity through its ability to suppress ACR levels [14]. Future studies are clearly warranted to identify the most appropriate hydrazino compounds for preventing FDP-lysine accumulation in the diabetic retina.
The AGE–RAGE axis in diabetic retinopathy
The receptor for AGEs (RAGE) is the most-established AGE-binding protein and acts as a signalling receptor for the common AGE CML [41] and hydroimidazolone adducts [29]. RAGE is a multi-ligand receptor and binds to many molecules including S100B, high-mobility group box-1, amyloid-β and Mac-1 [82] and regulates signalling in a range of cells and tissues. Ligand-binding and signal transduction activate transcription of NFκB and induction of adhesion molecules, cytokines and/or oxidative stress. In the context of the diabetic retina, this is significant since it is now widely appreciated that the retinal expression of pro-inflammatory cytokines and elaboration of adhesion molecules on the microvasculature are linked to leukostasis and possible occlusion of capillaries [37]. In the diabetic retina, there is a clear imbalance of cytokines, and it seems likely that factors such as IL-1α, IL-1β, IL-6 and TNF-α are linked to inflammatory processes including microglial activation and infiltration of monocytes [86, 87].
RAGE is expressed in many retinal cells, although the highest expression levels seem to be in Müller glia where it is constitutively expressed at low levels. During diabetes there is significant upregulation of this receptor where it co-localises with GFAP and vimentin [87, 88]. It is worth noting that some of the other non-AGE RAGE ligands also occur in the retina, and these may be capable of inducing pro-inflammatory signalling. For example, S100B is found in several retinal cell types including photoreceptors and Müller glia [20]. S100B has neurotrophic role at low levels although upregulation occurs in the Müller glia of diabetic animal models where it can induce inflammatory cytokine expression [89].
Blockade of RAGE may be a useful therapeutic strategy. For example, it has been shown that the soluble RAGE fragment (known as “sRAGE”) can prevent Muller cell dysfunction [5] during diabetes and retinal capillary leukostasis in AGE-infused (non-diabetic) mice [51]. RAGE blockade using various agents shows great potential for preventing diabetic retinopathy, and the coming years should bring new efficacious agents that can regulate activation of this receptor in the retina.
Conclusion
There is no question that management of retinopathy in patients will involve precise regulation of glycaemic, vasotensive and lipidemic profiles. As we go forward, there is expectation that new therapeutic agents will become available to prevent key biochemical and metabolic abnormalities that are definitively linked to neuroglial and vascular pathology. AGE/ALE formation and activation of AGE receptors appear to play an important role in diabetic retinopathy, and if we can regulate these pathways, there is hope that initiation and/or progression of this important complication can be prevented.
Acknowledgements
The authors would like to acknowledge the Juvenile Diabetes Research Foundation (JDRF) and Fight for Sight. We also thank Dr. Phaik-Har Yong for her input to this work.
References
- 1.UKPDS Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 2.Aiello LP, Gardner TW, King GL, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 3.Aldini G, Dalle-Donne I, Facino RM, et al. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med Res Rev. 2007;27:817–868. doi: 10.1002/med.20073. [DOI] [PubMed] [Google Scholar]
- 4.Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 5.Barile GR, Pachydaki SI, Tari SR, et al. The RAGE axis in early diabetic retinopathy. Investig Ophthalmol Vis Sci. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- 6.Bhatwadekar A, Glenn JV, Figarola JL, et al. A new advanced glycation inhibitor, LR-90, prevents experimental diabetic retinopathy in rats. Br J Ophthalmol. 2008;92:545–547. doi: 10.1136/bjo.2007.127910. [DOI] [PubMed] [Google Scholar]
- 7.Biedermann B, Skatchkov SN, Brunk I, et al. Spermine/spermidine is expressed by retinal glial (Muller) cells and controls distinct K+ channels of their membrane. Glia. 1998;23:209–220. doi: 10.1002/(SICI)1098-1136(199807)23:3<209::AID-GLIA4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Bolton WK, Cattran DC, Williams ME, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 9.Bringmann A, Uckermann O, Pannicke T, et al. Neuronal versus glial cell swelling in the ischaemic retina. Acta Ophthalmol Scand. 2005;83:528–538. doi: 10.1111/j.1600-0420.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 10.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M, Vlassara H, Kooney A, et al. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 12.Burcham PC, Fontaine FR, Kaminskas LM, et al. Protein adduct-trapping by hydrazinophthalazine drugs: mechanisms of cytoprotection against acrolein-mediated toxicity. Mol Pharmacol. 2004;65:655–664. doi: 10.1124/mol.65.3.655. [DOI] [PubMed] [Google Scholar]
- 13.Burcham PC, Kaminskas LM, Fontaine FR, et al. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology. 2002;181–182:229–236. doi: 10.1016/S0300-483X(02)00287-1. [DOI] [PubMed] [Google Scholar]
- 14.Burcham PC, Raso A, Thompson CA. Toxicity of smoke extracts towards A549 lung cells: role of acrolein and suppression by carbonyl scavengers. Chem Biol Interact. 2010;183:416–424. doi: 10.1016/j.cbi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Canning P, Glenn JV, Hsu DK, et al. Inhibition of advanced glycation and absence of galectin-3 prevent blood-retinal barrier dysfunction during short-term diabetes. Exp Diabetes Res. 2007;2007:51837. doi: 10.1155/2007/51837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen BH, Jiang DY, Tang LS. Advanced glycation end-products induce apoptosis involving the signaling pathways of oxidative stress in bovine retinal pericytes. Life Sci. 2006;79:1040–1048. doi: 10.1016/j.lfs.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Chibber R, Molinatti PA, Rosatto N, et al. Toxic action of advanced glycation end products on cultured retinal capillary pericytes and endothelial cells: relevance to diabetic retinopathy. Diabetologia. 1997;40:156–164. doi: 10.1007/s001250050657. [DOI] [PubMed] [Google Scholar]
- 18.Curtis TM, Hamilton R, Yong PH, et al. Muller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products. Diabetologia. 2011;54:690–698. doi: 10.1007/s00125-010-1971-x. [DOI] [PubMed] [Google Scholar]
- 19.No authors listed. Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes 1993;46:271–286 [PubMed]
- 20.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. doi: 10.1016/S1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 21.Druey J, Marxer A. Hypotensive hydrazinophthalazines and related compounds. J med pharm chem. 1959;1:1–21. doi: 10.1021/jm50002a001. [DOI] [PubMed] [Google Scholar]
- 22.Fosmark DS, Bragadottir R, Stene-Johansen I, et al. Increased vitreous levels of hydroimidazolone in type 2 diabetes patients are associated with retinopathy: a case-control study. Acta Ophthalmol Scand. 2007;6:618–622. doi: 10.1111/j.1600-0420.2007.00913.x. [DOI] [PubMed] [Google Scholar]
- 23.Fosmark DS, Torjesen PA, Kilhovd BK, et al. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metabolism. 2006;55:232–236. doi: 10.1016/j.metabol.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Furuhata A, Nakamura M, Osawa T, et al. Thiolation of protein-bound carcinogenic aldehyde. An electrophilic acrolein-lysine adduct that covalently binds to thiols. J Biol Chem. 2002;277:27919–27926. doi: 10.1074/jbc.M202794200. [DOI] [PubMed] [Google Scholar]
- 25.Gardiner TA, Anderson HR, Stitt AW. Inhibition of advanced glycation end-products protects against retinal capillary basement membrane expansion during long-term diabetes. J Pathol. 2003;201:328–333. doi: 10.1002/path.1429. [DOI] [PubMed] [Google Scholar]
- 26.Gardiner TA, Archer DB, Curtis TM, et al. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation. 2007;14:25–38. doi: 10.1080/10739680601072123. [DOI] [PubMed] [Google Scholar]
- 27.Genuth S, Sun W, Cleary P, et al. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes. 2005;54:3103–3111. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glomb MA, Pfahler C. Amides are novel protein modifications formed by physiological sugars. J Biol Chem. 2001;276:41638–41647. doi: 10.1074/jbc.M103557200. [DOI] [PubMed] [Google Scholar]
- 29.Goldin A, Beckman JA, Schmidt AM, et al. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 30.Griesmacher A, Kindhauser M, Andert SE, et al. Enhanced serum levels of thiobarbituric-acid-reactive substances in diabetes mellitus. Am J Med. 1995;98:469–475. doi: 10.1016/S0002-9343(99)80347-7. [DOI] [PubMed] [Google Scholar]
- 31.Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem. 2009;111:1348–1356. doi: 10.1111/j.1471-4159.2009.06395.x. [DOI] [PubMed] [Google Scholar]
- 32.Hammes HP, Martin S, Federlin K, et al. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc Natl Acad Sci (USA) 1991;88:11555–11558. doi: 10.1073/pnas.88.24.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammes HP, Alt A, Niwa T, et al. Differential accumulation of advanced glycation end products in the course of diabetic retinopathy. Diabetologia. 1999;42:728–736. doi: 10.1007/s001250051221. [DOI] [PubMed] [Google Scholar]
- 34.Hammes HP, Brownlee M, Edelstein D, et al. Aminoguanidine inhibits the development of accelerated diabetic retinopathy in the spontaneous hypertensive rat. Diabetologia. 1994;37:32–35. doi: 10.1007/BF00428774. [DOI] [PubMed] [Google Scholar]
- 35.Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 36.Hughes SJ, Wall N, Scholfield CN, et al. Advanced glycation endproduct modified basement membrane attenuates endothelin-1 induced [Ca2+]i signalling and contraction in retinal microvascular pericytes. Mol Vis. 2004;10:996–1004. [PubMed] [Google Scholar]
- 37.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 38.Kern TS, Engerman RL. Pharmacological inhibition of diabetic retinopathy: aminoguanidine and aspirin. Diabetes. 2001;50:1636–1642. doi: 10.2337/diabetes.50.7.1636. [DOI] [PubMed] [Google Scholar]
- 39.Khalifah RG, Baynes JW, Hudson BG. Amadorins: novel post-Amadori inhibitors of advanced glycation reactions. Biochem Biophys Res Commun. 1999;257:251–258. doi: 10.1006/bbrc.1999.0371. [DOI] [PubMed] [Google Scholar]
- 40.Kilhovd BK, Giardino I, Torjesen PA, et al. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism. 2003;52:163–167. doi: 10.1053/meta.2003.50035. [DOI] [PubMed] [Google Scholar]
- 41.Kislinger T, Fu C, Huber B, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 42.Kuhla B, Luth HJ, Haferburg D, et al. Methylglyoxal, glyoxal, and their detoxification in Alzheimer’s disease. Ann N Y Acad Sci. 2005;1043:211–216. doi: 10.1196/annals.1333.026. [DOI] [PubMed] [Google Scholar]
- 43.Lecleire-Collet A, Tessier LH, Massin P, et al. Advanced glycation end products can induce glial reaction and neuronal degeneration in retinal explants. Br J Ophthalmol. 2005;89:1631–1633. doi: 10.1136/bjo.2005.079491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu B, Bhat M, Padival AK, et al. Effect of dicarbonyl modification of fibronectin on retinal capillary pericytes. Investig Ophthalmol Vis Sci. 2004;45:1983–1995. doi: 10.1167/iovs.03-0995. [DOI] [PubMed] [Google Scholar]
- 45.Losada M, Alio JL. Malondialdehyde serum concentration in type 1 diabetic with and without retinopathy. Doc Ophthalmol. 1996;93:223–229. doi: 10.1007/BF02569062. [DOI] [PubMed] [Google Scholar]
- 46.Mamputu JC, Renier G. Signalling pathways involved in retinal endothelial cell proliferation induced by advanced glycation end products: inhibitory effect of gliclazide. Diabetes Obes Metab. 2004;6:95–103. doi: 10.1111/j.1462-8902.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- 47.Miller AG, Smith DG, Bhat M, et al. Glyoxalase I is critical for human retinal capillary pericyte survival under hyperglycemic conditions. J Biol Chem. 2006;281:11864–11871. doi: 10.1074/jbc.M513813200. [DOI] [PubMed] [Google Scholar]
- 48.Monnier VM, Bautista O, Kenny D, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes. 1999;48:870–880. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monnier VM, Mustata GT, Biemel KL, et al. Cross-linking of the extracellular matrix by the maillard reaction in aging and diabetes: an update on “a puzzle nearing resolution”. Ann N Y Acad Sci. 2005;1043:533–544. doi: 10.1196/annals.1333.061. [DOI] [PubMed] [Google Scholar]
- 50.Monnier VM, Sell DR, Genuth S. Glycation products as markers and predictors of the progression of diabetic complications. Ann N Y Acad Sci. 2005;1043:567–581. doi: 10.1196/annals.1333.065. [DOI] [PubMed] [Google Scholar]
- 51.Moore TC, Moore JE, Kaji Y, et al. The role of advanced glycation end products in retinal microvascular leukostasis. Investig Ophthalmol Vis Sci. 2003;44:4457–4464. doi: 10.1167/iovs.02-1063. [DOI] [PubMed] [Google Scholar]
- 52.Morcos M, Du X, Pfisterer F, et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell. 2008;7:260–269. doi: 10.1111/j.1474-9726.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 53.Murata T, Nagai R, Ishibashi T, et al. The relationship between accumulation of advanced glycation end products and expression of vascular endothelial growth factor in human diabetic retinas. Diabetologia. 1997;40:764–769. doi: 10.1007/s001250050747. [DOI] [PubMed] [Google Scholar]
- 54.O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 55.Ono Y, Aoki S, Ohnishi K, et al. Increased serum levels of advanced glycation end-products and diabetic complications. Diabetes Res Clin Pract. 1998;41:131–137. doi: 10.1016/S0168-8227(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 56.Padayatti PS, Jiang C, Glomb MA, et al. High concentrations of glucose induce synthesis of argpyrimidine in retinal endothelial cells. Curr Eye Res. 2001;23:106–115. doi: 10.1076/ceyr.23.2.106.5472. [DOI] [PubMed] [Google Scholar]
- 57.Phipps JA, Fletcher EL, Vingrys AJ. Paired-flash identification of rod and cone dysfunction in the diabetic rat. Investig Ophthalmol Vis Sci. 2004;45:4592–4600. doi: 10.1167/iovs.04-0842. [DOI] [PubMed] [Google Scholar]
- 58.Ren XY, Li YN, Qi JS, et al. Peroxynitrite-induced protein nitration contributes to liver mitochondrial damage in diabetic rats. J Diabetes Complications. 2008;22:357–364. doi: 10.1016/j.jdiacomp.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Roy MS, Gunkel RD, Podgor MJ. Color vision defects in early diabetic retinopathy. Arch Ophthalmol. 1986;104:225–228. doi: 10.1001/archopht.1986.01050140079024. [DOI] [PubMed] [Google Scholar]
- 60.Schalkwijk CG, Ligtvoet N, Twaalfhoven H, et al. Amadori albumin in type 1 diabetic patients: correlation with markers of endothelial function, association with diabetic nephropathy, and localization in retinal capillaries. Diabetes. 1999;48:2446–2453. doi: 10.2337/diabetes.48.12.2446. [DOI] [PubMed] [Google Scholar]
- 61.Schlotterer A, Kukudov G, Bozorgmehr F, et al. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes. 2009;58:2450–2456. doi: 10.2337/db09-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shinohara M, Thornalley PJ, Giardino I, et al. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101:1142–1147. doi: 10.1172/JCI119885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sokol S, Moskowitz A, Skarf B, et al. Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol. 1985;103:51–54. doi: 10.1001/archopht.1985.01050010055018. [DOI] [PubMed] [Google Scholar]
- 64.Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol. 2001;85:746–753. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stitt AW, Gardiner TA, Alderson NL, et al. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- 66.Stitt AW, Li YM, Gardiner TA, et al. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am J Pathol. 1997;150:523–531. [PMC free article] [PubMed] [Google Scholar]
- 67.Stitt AW, Mcgoldrick C, Rice-Mccaldin A, et al. Impaired retinal angiogenesis in diabetes: role of advanced glycation end products and galectin-3. Diabetes. 2005;54:785–794. doi: 10.2337/diabetes.54.3.785. [DOI] [PubMed] [Google Scholar]
- 68.Stitt AW, Moore JE, Sharkey JA, et al. Advanced glycation end products in vitreous: structural and functional implications for diabetic vitreopathy. Investig Ophthalmol Vis Sci. 1998;39:2517–2523. [PubMed] [Google Scholar]
- 69.Sugiyama S, Miyata T, Ueda Y, et al. Plasma levels of pentosidine in diabetic patients: an advanced glycation end product. J Am Soc Nephrol. 1998;9:1681–1688. doi: 10.1681/ASN.V991681. [DOI] [PubMed] [Google Scholar]
- 70.Thornalley PJ. The potential role of thiamine (vitamin B1) in diabetic complications. Curr Diabetes Rev. 2005;1:287–298. doi: 10.2174/157339905774574383. [DOI] [PubMed] [Google Scholar]
- 71.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344(Pt 1):109–116. doi: 10.1042/0264-6021:3440109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25:275–281. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 73.Tomitori H, Usui T, Saeki N, et al. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36:2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 74.Toyokuni S, Yamada S, Kashima M, et al. Serum 4-hydroxy-2-nonenal-modified albumin is elevated in patients with type 2 diabetes mellitus. Antioxid Redox Signal. 2000;2:681–685. doi: 10.1089/ars.2000.2.4-681. [DOI] [PubMed] [Google Scholar]
- 75.Uchida K, Kanematsu M, Morimitsu Y, et al. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 76.Voziyan PA, Metz TO, Baynes JW, et al. A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J Biol Chem. 2002;277:3397–3403. doi: 10.1074/jbc.M109935200. [DOI] [PubMed] [Google Scholar]
- 77.Wagner Z, Wittmann I, Mazak I, et al. N(epsilon)-(carboxymethyl)lysine levels in patients with type 2 diabetes: role of renal function. Am J Kidney Dis. 2001;38:785–791. doi: 10.1053/ajkd.2001.27695. [DOI] [PubMed] [Google Scholar]
- 78.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 79.Xu X, Li Z, Luo D, et al. Exogenous advanced glycosylation end products induce diabetes-like vascular dysfunction in normal rats: a factor in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2003;241:56–62. doi: 10.1007/s00417-002-0575-7. [DOI] [PubMed] [Google Scholar]
- 80.Yamagishi S, Fujimori H, Yonekura H, et al. Advanced glycation endproducts accelerate calcification in microvascular pericytes. Biochem Biophys Res Commun. 1999;258:353–357. doi: 10.1006/bbrc.1999.0625. [DOI] [PubMed] [Google Scholar]
- 81.Yamaguchi M, Nakamura N, Nakano K, et al. Immunochemical quantification of crossline as a fluorescent advanced glycation endproduct in erythrocyte membrane proteins from diabetic patients with or without retinopathy. Diabet Med. 1998;15:458–462. doi: 10.1002/(SICI)1096-9136(199806)15:6<458::AID-DIA601>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 82.Yan SD, Chen X, Fu J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 83.Yao D, Taguchi T, Matsumura T, et al. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem. 2007;282:31038–31045. doi: 10.1074/jbc.M704703200. [DOI] [PubMed] [Google Scholar]
- 84.Yonemura D, Aoki T, Tsuzuki K. Electroretinogram in diabetic retinopathy. Arch Ophthalmol. 1962;68:19–24. doi: 10.1001/archopht.1962.00960030023005. [DOI] [PubMed] [Google Scholar]
- 85.Yong PH, Zong H, Medina RJ, et al. Evidence supporting a role for N-(3-formyl-3,4-dehydropiperidino)lysine accumulation in Muller glia dysfunction and death in diabetic retinopathy. Mol Vis. 2010;16:2524–2538. [PMC free article] [PubMed] [Google Scholar]
- 86.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 87.Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000;17:463–471. doi: 10.1017/S0952523800173122. [DOI] [PubMed] [Google Scholar]
- 88.Zhang X, Lai Y, Mccance DR, et al. Evaluation of N (epsilon)-(3-formyl-3,4-dehydropiperidino)lysine as a novel biomarker for the severity of diabetic retinopathy. Diabetologia. 2008;51:1723–1730. doi: 10.1007/s00125-008-1071-3. [DOI] [PubMed] [Google Scholar]
- 89.Zong H, Ward M, Madden A, et al. Hyperglycaemia-induced pro-inflammatory responses by retinal Muller glia are regulated by the receptor for advanced glycation end-products (RAGE) Diabetologia. 2010;53:2656–2666. doi: 10.1007/s00125-010-1900-z. [DOI] [PubMed] [Google Scholar]
- 90.Jackson B, Brocker C, Thompson DC, Black W, Vasiliou K, Nebert DW, Vasiliou V. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum Genomics. 2011;5(4):283–303. [DOI] [PMC free article] [PubMed]
- 91.Berner AK, Brouwers O, Pringle R, Klaassen I, Colhoun L, McVicar C, Brockbank S, Curry JW, Miyata T, Brownlee M, Schlingemann RO, Schalkwijk C, Stitt AW. Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia. 2011, doi:10.1007/s00125-011-2393-0. [DOI] [PubMed]