Abstract
Embryonic zebrafish were used to assess the impact of solution ion concentrations on agglomeration and resulting in vivo biological responses of gold nanoparticles (AuNPs). The minimum ion concentration necessary to support embryonic development was determined. Surprisingly, zebrafish exhibit no adverse outcomes when raised in nearly ion-free media. During a rapid throughput screening of AuNPs, 1.2-nm 3-mercaptopropionic acid-functionalized AuNPs (1.2-nm 3-MPA-AuNPs) rapidly agglomerate in exposure solutions. When embryos were exposed to 1.2-nm 3-MPA-AuNPs dispersed in low ionic media, both morbidity and mortality were induced, but when suspended in high ionic media, there was little to no biological response. We demonstrated that the media ionic strength greatly affects agglomeration rates and biological responses. Most importantly, the insensitivity of the zebrafish embryo to external ions indicates that it is possible, and necessary, to adjust the exposure media conditions to optimize NP dispersion prior to assessment.
Keywords: Danio rerio, ion concentration, nanomaterial–biological interactions, toxicity
Introduction
The use and incorporation of nanoparticles (NPs) into industrial and consumer products is increasing. At the present, their impact on the environment and human health remains largely unknown. Although many studies have investigated NP effects, the materials used, the experimental assays, the model and platform (in vivo or in vitro) are highly diverse. These non-systematic approaches make it difficult to interpret the results and understand potential health and environmental implications of NPs. Without toxicological data collected systematically (with similar materials, relevant platforms and assays), it will be challenging to identify potential risks associated with NP exposure. Efficient, relevant and reliable toxicological models will help acquire these data.
One means of bridging this knowledge gap is by assessing NP toxicity in complex biological systems. Toxicological assessments can utilize both in vitro and in vivo methodologies. While cell culture-based approaches are rapid, cost-effective and amendable to high-throughput analysis, the utility and predictivity of in vitro data is limited as individual cells in artificial culture environments lack the complexity of whole animal systems. Commonly used in vivo systems, such as laboratory rodents (Paigen 2003), are likely more relevant and are extensively used for hazard identification as part of a risk assessment process. However, for the evaluation of numerous NPs, the animal- and labour-related cost and the high-test material demands lend rodent-based studies incompatible for high-throughput data collection. Rapid, applicable toxicity screens are necessary to assess the backlog of untested NPs and to begin defining the basic NP characteristics that drive biological responses.
An alternative model to help understand the influence of NP stability on biological responses is the embryonic zebrafish model (Parng 2005; Bowman & Zon 2010). As a widely accepted model for mechanistic-based toxicological studies, the embryonic zebrafish offer a rapid, high-throughput platform to assess chemical and biological system interactions (Yang et al. 2009;Furgeson & Fako 2009; Rubinstein 2003). Female zebrafish produce a few hundred embryos each spawn, which allows for large sample sizes and rapid assessments. Embryos develop externally and are optically clear, allowing for non-invasive evaluations. While other researchers have used zebrafish to assess NPs toxicity (Lee et al. 2007; Bar-Ilan et al. 2009), our group has developed rapid methods using this model to quickly evaluate NP responses in a multi-well plate format in a systematic manner (Harper et al. 2008; Truong et al. In press; Usenko et al. 2007). Using this in vivo platform, the impact of NP exposure on mortality, morbidity and complex central nervous system function can be rapidly assessed. These assessments are simultaneous and allow for evaluations using a minimal amount of nanomaterials, which further favours rapid throughput data collection.
Most in vivo and in vitro screening approaches utilize media that are rich in ionic species. These ions are often critical for viability and cellular function. It is well established that the suspension of NPs in ion-rich aqueous solution often agglomerates resulting in a loss of NPs monodispersion (Murdock et al. 2008). This issue of NP agglomeration in aqueous conditions extends to effect the assessment of NPs in other systems such as cell culture (Jin et al. 2010) and in vivo models such as embryonic zebrafish. Agglomeration of NPs is problematical since the resulting surface area, charge and sizes are drastically different compared with the synthesized particle. Clearly, these parameters influence resulting toxicological outcome.
At present, most researchers assess NP stability following synthesis in deionized or reverse osmosis (RO) water (Sayes & Warheit 2009), while toxicological studies are often evaluated in ion-rich assay systems. Many groups have tackled the dispersion challenge by coating NPs with various moieties. These include surfactants and compounds such as sodium dodecyl sulphate, sodium citrate, gum arabic, poly-vinylpyrrolidone, or ligands and polymers (Olenin et al. 2008; Tolaymat et al. 2008). Although these agents are effective in favouring dispersion, they dramatically alter NP surface properties. Coating types on quantum dots are the primary determinant of cytotoxicity and immunotoxicity in HEK cell lines (Ryman-Rasmussen et al. 2007). Alteration to the surface coatings results in significantly varied cytotoxic response to iron oxide NPs (Ying & Hwang 2010). When natural organic matter is added, the surface properties and size characteristics are dramatically altered. For example, Suwannee River humic acid and fulvic acid, when added to C60 fullerenes, agglomerate size, and morphology significantly changed (Xie et al. 2008). The widespread use of coatings is a major concern as they are used to modify the surface properties of the suspended test material. Since NP surface properties are major drivers of the NP–biological interface, NP coating for experimental convenience may complicate data interpretation. We propose that the zebrafish offer an alternative method to assessing NP toxicity by overcoming some of these limitations. Zebrafish are brackish fish that can tolerate a wide range of ion concentrations (Lawrence 2007; Uliano et al. 2010), but the tolerable range is not well defined for embryonic development. Embryos can tolerate an increased salinity level of 0.196 parts per thousand (ppt) up to 2 ppt (Sawant et al. 2001).
With this information, we instead focused on defining the minimum ion concentration necessary to support normal embryonic zebrafish development. We reasoned that if zebrafish could develop normally in low ionic concentration media, more classes of NPs could be assessed because NP agglomeration could be minimized during the exposure period. In previous studies, we assessed NP–biological interactions from a library of gold NPs (AuNPs). During that screening, 1.2-nm 3-mercaptopropionic acid-functionalized AuNPs (1.2-nm 3-MPA-AuNPs) rapidly agglomerated and fell out of solution. The goal of the current proof of concept study was to determine the influence of ionic strength on agglomeration and resulting biological responses. We determined that zebrafish can develop normally up to 120 h post-fertilization (hpf) in the absence of externally added ions and that the ionic concentration of the media greatly influenced the agglomeration rate and biological responses of 1.2-nm 3-MPA-AuNPs. The tolerance of zebrafish embryos to various ionic strength media will have the practical advantage in extending the range of materials that can be more accurately assessed in this powerful in vivo model.
Materials and methods
Nanoparticles
Materials
Hydrogen tetrachloroaurate (HAuCl4•H2O) was purchased from Strem (Newburyport, MA). Dichloromethane and tetrahydrofuran (THF) were purchased from Mallinckrodt Chemicals (Phillipsburg, NJ). All other compounds were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO). All chemicals were used as received. Nanopure water (18.2 MΩ•cm resistivity) was prepared with a Barnstead Nanopure filtration system and used for all aqueous samples. Polyethersulfone diafiltration membranes Omega TI10K were obtained from Pall Life Sciences (Ann Arbor, MI). The amine-functionalized SMART grids for transmission electron microscopy (TEM) imaging were purchased from Dune Sciences (Eugene, OR).
Procedure for the preparation of 3-MPA-protected AuNP
Water-soluble, 3-mercaptopropionic stabilized NPs were prepared through interfacial ligand exchange reaction between 1.5-nm phosphine-stabilized NPs (Au101(PPh)21Cl5) dissolved in dichloromethane/THF mixture with 3-MPA in water using the literature procedure (Woehrle et al. 2005). Briefly, a solution of 45 mg of 1.5-nm Au101(PPh)21Cl5 in 20 ml of dichloromethane/THF (1:1 mixture) was added to a solution of 23 mg of 3-MPA in 30 ml of phosphate KH2PO4/K2HPO4 buffer (10 mM, pH = 8). The biphasic reaction mixture was stirred rapidly at room temperature for 4 h. The reaction was completed when dark-coloured NPs transferred from the organic to aqueous phase. The layers were separated, and organic impurities were removed by washing the aqueous layer with dichloromethane. Solvents were removed under reduced pressure at room temperature and crude material was purified from excess ligand by diafiltration using 10 kDa membrane with 50 volumes of nanopure water. After lyophilization, the powdered material was obtained and characterized.
Analytical procedures
Proton NMR spectra were collected at 25°C on a Varian Unity Inova 300-MHz instrument in D2O. Chemical shifts are in δ units (ppm) with residual solvent peak (D2O δ4.65) as the internal standard. Ultraviolet-visible (UV-Vis) spectra were obtained on a Hewlett-Packard 8453 diode array instrument with a fixed slit width of 1 nm using 1-cm quartz cuvettes. TEM images were collected at 300 kV with an FEI Titan using a Cs aberration corrector. NP samples were prepared on amine-functionalized SMART grids by soaking the grid in a dilute NP solution (0.2 mg/ml) and then in nanopure water for 2 min each. The grid was then dried in the air.
Zebrafish
Exposure protocol
Adult Tropical 5D strain of zebrafish (Danio rerio) were kept at standard laboratory conditions of 28°C on a 14-h light/10-h dark photoperiod in fish water (FW) consisting of RO water supplemented with a commercially available salt solution (0.6% Instant Ocean®). Zebrafish were housed and reared at Sinnhuber Aquatic Research Laboratory at Oregon State University. Adult zebrafish were group-spawned and embryos were collected and staged (Kimmel et al. 1995). To increase the bioavailability, at 4 hpf, the chorion, an acellular envelope surrounding the embryo, was removed by pronase. Briefly, embryos were placed in 25 ml of FW with 50 μl of 41 mg/ml pronase (Sigma-Aldrich, St. Louis, MO) and gently agitated for 6.5 min; the water was decanted and replenished with fresh FW for 10 min. Embryos were rested for 30 min prior to transferring into exposure solution. After resting, dechorionated embryos were transferred to individual wells of a 96-well plate with 100 μL of exposure solution (n = 16, three replicates). Exposures were static and continued under standard laboratory conditions in sealed plates and kept in the dark until 120 hpf. At 24 hpf, each individual embryo was scored for mortality and developmental progression. By 120 hpf, each embryo was assessed for mortality, and 15 morphological malformations (Truong et al. 2010). The per cent of mortality and total morbidity were calculated and graphed as the mean of three replicates with standard error bars.
Exposure solutions
The 1.2-nm 3-MPA-AuNPs were suspended with varying ionic concentrations of embryo media (EM); these solutions were used to create working solutions with a concentration of 50 μg/ml. A Calipher Zephyr Liquid Handler was used to create fivefold serial dilutions (0–50 μg/ml, five concentrations) for each ionic strength media in 96-well plates. Embryo media consisted of 15 mM NaCl, 0.5 mM KCl, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM Na2HPO4 and 0.7 mM NaHCO3 (Westerfield 2000). Six ionic strength media were made by diluting 100% EM with RO water [0% (11 μS, 0.007 ppt), 0.16% (14 μS, 0.01 ppt), 0.8% (34 μS, 0.024 ppt), 4% (113 μS, 0.08 ppt), 20% (480 μS, 0.34 ppt) and 100% (2420 μS, 1.7 ppt) EM]. Since the buffering capacity of the solution would be reduced at the lower ionic strength solutions, the pH of 1.2-nm 3-MPA-AuNPs suspended in the various ionic concentrations was measured and did not vary more than 0.5 units from an average pH of 6.5.
Behaviour assay
Using Viewpoint LifeScience Zebrabox Quantization System, behavioural responses were evaluated in exposed embryos prior to the toxicity assessment. Prior to evaluation, embryos were acclimated to light for 20 min, after which the lights were turned off (dark period) for 10 min, and then on for 5 min (light period). The output data files were processed using a custom Perl script to average total movement (pixel changes per second) for the dark period. Sixteen embryos were used per concentration and three replicates were completed.
Statistics
All analyses were compiled using SigmaPlot 11 (SPSS, Inc., Chicago, IL, USA). One-way ANOVA (p < 0.05) and Dunnett’s post hoc test were used to assess the mortality, morbidity and behavioural changes. Each exposure group for each concentration consisted of 16 individual-exposed embryos (n = 16) and three replicates.
Results
Characterization of 1.2-nm 3-MPA-AuNPs
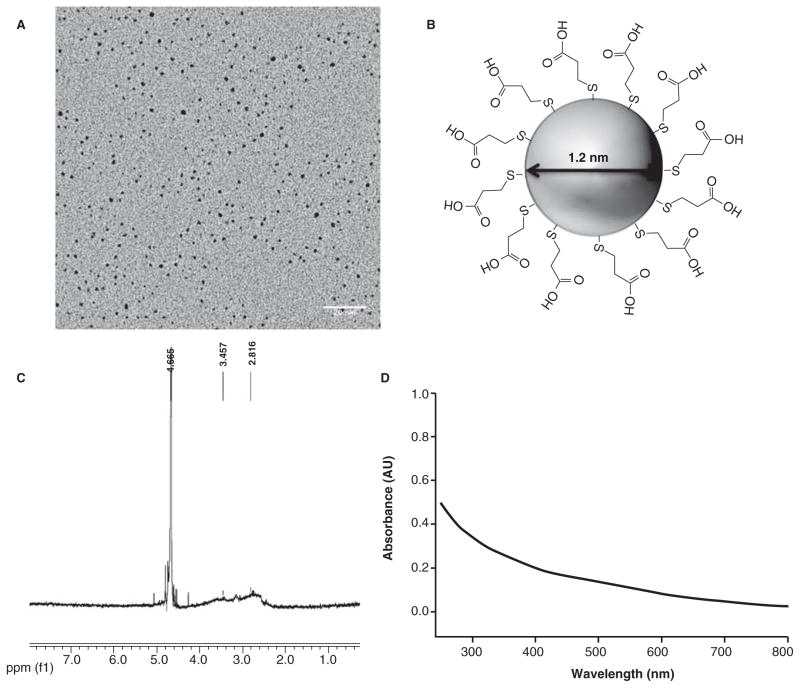
Each toxicological assessment was performed using the same parent batch of 1.2-nm 3-MPA-AuNPs. After synthesis, characterization was completed to define the core size and surface functionalization. TEM was used to calculate the average size of the NPs. Size analysis (Figure 1A) revealed that the average particle diameter was 1.2 ± 0.3 nm (N = 399). Proton NMR showed a broad peak at 2.4–4.2 ppm (Figure 1C) confirming that the 3-MPA ligand (Figure 1B) was attached to the gold core and no impurities (which would appear as sharp signals) were detected. Additionally, UV-Vis absorption spectroscopy was used to confirm the core size and degree of agglomeration of 1.2-nm 3-MPA-AuNPs in nanopure water (Figure 1D). These methods demonstrate that these AuNPs are free of molecular impurities and are precisely engineered 1.2-nm gold particles functionalized with 3-MPA ligands.
Figure 1.
(A) Transmission electron microscopy (TEM) image of 1.2-nm 3-MPA-AuNPs with a scale bar of 20 nm. Analysis of 399 individual particles yields an average particle core size of 1.2 ± 0.3 nm. (B) Structure of AuNPs with 3-MPA ligands. (C) 1HNMR analysis demonstrating that the 3-MPA is attached to the surface of the nanoparticle. Impurities, if present would lead to sharp signals at <4 ppm chemical shift. The sharp signals at higher than 4 ppm are due to the NMR measurement solvent. (D) UV-visible spectrum of 1.2-nm 3-MPA-AuNPs dissolved in nanopure water.
Embryo media causes the precipitation of 1.2-nm 3-MPA-AuNPs
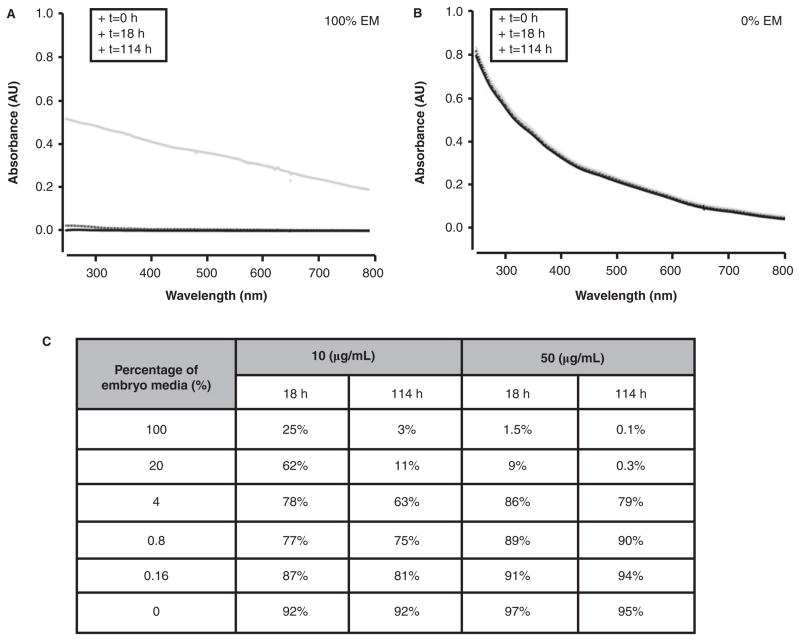
Embryos were exposed to 1.2-nm 3-MPA-AuNPs suspended in 100% embryo media over a fivefold concentration range (0.08–50 μg/ml) to determine whether the NPs elicited mortality or whether they induced developmental malformations. Upon suspension of the dried 1.2-nm 3-MPA-AuNPs, no precipitation was immediately visible. However, when the embryos were assessed at 24 hpf, NP precipitants were detected on the bottom of the wells and surrounding the animal. This exposure scenario continued until 120 hpf, when the zebrafish were evaluated. We tracked the agglomeration state of 1.2-nm 3-MPA-AuNPs using UV-Vis spectrum and found that, after 18 h, most of the AuNPs were no longer in solution (Figure 2A). Under these conditions, exposure to 1.2-nm 3-MPA-AuNPs did not increase mortality or malformations. However, at 50 μg/mL, 100% of the exposed embryos lacked a touch response (data not shown). Although the NPs had agglomerated, they still induced a subtle adverse biological response.
Figure 2.
Stability of solutions of 1.2-nm 3-MPA-AuNPs at 50 μg/ml in (A) 100% embryo media (EM) and (b) 0% EM at 0, 18 and 114 h using UV-Vis spectrum. The decrease in absorbance across the spectra to zero in (A) indicates complete loss of nanoparticles from solution, as opposed (B) where essentially no loss of particles is observed. (C) Table of nanoparticles that remain in solution at both 10 and 50 μg/ml over time when suspended in varying concentration of embryo media.
We took the approach to identify a dilution of embryo media (EM) with a level of ions that could support a stable NP dispersion, which should favour an increase in NP bioavailability throughout an extended exposure period. We evaluated NP agglomeration at the two highest concentrations (10 and 50 μg/mL) over 6% of EM (0–100%). Absorbance was measured at 18 and 114 h, which corresponded to the time points when exposed zebrafish would be evaluated for toxicological responses. As illustrated in Figure 2B, in 0% EM, the 1.2-nm 3-MPA-AuNPs did not agglomerate. On the other hand, when 1.2-nm 3-MPA-AuNPs were suspended in 100% EM, only 3% and 0.1% of the particles remained in suspension at 114 h, at 10 and 50 μg/mL, respectively (Figure 2C). At concentrations between 4% and 100% EM, there was a low percentage of dispersed 1.2-nm 3-MPA-AuNPs. With the decreased ionic concentration, the amount of suspended AuNPs increased. At concentrations between 0% and 0.8% EM, the amount of dispersed AuNPs was typically >90%. This demonstrates that the concentration of ions in the test media indeed plays a major role in the degree of 1.2-nm 3-MPA-AuNPs agglomeration.
Dechorionated zebrafish embryos can tolerate low ion concentrations
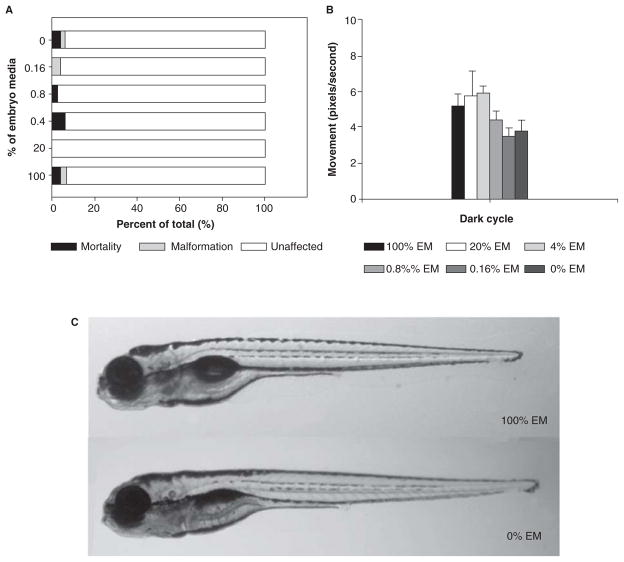
Although embryonic zebrafish are sensitive to toxicants and well suited for mechanistic-based toxicological studies, the importance of media ionic strength for embryonic development in the absence of the chorion is unknown. To reveal the minimum ionic strength required to support embryonic development, embryos were dechorionated and exposed to six different ion concentration media (fivefold serial dilution: 0, 0.16, 0.8, 4, 20, 100% EM in RO water) at 6 hpf. The initial assessments at 24 hpf revealed no differences between the groups when scoring for mortality or changes in developmental progression. At 120 hpf, exposed embryos were evaluated for mortality and a full suite of complex morphological endpoints. As Figure 3A illustrates, even at the lower concentrations of EM (0–0.8%), the incidence of mortality and malformation at 120 hpf was not statistically different. To determine whether varying ion concentration affected central nervous system function, exposed embryos were assessed for motor activity in the dark using ViewPoint LifeScience Zebralab. Larvae in 100% EM had a movement level of 5 pixels/s in the dark (Figure 3B). At the lower concentrations (0% and 0.16% EM), there was only a modest and not statistically significant decrease in pixels per second (~4). Visually, the embryos exposed to 0% and 100% EM were morphologically indistinguishable (Figure 3C). These studies indicate that dechorionated embryos can tolerate low ionic strength solutions and develop normally to at least 120 hpf.
Figure 3.
Embryos were dechorionated and exposed to various percentage of embryo media (0%, 0.16%, 0.8%, 4%, 20%, and 100%) exhibited low mortality and malformation (A). Exposed 120 h post-fertilization (hpf) embryos did not exhibit a statistically significant change in behaviour (B). Images were taken of embryos exposed to 0% and 100% EM at 120 hpf (C).
1.2 -nm 3-MPA-AuNPs developmental toxicity
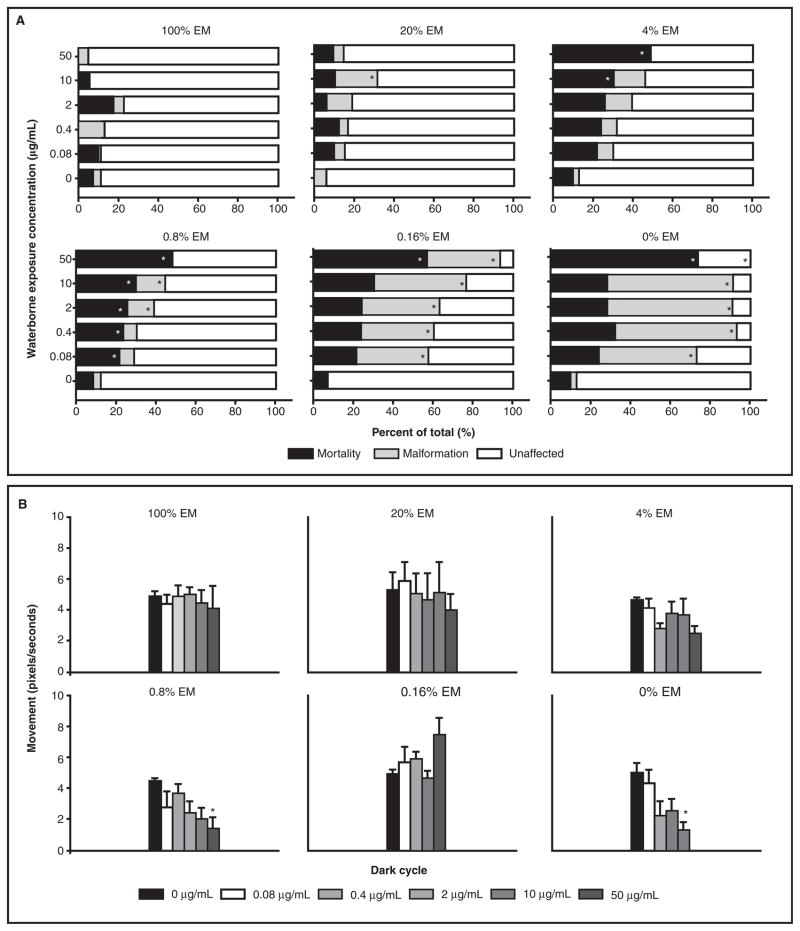
To investigate whether the agglomeration of 1.2-nm 3-MPA-AuNPs was masking toxic potential, the AuNPs were prepared at five concentrations (0–50 μg/mL) in various ionic strength media. These solutions were then continuously and statically exposed to 6 hpf dechorionated embryos until 120 hpf. As Figure 4A illustrates, at the higher ionic concentrations (20–100% EM), there was little mortality and malformation above background (<13%). This correlates well with the stability data showing that <20% of the AuNPs was in solution by 120 hpf. For the other ion concentrations (0–4% EM), the per cent of mortality and malformations increased as the ion concentration decreased. For each ionic concentration, a dose-dependent increase in mortality and malformation was observed. At 120 hpf, exposed embryos were assessed for behavioural abnormalities. Data generated from dead or malformed embryos were removed prior to processing. At the higher ion concentrations (4–100% EM), there were no statistically significant differences in the motor activity level between the groups, while the lower concentration (0–0.8% EM) motor deficits were significant at the higher NP concentrations (Figure 4B). Collectively, as the ionic concentration decreased, more 1.2-nm 3-MPA-AuNPs remained in solution and there was a corresponding increase in the per cent of mortality, malformation and behavioural deficits in the exposed embryos.
Figure 4.
Dechorionated embryos were exposed to five concentrations of 1.2-nm 3-MPA-AuNPs and six different solutions with varying ionic concentrations. As the ionic concentration decreased, mortality and malformations increased (A). Surviving embryos at 120 hpf were assessed for behavioural effects. Similarly, the lower concentrations (0–0.8%) caused behavioural changes at higher concentration, while the higher ionic concentrations (4–100%) did not (B). Significance was determined using one-way ANOVA and a Dunnett’s post hoc test (p < 0.05).
Discussions
In this in vivo study, we report that dechorionated zebrafish embryos develop normally up to 120 hpf in low ionic strength medium. Furthermore, we report that the response to 1.2-nm 3-MPA-AuNPs exposure is highly dependent on the medial constituents. When suspended in high ionic strength medium, agglomeration occurs within 18 h and the AuNPs precipitate and elicit little to no adverse biological responses. On the other hand, when suspended in low ionic strength, the AuNPs remain dispersed in solution and induce significant morbidity and mortality.
One potential explanation for the increased sensitivity to 1.2-nm 3-MPA-AuNPs in low ionic strength solutions is that the low salt conditions lead to a general increase in embryonic stress, and the changes in agglomeration state play little or no role in the differential toxicological response. To directly address this possibility, we exposed embryonic zebrafish to AuNPs with a 1.5-nm core and functionalized either with MES (2-mercaptoethanesulfonic acid) or TMAT (2-mercapto-N,N,N-trimethylethanaminium). Previous studies have demonstrated that these materials induce differential biological responses in zebrafish, but they do not agglomerate over time in standard high ionic strength medium (Harper et al. In press; Truong et al. (n.d)). When these particular NPs were suspended in RO water, the biological responses for 1.5-nm TMAT-AuNPs (unpublished data) and MES-AuNPs (Supplemental Figure 1) were indistinguishable from the responses observed when the particles were suspended in high ionic strength medium. This strongly suggests that altering the medium’s ionic strength does not lead to a general stress response, and the increase in 1.2-nm 3-MPA-AuNPs toxicity is attributed to changes in the particle properties themselves. The embryo media used for most embryonic zebrafish toxicity studies typically contains high concentrations of divalent ions, and these ions are known to lead to NPs agglomeration and produce complex particle behaviours (Saleh et al. 2008; Liu et al. 2009). By diluting the EM with RO water, we found that ion concentration of media influenced agglomeration rate, which is consistent with what was found for cell culture (Jin et al. 2010) and other media (Elimelech & Omelia 1990). When NPs agglomerate, their dissolution is impacted. Agglomeration of silver NP causes dissolution rate to be reduced, but this is highly dependent on the electrolytes and its concentration in the media (Li et al. 2010). The number of available particles is drastically different when solutions are monodispersed versus clustering together. When NPs agglomerate, this effectively changes the surface area to volume ratio. Particle surface area is a major player in NP-induced toxicity. For example, particle surface area was the principle driver in producing differential gene expression changes than was particle mass or number in alveolar macrophages (Waters et al. 2009). Cytotoxicity is also induced by iron oxide in a surface area-dependent manner (Ying & Hwang 2010). When NPs agglomerate, there is a wide distribution of sizes produced. It is currently impossible to understand what fractional agglomerate size is responsible for producing a biological response. In a recent study, cytotoxicity was size-dependent when exposed to 0.8–15 nm gold NPs (Pan et al. 2007) and 5, 20 and 50 nm silver NPs (Liu et al. 2010) where the smaller NPs were more toxic than their larger counterpart. Gold and silver NPs regulate membrane receptor internalization in a size-dependent manner (Jiang et al. 2008). These studies suggest that particle size influences the biological response. So effectively, agglomeration of NP causes a change in concentration, dissolution, surface area and particle size, which ultimately results in complex differential responses.
We found, for the first time, that dechorionated embryos develop morphologically normal and display normal CNS function up to at least 120 hpf when raised in RO water. These findings will provide new opportunities to exploit embryonic zebrafish to identify the physicochemical NP properties that are important to produce specific biological responses. With this flexibility, we can now adapt the testing model to conditions ideal for the NPs rather than the other way around. For example, researchers are routinely adding agents to the NP solutions that favour dispersion, but after addition of these agents, the NP surface properties are drastically changed. Although these coating agents help to disperse NPs in aqueous media, the addition of capping agents at the very least complicates the interpretation of toxicological response data with the added variability.
In summary, since embryonic and larval zebrafish can tolerate wide ranges of ionic strength, we recommend an approach where initial NP agglomeration studies are performed under multiple ionic strengths covering the exposure duration. Adjusting the assay to match optimal dispersion conditions will enhance bioavailability and thus increase assay sensitivity. Controlling agglomeration without the addition of arbitrary capping agents will allow the assessment of a more broad range of NPs and move us one step closer to identifying the key physicochemical NP properties that drive specific biological responses.
Supplementary Material
Acknowledgments
The authors thank Sinnhuber Aquatic Research Laboratory for the embryos, Jane La Du for her assistance in preparation of this manuscript and John Miller for helpful discussions regarding nanoparticle preparation. These studies were partially supported by National Institute of Environmental Health Sciences (NIEHS), R01ES016896, P3000210, the Air Force Research Laboratory (AFRL) under agreement number FA8650-05-1-5041 and Environmental Protection Agency (EPA) RD-833320. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of NIEHS, AFRL, EPA or the U.S. Government.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;5:1897–1910. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- Bowman TV, Zon LI. Swimming into the Future of Drug Discovery: In Vivo Chemical Screens in Zebrafish. ACS Chemical Biology. 2010;5:159–161. doi: 10.1021/cb100029t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elimelech M, Omelia CR. Effect of Electrolyte Type on the Electrophoretic Mobility of Polystyrene Latex Colloids. Colloids and Surfaces. 1990;44:165–178. [Google Scholar]
- Furgeson D, Fako V. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Advanced Drug Delivery Reviews. 2009;61:478–486. doi: 10.1016/j.addr.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Harper SL, Dahl JA, Maddux BLS, Tanguay RL, Hutchison JE. Proactively designing nanomaterials to enhance performance and minimise hazard. International Journal of Nanotechnology. 2008;5:124–142. [Google Scholar]
- Harper SL, Carriere J, Miller J, Hutchison JE, Maddux BLS, Tanguay RL. Systematic Evaluation of Nanomaterials Toxicity: Utility of Standarized Materials and Rapid Assays. doi: 10.1021/nn200546k. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Li M, Wang J, Marambio-Jones C, Peng F, Huang X, et al. High-Throughput Screening of Silver Nanoparticle Stability and Bacterial Inactivation in Aquatic Media: Influence of Specific Ions. Environmental Science & Technology. 2010;44:7321–7328. doi: 10.1021/es100854g. [DOI] [PubMed] [Google Scholar]
- Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nature Nanotechnology. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XH. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1:133–43. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Aruguete DM, Murayama M, Hochella MF. Influence of Size and Aggregation on the Reactivity of an Environmentally and Industrially Relevant Manomaterial (PbS) Environmental Science & Technology. 2009;43:8178–8183. doi: 10.1021/es902121r. [DOI] [PubMed] [Google Scholar]
- Li X, Lenhart JJ, Walker HW. Dissolution-Accompanied Aggregation Kinetics of Silver Nanoparticles. Langmuir. 2010;26:16690–16698. doi: 10.1021/la101768n. [DOI] [PubMed] [Google Scholar]
- Liu W, Wu Y, Wang C, Li H, Wang T, Liao C, et al. Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology. 2010;4:319–330. doi: 10.3109/17435390.2010.483745. [DOI] [PubMed] [Google Scholar]
- Lawrence C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture. 2007;269:1–20. [Google Scholar]
- Murdock RC, Braydich-Stolle L, Schrand AM, Schlager JJ, Hussain SM. Characterization of nanomaterial dispersion in solution prior to In vitro exposure using dynamic light scattering technique. Toxicological Sciences. 2008;101:239–253. doi: 10.1093/toxsci/kfm240. [DOI] [PubMed] [Google Scholar]
- Olenin AY, Krutyakov YA, Kudrinskii AA, Lisichkin GV. Formation of surface layers on silver nanoparticles in aqueous and water-organic media. Colloid Journal. 2008;70:71–76. [Google Scholar]
- Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, et al. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- Paigen K. One hundred years of mouse genetics: An intellectual history. I. The classical period (1902–1980) Genetics. 2003;163:1–7. doi: 10.1093/genetics/163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parng C. In vivo zebrafish assays for toxicity testing. Curr Opin Drug Discov Devel. 2005;8:100–6. [PubMed] [Google Scholar]
- Rubinstein AL. Zebrafish: from disease modeling to drug discovery. Curr Opin Drug Discov Devel. 2003;6:218–23. [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. Journal of Investigative Dermatology. 2007;127:143–153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- Sawant MS, Zhang S, Li L. Effect of salinity on development of zebrafish, Brachydanio rerio. Current Science. 2001;81:1347–1350. [Google Scholar]
- Saleh N, Kim H-J, Phenrat T, Matyjaszewski K, Tilton RD, Lowry GV. Ionic Strength and Composition Affect the Mobility of Surface-Modified Fe0 Nanoparticles in Water-Saturated Sand Columns. Environmental Science & Technology. 2008;42:3349–3355. doi: 10.1021/es071936b. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Warheit DB. Characterization of nanomaterials for toxicity assessment. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009;1:660–670. doi: 10.1002/wnan.58. [DOI] [PubMed] [Google Scholar]
- Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Science of the Total Environment. 2008;408:999–1006. doi: 10.1016/j.scitotenv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Truong L, et al. Surface Functionalities of Gold Nanoparticles (AuNPs) Impact Gene Expression in the Developing Zebrafish. n.d. Submitted. [Google Scholar]
- Truong L, Moody I, Stankus D, Nason J, Lonergan M, Tanguay R. Differential stability of lead sulfide nanoparticles influences biological responses in embryonic zebrafish. Archives of Toxicology. 2010:1–12. doi: 10.1007/s00204-010-0627-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon N Y. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliano E, Cataldi M, Carella F, Migliaccio O, Iaccarino D, Agnisola C. Effects of acute changes in salinity and temperature on routine metabolism and nitrogen excretion in gambusia (Gambusia affinis) and zebrafish (Danio rerio) Comparative Biochemistry and Physiology–Part A: Molecular & Integrative Physiology. 2010;157:283–290. doi: 10.1016/j.cbpa.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Woehrle GH, Brown LO, Hutchison JE. Thiol-functionalized, 1.5-nm gold nanoparticles through ligand exchange reactions: Scope and mechanism of ligand exchange. Journal of the American Chemical Society. 2005;127:2172–2183. doi: 10.1021/ja0457718. [DOI] [PubMed] [Google Scholar]
- Westerfield M. U.o.O.Press. The Zebrafish Book. Eugene, OR: 2000. [Google Scholar]
- Waters KM, Masiello LM, Zangar RC, Karin NJ, Quesenberry RD, Bandyopadhyay S, et al. Macrophage Responses to Silica Nanoparticles are Highly Conserved Across Particle Sizes. Toxicological Sciences. 2009;107:553–569. doi: 10.1093/toxsci/kfn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B, Xu Z, Guo W, Li Q. Impact of Natural Organic Matter on the Physicochemical Properties of Aqueous C60 Nanoparticles. Environmental Science & Tech nology. 2008;42:2853–2859. doi: 10.1021/es702231g. [DOI] [PubMed] [Google Scholar]
- Yang LX, Ho NY, Alshut R, Legradi J, Weiss C, Reischl M, et al. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reproductive Toxicology. 2009;28:245–253. doi: 10.1016/j.reprotox.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Ying E, Hwang H-M. In vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytes. Science of the Total Environment. 2010;408:4475–4481. doi: 10.1016/j.scitotenv.2010.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.