Abstract
Objective
To understand a possible role for transient potential receptor vanilloid 1 (TRPV1) ion channels in sumatriptan relief of pain mediated by trigeminal nociceptors.
Background
TRPV1 channels are expressed in small nociceptive sensory neurons. In dorsal root ganglia (DRG), TRPV1-containing nociceptors mediate certain types of inflammatory pain. Neurogenic inflammation of cerebral dura and blood vessels in the trigeminal nociceptive system is thought to be important in migraine pain, but the ion channels important in transducing migraine pain are not known. Sumatriptan is an agent effective in treatment of migraine and cluster headache. We hypothesized that sumatriptan might modulate activity of TRPV1 channels found in the trigeminal nociceptive system.
Methods
We used immunohistochemistry to detect the presence of TRPV1 channel protein, whole cell recording in acutely dissociated trigeminal ganglia (TG) to detect functionality of TRPV1 channels, and whole cell recording in trigeminal nucleus caudalis (TNC) to detect effects on release of neurotransmitters from trigeminal neurons onto second order sensory neurons. Effects specifically on TG neurons that project to cerebral dura were assessed by labeling dural nociceptors with DiI.
Results
Immunohistochemistry demonstrated that TRPV1 channels are present in cerebral dura, trigeminal ganglion, and in the trigeminal nucleus caudalis. Capsaicin, a TRPV1 agonist, produced depolarization and repetitive action potential firing in current clamp recordings and large inward currents in voltage clamp recordings from acutely dissociated TG neurons, demonstrating that TRPV1 channels are functional in trigeminal neurons. Capsaicin increased spontaneous excitatory postsynaptic currents (sEPSCs) in neurons of layer II in TNC slices, showing that these channels have a physiological effect on central synaptic transmission. Sumatriptan (10 μM), a selective anti-migraine drug inhibited TRPV1-mediated inward currents in TG. and capsaicin-elicited sEPSCs in TNC slices. The same effects of capsaicin and sumatriptan were found in acutely dissociated DiI-labeled TG neurons innervating cerebral dura.
Conclusion
Our results build on previous work indicating that TRPV1 channels in trigeminal nociceptors play a role in craniofacial pain. Our findings that TRPV1 is inhibited by the specific antimigraine drug sumatriptan, and that TRPV1 channels are functional in neurons projecting to cerebral dura suggests a specific role for these channels in migraine or cluster headache.
Keywords: Transient receptor potential, vanilloid receptor, pain, headache, trigeminal ganglion, trigeminal nucleus caudalis
INTRODUCTION
Transient receptor potential (TRP) channels are a group of ion channels found in neurons and other cell types. Thirty-three different TRP channels in seven different families have been identified. They are found in vertebrate and invertebrate species, and are sensitive to various environmental stimuli. They have diverse roles in pain, vision, osmosensation, olfaction, mechanosensation, apoptosis, necrosis and vasorelaxation 1.
TRPV1 is a nonselective cationic channel activated by exogenous and endogenous physical and chemical stimuli, including heat greater than 43°C, low pH, the endocannabinoids anandamide and N-arachidonoyl-dopamine, and capsaicin, the active ingredient of hot chili peppers 2. In mammals, TRPV1 channels are found in the somatic sensory system, and are especially involved in the transmission and modulation of pain from injurious heat and inflammation 3–5. Activation of TRPV1 in dendrites and axon terminals of nociceptors causes release of inflammatory neuropeptides and neurotransmitters from their peripheral and central terminals, which may account for the involvement of TRPV1 in inflammatory pain 4. Although much is known of TRPV1 in the somatic pain system, its roles in the craniofacial pain system have not been well studied.
Cranial pain is sensed by nociceptors with their cell bodies in the trigeminal ganglia, and migraine is a neurovascular disorder associated with trigeminal activation. Although the pathophysiology of migraine is still not clear, excitation of the trigeminovascular system followed by neurogenic inflammation in the dura mater has been implicated in the development of migraine headaches. Recently, the TRPV1 channel has been shown to be expressed in dura mater. These findings indicate a possible association between the TRPV1 channel and migraine headaches. Furthermore, it suggests that TRPV1 may be a useful therapeutic target in migraine 6.
Sumatriptan and similar “triptan” drugs are clinically very effective in treatment of migraine, and are selective 5-Hydroxytryptamine type 1 B/D (5-HT1B/D) receptor agonists. These receptors are present in large cerebral arteries, small arteries of the cerebral dura, trigeminal sensory fibers (both central and peripheral) and in trigeminal ganglia. Sumatriptan may be useful in migraine because of 5-HT receptor mediated effects on trigeminal nerve fibers, such as by reduction of inflammatory neuropeptide release7.
In the present study we used immunohistochemistry to determine the anatomical locations of TRPV1 in the trigeminal ganglion and its projections to dura and brainstem. We used whole cell recording from the cell bodies of the primary craniofacial sensory neurons in the trigeminal ganglion and secondary neurons in the trigeminal nucleus caudalis to determine the functionality of TRPV1 and its modulation by sumatriptan.
METHODS AND MATERIALS
Animals
All experimental procedures were approved by the Southern Illinois University School of Medicine Laboratory Animal Care and Use Committee. Experiments used two to five week old male or female Sprague-Dawley (SD) rats obtained from Harlan Laboratories (Indianapolis, IN) or bred locally.
Materials
Intracellular and extracellular solutions and stock solutions of drugs were made in nanopure water using a NANOpure Infinity UF system (Barnstead International, Dubuque, Iowa). Stock solutions were then further diluted in extracellular solution for final application. Capsaicin stock solutions were made in 100% ethanol. Sumatriptan succinate stock concentration was 12 mg/ml. Final concentrations were capsaicin 50 nM-2 μM, sumatriptan 10–20 μM. All drugs and chemicals were purchased from Sigma Chemical Company (St Louis, MO), except sumatriptan from Glaxo-Smith Kline.
Immunohistochemistry
Rats were anesthetized using ketamine (85 mg/kg i.p.) and xylazine (5 mg/kg i.p.). Freshly made fixative (4% paraformaldehyde) was perfused transcardially. Dura, trigeminal ganglia, and brain medulla and upper cervical cord were harvested and kept in the same fixative for three hours, followed by incubation with 15% and 30% sucrose for 24 hours each. Samples were embedded with embedding medium (Triangle Biomedical Sciences, Durham, NC) and quickly frozen with liquid nitrogen. Ganglia and brain medulla were cut into 20 uM sections using a cryostat (Leica CM 1850, Nussloch, Germany). After permeabilizing with PBS containing 0.1% Triton X-100 for 20 minutes and blocking with 10% donkey serum for 30 minutes, the sections were incubated with polyclonal rabbit anti-TRPV1 antibody (Affinity Bioreagents PA1-747, 1:500) and monoclonal mouse anti-NeuN antibody (Chemicon, MAB377, 1:100) for two hours at room temperature, incubated with Rhodamine Red (TM)-X donkey anti-rabbit IgG (Jackson 711-295-152, 1: 100) and FITC donkey anti-mouse IgG (Jackson, 715-095-151, 1: 100) for one hour at room temperature, then spread on slides and mounted with mounting medium (Biomeda Gel/Mount) and cover slips. Images were taken using a confocal microscope (Olympus Fluoview Confocal Laser Scanning Microscope IX70).
Specificity of the antibody to TRPV1 has previously been confirmed by complete lack of staining in TRPV1 knockout mice8. Peptide raised against the antibody binding epitope on TRPV1 also prevented staining (data not shown).
Trigeminal neuron dissociation
Acutely dissociated trigeminal ganglion neurons were used. Rats were anesthetized with isoflurane and then decapitated. The trigeminal ganglia were removed bilaterally and dissociated in a 15 ml centrifuge tube containing Hanks Balanced Salt Solution with 1 mg/ml trypsin (Worthington Biochemical, Lakewood, NJ) and 1 mg/ml collagenase (Roche, Indianapolis, IN). After 30 minutes incubation at 37°C in a Precision 2874 shaking water bath (Thermo Scientific, Marietta OH) the resulting cell suspension was centrifuged at 500 rpm for 5 minutes. The supernatant was discarded and the tissue pellet resuspended in 2 ml of Neurobasal (Invitrogen, Carlsbad CA) medium containing B27 and L-glutamine. Centrifugation was repeated and the resuspended cells triturated with a Pasteur pipette until cells were dispersed evenly. Neurons were plated on glass cover slips coated with poly-D-lysine and kept in an incubator (Forma Scientific Inc., Marietta, OH) at 37°C with an atmosphere of 5% CO2 and 9% O2. Neurons were used for experiments within 30 hours of plating without any medium change.
Labeling of TG neurons projecting to cerebral dura
Trigeminal ganglion neurons projecting to cerebral dura were labeled in four week old SD rats using the retrograde tracer 1,1′-dilinoleyl-3,3,3′,3′-tetramethylindocarbocyanine, 4-chlorobenzenesulfonate (FAST DiI) (Invitrogen, Oregon, USA). The rat was deeply anesthetized with isoflurane, the head shaved and disinfected. A 1 cm scalp incision exposed the skull 2–4 mm anterior to lambda. A window of dura was exposed over the superior sagittal sinus by careful drilling with a small burr 9. A tiny piece of FAST DiI was put in the window, which was then packed with Gelfoam® to avoid dye movement. The skull was covered with a rubber dental dam, sealed with tissue glue, and the incision closed with stitches. Rats were allowed to recover for five to seven days then labeled trigeminal ganglia were collected and dissociated. Dural neurons were detected using a fluorescence microscope with a rhodamine filter.
Whole cell recording in dissociated neurons
Recordings were performed using whole cell voltage clamp and current clamp methods. Recording electrodes with a resistance of 2 to 4 MΩ were pulled from glass pipettes (i.d. 1.00, o.d. 1.50, King Precision Glass Inc., Claremont, CA) using a P-97 horizontal micropipette puller (Sutter Instrument, Novato, CA). An Axopatch 200B amplifier and Clampex 10 software (Axon Instrument Inc., Foster City, CA) were used for data acquisition. Neurons were visualized with an Olympus IMT-2 inverted microscope. Drugs were applied from a multi-barreled pipette held close to the neuron under study using a SF-77B Perfusion Fast-Step system (Warner Instruments, Hamden, CT). Application times and order were programmed with a Master 8 pulse generator (AMPI, Jerusalem, Israel) and controlled by a VC-6 six channel valve controller (Warner Instruments, Hamden, CT). The bath solution contained (in mM) 140 sodium gluconate, 5 potassium chloride, 10 HEPES, 2 magnesium chloride, 2 calcium chloride or 2 ethylene glycol tetraacetic acid (EGTA), pH 7.4. The intracellular solution contained (in mM) 130 potassium gluconate, 5 potassium chloride, 2 magnesium chloride, 10 EGTA, 10 HEPES, 2 magnesium-ATP, 0.5 GTP, pH 7.35. Membrane currents were measured under voltage clamp, with neurons held at −60 mV. Seal resistance, input resistance and series resistance were measured and series resistance was compensated 60%. Control TRPV1 responses were obtained by repeated application of capsaicin (100–250 nM) for 30 seconds. Neurons that did not exhibit significant desensitization to two consecutive capsaicin applications were selected for determining drug effects on TRPV1. The peak values of inward currents were recorded in gap-free mode and analyzed using Clampfit (Axon Instruments) and Origin 6.1 software (OriginLab Corporation, Northampton, MA). Depolarization and action potential firing were measured in current clamp at resting membrane potential.
Trigeminal nucleus caudalis slice preparation
Horizontal TNC slices were prepared using methods similar to ones previously described 10. Sprague Dawley rats between 10 and 20 days old were anesthetized with isoflurane and decapitated with a guillotine. The entire brain was removed and placed in ice cold sucrose solution: (in mM) 209 sucrose, 2 KCl, 1.25 NaH2PO4, 5 MgCl2, 0.5 CaCl, 26 NaHCO3 and 10 D glucose; 290 mOsm; pH 7.4; aerated with 95% O2/5% CO2 gas for 2 min. The medulla was isolated from the brainstem and was cut rostrally at the obex and caudally at the cervical spinal cord in order to obtain slices containing the caudal spinal trigeminal nucleus. An OTS 3000 vibrating tissue slicer was used to make 250 μm horizontal slices of the medulla in ice cold aerated sucrose. Slices were immediately harvested and placed in an incubation chamber containing oxygenated Hibernate A(Invitrogen) at 32°C. The temperature of the incubation chamber was allowed to fall to room temperature as the slices incubated. Slices were allowed to recover for 1 hr before being placed in the recording chamber for study.
Whole cell recording in brain slices
Pipettes with tips of 2–5 MΩ were pulled from thick walled borosilicate glass (World Precision Instruments, Inc. 1B150F-4) with a horizontal pipette puller (Sutter Instruments, Co. P-97). Slices were visualized with an upright microscope (Olympus BX 50W) and Gibraltar stage (Burleigh Instruments) using a 40 X objective and infrared filter. The image was captured using an infrared camera and visualized on a video monitor. For voltage clamp studies, a pipette solution containing (in mM) 140 CsMeSO3, 10 EGTA, 10 HEPES, 5 CsCl, 5 MgCl2, 5 MgATP, 1 LiGTP, adjusted to pH 7.4 with CsOH was used. Whole-cell currents were recorded using a patch clamp amplifier (HEKA EPC 10) and acquisition software (Pulse 8.6). Data was low-pass filtered at 2.5 kHz (−3 db frequency with an 8 pole low pass filter, LP10, Warner Instruments), digitized at 5 kHz (VR-10B Instrutech Corp, Great Neck, NY) and stored on a hard disk using Labview (National Instruments) based software. The recording chamber (Warner PH1) was perfused at 3 ml/min with extracellular solution: (in mM) 126 NaCl, 2.5 KCl, 1.4 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 25 NaHCO3 and 10 glucose; 290 mOsm; pH 7.4; aerated with 95%O2/5% CO2 gas. Temperature was held at 28° C using a glass water jacket and circulating water bath (VWR 1130). Access resistance and input resistance were monitored by applying a series of −10 mV voltage commands for 250 ms with the cell in voltage clamp.
Data analysis and statistics
Data are shown as mean ± standard error of the mean in the text and mean ± standard deviation in the figures. Statistical testing used student paired t tests or one-way analysis of variance (ANOVA). P<0.05 was considered a significant difference.
RESULTS
TRPV1 channels are present in cerebral dura, TG, and TNC
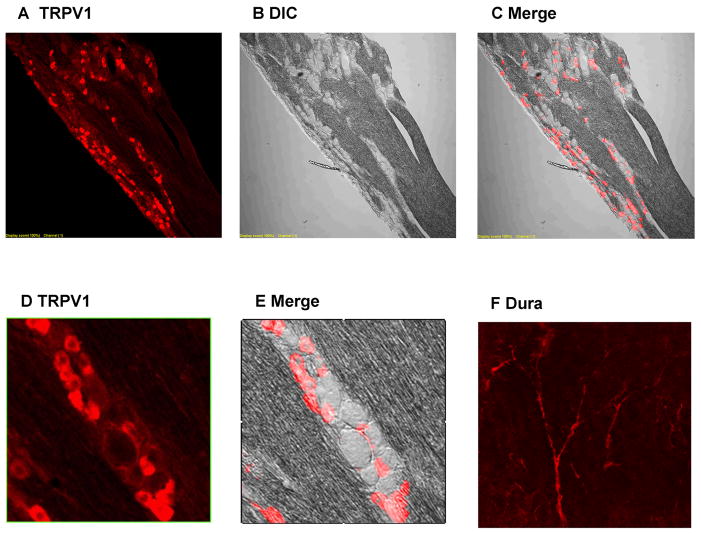
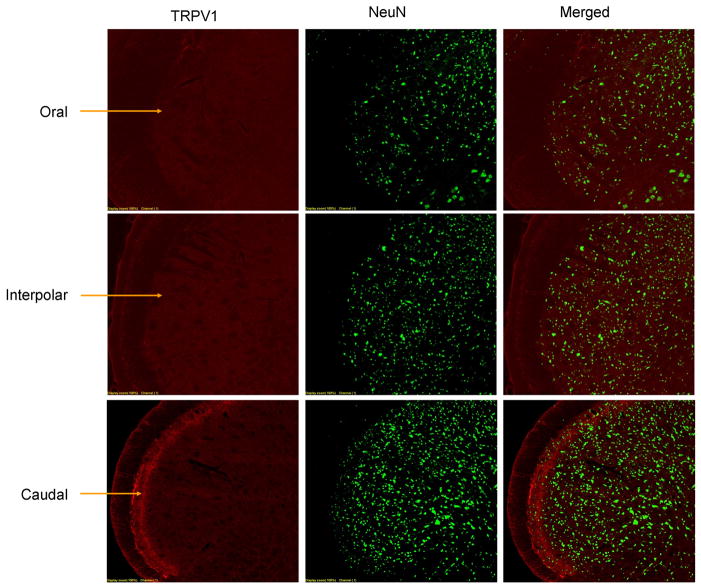
We performed immunohistochemical labeling of TRPV1 in frontal dura mater, in TG and in TNC (Figure 1). In frontal dura, TRPV1 staining appeared to follow the course of blood vessels, and could be localized either to nerve fibers innervating the vessels, or to arteriolar smooth muscle11. In trigeminal ganglion, TRPV1 staining was found in cell bodies. Neurons were counted manually and both neurons and TRPV1 staining were easy to discern. 36% of TG neurons were labeled by TRPV1 antibody. Our visual impression is that TRPV1 positive neurons were primarily small neurons, but this was not further quantified. In the brainstem trigeminal nucleus caudalis, TRPV1 labeling was abundant in fibers present in lamina II, but not found in neuronal cell bodies. TRPV1 labeling was selective for the pars caudalis of the spinal trigeminal nucleus, and not found in pars interpolaris and pars oralis.
Figure 1. TRPV1 immunohistochemistry in trigeminal ganglion and dura.
(A to C) Trigeminal ganglion is labeled for TRPV1 and seen in fluorescent, differential interference contrast (DIC), and merged images. Large numbers of cell bodies express TRPV1. (D and E) Magnified sections show TRPV1 labeling is in a minority of neurons, primarily small neurons. (F) Cerebral dura has abundant TRPV1 signal especially near blood vessels.
Capsaicin activates TRPV1 in TG
Nociceptors are small unmyelinated neurons expressing TRPV1 channels. Small and medium-sized dissociated trigeminal ganglion neurons 25 to 40 μm in diameter were studied. To activate TRPV1 channels, capsaicin (50–250 nM) was applied by perfusion for 30 or more seconds.
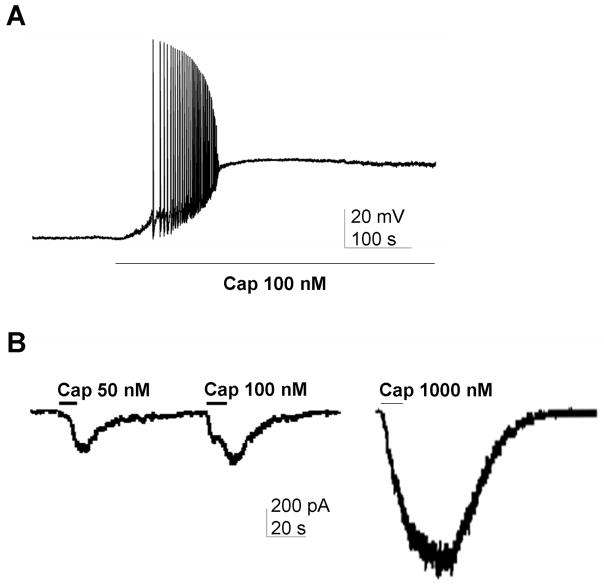
In TG neurons studied in current clamp, capsaicin elicited large depolarizations and transient action potential firing (Figure 2A). We did not attempt to randomly sample neurons, but of the small and medium-sized neurons selected for study, about 70% responded to capsaicin.
Figure 2. TRPV1 immunohistochemistry in trigeminal nucleus.
Coronal slices of spinal trigeminal nucleus are shown at the level of the oral (top), interpolar (middle) and caudal (bottom) subnuclei. At each level, immunoreactivity for TRPV1 (left), NeuN (middle) and merged images are shown. The caudal subnucleus has TRPV1-containing fibers in the superficial layers (arrows), but there is no TRPV1 immunoreactivity in the oral and interpolar subnuclei. TRPV1 immunopositive cell bodies are absent in all parts of the spinal trigeminal nucleus.
In TG neurons studied in voltage clamp capsaicin produced large inward currents. Capsaicin currents were relatively rapid, had a clear maximum, and could be elicited repeatedly. Capsaicin responses were concentration-dependent (Figure 2B), and concentration-response curves indicated an EC50 of approximately 250 nM (not shown).
TRPV1 modulates transmitter release in trigeminal nucleus caudalis
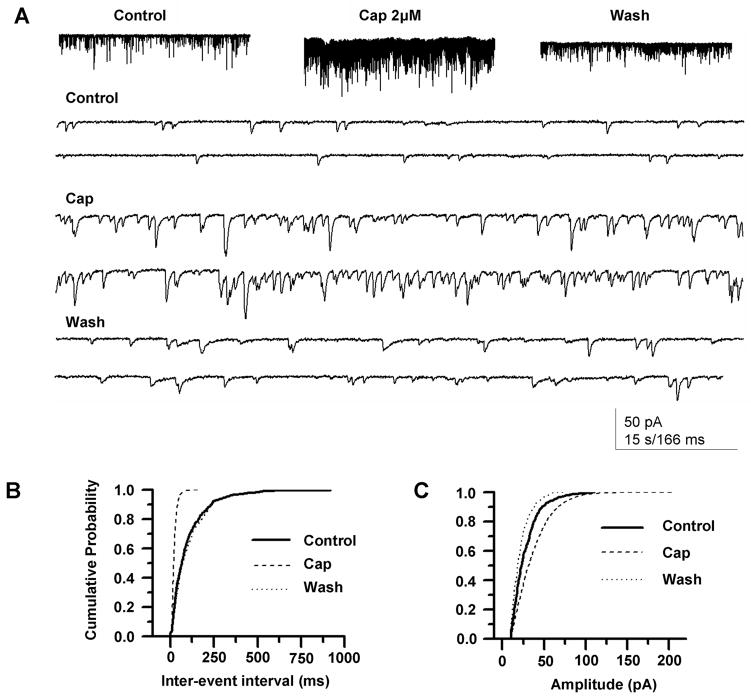
Consistent with the presence of TRPV1 in fibers of TNC lamina II, recording from lamina II TNC neurons showed that capsaicin markedly increased the frequency of spontaneous EPSCs (Figures 3A and 3B). EPSC amplitude was little changed (Figure 3C) and time course was unchanged (not shown), suggesting that capsaicin’s effect is on TRPV1-containing presynaptic terminals synapsing in TNC. Except for abundant and overlapping sEPSCs, capsaicin did not produce ionic currents in TNC neurons, indicating that, consistent with the immunohistochemistry, TRPV1 channels are not present in the second-order sensory neurons themselves.
Figure 3. TRPV1 depolarization and inward currents in trigeminal ganglion neurons.
(A) The TRPV1 agonist capsaicin (Cap, 100 nM) produced a strong depolarization and action potential firing. (B) Capsaicin produced concentration-dependent inward currents.
Sumatriptan inhibits TRPV1 in trigeminal ganglia neurons
Sumatriptan, an agent effective for treatment of migraine and cluster headaches, was applied to trigeminal ganglia neurons using a flow system that allowed for quick solution changes around the neuron under study. Sumatriptan 10 μM quickly and reversibly inhibited TRPV1 currents (Figure 4A). Repeated application of large concentrations of capsaicin for prolonged times produces an activity-dependent reductions in responding, or tachyphlaxis. We minimized tachyphylaxis by using brief applications in relatively low concentrations. Using this method, approximately 70% inhibition of TRPV1 currents elicited by capsaicin was observed (Figure 4B). Statistical analysis of all neurons to which sumatriptan 10–20 μM was applied showed significant effects on capsaicin currents (N = 32; control 1476 ± 214 pA; sumatriptan 825 ± 145 pA; P = 0.000005 t-test).
Figure 4. TRPV1 modulates spontaneous EPSCs in neurons of the trigeminal nucleus caudalis (TNC).
(A) Capsaicin reversibly increased the frequency of sEPSCs. Top figures show the increase on a slow time scale, and lower figures on a faster time scale. (B) The increase in sEPSCs is associated with a marked increase in frequency of sEPSCs but (C) not in amplitude. If synaptic potentials were blocked, capsaicin had no effect on neurons of the TNC (data not shown).
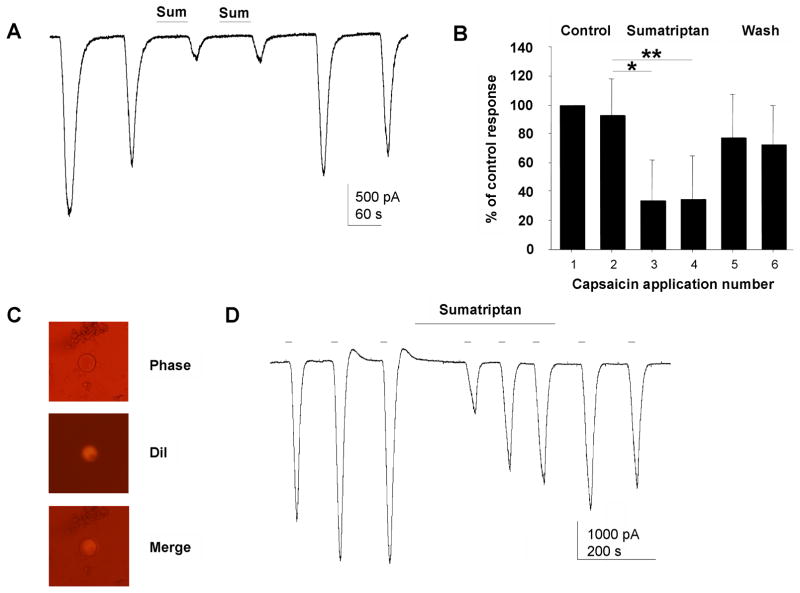
Trigeminal ganglion neurons projecting to cerebral dura were identified using DiI applied to dura. Dural neurons identified by fluorescence (Figure 4C) responded robustly to capsaicin and capsaicin currents were inhibited by sumatriptan (Figure 4D). Four of six dural neurons studied showed a strong trend toward inhibition by capsaicin (N = 6; control 2126 ± 686 pA; sumatriptan 1022 ± 241 pA; P= 0.08 t test).
Sumatriptan reduces capsaicin-induced sEPSCs in TNC
Capsaicin reversibly increased the frequency and amplitude of spontaneous EPSCs recorded in second order neurons, as indicated by cumulative probability histograms for inter-event interval and amplitude (Figure 3). The effect of capsaicin on sEPSC frequency suggests capsaicin acts presynaptically to enhance glutamate release. Sumatriptan treatment significantly reduced the sEPSC frequency change elicited by capsaicin (Figure 5).
Figure 5. Sumatriptan inhibits TRPV1 currents in TG neurons.
(A) Sumatriptan (10 μM) inhibited inward currents evoked by capsaicin (100 nM) in a TG neuron. (B) The mean inhibition of capsaicin currents was 70% (N = 13 TG neurons). Error bars indicate SD. Sumatriptan inhibition of capsaicin currents was statistically significant by ANOVA (P = 0.016 by ANOVA). * indicates paired t test P = 0.0009, ** indicates P = 0.0003. (C) TG neurons innervating cerebral dura were labeled with DiI (D) Capsaicin currents in labeled dural TG neurons showed robust inhibition by sumatriptan.
DISCUSSION
We found that TRPV1 channels are present and functional in the craniofacial pain system. Our results agree with others in that they cause depolarization and inward currents in trigeminal ganglion neurons and they increase spontaneous transmitter release at the first sensory synapse in the trigeminal nucleus caudalis. We have extended this work to show that TRPV1 channels are functional in neurons specifically projecting to cerebral dura, and to show that the anti-migraine drug sumatriptan inhibits TRPV1 channels in the craniofacial pain system.
Migraine headache is a very common and very painful condition affecting approximately 15% of adults in the U.S. Pain is thought to originate in intracranial dura or blood vessels 12, with brain itself being pain-insensitive 13. A leading candidate for the pathophysiological mechanism of migraine headache is neurogenic inflammation caused by release of inflammatory mediators in the dura mater, with sensitization of trigeminal afferents to dura 14.
There are a number of ways TRPV1 could be involved in migraine. TRPV1 is present in cerebral dura 15,16, and TG neurons respond to capsaicin 17. TRPV1 is found in nearly half of TG afferents that originate in dura, and these TRPV1-containing dural nerve fibers also contain calcitonin gene related peptide (CGRP)18. CGRP and substance P are thought to play a role in neurogenic inflammation, with CGRP responsible for cerebral vasodilation. The CGRP antagonist telcagepant is useful for treatment of acute migraine attacks 19. TRPV1 agonists can cause release of CGRP and substance P, indicating a possible role in neurogenic inflammation. In keeping with this, TRPV1 was found to play a role in dural vasodilation, a component of neurogenic inflammation 20,21. Consistent with a possible role of TRPV1, studies suggest that TRPV1 antagonists may be useful in migraine pain 22. The means by which TRPV1 might be activated in migraine is unknown, but could be multiple. TRPV1 can be sensitized by kinases PKC and PKAand by nociceptive peptides that work through them such as bradykinin and prostaglandins. TRPV1 can also be directly activated by acidosis and by the endogenous TRPV1 agonists anandamide and NADA 1–4,23.
Although TRPV1 may be involved in migraine, it may not be required for every type of headache, or even every etiology of migraine. Summ et al.24 found that a TRPV1 antagonist did not affect responding to mechanically induced cortical spreading depression or to electrical stimulation of the middle meningeal artery/dura mater, although it did affect response to capsaicin stimulation. Arulmani et al.25 found that sumatriptan did not inhibit CGRP release from intracarotid capsaicin injections and concluded that sumatriptan’s main effect must then be to promote cranial vasoconstriction. Yan et al.26 found that acid-sensitive currents in dural neurons were not modulated by capsaicin, a TRPV1 antagonist, but were strongly affected by amiloride, an ASIC antagonist. These results suggest that TRPV1 is not involved in every possible cause of dural, pain.
The possible involvement of TRPV1 in migraine pain is emphasized by the effects of sumatriptan on these channels. Sumatriptan and other “triptan” drugs are very effective migraine abortive agents, but they are not effective for non-migraine pain 27,28. The mechanism of sumatriptan migraine relief remains uncertain although there is evidence that 5-HT1B/D receptors are involved. Sumatriptan inhibits the vasodilation and protein extravasation that underlies neurogenic inflammation and possibly headache 29,30. Sumatriptan is known to constrict cerebral blood vessels 31 and to inhibit peptide release from meningeal sensory neurons 32,33, specifically CGRP34. Given the possible role of TRPV1 in vasodilation, neurogenic inflammation, and increased peptide release, our finding that sumatriptan inhibits TRPV1 suggests this could be an important mechanism of sumatriptan’s effects on headache pain.
The cellular mechanism by which sumatriptan affects TRPV1 is not yet known, but could be due to 5-HT1B/D receptors. 5-HT1B/D receptors negatively modulate cAMP which could in turn negatively modulate TRPV1 through reduced PKA-mediated sensitization35.
Sumatriptan does not cross the intact blood brain barrier, but does affect transmitter release in the TNC in isolated brainstem slices. Jennings et al. 36 have shown that sumatriptan can inhibit evoked transmitter release in the TNC. We have confirmed previous results that TRPV1 is present in the trigeminal nucleus caudalis, and can modulate transmitter release at that location37,38. We found that sumatriptan inhibits TNC spontaneous EPSCs elicited by capsaicin. These data suggest that triptans that can cross the blood brain barrier, such as zolmitriptan, could inhibit excitatory transmitter release within the TNC.
If indeed sumatriptan works in part through inhibition of TRPV1, its effects should be detectable with sumatriptan doses used clinically. We did not do dose-response curves to define the EC50 for these effects, but sumatriptan inhibited capsaicin currents by an average of 70%, suggesting that detectable inhibition is likely to occur with lower concentrations. The concentration of sumatriptan we usually used, 10 μM, is similar to the 7.25 μM that would result if a standard subcutaneous dose of 6 mg sumatriptan (base) were dissolved in the plasma volume of a normal 70 kg adult male. This could be obtained by intravenous push loading of sumatriptan. This concentration is far less than the LD50 of subcutaneous sumatriptan in mice (1680 mg/kg), however, it is several-fold greater than that actually measured in humans after sumatriptan subcutaneous injection. After a single 6 mg subcutaneous injection in healthy males, the mean maximal concentration was 72 ng/mL 39 (approximately 0.18 μM) and the time to maximal concentration was 12 minutes, with a terminal half life of 115 minutes. Presumably the site of action of sumatriptan is in cerebral dura, cerebral arteries, or in the brainstem. Its concentrations at those locations are unknown, but exposure in these areas is likely to be at much lesser concentrations, for much longer times than the 1 to 5 minute applications we used. Further experiments will be necessary to clearly determine whether sumatriptan effects on TRPV1 channels are biologically relevant.
TRPV1 channels are known to be important in somatic inflammatory pain, but their role in pain originating in the head is unknown. We have demonstrated the presence and functionality of TRPV1 in the craniofacial pain system, including in the cerebral dura, which is a possible location for the intracranial pain of migraine. They cause depolarization of small trigeminal neurons and release of excitatory transmitter from their central terminals, and thus are likely to increase pain originating in trigeminal nociceptors. We found that both these phenomena are inhibited by sumatriptan, a drug selectively effective in migraine pain. The possible involvement of these channels in sumatriptan-responsive migraine and cluster headache pathophysiology merits further study.
Figure 6. Sumatriptan inhibits TRPV1-mediated sEPSCs in TNC.
(A) In most neurons, repeated application of capsaicin caused a decrease in sEPSC response, as shown. (B) In this neuron, the frequency of sEPSCs is significantly reduced with the second application of capsaicin. (C) A histogram summarizing average results for repeated capsaicin application. (D) To control for activity-dependent decreases in capsaicin responses, capsaicin was applied first with sumatriptan, then again without sumatriptan, since without sumatriptan the first response is expected to be larger. In the example shown, sumatriptan inhibited the first capsaicin response, and the second response is larger. (E) sEPSC frequency was decreased by sumatriptan, but (F) the amplitude of sEPSCs was not affected by sumatriptan. (G) Histogram summarizing sumatriptan inhibition of capsaicin-mediated sEPSCs. Error bars indicate SD, * indicates P = 0.049 t test.
Acknowledgments
This work was supported in part by grants from the Southern Illinois University Central Research Committee to M. Steven Evans and the National Institutes of Health (DA028017) to Louis S. Premkumar. The authors thank Steven R. Johnson for the example shown in figure 3A.
List of Abbreviations
- CGRP
calcitonin gene related peptide
- DRG
dorsal root ganglion
- EPSC
excitatory postsynaptic currents
- LD50
50% lethal dose
- SD
standard deviation
- TG
trigeminal ganglion
- TNC
trigeminal nucleus caudalis
- TRPV1
transient receptor potential vanilloid 1
- sEPSC
spontaneous EPSC
Footnotes
Conflicts of Interest:
No conflict for any author
References
- 1.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–98. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 2.Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271:1814–19. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 3.Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 4.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 5.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 6.Meents JE, Neeb L, Reuter U. TRPV1 in migraine pathophysiology. Trends Mol Med. 2010;16:153–59. doi: 10.1016/j.molmed.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Link AS, Kuris A, Edvinsson L. Treatment of migraine attacks based on the interaction with the trigemino-cerebrovascular system. J Headache Pain. 2008;9:5–12. doi: 10.1007/s10194-008-0011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pabbidi RM, Cao DS, Parihar A, Pauza ME, Premkumar LS. Direct Role of Streptozotocin in Inducing Thermal Hyperalgesia by Enhanced Expression of TRPV1 in Sensory Neurons. Mol Pharmacol. 2008 doi: 10.1124/mol.107.041707. [DOI] [PubMed] [Google Scholar]
- 9.Lipton JM. Superior sagittal sinus as a chronic venous route in the rat. J Appl Physiol. 1972;32:701–2. doi: 10.1152/jappl.1972.32.5.701. [DOI] [PubMed] [Google Scholar]
- 10.Grudt TJ, Williams JT. mu-Opioid agonists inhibit spinal trigeminal substantia gelatinosa neurons in guinea pig and rat. J Neurosci. 1994;14:1646–54. doi: 10.1523/JNEUROSCI.14-03-01646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavanaugh DJ, Chesler AT, Jackson AC, et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–77. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blau JN, Dexter SL. The site of pain origin during migraine attacks. Cephalalgia. 1981;1:143–47. doi: 10.1046/j.1468-2982.1981.0103143.x. [DOI] [PubMed] [Google Scholar]
- 13.Ray BS, Wolff HG. Experimental studies on headache. Pain-sensitive structures of the head and their significance in headache. Arch Surg. 1940;41:813–856. [Google Scholar]
- 14.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–10. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 15.Bae YC, Oh JM, Hwang SJ, Shigenaga Y, Valtschanoff JG. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol. 2004;478:62–71. doi: 10.1002/cne.20272. [DOI] [PubMed] [Google Scholar]
- 16.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–58. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Simon SA. Capsaicin, acid and heat-evoked currents in rat trigeminal ganglion neurons: relationship to functional VR1 receptors. Physiol Behav. 2000;69:363–78. doi: 10.1016/s0031-9384(00)00209-2. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu T, Toriumi H, Sato H, et al. Distribution and origin of TRPV1 receptor-containing nerve fibers in the dura mater of rat. Brain Res. 2007;1173:84–91. doi: 10.1016/j.brainres.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 19.Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics. 2010;7:164–75. doi: 10.1016/j.nurt.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akerman S, Kaube H, Goadsby PJ. Anandamide is able to inhibit trigeminal neurons using an in vivo model of trigeminovascular-mediated nociception. J Pharmacol Exp Ther. 2004;309:56–63. doi: 10.1124/jpet.103.059808. [DOI] [PubMed] [Google Scholar]
- 21.Dux M, Santha P, Jancso G. Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J Physiol. 2003;552:859–67. doi: 10.1113/jphysiol.2003.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szallasi A, Cruz F, Geppetti P. TRPV1: a therapeutic target for novel analgesic drugs? Trends Mol Med. 2006;12:545–54. doi: 10.1016/j.molmed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Jennings EA, Vaughan CW, Roberts LA, Christie MJ. The actions of anandamide on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2003;548:121–29. doi: 10.1113/jphysiol.2002.035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summ O, Holland PR, Akerman S, Goadsby PJ. TRPV1 receptor blockade is ineffective in different in vivo models of migraine. Cephalalgia. 2011;31:172–80. doi: 10.1177/0333102410375626. [DOI] [PubMed] [Google Scholar]
- 25.Arulmani U, Heiligers JP, Garrelds IM, et al. Effects of sumatriptan on capsaicin-induced carotid haemodynamic changes and CGRP release in anaesthetized pigs. Cephalalgia. 2004;24:717–27. doi: 10.1111/j.1468-2982.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 26.Yan J, Edelmayer RM, Wei X, De FM, Porreca F, Dussor G. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain. 2011;152:106–13. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dao TT, Lund JP, Remillard G, Lavigne GJ. Is myofascial pain of the temporal muscles relieved by oral sumatriptan? A cross-over pilot study. Pain. 1995;62:241–44. doi: 10.1016/0304-3959(95)00025-N. [DOI] [PubMed] [Google Scholar]
- 28.Harrison SD, Balawi SA, Feinmann C, Harris M. Atypical facial pain: a double-blind placebo-controlled crossover pilot study of subcutaneous sumatriptan. Eur Neuropsychopharmacol. 1997;7:83–88. doi: 10.1016/s0924-977x(96)00385-9. [DOI] [PubMed] [Google Scholar]
- 29.Carmichael NM, Charlton MP, Dostrovsky JO. Activation of the 5-HT1B/D receptor reduces hindlimb neurogenic inflammation caused by sensory nerve stimulation and capsaicin. Pain. 2008;134:97–105. doi: 10.1016/j.pain.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 30.Schuh-Hofer S, Tayefeh M, Reuter U, Dirnagl U, Arnold G. Effects of parecoxib on plasma protein extravasation and c-fos expression in the rat. Headache. 2006;46:276–85. doi: 10.1111/j.1526-4610.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 31.Waeber C, Moskowitz MA. Therapeutic implications of central and peripheral neurologic mechanisms in migraine. Neurology. 2003;61:S9–20. doi: 10.1212/wnl.61.8_suppl_4.s9. [DOI] [PubMed] [Google Scholar]
- 32.Durham PL, Russo AF. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. J Neurosci. 1999;19:3423–29. doi: 10.1523/JNEUROSCI.19-09-03423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buzzi MG, Moskowitz MA. Evidence for 5-HT1B/1D receptors mediating the antimigraine effect of sumatriptan and dihydroergotamine. Cephalalgia. 1991;11:165–68. doi: 10.1046/j.1468-2982.1991.1104165.x. [DOI] [PubMed] [Google Scholar]
- 34.Jansen-Olesen I, Mortensen A, Edvinsson L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia. 1996;16:310–316. doi: 10.1046/j.1468-2982.1996.1605310.x. [DOI] [PubMed] [Google Scholar]
- 35.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–31. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 36.Jennings EA, Ryan RM, Christie MJ. Effects of sumatriptan on rat medullary dorsal horn neurons. Pain. 2004;111:30–37. doi: 10.1016/j.pain.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Davies AJ, North RA. Electrophysiological and morphological properties of neurons in the substantia gelatinosa of the mouse trigeminal subnucleus caudalis. Pain. 2009;146:214–21. doi: 10.1016/j.pain.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 38.Jennings EA, Christie MJ, Sessle BJ. ATP potentiates neurotransmission in the rat trigeminal subnucleus caudalis. Neuroreport. 2006;17:1507–10. doi: 10.1097/01.wnr.0000234740.97076.95. [DOI] [PubMed] [Google Scholar]
- 39.GlaxoSmithKline. Package Insert. 2010. Imitrex INJECTION Package Insert. [Google Scholar]