Abstract
Background
The annual incidence of chronic myeloid leukemia (CML) in the United States is about 4800 cases. With the success of tyrosine kinase inhibitor (TKI) therapy, the all-cause annual mortality rate was reduced to 2%. The prevalence of CML is therefore increasing over time. Estimating the CML prevalence and plateau prevalence is important in the implementation of healthcare strategies and future therapeutic trials.
Aims
Estimate the increasing prevalence and plateau prevalence of CML in future years.
Methods
The prevalence of CML was estimated based on several parameters: annual mortality rate on TKI therapy compared to age-matched normal population, incidence of CML, anticipated population growth in the United States, aging of the population.
Results
Based on these calculations, the mortality ratio of patients with CML compared to an age-matched normal population is about 1.53. The prevalence of CML is estimated to be about 70,000 in 2010, 112,000 in 2020, 144,000 in 2030, 167,000 in 2040 and 181,000 in 2050 when it reaches a near plateau prevalence.
Conclusions
The prevalence of CML will continue to increase to reach a near plateau prevalence 35 times the annual incidence. These estimates should be considered in healthcare policies and in the design of future studies in CML.
INTRODUCTION
The annual incidence of newly diagnosed cases of chronic myeloid leukemia (CML) in the United States is estimated to be about 1 – 1.3 cases per 100,000, or about 4,800 to 5,200 new cases (1). Before the advent of therapy with imatinib mesylate, a selective Bcr-Abltyrosine kinase inhibitor(TKI), and second generation TKIs (dasatinib, nilotinib), the median survival of patients was approximately 6 years(2,3). Therefore, the estimated prevalence of cases of CML in the United States was approximately 25,000–30,000. The success of imatinib therapy has changed the demographics of CML drastically. While the annual incidence remains essentially unchanged, the estimated annual mortality, which was approximately 10% for the first two years, and 20–25% in subsequent years, has now been reduced to an estimated all-cause mortality of 2% annually for the first 10 years of follow-up (4–7). As a consequence of this very favorable therapeutic outcome, the prevalence of CML has started to increase annually. Based on average estimates, it has been suggested that the plateau prevalence of CML might be reached in the year 2040 with a plateau prevalence (i.e., when incidence and mortality balance each other, and no further significant accumulation of CML cases are expected) of 150,000 to 250,000 cases (6,7). However, there has been no formal analysis of the rate of increase in prevalence and the plateau prevalence of CML. This is the purpose of this analysis.
METHODS
For the calculations of the increased prevalence of CML and the time periods associated with the estimated prevalence, as well as the calculations of the plateau prevalence, we considered the following factors as reported in the literature: 1) the annual mortality rate of patients with CML treated with imatinib; 2) the incidence of CML; 3) the anticipated population growth in the United States; and 4) the aging of the population in the United States.
The annual all-cause mortality rate of patients with CML treated with imatinib is about 1–2% in the first 8–10 years of follow-up (4–7). This estimate is based on a patient population with a median age of 40–50 years. However, as patients age, the all-cause mortality rate is anticipated to increase. Since the follow-up time with imatinib is only 10 years for patients with CML treated with imatinib, an extrapolation is necessary for the prediction of survival beyond 10 years. We therefore compared the mortality rates of 415 patients with newly diagnosed CML referred to our institution from 2001 to 2010 with that of a general population of the same age distribution. A Cox proportional hazards model was used to estimate the hazard(or mortality) ratio between these two populations (8). The mortality ratio of CML patients (in their first 10 years since the diagnosis of CML) vs. the general population with the same age distribution was calculated to be 1.53. We then assumed that this trend will remain the same for the rest of the life times of these two populations. Based on mortality rates of a general population by age groups(1), extrapolation of the mortality rates for patients with CML for all the years after diagnosis was computed. We calculated the prevalence of CML in 2000 to be about 25,000 cases. A total of 4,870 new cases of Philadelphia chromosome-positive CML are diagnosed every year before considering the population growth (9). Less than 5% of patients will present in a transformed phase; these patients have a short median survival regardless of TKIs therapy. Therefore, the estimated annual incidence of newly diagnosed CML in chronic phase is about 4,700.
For the growth of the United States population, the Census Bureau published 3 predictions (lowest, middle, and highest). We have used the middle projection, which predicts 392 million individuals in the Year 2050, a rough growth of 40% over the estimated number of 281 million at the Year 2000(10). The median age of the population will increase by 5 years. This translates approximately into a 10% increase in the incidence of CML.
RESULTS
Without considering population growth and the effect of aging, but including the effect of the CMLvs. normal population mortality ratio of 1.53, the estimated plateau prevalence of CML was calculated to be 105,000 cases and would be reached by 2035. A previous assumption of a constant 2% annual mortality for the whole remaining lifetime of patients with CML would have projected a plateau-prevalence of CML of about 240,000 cases, but this would be reached after 2110. This assumption would be incorrect though. Under this assumption, the long-term survival of a patient with CML would in time become longer than that of a normal aging person whose annual mortality would obviously exceed 2% in later years. We instead estimated that the mortality rate ratio between the CML study group versus general population to be 1.53 by a Cox proportional hazards model (8). Assuming the trend will continue for the lifetimes of these two populations, with such calculation, the median remaining lifetime for a patient with newly diagnosed CML would be about 22 years.
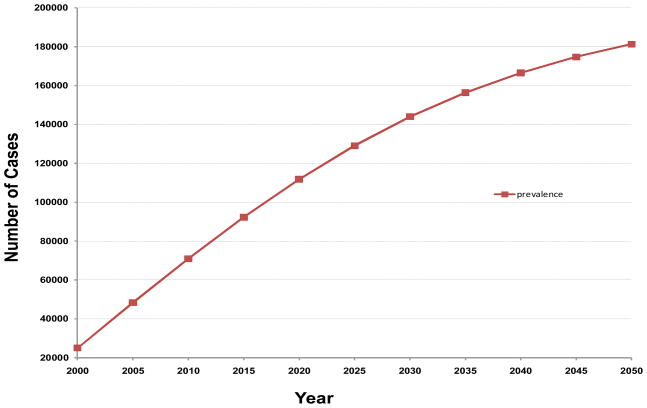
Combining these considerations, our projection of the future prevalence of CML in time is shown in Figure 1.
Figure 1.
Estimated prevalence of chronic myeloid leukemia in the United States by calendar year.
Based on the analysis, the projected number of patients with CML is roughly 70,000 in 2010, 112,000 in 2020, 144,000 in 2030, 167,000 in 2040, and 181,000 in 2050, when it reaches a close to plateau prevalence. For extrapolation purposes, the plateau prevalence of CML in other countries is approximately 35–40 times the incidence of CML for the particular country if similar assumptions can be made.
DISCUSSION
In cancer, the implied definition of cure is the clinical elimination of disease for a long period of time (usually more than 5 to 10 years) after completion of therapy. In other words, it is a disease-free status offering the patient a life expectance similar to the normal population. In most cancers, therapy is completed within a short period of time (e.g. less than 6–12 months; with rare exceptions like acute lymphocytic leukemia). Few cancers have an indolent course requiring no interventions, or treatment with therapies at reasonable costs. These include indolent lymphomas, chronic lymphocytic leukemia, polycythemia vera, essential thrombocytopenia, and others. Chronic myeloid leukemia is unique in combining the need for an expensive long-term therapy (daily TKIs) that results in clinical elimination of the disease only with continued therapy in most instances. Thus, patients with CML resemble more patients with benign conditions (rather than patients with cancer) such as diabetes, hypertension, or coronary heart disease, where continuous effective therapy results in a “functional” rather than “molecular” cures. This increases dramatically the prevalence of CML, which, in this analysis, is estimated to plateau at about 35 times its incidence by 2050, about 180,000 cases in the United States.
The estimation of the increasing prevalence and of the plateau prevalence of CML is important for economic considerations and for the design of future trials.
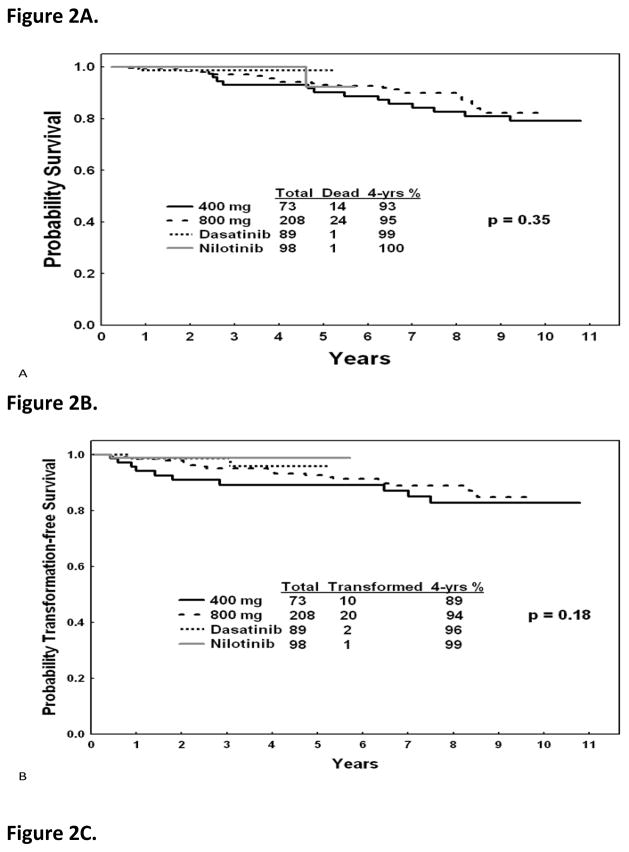
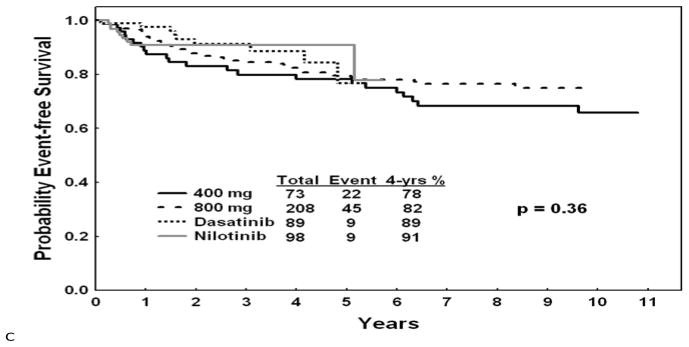
The estimated cost of imatinib today is about $54,000 annually while that of second generation TKIs is $80–90,000. If second generation TKIs replace imatinib as frontline therapy, and if TKI therapy is considered as a lifetime necessary treatment for most patients, then, depending on the year of analysis, the cost of care of patients with CML can be prohibitive in most emerging countries and even in more devloped countries. For example, once the prevalence of CML reaches 100,000 cases, the cost of frontline second generation TKI therapy will be about $8 billion in the U. S. However, imatinib will become generic after the year 2014. The cost of generic imatinib is unknown, but may range from $2,000 to $10,000 per year. If generic imatinib remains frontline CML therapy, then the same estimations will result in a range of cost of care of CML annually of $180 million to $1 billion, a more acceptable burden on the healthcare programs. Whether imatinib or second generation TKIs are established as frontline therapy will depend on the 5–10 year follow-up of the ongoing randomized trials of ENEST-nd and DASISION, comparing nilotinib and dasatinib to imatinib (11,12)and the possible emergence of other drugs that may also be used as initial therpay. If the 5–10 year follow-up data show significant differences in overall survival (perhaps more than 5–10%), progression-free survival (PFS) and event-free survival(EFS)(perhaps more than 10—15%), then using second generation TKIs as frontline therapy may be justifiable in terms of the life-years saved, with the accompanying productivity, despite the differences in cost of care. Such decisions may depend on the perceptions of the differences in benefit versus differences in cost of care, as judged by health authorities in various countries, as well as by physicians and patients. For estimation purposes, Figure 2 shows the longer-term outcomes with imatinib and second-generation TKI from our historical institutional data with the current follow-up.
Figure 2.
Survival, progression-free survival, and event-free survival with imatinib and second generation tyrosine kinase inhibitors as frontline therapy in newly diagnosed chronic phase chronic myeloid leukemia. (MD Anderson data)
The increasing prevalence of CML also highlights the need for strategies to eradicate CML, and to produce molecular cures (allowing discontinuation of therapy) as opposed to functional cures (maintenance of close to normal lifespan with continuation of TKI therapy). At present, 10–30% of patients achieve a status of undetectable molecular disease (ratio of BCR-ABL/ABL < 4.5 log) for more than 2 years. Among such patients, discontinuation of imatinib therapy shows a molecular relapse rate of about 60%, mostly in the first year after discontinuation, and a 40% maintenance of absence of molecular disease(13). Thus with current strategies, less than 5% of the patients maybe able to discontinue imatinib therapy. This figure may increase with the use of second generation TKIs as frontline therapy. This highlights the need to develop additional investigational strategies, potentially implemented at the time of minimal molecular disease, to increase significantly the proportion of patients with durable undetectable molecular disease. Such strategies may include old agents like pegylated interferon (pegasys), omacetaxine, decitabine or others, or newer targeted therapies such as Hedgehog inhhibitors that may also eradicate the dormant CML stem cells.
Based on an incidence of 1 case per 100,000 population in the West, the current study estimates the prevalence of CML to plateau at 35 times the incidence(14). This however may not apply to all countries, and will depend on whether there are known variations of the incidence of CML in particular geographic areas and on the presentation and course of CML in different regions. For example, the incidence of CML in India ranges from 0.8 to 2.2 cases per 100,000 population, similar to Western studies. However, some studies from India also report sometimes higher frequency of CML among leukemias, 40–80% compared with 25% in the U. S. Patients with CML from India are also younger(median age range of 35 to 40 years compared with 50 to 60 years in Western countries) (15). Some studies have also reported a worse prognosis of CML in India in the pre-imatinib era, which might be attributed to delayed diagnosis and a higher proportion of patients with high-risk chronic phase CML or with advanced phases of CML at presentation. Thus, the extrapolation of the prevalence of CML from this study to other nations or geographic areas, and the potential financial implications, have to be reinterpreted in the context of such differences in incidence, aggressiveness of presentations, response to modern therapy, and annual mortality with new TKI therapy.
Acknowledgments
Dr. Hagop Kantarjian has research grants with Novartis, BMS, and Pfizer. He is also a consultant for Novartis. Dr. Jorge Cortes has research grants and is a consultant for Novartis, BMS, Pfizer and Ariad. Dr. Xuelin Huang has no conflicts or financial disclosures.
This work was supported by P01 CA049639 The Therapy of CML.
References
- 1.Arias E. United State Life Tables. National Vital Statistic Reports. 2010;58(21) http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_21.pdf. [PubMed] [Google Scholar]
- 2.Tura S, Baccarani M, Zuffa E, et al. Interferon alfa-2a as compared with conventional chemotherapy for the treatment of chronic myeloid leukemia. N Engl J of Med. 1994;330:820–5. doi: 10.1056/NEJM199403243301204. [DOI] [PubMed] [Google Scholar]
- 3.Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-α with busulfan and hydroxyurea in chronic myelogenous leukemia. Blood. 1994;84 (12):4064–4077. [PubMed] [Google Scholar]
- 4.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355 (23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 5.Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs. ST1571 (IRIS) 8-year follow-up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase treated with imatinib. Blood. 2009;114(22; Suppl):462. (abstract 1126) [Google Scholar]
- 6.Cortes J, Silver R, Kantarjian H. Chronic Myeloid Leukemia. In: Abeloff M, Armitage J, Niederhuber J, Kastan M, McKenna G, editors. Abeloff’s Clinical Oncology. 4. Churchill Livingstone Elsevier; Philadelphia, PA USA: 2008. pp. 2279–2293. [Google Scholar]
- 7.Kantarjian H, O’Brien S. The Chronic Leukemias: Chronic Myelogenous Leukemia. In: Goldman L, Ausiello D, editors. Cecil Medicine. 23. Saunders Elsevier USA: Philadelphia, PA USA; 2008. pp. 1397–1408. [Google Scholar]
- 8.Cox DR. Regression models and life tables (with discussion) Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 9.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:000–000. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 10.Day JC. National Population Projections. Available at http://www.census.gov/population/www/pop-profile/natproj.html.
- 11.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, Nakamae H, Huguet F, Boque C, Chuah C, Bleickardt E, Bradley-Garelik MB, Zhu C, Szatrowski T, Shapiro D, Baccarani M. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. The New England Journal of Medicine. 2010;362:2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 12.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP, Gallagher N, Hoenekopp A, Dong M, Haque A, Larson RA, Kantarjian HM. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. The New England Journal of Medicine. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 13.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11 (11):1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 14.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2002;205(55):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 15.Kumar L. Chronic myelogenous leukaemia (CML): an update. National Medical Journal of India. 2006;19:255–263. [PubMed] [Google Scholar]