Abstract
Objective
Waist-to-hip ratio (WHR) is strongly associated with prevalent atherosclerosis. We analyzed the associations of baseline serum levels of testosterone (T), estradiol (E2), sex hormone binding globulin (SHBG), and dehydroepiandrosterone (DHEA) with WHR in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort.
Subjects
Baseline data was available for 3144 men and 2038 postmenopausal women, who were non-users of hormone therapy, who were 45–84 years of age, and of White, Chinese, Black or Hispanic racial/ethnic groups. Of these, 2708 men and 1678 women also had longitudinal measurements of WHR measured at the second and/or the third study visits (median follow-up 578 days, and 1135 days, respectively).
Results
In cross-sectional analyses adjusted for age, race, and cardiovascular disease risk factors, T was negatively associated with baseline WHR in men, while in both sexes, E2 was positively associated and SHBG was negatively associated with WHR (all p<0.001). In longitudinal analyses, further adjusted for follow-up time and baseline WHR, baseline T was negatively associated with WHR at follow-up (p=0.001) in men, while in both sexes, E2 was positively associated (p=0.004), and SHBG was negatively associated with WHR (p<0.001). The longitudinal association of E2, but not T, was independent of SHBG. In both cross-sectional or longitudinal analyses, there were no associations between DHEA and WHR in either men or women.
Conclusion
Sex hormones are associated with WHR at baseline and also during follow-up above and beyond their baseline association. Future research is needed to determine if manipulation of hormones is associated with changes in central obesity.
Keywords: Sex Hormones, epidemiology, waist to hip ratio
Introduction
Obesity has been identified as a major modifiable risk factor associated with morbidity1, 2 and mortality3, 4,5 The are differences between men and women regarding body fat distribution and its changes over time,6 suggesting that sex hormones may play a role in changes in body composition in both sexes. Cross sectional studies show that obesity is associated with lower levels of free and bioavailable testosterone (T) and sex hormone binding globulin (SHBG) in men,7, 8 but higher levels of estradiol (E2).8 In women, SHBG has also been shown to be lower in obese women while T levels are higher,9 and estradiol (E2) has been shown to be higher in overweight or obese women.10 There are limited data available from studies with longitudinal measures of central obesity. In a cohort consisting of predominantly white men aged 40–70 years, an increase in obesity during follow-up was associated with a decrease in total T, free T and SHBG.11, 12 In younger men aged 24 to 31 yrs at the time of first hormone measurement, only those with reduction in BMI over 8 yrs of follow-up had an increase in SHBG level with age, while those with a gain in BMI during the followup period had a decrease in total T (but not bioavailable T) associated with age.13 In the Penn Ovarian aging study (aged 35–47 years), among premenopausal women, overweight and obese women tended to have lower E2 levels, but this association was reversed after menopause,14 while in the Study of Women Across the Nation in 8–9 years of follow-up, perimenopausal women decreasing SHBG and an increase in bioavailable T and free androgen index were associated with increasing obesity.15, 16 Among premenopausal women in the CARDIA study, only decreasing SHBG levels but not T levels were associated with increasing BMI.17 Given these dynamic relationships around menopause, it is of interest to examine longitudinal relationships during the postmenopausal period.
We examined the cross sectional relationships of baseline endogenous sex hormones with baseline abdominal obesity and with longitudinal changes in abdominal obesity in men and women using data collected in the Multi-Ethnic Study of atherosclerosis (MESA).
Methods
MESA is a multicenter cohort study of the progression of subclinical atherosclerosis among men and women of four racial/ethnic groups within the United States: White, Chinese, Black, and Hispanic. The study design, detailing the recruitment from the population of individuals without clinical cardiovascular disease at baseline has been described previously.18 The institutional review boards of all participating institutions approved the research protocol. Of 3213 men and 3601 women from the MESA baseline, 3161 men and 3009 postmenopausal women had measurement of the sex hormones total testosterone (T), estradiol (E2), dehydroepiandrosterone (DHEA) and sex hormone binding globulin (SHBG). We excluded 958 women who were on current hormone replacement therapy and 15 men and 15 women who had missing data for covariates, leaving 3144 men and 2038 women in the cross sectional analysis. For the longitudinal analysis, 2954 men and 1856 women who had follow-up anthropometric data from at least one follow-up visit were included. The median and interquartile ranges of the time to the second visit were 578 days (498 to 663 days), and to the third visit were 1135 days (1084 to 1219 days), respectively.
Sex hormone measurements (Baseline)
Serum samples obtained at the MESA baseline examination and stored at −70°C were used to assay sex hormone levels at the University of Massachusetts Medical Center in Worcester, MA. Total T and DHEA were measured directly using radioimmunoassay kits, and SHBG was measured by chemiluminescent enzyme immunometric assay using Immulite kits obtained from Diagnostic Products Corporation (Los Angeles, CA). E2 was measured using an ultra-sensitive radioimmunoassay kit from Diagnostic System Laboratories (Webster, TX). The intra-assay coefficients of variation for total T, SHBG, DHEA, and E2 were 12.3%, 9.0%, 11.2%, and 10.5%, respectively.
Anthropometric measurements (Baseline and follow-up)
All participants were measured wearing light clothing and no shoes. Height was measured using Accu-Hite® stadiometers, rounded to the nearest mm. Body weight was measured using a Detecto Platform Balance Scale, rounded to the nearest pound. Waist and hip girth measurements were made in the standing position using Gulick II 150 cm anthropometric tape measures keeping the tape within a horizontal plane. No measurement was taken around clothing. Waist circumference was measured at the level of the umbilicus. Hip circumference was measured around the widest circumference of the buttocks. Waist-Hip ratio (WHR) was calculated from waist and hip girth measurements and used as our index of central obesity because it is the most strongly associated with prevalent atherosclerosis.19
Covariate assessment
A complete medical history questionnaire including medication use, smoking and alcohol consumption was obtained. Persons were considered current smokers if they had smoked cigarettes in the last 30 days. Seated blood pressure was measured using Dinamapp® apparatus, and the mean of the last two of three measurements was reported. Fasting blood samples were drawn between 7:30 am and 10:30 am. Fasting plasma glucose levels and serum triglyceride, total and high density lipoprotein (HDL) cholesterol levels were assayed. Serum low-density lipoprotein (LDL) cholesterol levels were calculated using the Friedewald equation.20 The insulin sensitivity measure QUICKI was calculated as 1/(log[fasting insulin in µU/mL]+log[fasting glucose mg/dL]),21 i.e., an inverse log transformation of the homeostatic model assessment of insulin resistance [HOMA]22). Diabetes was defined as a fasting plasma glucose >= 126 mg/dL or use of anti-diabetic medications.
Statistical Methods
Continuous variables were tabulated as mean (± standard deviation) and sex differences tested using t-tests, or as median [interquartile range] and tested using rank sum tests if the distribution was skewed. Categorical variables were tabulated as percentages and sex differences were tested using χ2 tests. Differences in these variables were also tested between those participants who had any follow-up data versus those that had no follow-up data for either follow-up visit.
The associations of quintiles of sex hormone levels with WHR adjusted for age and race were estimated separately among men and women using ANOVA, and linear trends of these associations were estimated by linear regression. Age and race-adjusted regression analysis with log(sex hormone levels) were done in men and women. For E2, SHBG, and DHEA, pooled models were analyzed in men and women were analyzed if there was no statistically significant interaction by sex. Fasting plasma glucose, triglycerides, total and HDL cholesterol, systolic blood pressure, smoking and reported weekly moderate and vigorous exercise levels were examined as potential confounders. Parsimonious regression models included only those covariates that changed the beta coefficient for any sex-hormone/WHR association by 10% or more. All models presented included age and race as covariates, and sex was a covariate in models including both sexes. Regression models included either single hormones, or multiple hormone variables as covariates. Adjustment for SHBG was performed rather than calculated bioavailable T or E2 fractions because the calculated values for women do not correlate as well with measured free hormone levels.23 SHBG associations were tested for confounding by QUICKI. We used generalized estimating equations (GEE) to assess the longitudinal associations of WHR from all follow-up visits, accounting for within-subject correlations, with sex hormone variables as the exposure variable, adjusting for baseline WHR and follow-up time in days. All other covariates and sex strata were included as described for the cross sectional analysis. Associations were considered significant at p<0.05. The statistical interactions for any heterogeneity by any race/ethnicity of the 16 WHR-sex hormone associations were assessed and considered significant at the Bonferroni corrected level of p<0.003. We performed sensitivity analyses for the race and age-adjusted association of WHR and each hormone for confounding by examination center, reported education level, and reported habitual moderate to vigorous weekly exercise levels, by including each of the possible confounders as covariates in regression.
Results
Study population
No follow-up data were available from 6% of men and 8.9% of women (Table 1). Compared to those with follow-up, those without follow-up data included a greater proportion of Blacks and Hispanics, and at baseline were slightly older and had slightly higher waist-hip ratios. However there was no significant differences in the baseline sex hormone levels between those with and without follow-up (data not shown).
Table 1.
Baseline characteristics of men and women in the study population (Multi-Ethnic Study of Atherosclerosis baseline examination 1998–2000)
| Men | Women | p | ||
|---|---|---|---|---|
| N at baseline | 3144 | 2038 | ||
| Data available from both follow-up visits | 2708 (86.1%) | 1678 (82.3%) | ||
| Follow-up data available from only one visit | 246 (7.8%) | 178 (8.7%) | ||
| No follow-up data available | 190 (6.0%) | 182 (8.9%) | ||
| Age (years) | 62.2±10.2 | 65.6±9.2 | <0.001 | |
| Race/Ethnicity | ||||
| White | 39.3% | 30.3% | <0.001 | |
| Chinese | 12.3% | 13.5% | ||
| Black | 25.7% | 31.0% | ||
| Hispanic | 22.7% | 25.2% | ||
| Cardiovascular Risk Factors | ||||
| Systolic BP (mmHg) | 125.9±19.3 | 130.7±23.9 | <0.001 | |
| Diastolic BP (mmHg) | 75.0±9.4 | 69.6±10.4 | <0.001 | |
| Diabetes (%) | 15.5% | 15.8% | 0.764 | |
| Current Smokers (%) | 14.3% | 10.6% | <0.001 | |
| Current Alcohol users (%) | 62.6% | 42.4% | <0.001 | |
| BP medication use (%) | 30.5% | 38.5% | <0.001 | |
| Lipid medication use (%) | 17.6% | 20.6% | 0.008 | |
| Biochemical assays (mg/dL) | ||||
| Fasting Plasma Glucose | 107.2±33.5 | 105.3±29.7 | 0.034 | |
| Serum Total Cholesterol | 188±35 | 203±37 | <0.001 | |
| Serum LDL Cholesterol | 117±31 | 122±32 | <0.001 | |
| Serum HDL Cholesterol | 45.0±11.8 | 54.5±14.4 | <0.001 | |
| Serum Triglycerides | 113 [79 to 166] | 110 [77 to 157] | 0.026 | |
| Serum Insulin (µU/mL) | 5.4 [3.5 to 8.5] | 5.8 [3.9 to 8.8] | <0.001 | |
| Anthropometry | ||||
| BMI (kg/m2) | 27.9±4.5 | 29.0±6.1 | <0.001 | |
| Waist Girth (cm) | 99.3±12.2 | 98.6±15.5 | 0.094 | |
| Waist-Hip Ratio (%) | 95.8±6.6% | 91.7±8.0% | <0.001 | |
| Sex Hormone variables | ||||
| Total Testosterone (nmol/L) | 14.2 [11.4 to 17.8] | 0.97 [0.62 to 1.39] | <0.001 | |
| Bioavailable Testosterone (nmol/L) | 5.2 [4.2 to 6.5] | 0.24 [0.17 to 0.38] | <0.001 | |
| Estradiol (nmol/L) | 0.114 [0.088 to 0.140] | 0.059 [0.040 to 0.084] | <0.001 | |
| DHEA (nmol/L) | 12.5 [9.1 to 17.1] | 11.0 [7.5 to 15.3] | <0.001 | |
| SHBG (nmol/L) | 40.8 [31.4 to 52.7] | 50.3 [36.7 to 69.9] | <0.001 | |
In MESA, there were no differences between men and women in mean age at baseline or in race/ethnic distributions.18 However, as this analysis was restricted to postmenopausal women not using hormone therapy (HT) the women in the cohort included in this analysis were older than the men and less likely to be white. On average, women had higher systolic pressure, total and HDL-cholesterol, triglycerides and BMI, but lower fasting plasma glucose levels than men. There was no sex difference in diabetes prevalence, and a smaller percentage of women than men were current smokers or regular alcohol consumers. The average WHR was lower in women compared to men, as expected. The range of total T levels in men was widely separated from those in women, while there was a small overlap in the range of E2 levels. On average, men had higher DHEA levels and lower average SHBG levels compared to women.
Cross-sectional association of WHR with sex hormones at baseline
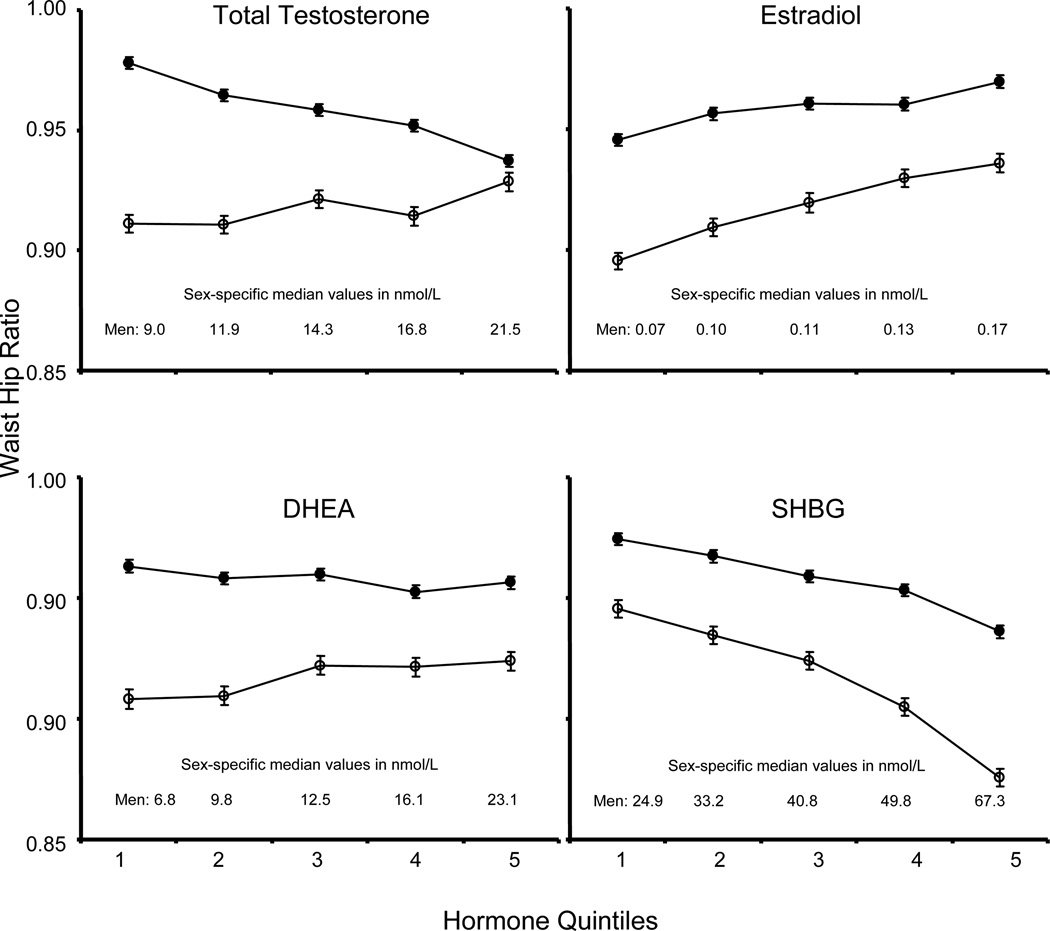
Figure 1 shows the baseline association of WHR with the quintiles of sex hormones, adjusted for age and race, among men and women. There was a positive relationship between T level and WHR in women whereas there was a strong inverse relationship in men. Similarly, but to a lesser degree, there was a non-significant positive trend for DHEA in women, and an inverse trend in men. In both men and women, higher E2 levels are associated with greater WHR, and higher levels of SHBG are associated with lower WHR.
Figure 1.
Age and race adjusted waist-hip ratio at baseline by quintile of sex hormone in men (closed circles) and women (open circles) at MESA baseline examination (1998–2000). The graphed values (with standard error bars) are regression estimates standardized using the mean age of 62.2 years in men and 65.6 years in women, with the proportions of racial/ethnic groups White – 39.3%, Chinese – 12.3%, African-American – 25.7%, Hispanic – 22.7% in men, and White – 30.3%, Chinese – 13.6%, African-American – 31.0%, Hispanic – 25.2% in women. DHEA: Dehydroepiandrosterone, SHBG: Sex Hormone Binding Globulin. The heterogeneity of WC by hormone quintiles of Total Testosterone, Estradiol and SHBG, and also the linear trend by the median level of each quintile, are all statistically significant at p<0.001; heterogeneity by DHEA quintile in men, p = 0.006, in women, p = 0.084; linear trend by median DHEA of each quintile in men, p = 0.001, in women, p = 0.091
Regression analysis of cross sectional data
Table 2 shows various adjusted models of the association of log transformed sex hormones with WHR. The associations that were seen with the quintiles are all confirmed in regression analysis adjusted for age and race. After further adjustment for metabolic risk factors, some of the associations are slightly attenuated, and the association of WHR with both T and with DHEA loses statistical significance. Adjustment of models for T and E2 by SHBG levels shows that SHBG explains much, but not all of the association of these hormones with WHR. Conversely, adjustment for these steroid hormone levels does attenuate the association of SHBG with WHR either in men or women, but it is still very strongly significant. Adjustment for QUICKI (a monotonic transformation of the HOMA measure of insulin resistance) rather than glucose results in a marked attenuation of the associations of Total T and SHBG with WHR. The association of DHEA with WHR becomes non-significant on adjustment for Total T levels, but not on adjustment for E2 levels.
Table 2.
Adjusted cross-sectional difference in waist hip ratio (×10−2) and 95% confidence intervals associated with a 1-log unit of sex hormones at baseline (Multi-Ethnic Study of Atherosclerosis baseline examination 1998–2000; N=3144 men and 2038 women)
| Men | Women | p* | |
|---|---|---|---|
| Single hormone adjusted for age, race | |||
| Total T | −2.60 (−3.09 to −2.12) | 0.72 (0.19 to 1.25) | † |
| E2 | 1.86 (1.29 to 2.42) | 1.67 (1.19 to 2.16) | 0.335 |
| 1.71 (1.35 to 2.06) | |||
| SHBG | −3.32 (−3.90 to −2.76) | −5.03 (−5.71 to −4.36) | 0.001 |
| DHEA | −0.58 (−1.10 to −0.07) | 1.08 (0.42 to 1.74) | <0.001 |
| Single hormone adjusted for age, race, HDL, log(triglycerides), SBP, glucose | |||
| Total T | −1.86 (−2.33 to −1.39) | 0.41 (−0.10 to 0.91) | † |
| E2 | 1.16 (0.61 to 1.71) | 1.11 (0.64 to 1.59) | 0.934 |
| 1.13 (0.79 to 1.48) | |||
| SHBG | −1.87 (−2.46 to −1.29) | −3.42 (−4.15 to −2.69) | 0.001 |
| DHEA | −0.58 (−1.07 to −0.09) | 0.52 (−0.11 to 1.15) | 0.004 |
| Sex Hormone adjusted for SHBG, age, race, HDL, log(triglycerides), SBP, glucose | |||
| Total T | −1.48 (−2.01 to −0.96) | 0.43 (−0.07 to 0.92) | † |
| E2 | 1.14 (0.60 to 1.68) | 1.09 (0.63 to 1.55) | 0.571 |
| 1.08 (0.74 to 1.42) | |||
| SHBG (adjusted for T, E2), age, race, HDL, log(triglycerides), SBP, glucose | |||
| SHBG | −0.79 (−1.44 to −0.14) | −3.40 (−4.13 to −2.68) | <0.001 |
| Hormone adjusted for age, race, HDL, log(triglycerides), SBP, QUICKI | |||
| Total T (adjusted for SHBG) | −1.04 (−1.54 to −0.54) | 0.16 (−0.32 to 0.64) | † |
| SHBG (adjusted for Total T, E2) | −0.57 (−1.20 to 0.05) | −2.39 (−3.12 to −1.67) | <0.001 |
| DHEA (adjusted for T or E2), age, race, HDL, log(triglycerides), SBP, glucose | |||
| DHEA (T) | −0.37 (−0.86 to 0.11) | 0.41 (−0.25 to 1.06) | 0.042 |
| DHEA (E2) | −0.88 (−0.14 to −0.37) | 0.14 (−0.51 to 0.79) | 0.021 |
Associations are considered statistically significant if the 95% confidence intervals that do not include zero. T/ Bio T – total/bioavailable testosterone, E2 – estradiol, SHBG – Sex hormone binding globulin, DHEA – dehydroepiandrosterone;
p for interaction by sex.
There is minimal overlap between the range of T levels between men and women; hence interaction p-value cannot be interpreted.
(log-base-e done)
Regression analysis for longitudinal data
In longitudinal analyses (Table 3) adjusted for age, race and baseline WHR, higher baseline levels of T in men and SHBG in men and women was associated with lower WHR during the follow-up visit. Because baseline WHR was held constant for the comparison by hormone levels, this was interpreted as an association of baseline hormone levels with change in WHR over the follow-up period. In both men and women with the same WHR at baseline, higher levels of E2 at baseline are associated with greater WHR at follow-up. All of these associations are maintained on adjustment of baseline metabolic factors. However, after adjustment for QUICKI, the association of baseline T with follow-up WHR was no longer significant. The longitudinal association of baseline DHEA with follow-up WHR (for persons with the same baseline WHR) was significant and negative on adjustment for baseline E2 levels.
Table 3.
Adjusted difference in waist hip ratio at follow-up (×10−2) and 95% confidence intervals associated with a 1-log unit of sex hormones at baseline, adjusting for follow-up time for individuals with the same baseline waist hip ratio (Multi-Ethnic Study of Atherosclerosis baseline examination 1998–2000, follow-up 2002 to 2005; N= 2954 men, 1856 women)
| Men | Women | p* | |
|---|---|---|---|
| Single hormone adjusted for age, race | |||
| Total T | −0.72 (−1.03 to −0.41) | 0.01 (−0.32 to 0.34) | † |
| E2 | 0.54 (0.19 to 0.89) | 0.44 (0.12 to 0.75) | 0.779 |
| 0.47 (0.24 to 0.69) | |||
| SHBG | −1.17 (−1.53 to −0.81) | −1.78 (−2.24 to −1.32) | 0.014 |
| DHEA | −0.13 (−0.44 to 0.18) | −0.19 (−0.61 to 0.23) | 0.714 |
| −0.12 (−0.38 to 0.12) | |||
| Single hormone adjusted for age, race, HDL, log(triglycerides), SBP, glucose | |||
| Total T | −0.53 (−0.84 to −0.22) | −0.05 (−0.38 to 0.28) | † |
| E2 | 0.38 (0.03 to 0.72) | 0.30 (−0.01 to 0.61) | 0.951 |
| 0.33 (0.11 to 0.55) | |||
| SHBG | −0.80 (−1.17 to −0.42) | −1.43 (−1.92 to −0.93) | 0.008 |
| DHEA | −0.14 (−0.44 to 0.17) | −0.33 (−0.75 to 0.08) | 0.529 |
| −0.19 (−0.44 to 0.06) | |||
| Sex Hormone adjusted for SHBG, age, race, HDL, log(triglycerides), SBP, glucose | |||
| Total T | −0.29 (−0.63 to 0.05) | −0.05 (−0.37 to 0.28) | † |
| E2 | 0.38 (0.03 to 0.73) | 0.30 (−0.01 to 0.61) | 0.701 |
| 0.32 (0.10 to 0.54) | |||
| SHBG (adjusted for T, E2), age, race, HDL, log(triglycerides), SBP, glucose | |||
| SHBG | −0.56 (−0.98 to −0.14) | −1.43 (−1.92 to −0.93) | 0.005 |
| Hormone adjusted age, race, HDL, log(triglycerides), SBP, QUICKI | |||
| T (adjusted for SHBG) | −0.14 (−0.48 to 0.20) | −0.11 (−0.43 to 0.22) | † |
| SHBG (adjusted for T and E2) | −0.54 (−0.95 to −0.12) | −1.22 (−1.72 to −0.72) | 0.018 |
| DHEA (adjusted for T or E2), age, race, HDL, log(triglycerides), SBP, glucose | |||
| DHEA (T) | −0.09 (−0.40 to 0.22) | −0.34 (−0.76 to 0.09) | 0.435 |
| −0.17 (−0.42 to 0.09) | |||
| DHEA (E2) | −0.23 (−0.55 to 0.09) | −0.46 (−0.88 to −0.03) | 0.350 |
| −0.29 (−0.55 to −0.04) | |||
Associations are considered statistically significant if the 95% confidence intervals that do not include zero. T/ Bio T – total/bioavailable testosterone, E2 – estradiol, SHBG – Sex hormone binding globulin, DHEA – dehydroepiandrosterone;
p for interaction by sex.
There is minimal overlap between the range of T levels between men and women; hence interaction p-value cannot be interpreted.
Interactions by race/ethnicity
All cross sectional and longitudinal models, stratified by sex and adjusted for age were examined for the association of each sex hormone with WHR. For 16 such exploratory models, none of the associations showed significant interactions by race/ethnicity that was significant at the Bonferroni corrected levels.
Sensitivity Analyses
The associations of hormones with WHR at baseline or at follow-up were not confounded by examination site, education level as a proxy for socioeconomic status, and for reported habitual physical activity.
Discussion
In this study we report the cross-sectional and longitudinal associations of sex hormones with WHR in a large multi-ethnic population. We principally focused on WHR as an anthropometric measure of central obesity because it has been shown in meta-analyses to be much more strongly associated with prevalent coronary atherosclerosis,19 and as strongly associated with incident diabetes24 when compared to waist circumference or BMI.
In both men and women, higher levels of E2 are associated with larger baseline and follow-up WHR measures. Adjustment for SHBG does not affect the associations of E2 with WHR. This is the first study showing such a strong association of baseline E2 with the longitudinal changes in WHR in a large age range of postmenopausal women, and extends the observation in the immediate postmenopausal period reported for the POAS study.14 The strong cross sectional association we found is consistent with those reported in the Women’s Ischemia Syndrome Evaluation study,25 where there was an independent association of both BMI and waist circumference with E2 levels.
It has been suggested that an androgenic state per se may be associated with greater cardiovascular risk factors in women while the opposite may be true in men.26–28 Though our cross-sectional findings for WHR are consistent with the above studies, when we consider the continued association over the trajectory of WHR over time, such an association was found only in men but not women. Further, in men, the longitudinal association of baseline T levels with WHR was explained by metabolic factors and SHBG levels. The significant cross-sectional associations are only partially explained by the correlation of T with SHBG levels, and remain significant when T was adjusted for SHBG, suggesting that the association is not with the T that is carried tightly bound to SHBG. Our observation of the increasing obesity over follow-up in men with lower SHBG at baseline is consistent with the results from the Massachusetts Male Aging Study that showed an increasing incidence of newly detected metabolic syndrome in men with lower SHBG.29 Also consistent with the metabolic syndrome associations seen in that study, in our analysis, higher SHBG levels at baseline were associated with lower WHR during follow-up, after accounting for WHR at baseline.
Previous cross sectional studies show that obesity is associated with lower levels of free and bioavailable testosterone (T) and sex hormone binding globulin (SHBG) in men.7 In women, SHBG has also been shown to be lower in obese women while testosterone levels are higher9, and estradiol (E2) has been shown to be higher in overweight or obese women.10 Our study confirms and extends the results of published longitudinal studies. Previous studies show an association between lower SHBG levels and obesity measured 12–15 years after baseline hormone measurements, larger WHR being associated with lower androgens in men,27 but not women,28 though these studies did not assess change in WHR. Recent results from the Massachusetts Male Aging study show that greater baseline obesity, and greater increases in obesity over time, was associated with a greater decrease in androgens, presumably linked to a greater decrease in SHBG.11, 12 In younger men aged 24 to 31 yrs at the time of first hormone measurement, the change in BMI over 8 yrs of follow-up modified the relationships of age with change in SHBG and total T but not bioavailable T.13 It has been shown that among obese men, a decrease in weight achieved by a very low energy diet increases SHBG and total testosterone levels.30 While some studies of weight loss trials in obese adolescent girls31 and amenorrhic women32 have reported reductions in androgens, and population based studies in pre and perimenopausal women have reported the strong longitudinal association of SHBG with obesity, and inconsistent relationships with testosterone levels,15–17 ours is the first study reporting longitudinal WHR findings in a population-based sample of postmenopausal women.
SHBG, in addition to its role as a sex hormone transporter, is thought to modulate sex hormone actions through its membrane bound receptor.33 The secretion of SHBG from the liver is sensitive to nutritional status.34 Insulin is known to suppress the secretion of SHBG in vitro35 and may do so in vivo.36 SHBG levels have been shown to be associated with insulin sensitivity rather than insulin secretion during glucose challenge.37 Consistent with this, we found that the association of SHBG with WHR is much attenuated on adjustment for glucose levels and further by adjustment for the insulin sensitivity measure QUICKI. However in all models, SHBG remains significantly associated with WHR, with the exception of the cross-sectional association in men. In this observational study, it cannot be determined whether this is due to mediation of insulin sensitivity in a mechanistic pathway between SHBG and WHR. However, the residual association of SHBG suggests an independent role for SHBG, by the mechanisms discussed above. In this longitudinal epidemiologic cohort study, insulin sensitivity was assessed by QUICKI, which only uses fasting blood sample measures and not more accurate but invasive and burdensome methods such as insulin and glucose clamps. It is therefore possible that there may be residual confounding by insulin sensitivity.
It is likely that measures of abdominal adiposity track sex hormone levels throughout life. Studies have shown that among young men aged 24–31 years who lost weight over 8 years there was a steep age-related increase in SHBG levels, while among those that gained weight there was an age-related decrease in T levels.13 Our results assess these associations later in the lifespan, showing that that among men 45–84 years of age, those with higher SHBG and T at baseline continue to have lower WHR as they age.
Our study raise some interesting, though not definitive, hypotheses regarding the association of the levels of the adrenal sex steroid DHEA with WHR. In our analysis, the associations of DHEA roughly paralleled those of T, and were attenuated in models adjusting for T. Adjustment for E2, on the other hand, accentuated the parallelism between T and DHEA, especially in men. This suggests that insofar as DHEA levels correlated with T, it was associated with lower WHR in men, and longitudinally in women, but insofar as DHEA levels correlated with E2, they were associated with higher WHR. DHEA is metabolized peripherally both into androgenic hormones including T and estrogenic hormones, including E2, and is thus expected to be correlated with both T and E2. Future studies that include measurements of intermediate metabolites of DHEA may provide more insight into these possibilities.
Another interesting hypothesis raised is whether exogenous hormones may also have effects on central obesity in the direction on the observed associations with endogenous hormones. However, these questions can only be definitively answered in controlled intervention studies.
The longitudinal nature of this multi-center study with rigorous anthropometric measurements, with adequate representation of both men and women, is a significant strength of this study. The large sample size and availability of many measured variables allowed for statistical adjustment of multiple possible confounders and exploration of different mediator variables. A potential weakness is the availability of sex hormones only at the baseline visit, which precludes assessing any changes in sex hormone levels that may have accompanied changes in abdominal adiposity. Further, levels of estrone, the major estrogen in postmenopausal women, were not available in MESA. However, we analyzed the levels of E2, the more potent circulating endogenous estrogen. Further, the cross-sectional associations of estrone and E2 with waist circumference have been shown to be similar in another study.25 Thus we believe that the interpretation of our results are valid.
In summary, we have shown that the strong cross-sectional association of androgens with greater WHR in women but lesser WHR in men does not result in continued longitudinal trends in WHR trajectory, especially when adjusted for levels of SHBG. However, in both men and women endogenous E2 levels are associated with a higher level of central obesity cross-sectionally and an increasing trend in obesity over follow-up. Low SHBG levels are strong correlates of current adiposity and a predictor of future increases in adiposity. This is only partially explained by being a marker of insulin resistance or a transporter for sex hormones. Future research is needed to investigate if SHBG directly affects adipose tissue metabolism and to determine if manipulation of hormones is associated with changes in central obesity.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. This research was supported by RO1 HL074406, RO1 HL-74338, and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. DV was supported by the National Center for Research Resources (grant No UL1 RR 025005), a component of the National Institutes of Health.
The funding body did not have a role in the design of the study. The Multi-Ethnic Study of Atherosclerosis Publications and Presentations Committee reviews publications independently of the NIH. DV received compenstation as consultant for Excet Inc.
Footnotes
Conflict of Interest:
No other conflicts of interest are noted.
References
- 1.Dixon JB, Dixon ME, O'Brien PE. Depression in association with severe obesity: changes with weight loss. Arch Intern Med. 2003;163(17):2058–2065. doi: 10.1001/archinte.163.17.2058. [DOI] [PubMed] [Google Scholar]
- 2.Pi-Sunyer FX. Comorbidities of overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31(11 Suppl):S602–S608. doi: 10.1097/00005768-199911001-00019. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, et al. Body weight and mortality among women. N Engl J Med. 1995;333(11):677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 5.Smith SC, Jr, Blair SN, Bonow RO, Brass LM, Cerqueira MD, Dracup K, et al. AHA/ACC Guidelines for Preventing Heart Attack and Death in Patients With Atherosclerotic Cardiovascular Disease: 2001 update. A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol. 2001;38(5):1581–1583. doi: 10.1016/s0735-1097(01)01682-5. [DOI] [PubMed] [Google Scholar]
- 6.Peeters A, Magliano DJ, Stevens J, Duncan BB, Klein R, Wong TY. Changes in abdominal obesity and age-related macular degeneration: the Atherosclerosis Risk in Communities Study. Arch Ophthalmol. 2008;126(11):1554–1560. doi: 10.1001/archopht.126.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, Ble A, et al. Association between hormones and metabolic syndrome in older Italian men. J Am Geriatr Soc. 2006;54(12):1832–1838. doi: 10.1111/j.1532-5415.2006.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohrmann S, Shiels MS, Lopez DS, Rifai N, Nelson WG, Kanarek N, et al. Body fatness and sex steroid hormone concentrations in US men: results from NHANES III. Cancer Causes Control. 2011;22(8):1141–1151. doi: 10.1007/s10552-011-9790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappola AR, Ratcliffe SJ, Bhasin S, Blackman MR, Cauley J, Robbins J, et al. Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab. 2007;92(2):509–516. doi: 10.1210/jc.2006-1399. [DOI] [PubMed] [Google Scholar]
- 10.Mahabir S, Baer DJ, Johnson LL, Hartman TJ, Dorgan JF, Campbell WS, et al. Usefulness of body mass index as a sufficient adiposity measurement for sex hormone concentration associations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2502–2507. doi: 10.1158/1055-9965.EPI-06-0499. [DOI] [PubMed] [Google Scholar]
- 11.Mohr BA, Bhasin S, Link CL, O'Donnell AB, McKinlay JB. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. Eur J Endocrinol. 2006;155(3):443–452. doi: 10.1530/eje.1.02241. [DOI] [PubMed] [Google Scholar]
- 12.Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 2006;65(1):125–131. doi: 10.1111/j.1365-2265.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- 13.Gapstur SM, Kopp P, Gann PH, Chiu BC, Colangelo LA, Liu K. Changes in BMI modulate age-associated changes in sex hormone binding globulin and total testosterone, but not bioavailable testosterone in young adult men: the CARDIA Male Hormone Study. Int J Obes (Lond) 2007;31(4):685–691. doi: 10.1038/sj.ijo.0803465. [DOI] [PubMed] [Google Scholar]
- 14.Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17(4):718–726. doi: 10.1097/gme.0b013e3181cec85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med. 2008;168(14):1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton-Tyrrell K, Zhao X, Santoro N, Lasley B, Sowers M, Johnston J, et al. Reproductive hormones and obesity: 9 years of observation from the Study of Women's Health Across the Nation. Am J Epidemiol. 2010;171(11):1203–1213. doi: 10.1093/aje/kwq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sternfeld B, Liu K, Quesenberry CP, Jr, Wang H, Jiang SF, Daviglus M, et al. Changes over 14 years in androgenicity and body mass index in a biracial cohort of reproductive-age women. J Clin Endocrinol Metab. 2008;93(6):2158–2165. doi: 10.1210/jc.2007-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50(8):752–759. doi: 10.1016/j.jacc.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 21.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 25.Olson MB, Shaw LJ, Kaizar EE, Kelsey SF, Bittner V, Reis SE, et al. Obesity distribution and reproductive hormone levels in women: a report from the NHLBI-sponsored WISE Study. J Womens Health (Larchmt) 2006;15(7):836–842. doi: 10.1089/jwh.2006.15.836. [DOI] [PubMed] [Google Scholar]
- 26.Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23(7):912–918. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 27.Khaw KT, Barrett-Connor E. Lower endogenous androgens predict central adiposity in men. Ann Epidemiol. 1992;2(5):675–682. doi: 10.1016/1047-2797(92)90012-f. [DOI] [PubMed] [Google Scholar]
- 28.Goodman-Gruen D, Barrett-Connor E. Total but not bioavailable testosterone is a predictor of central adiposity in postmenopausal women. Int J Obes Relat Metab Disord. 1995;19(5):293–298. [PubMed] [Google Scholar]
- 29.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91(3):843–850. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 30.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11(6):689–694. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- 31.Wabitsch M, Hauner H, Heinze E, Bockmann A, Benz R, Mayer H, et al. Body fat distribution and steroid hormone concentrations in obese adolescent girls before and after weight reduction. J Clin Endocrinol Metab. 1995;80(12):3469–3475. doi: 10.1210/jcem.80.12.8530585. [DOI] [PubMed] [Google Scholar]
- 32.Pasquali R, Antenucci D, Casimirri F, Venturoli S, Paradisi R, Fabbri R, et al. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab. 1989;68(1):173–179. doi: 10.1210/jcem-68-1-173. [DOI] [PubMed] [Google Scholar]
- 33.Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175(1):113–120. doi: 10.1677/joe.0.1750113. [DOI] [PubMed] [Google Scholar]
- 34.Pascal N, Amouzou EK, Sanni A, Namour F, Abdelmouttaleb I, Vidailhet M, et al. Serum concentrations of sex hormone binding globulin are elevated in kwashiorkor and anorexia nervosa but not in marasmus. Am J Clin Nutr. 2002;76(1):239–244. doi: 10.1093/ajcn/76.1.239. [DOI] [PubMed] [Google Scholar]
- 35.Plymate SR, Jones RE, Matej LA, Friedl KE. Regulation of sex hormone binding globulin (SHBG) production in Hep G2 cells by insulin. Steroids. 1988;52(4):339–340. doi: 10.1016/0039-128x(88)90136-5. [DOI] [PubMed] [Google Scholar]
- 36.Pugeat M, Crave JC, Elmidani M, Nicolas MH, Garoscio-Cholet M, Lejeune H, et al. Pathophysiology of sex hormone binding globulin (SHBG): relation to insulin. J Steroid Biochem Mol Biol. 1991;40(4–6):841–849. doi: 10.1016/0960-0760(91)90310-2. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Real JM, Grasa M, Casamitjana R, Pugeat M, Barret C, Ricart W. Plasma total and glycosylated corticosteroid-binding globulin levels are associated with insulin secretion. J Clin Endocrinol Metab. 1999;84(9):3192–3196. doi: 10.1210/jcem.84.9.5946. [DOI] [PubMed] [Google Scholar]