Abstract
β-site APP cleaving enzyme 1 (BACE1) is the transmembrane aspartyl protease that catalyzes the first cleavage step in the proteolysis of the amyloid β-protein precursor (APP) to the amyloid β-protein (Aβ), a process involved in the pathogenesis of Alzheimer disease. BACE1 pre-mRNA undergoes complex alternative splicing, the regulation of which is not well understood. We identified a G-rich sequence within exon 3 of BACE1 involved in controlling splice site selection. Mutation of the G-rich sequence decreased use of the normal 5′ splice site of exon 3, which leads to full-length and proteolytically active BACE1, and increased use of an alternative splice site, which leads to a shorter, essentially inactive isoform. Nuclease protection assays, nuclear magnetic resonance, and circular dichroism spectroscopy revealed that this sequence folds into a G-quadruplex structure. Several proteins were identified as capable of binding to the G-rich sequence, and one of these, heterogeneous nuclear ribonucleoprotein H (hnRNP H), was found to regulate BACE1 exon 3 alternative splicing and in a manner dependent on the G-rich sequence. Knockdown of hnRNP H led to a decrease in the full-length BACE1 mRNA isoform as well as a decrease in Aβ production from APP, suggesting new possibilities for therapeutic approaches to AD.
Keywords: cis-acting elements, trans-factor proteins, mRNA splicing, protease, Alzheimer disease
INTRODUCTION
Alzheimer’s disease (AD) is characterized by a progressive neurodegeneration associated with production of the aggregation-prone amyloid β-peptide (Aβ), with small, soluble aggregates of Aβ thought to be the primary neurotoxic species (Walsh & Selkoe 2007). Production of this peptide occurs through sequential cleavage of the Aβ precursor protein (APP) by the β-site APP cleaving enzyme 1 (BACE1) and γ-secretase. Because BACE1 is required for Aβ generation, it is considered a major target for drug discovery. Indeed, it was shown in APP transgenic mice that even a partial reduction of BACE1 can effectively decrease production of Aβ and synaptic deficits (Laird et al. 2005, Singer et al. 2005, McConlogue et al. 2007).
The major isoform of BACE1 is the full-length 501-amino acid protein. However, BACE1 pre-mRNA is subjected to alternative splicing to generate five shorter versions of the enzyme (Figure 1A). Alternative splicing within exons 3 and 4 leads to production of isoforms of 476, 457 and 432 amino acids. Skipping of exon 4 can also occur to generate isoform 455. Finally, isoform 127 has been detected but is usually subjected to nonsense-mediated decay (NMD) because it contains a premature termination codon (Tanahashi & Tabira 2007). Shortening the region between the two catalytic aspartic acid residues (encoded in exon 2 and 6) should be detrimental to the structure and affect proteolytic activity (Mowrer & Wolfe 2008). Indeed, we have shown that the full-length 501-amino acid protein is the only isoform with significant proteolytic activity when compared with the shorter isoforms 476, 457, 432 and 455. Moreover, targeting BACE1 pre-mRNA with antisense RNA oligonucleotides can shift alternative splicing away from the 501 isoform and toward inactive isoforms to significantly reduce secretion of Aβ peptides (Mowrer & Wolfe 2008).
Figure 1.
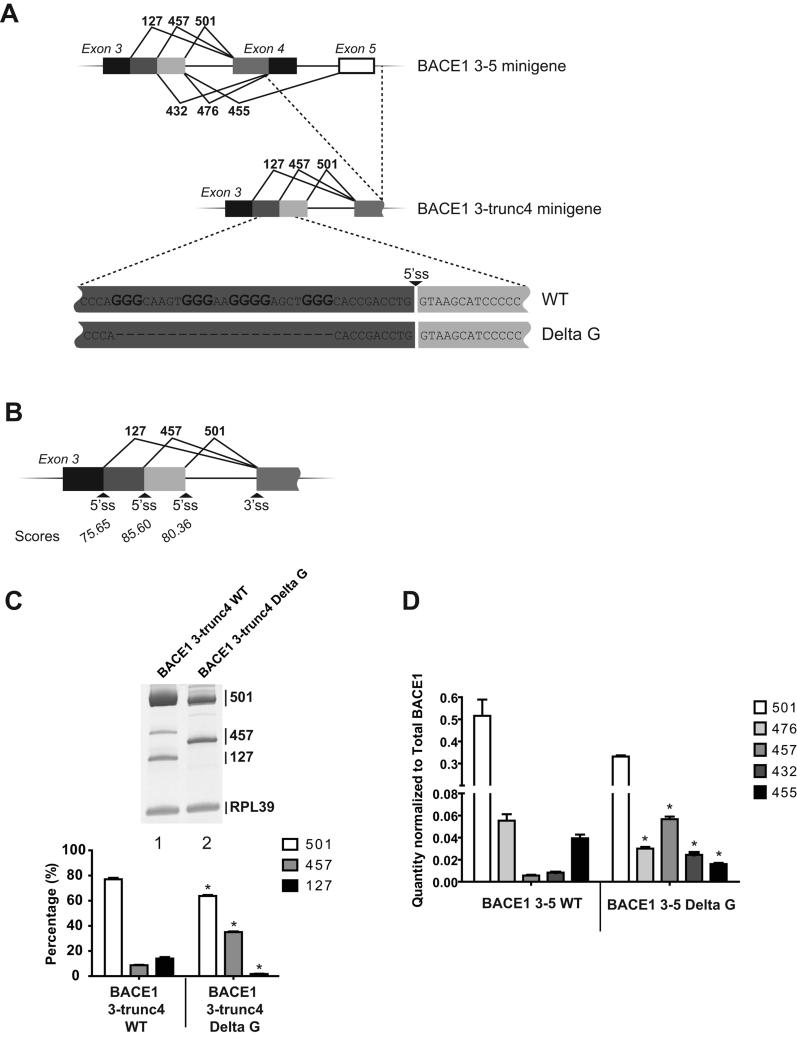
Deletion of the G-rich sequence changes selection of the 5′ ss in BACE1. (A) Representation of the pre-mRNAs used in the in vivo splicing assay. Exon 5 as well as part of exon 4 were deleted to generate BACE1 3-trunc4 minigene. A 24-nucleotide sequence was deleted from the BACE1 3-trunc4 WT and BACE1 3-5 WT constructs to generate BACE1 3-trunc4 Delta G and BACE 1 3-5 Delta G respectively. (B) The strength of the 5′ ss in exon 3 were evaluated using the Shapiro and Senepathy scoring method. (C) Splicing products from in vivo splicing assays in HEK 293 cells were amplified by RT-PCR and fractionated on gel. Amplification of ribosomal protein L39 (RPL39) was used as a control in each reaction. The percentage of each isoform was calculated and represented in the graph. The experiment was done in triplicate and is representative of the three experiments. (D) BACE1 3-5 WT and BACE1 3-5 Delta G minigenes were transfected in triplicate. After RNA purification, splicing products were amplified and quantified by real-time PCR. Quantities were normalized to total amount of BACE1. Quantity of each isoform was compared between the WT and Delta G constructs. * corresponds to a statistical difference (p value <0.05).
Alternative splicing plays a major role in proteomic diversity. Some 92 to 95% of human genes have been estimated to undergo alternative splicing (Pan et al. 2008, Wang et al. 2008). To achieve a high level of fidelity and regulation, cis- and trans-acting elements work together to coordinate selection of the proper splice sites. A variety of splicing factors can be recruited to the pre-mRNAs, and the balance of all these proteins can dictate spliceosome accessibility. Among these, the heterogeneous nuclear ribonucleoprotein H (hnRNP H) is a very well-characterized splicing regulator that was shown to bind G-rich motifs (Caputi & Zahler 2001) and activate (Garneau et al. 2005) or inhibit (Buratti et al. 2004) the use of alternative splice sites. Aside from recruiting proteins, sequences containing stretches of guanosines have the potential to fold into a G-quadruplex, a four-stranded structure consisting of planar arrangements of guanosines stabilized by Hoogsteen hydrogen bonding and K+ ion chelation (Burge et al. 2006). As an illustration that secondary structure can modulate splice sites selection, a G-quadruplex has been shown to negatively regulate splicing of the fragile X mental retardation 1 gene (Didiot et al. 2008).
Little is known about cis- and trans-acting elements that modulate BACE1 alternative splicing. Recently, a potential binding site for SC35 was identified and shown to modulate exon 4 inclusion (Mowrer & Wolfe 2009). We seek to better understand alternative splicing of BACE1 with the aim of identifying potential therapeutic targets that would lower production of the most active 501 isoform and consequently decrease secretion of the deleterious Aβ. Herein, we present evidence that strongly supports the ability of a G-rich region in exon 3 of BACE1 to form a G-quadruplex structure and to recruit the trans-factor hnRNP H to increase generation of the 501 isoform. Finally, we show that targeting this factor decreases secretion of Aβ.
MATERIALS AND METHODS
Plasmid constructs
Plasmid BACE1 3-5 has been described previously (Mowrer & Wolfe 2009). BACE1 3-5 delta G minigene was created by PCR amplification on BACE1 3-5 plasmid using primers B1 deltaG for.1 (5′-ACCGACCTGGTAAGCATCC-3′) and B1 deltaG rev.1 (5′-TGGGTGTAGGGCACATACAC-3′). PCR product was then purified and circularized by ligation. Mutants BACE1 3-5 m1, m2, m3 and m4 were generated using the same strategies. Primers BACE1 m.1 for (5′-CTACACCCAGAGCAAGTGGGA-3′), BACE1 m1 rev (5′-TCCCACTTGCTCTGGGTGTAG-3′), BACE1 m2 for (5′-GGGCAAGTGAGAAGGGGAG-3′), BACE1 m2 rev (5′-CTCCCCTTCTCACTTGCC-3′), BACE1 m3 for (5′-AGTGGGAAGAAGAGCTGGGA-3′), BACE1 m3 rev (5′-TCCCAGCTCTTCTTCCCACT-3′), BACE1 m4 for (5′-GGGAGCTGAGCACCGAC-3′) and BACE1 m4 rev (5′-GTCGGTGCTCAGCTCCC-3′) were used to amplify the different constructs. To generate BACE1 minigene 3-trunc4 WT and delta G, the regions containing the alternative 3′ ss from exon 4, the 5′ ss from exon 5 and intervening sequences were deleted. These constructs were made by PCR amplification of BACE1 3-5 and BACE1 3-5 delta G, respectively, with primers BACE1 delta 3ss for (5′-TGGACTGCAAGGAGCTCGAG-3′) and BACE1 delta 3ss rev (5′-GTGGGTCTGCTTTACCAGAGAGTC-3′). Plasmid DUP 5.1 was initially produced by D. Black (Modafferi & Black 1997) and modified by B. Chabot (Villemaire et al. 2003) to generate DUP 5.1-HpaI. Oligonucleotides G Dup (5′-CCCTACACCCAGGGCAAGTGGGAAGGGGAGCTGGGCACCGACCTGG-3′) and G Dup comp (5′-CCAGGTCGGTGCCCAGCTCCCCTTCCCACTTGCCCTGGGTGTAGGG-3′) were hybridized and inserted into the HpaI sites of plasmid DUP 5.1-HpaI to create construct DUP + G. Complementary oligonucleotides Gm.3 Dup (5′-CCCTACACCCAGGGCAAGTGGGAAGAAGAGCTGGGCACCGACCTGG-3′) and Gm.3 Dup comp (5′-CCAGGTCGGTGCCCAGCTCTTCTTCCCACTTGCCCTGGGTGTAGGG-3′) were inserted into the HpaI sites of DUP 5.1-HpaI to generate minigene DUP + m3.
RNase T1 protection assay
Mixtures containing 50 pmol of biotinylated RNA and 150 mM KCl, NaCl or LiCl were boiled for 2 min and cooled down for 2 h. 1 μg of yeast RNA and 0.6 U of RNase T1 were then added. After 0.5, 1, 2 and 5 min, inactivation/precipitation buffer and Glycoblue (Ambion) were added. Samples were incubated at −80 °C for 15 min and centrifuged at maximum speed for 15 min. Pellets were washed with 70% ethanol and dissolved in 10 μl of gel loading Buffer II (Ambion). Products were fractionated on a 15% acrylamide gel (29:1 acrylamide:bisacrylamide) containing 8 M urea. RNA fragments were transferred to a positively charged nylon membrane (Ambion) and detected using Brightstar Biodetect kit (Ambion).
Circular dichroism spectroscopy
The circular dichroism (CD) spectra were recorded at 25 °C on an Aviv Biomedical spectrometer (Model 410) equipped with a Peltier temperature controller. All experiments were carried out using a 1 mm path-length cuvette. 5 μM of RNA oligonucleotides diluted in 50 mM Tris-HCl pH 7.5 and 100 mM KCl were heated in boiling water for 2 min and cooled to room temperature for 2 h. The spectra were measured between 200-320 nm, corrected for solvent contributions and dilutions and smoothened using adjacent averaging in Origin 7.0.
RNA chromatography
Immobilization of RNA on adipic acid dihydrazide-agarose beads was done following published procedure (Caputi & Zahler 2001). The beads containing the linked RNA were incubated with 200 μg of HEK 293 nuclear extract (Active Motif) for 20 min at 30 °C. Beads were washed three times with chromatography buffer (20 mM HEPES-KOH, pH 7.6, 100 mM KCl, 5% glycerol, 0.2 mM EDTA and 0.5 mM DTT). Proteins were eluted with the same buffer containing 0.25, 0.5 and 1 M KCl. Subsequently, TCA precipitation was performed, and pellets were dissolved with 0.1 N NaOH. Mixtures were loaded on a 4-12% Bis-Tris gel and detected by silver staining. Protein bands were excised and analyzed by MS.
Pull-down assay
50 pmol of biotinylated RNA was incubated with 1 μl of HEK 293 nuclear extract (Active Motif) in chromatography buffer containing 20 mM HEPES-KOH, pH 7.6, 100 mM KCl, 5% glycerol, 0.2 mM EDTA and 0.5 mM DTT. After 20 min at 30 °C, 10 μl of Streptavidin agarose beads (Sigma) was added to the mixtures and incubated for 20 min at room temperature. Beads were then washed three times with chromatography buffer. Proteins attached to the RNA were eluted by heating the mixture at 70 °C for 10 min. Two other samples were prepared using the same procedure (except with additional washing steps). For the second sample, a fourth step of washing was added with chromatography buffer containing 250 mM KCl. The last sample was washed three times with 100 mM KCl, once with 250 mM and finally with 500 mM. The three samples were loaded on a 4-12 % Bis-Tris gel and transferred to a PVDF membrane. hnRNP H protein was detected using hnRNP H antibody (Santa Cruz).
Gel shift experiment
Biotinylated RNA (6.25 μM) was incubated with 1.5, 3 or 4 μM of recombinant hnRNP H protein (Chou et al. 1999) in the presence of 1 mg/ml of heparin for 5 min on ice. Mixtures were fractionated on a 5% Tris-Glycine gel (29:1 acrylamide:bisacrylamide). RNA was transferred to a positively charged nylon membrane (Ambion) and detected using Brightstar Biodetect kit (Ambion).
Transfection
Human embryonic kidney (HEK) 293 cells were transfected with 1 μg of plasmids BACE1 3-5, BACE1 3-trunc4 or DUP using Lipofectamine 2000 (Invitrogen) and incubated for 24 h. Knockdown of hnRNP H was achieved by two successive 48h transfections with 100 nM of siRNA (Santa Cruz). Cells were incubated for a total of 96 h. Stably transfected cells were created by transfection of 1 μg of plasmids expressing shRNA-hnRNP H, scrambled shRNA-A and scrambled shRNA-B (Santa Cruz) using a protocol provided by the company. 1 μg/ml of puromycin was added (lowest concentration that killed 100% of non-transfected cells) 48 h post-transfection and media was changed every 3 days. RNA was purified with RNAqueous Kit (Ambion) and reverse transcribed using Quantitect reverse transcription Kit (Qiagen). Reverse primer BGH (5′-TAGAAGGCACAGTCGAGG-3′) was used with BACE1 minigenes. Reverse transcription of the endogenous BACE1 and DUP 5.1 minigenes was carried with RT primer mix (Qiagen).
ELISA
The experiments were carried out as described previously (Mowrer & Wolfe 2008). Briefly, cell media were changed, and 16 h later media samples were collected for analysis. A dilution of 1:15 was performed, and levels of Aβ40 secreted were subsequently quantified using an ELISA kit (Invitrogen).
Semi-quantitative and real-time PCR
The experiments were carried out as described previously (Mowrer & Wolfe 2009). Briefly, amplification of DUP 5.1 minigene was carried out with primers Ge1 (5′-ACACAACTGTGTTCACTAGC-3′ and Ge2 (5′-AGTGGACAGATCCCCAAAGG-3′). To amplify BACE1 minigenes, primers annealing to exon 3 and BGH were used. Amplification of the endogenous BACE1 isoforms were carried out with primers that anneals to exon 3 and 5 respectively. Semi-quantitative PCR was performed with Advantage 2 polymerase (Clontech). Real-time PCR was performed with Power SYBR Green Master Mix (Applied Biosystems). Quantity of each product was normalized to the total amount of BACE1.
NMR
The 1D 1H NMR spectra were acquired either at 25°C on a 500 MHz Bruker AVANCE™ spectrometer. Water suppression was accomplished using the Watergate pulse sequence (Piotto et al. 1992). The RNA (0.6 mM) was prepared in 10 mM cacodylic acid pH 6.5 in the presence of 150 mM KCl at a 95%: 5% H2O:D2O ratio. To observe the G-quadruplex melting additional spectra were acquired at 48°C and 85°C, respectively.
Statistical analysis
All data were generated in triplicate and unpaired Student’s t-test was used to compare means of group samples. P value <0.05 was used to assert statistical difference.
RESULTS
Sequence containing four G elements controls the selection of the alternative 5′ splice sites in exon 3 of BACE1
With the aim of identifying cis-acting elements that modulate production of the most active BACE1 isoform (501), we first analysed the strength of the 5′ splice sites (ss) in exon 3 using the Shapiro and Senepathy scoring methods (Shapiro & Senapathy 1987). This algorithm is based on the degree of conservation at each position of the consensus 5′ ss and generates a score of 100 for a perfect fit. The proximal 5′ ss (relative to the 3′ ss of exon 4) has a score of 80.36. The middle and the distal donor sites have a score of 85.60 and 75.65 respectively (Figure 1B). In line with this observation, BACE1 3-trunc4 WT minigene was created (from the previously described BACE1 3-5 construct (Mowrer & Wolfe 2009)) to evaluate selection of the 5′ ss in exon 3 (Figure 1A). This simplified pre-mRNA, containing three 5′ ss in competition with each other in exon 3 but only one 3′ ss in exon 4, was subjected to in vivo splicing in HEK 293 cells. Interestingly, even though the middle 5′ ss has the highest score, a strong selection of the proximal 5′ ss was observed (77% of 501 isoform) compared with the 457 isoform (9%) and 127 isoform (14%) (Figure 1C, lane 1 and graph). These results suggest the presence of splicing regulators that would either activate selection of the proximal 5′ss and/or inhibit the use of the middle one.
A sequence containing three GGG motifs as well as one GGGG element is located 11 nucleotides upstream of the theoretically strongest 5′ ss. Because G-rich sequences have been reported to modulate splice site selection in numerous examples (Garneau et al. 2005, Fisette et al. 2010), we decided to determine the potential role of this motif in the selection of the 5′ ss in BACE1. Minigene BACE1 3-trunc4 Delta G was created (Figure 1A) and the splicing profile was compared with the wild-type construct. Deletion of the G-rich sequence promoted reduction of the 501 and 127 isoforms and an increased production of 457 isoform (Figure 1C). This first result suggests that the deleted motif silences the immediate downstream 5′ ss and activates selection of the donor sites that lead to the isoforms 127 and 501. In order to confirm the effect observed, this same deletion was created in the BACE1 3-5 minigene, which contains all of exons 3-5 and intervening sequences (Figure 1A). This time, quantitative PCR was performed to quantify each isoform. Using this more complex splicing unit, the same silencing and enhancing activity was observed: deletion of the G-rich sequence promoted production of the 457 and 432 isoforms as well as a decrease in the 501, 476 and 455 isoforms (Figure 1D), consistent with enhanced usage of the minor internal 5′ ss in exon 3 and decreased usage of the major 5′ ss. Although the decrease in the 501 isoform is not statistically significant, the p value (0.0665) was very close to the confidence level of 0.05. Isoform 127 has been shown to be degraded by NMD (Tanahashi & Tabira 2007) and use of BACE1 minigenes is a way to detect this kind of transcript. However, because any potential results regarding this transcript could not be verified and reproduced at the endogenous level, we considered evaluation of this transcript not relevant for further study here and therefore did not quantify this product. Overall, the experiments provided in Figure 1 suggest that the G-rich sequence has an effect on 5′ss selection.
The G-elements mediate 5′ ss selection in BACE1
We next investigated more precisely the motifs implicated in the selection of the 5′ ss in BACE1. Minigenes that contain mutations in the first (m1), second (m2), third (m3) and fourth (m4) G-elements were generated (Figure 2A, top panel). HEK 293 cells were transfected with each construct and real-time PCR was performed to quantify the isoforms. Mutation of the last three G-runs reduced production of the 501 isoform when compared with the BACE1 3-5 WT minigene (Figure 2A, bottom panel, compare WT with m2, m3 and m4). The biggest effect was observed with the m3 construct which was very close to statistical significance (p value 0.0654) when compared with the WT minigene. On the other hand, m1, m2, m3 and m4 constructs had a positive modulation on 457 and 432 isoforms.
Figure 2.
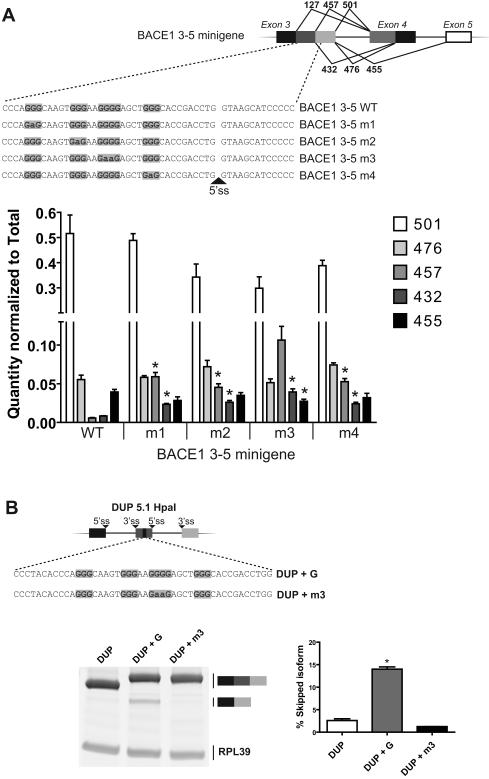
The G-elements are important for the selection of the 5′ ss. (A) Schematic representation of the minigenes transfected in HEK 293 cells (top panel). The first, second and fourth GGG elements were mutated to GAG to generate m1, m2 and m4 constructs respectively. The GGGG motif was modified to GAAG (m3). Following RNA purification, splicing products were quantified by real-time PCR and normalized to total amount of BACE1 (bottom panel). Each experiment was done in triplicate. Quantity of each isoform was compared relative to the WT construct and * represents a p value <0.05. (B) A 46-nucleotide sequence was inserted into the central exon of DUP 5.1 HpaI minigene (DUP + G). A control sequence in which the GGGG motif was mutated to GAAG was also inserted (DUP + m3). Semi-quantitative PCR was carried out, and amplicons were loaded on gel (bottom panel). The percentage of skipped isoforms were calculated and represented in the graph. The experiment was performed in triplicate. Mean corresponding to the percentage of skipped isoform of DUP + G minigene was compared with the DUP construct and * corresponds to a p value <0.05.
Based on these results, along with those in Figure 1, the G-motifs have a direct silencing activity on the immediate downstream 5′ ss. In order to confirm this observation, we inserted a 46-nucleotides sequence (containing the G-rich elements) into the central exon of the heterologous splicing unit DUP 5.1 (Figure 2B, top panel). Introducing this sequence promoted skipping of the second exon whereas insertion of the mutated sequence m3 abrogated the negative activity (Figure 2B, bottom panel). These results confirm the negative effect of the G-rich sequence on the downstream 5′ ss.
The G-rich sequence folds into a parallel G-quadruplex structure
To try to understand how this G-rich cis-acting element can mediate splicing modulation in BACE1, we first investigated formation of secondary structure. As a first approach, the web-based program QuadFinder (Scaria et al. 2006) was used and predicted formation of a G-quadruplex structure. Three different experiments were then initiated to confirm the presence of this unique arrangement of guanosine tracts. First, RNase T1 footprinting was performed to measure interaction between the G-repeats, using a 33-nucleotide (nt) sequence labelled with biotin on the 5′ end (Figure 3A, top). RNase T1 cleaves single-stranded G residues and does not require any metal ion for activity. Taken into consideration that G-quadruplex structures are stabilized by K+ cations, the experiment was carried out in the presence of KCl, NaCl or LiCl. The cleavage profiles indicated that guanosines 1 to 10 are protected in the presence of KCl (Figure 3A, lane 3 to 6) whereas incubation in NaCl- or LiCl-containing buffer leads to a much stronger digestion at these G residues (Figure 3A, lane 7 to 14). This result is consistent with the observed general trend of G quadruplex structure stabilization by K+>>Na+>Li + (Zhou et al. 2011). Unfortunately, because of degradation at the 3′ end (Figure 3A, lane 2), we could not compare cleavage profile for residues 13, 14 and 15. Overall, the RNase T1 assay results indicated K+-dependent protection of three G-elements, which supports the view that the sequence can fold into a G-quadruplex.
Figure 3.
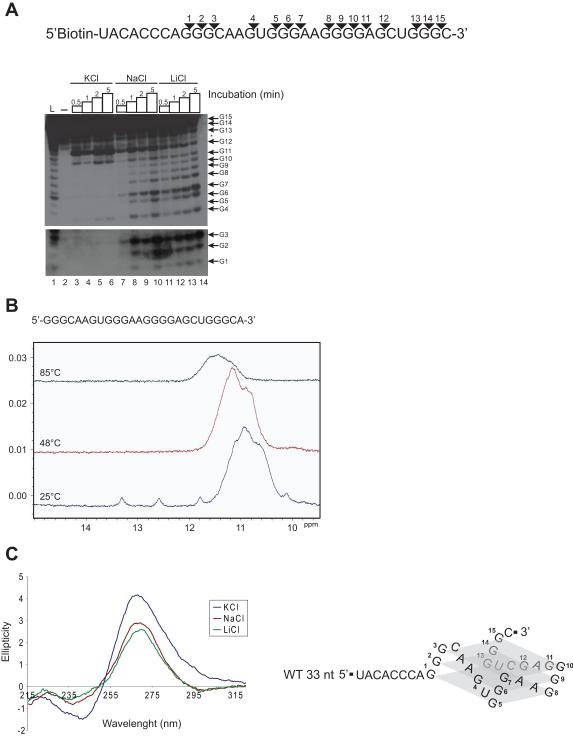
The G-rich sequence can fold into a G-quadruplex structure. (A) 5′ biotinylated RNA used in the RNase T1protection assay and fifteen potential cleavage sites are represented by triangles (top panel). RNA was digested in KCl-, NaCl- or LiCl-containing buffer for 0.5, 1, 2 and 5 min. Lanes designated L and – represent an alkaline hydrolysis and a control reaction (without enzyme) respectively. The position of the residues G1 to G15 is represented. * corresponds to a degradation product. The lower portion of the film has been exposed a little bit longer in order to visualize smaller fragments. (B) Spectral changes of the 1D 1H NMR imino proton region as a function of increasing the temperature. 0.6 mM RNA in 10 mM cacodylic acid pH 6.5 plus 150 mM KCl. (C) CD spectra of the WT 33-nt sequence in the presence of KCl, NaCl or LiCl. The parallel conformation proposed is represented on the left part of the panel.
The presence of a G-quadruplex structure was also confirmed by 1H-NMR spectroscopy. At 25 °C and in the presence of 150 mM KCl, a large broad resonance centered around 11.0 ppm was observed in the imino proton region, characteristic of G-quadruplex imino protons. Less intense and sharper resonances were observed at ~12.6 and 13.3 ppm, originating from Watson-Crick imino protons, and at ~10.2 and ~11.8 ppm, likely originating from the imino protons of a wobble G-U base pair. Increasing the temperature to 48 °C resulted in the disappearance of the smaller, sharper resonances but not the large, broad resonance, originating from the G-quadruplex imino. The persistence of these imino proton resonances even at elevated temperature is consistent with the known stability of G-quadruplex structures. As the temperature was raised further to 85 °C, the broad resonance substantially decreased in intensity, indicating the melting of the G-quadruplex. Upon re-cooling the RNA to 25 °C, the Watson-Crick and G-U imino proton peaks did not reappear (data not shown), suggesting that these imino protons represent a minor alternative conformation co-existing with the G-quadruplex structure. This minor alternative conformation is destroyed at elevated temperatures, with refolding upon cooling in the presence of 150 mM KCl favoring the G-quadruplex structure. Circular dichroism spectroscopy (CD) was used to characterize the fold of the RNA quadruplex-forming sequence. DNA quadruplexes can adopt a parallel conformation, with each strand having the same 5′-to-3′ directionality, or one of several possible antiparallel conformations. In contrast, RNA quadruplexes adopt only a parallel conformation due to the nature of the ribose (Joachimi et al. 2009). Antiparallel topologies present a positive band at 295 nm and a negative one at 265 nm, while a parallel structure gives a positive band at 265 nm and a negative one at 240 nm (Viglasky et al. 2010). When performing a CD experiment, identification of a G-quadruplex structure cannot rely only on a spectra acquired in the presence of KCl. Indeed, other RNA structures can also generate the same spectra. However, when the experiment is compared with NaCl- or LiCl-containing buffer, a KCl-dependent transition at 240 nm and 265 nm is indicative of a G-quadruplex (Beaudoin & Perreault 2010). In Figure 3C, the CD spectrum of the 33-nt wild-type RNA performed in the presence of KCl presented the typical signature of a parallel G-quadruplex. Moreover, when the experiment was done in buffers containing Na+ and Li+, which do not have the same stabilizing effect on the G quadruplex structure as K+ ions, the intensity of the 265 nm band shifted down and the 240 nm trough increased. Thus, taken together, the RNase T1 assay, NMR and CD spectroscopy results indicate that the RNA molecule folds into a G-quadruplex structure in the presence of KCl.
The G-rich sequence can recruit hnRNP H
With the goal of identifying potential splicing regulators that bind and mediate splicing regulation in BACE1, affinity chromatography was performed using a 46-nt RNA containing the G-rich sequence (Figure 4A, top). The oligonucleotide was immobilized onto agarose adipic acid dihydrazide beads and incubated with HEK 293 nuclear extract. Proteins attached to the beads were eluted at 100, 250, 500 and 1000 mM KCl. Mass spectrometric analysis identified three proteins: PARP-1, hnRNP L and hnRNP H (Figure 4A). Two other proteins could not be identified because of low abundance (Figure 4A, see asterisks). For three reasons, hnRNP H was selected for further investigation. First, as explained in the introduction, this protein can modulate splicing. Second, GGG motifs are important for binding of hnRNP H (Caputi & Zahler 2001). Finally, since we have shown that the G-elements are implicated in the splicing decision of BACE1, we have good reason to speculate that hnRNP H could mediate the effect observed.
Figure 4.
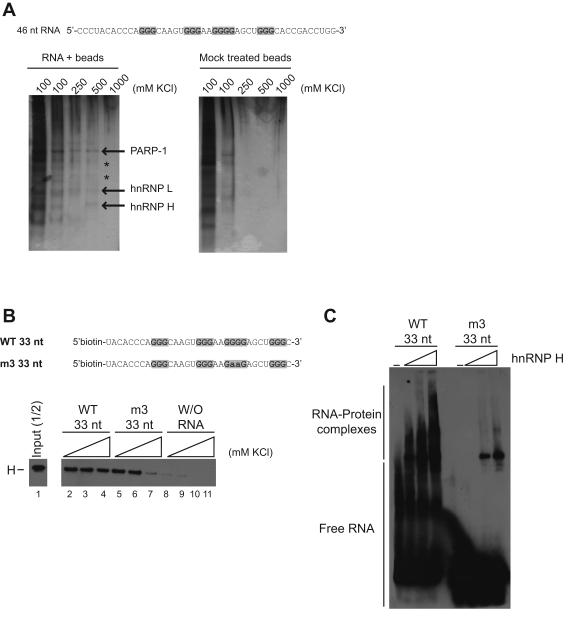
Identification of a potential trans-acting factor that binds the G-rich sequence. (A) RNA oligonucleotide containing the G-elements was immobilized on adipic acid dihydrazide-agarose beads. The RNA-bound beads were incubated in the presence of HEK 293 nuclear extract and proteins were eluted with 100 mM KCl (two times), followed by 250, 500 and 1000 mM. As a control, mock treated beads (without RNA) were used to detect non-specific binding of proteins. Because of low abundance, two proteins could not be identified by MS analysis (*). (B) Pull-down of biotinylated RNA oligonucleotides (WT 33 nt and m3 33 nt) with streptavidin beads after incubation in nuclear extract. After a series of washing steps, proteins that remained bound were eluted by heating the mixture. Incubation of streptavidin beads only (without RNA) was used as control. Proteins were loaded on gel, and detection of hnRNP H protein was carried out by western blot. (C) Electrophoretic mobility shift assay using 1.5, 3 and 4 μM of recombinant his-hnRNP H protein. Binding of hnRNP H to the G-rich sequence was tested by visualization of RNA-protein complexes following addition of the recombinant protein to the WT 33 nt or m3 33 nt RNA.
Binding of hnRNP H to the G-rich sequence was confirmed using a pull-down experiment. A shorter version of the G-rich sequence containing a biotin at the 5′ end was incubated with HEK 293 nuclear extract and pulled down with streptavidin beads. Three samples were prepared and washed with different concentrations of KCl. The proteins that remained attached to the RNA were eluted by heating the mixture. Western blot analysis indicated that hnRNP H can be recruited by the 33-nt wild-type RNA (Figure 4B, lane 2 to 4). When compared with the mutated (m3) RNA, hnRNP H remains attached more strongly to the wild-type oligonucleotide using the highest concentration of KCl (Figure 4B, compare lane 4 with 7). In addition, recombinant hishnRNP H was produced and used in an electrophoretic mobility shift experiment. Strong binding of hnRNP H protein was observed when the wild-type 33-nt RNA was used, whereas weaker binding occurred with the mutated oligonucleotide (Figure 4C).
Taken together, hnRNP H can be recruited by the G-rich sequence, and at least the third G-element is important for binding. Because we could still observe binding of hnRNP H to the mutated m3 33-nt RNA, we cannot rule out the possibility that the other G-elements may mediate the interaction.
hnRNP H activates production of the 501 isoform
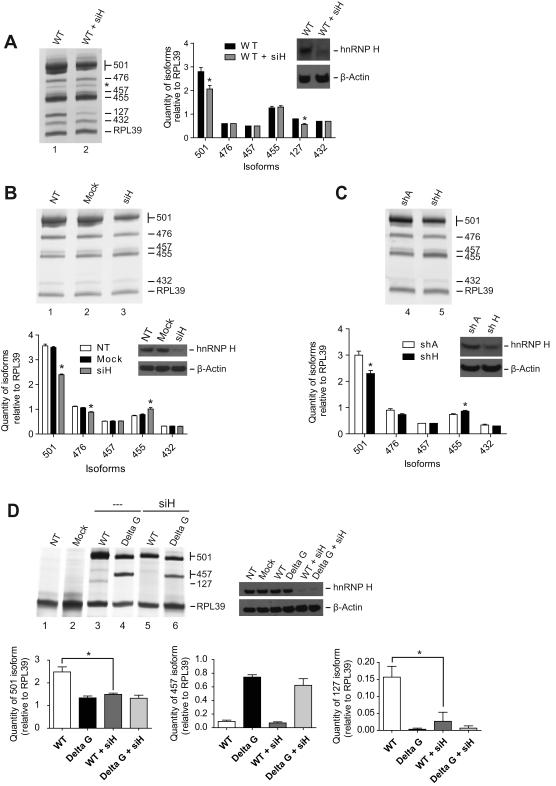
Because hnRNP H can be recruited to the G-rich sequence, we tested if this factor could affect the alternative splicing of BACE1. Following transfection of the BACE1 3-5 minigene and knockdown of hnRNP H, spliced products were reverse transcribed, amplified by PCR and loaded on acrylamide gel. When treated with siRNA against hnRNP H, decrease in the 501 isoform was observed (Figure 5A, compare lane 1 with 2 and see graph). This trend was also observed when the splicing profile of endogenous BACE1 was verified (Figure 5B, compare lane 1 and 2 with 3 and see graph). To ensure that the effect observed is specific to hnRNP H, we have generated two cell lines stably expressing shRNA, one against hnRNP H (shH) and the other a scrambled shRNA (shA). Quantifying BACE1 isoforms relative to RPL39 showed that targeting hnRNP H with shH promoted a strong reduction of the 501 isoform (Figure 5C, lane 4 and 5 and see corresponding graph).
Figure 5.
hnRNP H activates production of the 501 isoform. (A) In vivo splicing assay using BACE1 3-5 WT minigene and siRNA against hnRNP H. The quantity of each isoform is indicated in the graph. * represents a statistical difference (p value <0.05) between the WT and WT + siH. Standard errors of the mean are too small to be visualized on the graph for isoform 476, 457 and 432. (B) RT-PCR amplification of the endogenous BACE1 after knocking down hnRNP H. The isoforms were quantified relative to the internal control RPL39. Quantity of each isoform corresponding to the siH condition was compared with the NT cells. * correspond to a statistical difference (p value <0.05) between the NT and siH. (C) Cell lines that stably express shRNA against hnRNP H (shH) and scrambled shRNA (shA) were created. * correspond to a statistical difference (p value <0.05) between shA and shH. (D) RT-PCR results after cotransfection of BACE1 3-trunc4 WT or BACE1 3-trunc4 Delta G minigenes with siRNA against hnRNP H. Isoforms 501, 457 and 127 were quantified. Statistical comparison was calculated between the indicated groups. Experiments (A, B and C) were performed in triplicate and Western blot was generated to monitored knockdown of hnRNP H.
As we have shown previously, deletion and mutation of the G-rich sequence lowered the production of the 501 isoform. The same effect was observed when the concentration of hnRNP H was decreased. Based on that, we asked if hnRNP H protein modulates splicing directly via binding to the cis-acting element. To verify this hypothesis, we carried out knockdown of hnRNP H and examined the splicing profile of BACE1 3-trunc4 Delta G. Following knockdown of hnRNP H, no change in the level of the 501 isoform was observed (Figure 5D, compare lane 4 with 6 and see graph bottom panel), which is in contrast with the significant decreased of this isoform when the wild-type construct was used (Figure 5D, compare lane 3 with 5 and see graph). Product corresponding to the level of 127 disappeared completely after deletion of the G-rich sequence, and consequently, we were unable to evaluate the effect after knockdown of hnRNP H (Figure 5D, compare lane 3, 4 and 6). The amount of 457 isoform shifted down after knockdown of hnRNP H but was not statistically significant when compared with the non-treated sample (Figure 5D, see graph). This would suggest another factor or perhaps the G-quadruplex structure per se may be responsible for the modulation of this isoform (see discussion).
In light of these results, we conclude that hnRNP H activates production of the 501 isoform, and this activity depends on the presence of the G elements.
Targeting hnRNP H lowers production of Aβ40
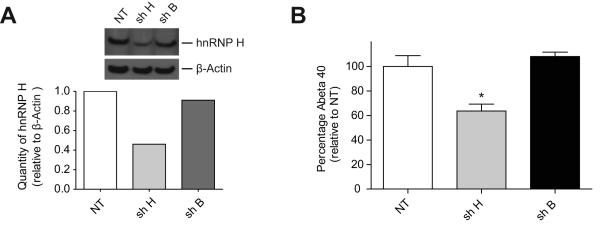
Reduction of hnRNP H protein decreased expression of the most active BACE1 isoform. Consequently, we could expect that production of Aβ would be affected as well. To address this hypothesis, we have used an HEK 293 cell line stably overexpressing Swedish-mutated APP (HEKswe). This KM-to-NL double mutation near the BACE1 cleavage site is associated with autosomal dominant early-onset Alzheimer’s disease and leads to increased production and secretion of Aβ compared to wild-type APP. From these cells, we stably transfected plasmid expressing shRNA against hnRNP H (shH) and plasmid expressing scrambled shRNA (shB). Densitometric analysis of hnRNP H level relative to β-actin showed a strong diminution of hnRNP H protein only when shH was used (Figure 6A). In order to quantify the amount of secreted Aβ40 (40-residue form of Aβ that is the most abundant), media was changed and then analyzed 16 hours later by ELISA. shRNA B failed to affect level of Aβ40 when compared with the non-treated cells (NT) (Figure 6B, compare NT and shB). However, knocking down hnRNP H substantially lowered secretion of Aβ40.
Figure 6.
Reduction of hnRNP H proteins decreases production of Aβ40. (A) HEK 293sw cell lines that stably express shRNA against hnRNP H (shH) and scrambled shRNA (shB) were created. Western blot densitometric analyses were performed to measure levels of hnRNP H relative to β-actin. Levels of hnRNP H were substantially reduced by shH (0.46) but only slightly reduced by shB (0.91) relative to NT (1.0). (B) To measure the amount of Aβ40 secreted, media was changed, and 16 h later samples were analysed by ELISA. Quantity of Aβ40 produced was compared with a non-treated cell line (NT) (Figure 6B). The experiment was performed in triplicate, and * corresponds to a statistical difference (p value <0.05).
DISCUSSION
BACE1 alternative splicing generates six isoforms, but only one is fully active. The proteolysis cascade of APP requires maintaining levels of the full-length 501 transcript. Consequently, inhibition of the shorter isoforms and activation of the full-length enzyme are most likely part of a very important splicing regulation process. To gain insight into this regulation, our study has dissected cis- and trans-acting elements that modulate production of the most active 501 isoform. We have provided strong evidence that a G-rich sequence can modulate selection of the alternative 5′ ss located in exon 3. A dual effect was observed when this region was deleted or mutated: use of the immediate downstream 5′ ss was enhanced, whereas use of the donor site producing the 501 isoform was inhibited. In an effort to better understand the mechanism underlying modulation of these 5′ ss, we have characterized the presence of a secondary structure and identified a splicing regulator. We have demonstrated that this repetition of G-elements can fold into a G-quadruplex structure. Furthermore, we have shown that this region can recruit hnRNP H and thereby activate production of the most active 501 isoform. Finally, we established that knocking down hnRNP H lowers production of Aβ40.
The silencing activity that we have observed with the G-rich sequence is probably part of a complex network of modulators that ensures repressed utilization of the internal 5′ ss located in exon 3. Indeed, in order to maintain high expression of the 501 isoform, repression of the internal 5′ ss could be a key mechanism, especially when considering that the 5′ ss leading to the 501 isoform is a weaker donor site than the one located immediately upstream. Interestingly, when the G-rich motif was positioned into the heterologous splicing unit DUP (Figure 2B), the silencing activity was reproduced, reinforcing the view that this G-rich region can act as a silencer by itself. Several scenarios can be ruled out to explain repression by the G-rich sequence. Based on our results provided in Figures 5 and 6, hnRNP H does not have any silencing effect: it only activates production of the 501 isoform. hnRNP L and PARP-1, which were also isolated from the RNA chromatography, were likewise tested and did not shift the splicing decisions of BACE1 (data not shown). FMRP has also been shown to bind G-quadruplexes and regulate negatively alternative splicing of its own pre-mRNA (Didiot et al. 2008). Although we observed binding of FMRP to the G-rich sequence located in BACE1, knocking down FMRP did not have any effect on BACE1 splice site decisions (data not shown). Two different mechanisms could explain how the cis-acting element inhibits 5′ ss selection. Perhaps one or both of the two unidentified proteins that were visualized on the gel in Figure 4A could contribute to the silencing activity. Another possibility could be that the structure alone directly influences the recruitment of the spliceosome by an unknown mechanism.
For some experiments (Figure 1D and 2A), reduction of the 501 isoform was very close to statistical significance. Nevertheless, the same trend was always observed when the G-elements were targeted: production of the most active BACE1 isoform was always affected. Results presented herein, using two different RNA interference approaches (siRNA and shRNA) clearly demonstrated that hnRNP H activates production of the most active isoform of BACE1 (501) and this effect was always statistically significant. This was shown by examining endogenous BACE1 as well as two different BACE1 minigenes (BACE1 3-5 and BACE1 3-trunc4). Moreover, we confirmed that the activity of hnRNP H depended on the presence of the G-rich sequence. How might hnRNP H activate production of the 501 isoform? The mechanism remains unknown. However, we suspect that this factor could be part of a splicing complex that activates the 5′ ss leading to the production of the 501 isoform. Similar situations have been documented in the literature. For example, hnRNP H can activate inclusion of exon 6D of HIV-1 by recruitment of U1 snRNP (Caputi & Zahler 2002), and this same mechanism might explain activation of the 501 isoform. However, because the isoforms 476 and 455, which are under the control of this 5′ss, are not activated by hnRNP H, we believe other cis- and trans-acting elements are involved in repressing production of these non-active proteins.
Our attempt to affect the level of the most active isoform of BACE1 was successfully achieved. Most importantly, knocking down the splicing factor hnRNP H was also followed by a significant reduction of Aβ40. This finding suggests a new therapeutic strategy. Many efforts have been deployed to develop compounds targeting nucleic acids, and some of these molecules were shown to bind specifically G-quadruplex structures (Satyanarayana et al. 2010). One could consider this kind of ligand to bind the BACE1 G-quadruplex structure and block hnRNP H accessibility, which would abrogate the activation of the 501 isoform. Antisense oligonucleotides represent another attractive strategy to manipulate splicing decisions by masking the binding site for hnRNP H. An example of this strategy has been described for spinal muscular atrophy, in which administration of a modified oligonucleotide into the CNS of mice was shown to redirect alternative splicing of a human survival of motor neuron 2 (SMN2) transgene and abrogate the necrotic phenotype caused by SMN deficiency (Hua et al. 2010). The development of compounds that selectively inhibit the BACE1 enzyme directly has been quite challenging, as these molecules typically have poor drug-like characteristics (Meredith et al. 2008). Targeting BACE1 at the pre-mRNA level offers a very different alternative toward lowering BACE1 proteolytic production of Aβ.
Messenger Origami: BACE1 mRNA Structure Controls Splicing.
Alternative splicing of pre-mRNA of BACE1 (aka, beta-secretase) can affect levels of active protease and, thereby, production of the amyloid β-protein (Aβ). A G-quadruplex structure in exon 3 and interaction with hnRNP H protein helps regulate BACE1 splicing. Targeting this regulatory mechanism can lower A levels, a major therapeutic goal for Alzheimer’s disease.
Acknowledgements
This work was supported by a Zenith Fellows Award (ZEN-08-91633) to M.S.W. from the Alzheimer’s Association and an NIH grant (2R15GM074660) to M.R.M. The authors thank Dr. Benoit Chabot (Université de Sherbrooke, Québec, Canada) and Dr. Douglas Black (University of California, California, United States) for their gift of plasmid DUP HpaI and plasmid expressing hnRNP H respectively.
Abbreviations
- BACE1
β-site APP-cleaving enzyme 1
- APP
β-amyloid precursor protein
- Aβ
amyloid β-peptide
- AD
Alzheimer disease
- CD
circular dichroism spectroscopy
- NMD
nonsense-mediated mRNA decay
- hnRNP H
heterogeneous nuclear ribonucleoprotein H
- HEK
human embryonic kidney
- RT
reverse transcription
- RPL39
ribosomal protein L39
Footnotes
The authors declare no conflicts of interest for this work.
REFERENCES
- Beaudoin JD, Perreault JP. 5′-UTR G-quadruplex structures acting as translational repressors. Nucleic Acids Res. 2010;38:7022–7036. doi: 10.1093/nar/gkq557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti E, Baralle M, De Conti L, Baralle D, Romano M, Ayala YM, Baralle FE. hnRNP H binding at the 5′ splice site correlates with the pathological effect of two intronic mutations in the NF-1 and TSHbeta genes. Nucleic Acids Res. 2004;32:4224–4236. doi: 10.1093/nar/gkh752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S. Quadruplex DNA: sequence, topology and structure. Nucleic Acids Res. 2006;34:5402–5415. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi M, Zahler AM. Determination of the RNA binding specificity of the heterogeneous nuclear ribonucleoprotein (hnRNP) H/H’/F/2H9 family. J Biol Chem. 2001;276:43850–43859. doi: 10.1074/jbc.M102861200. [DOI] [PubMed] [Google Scholar]
- Caputi M, Zahler AM. SR proteins and hnRNP H regulate the splicing of the HIV-1 tev-specific exon 6D. EMBO J. 2002;21:845–855. doi: 10.1093/emboj/21.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MY, Rooke N, Turck CW, Black DL. hnRNP H is a component of a splicing enhancer complex that activates a c-src alternative exon in neuronal cells. Mol Cell Biol. 1999;19:69–77. doi: 10.1128/mcb.19.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didiot MC, Tian Z, Schaeffer C, Subramanian M, Mandel JL, Moine H. The G-quartet containing FMRP binding site in FMR1 mRNA is a potent exonic splicing enhancer. Nucleic Acids Res. 2008;36:4902–4912. doi: 10.1093/nar/gkn472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisette JF, Toutant J, Dugre-Brisson S, Desgroseillers L, Chabot B. hnRNP A1 and hnRNP H can collaborate to modulate 5′ splice site selection. RNA. 2010;16:228–238. doi: 10.1261/rna.1890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau D, Revil T, Fisette JF, Chabot B. Heterogeneous nuclear ribonucleoprotein F/H proteins modulate the alternative splicing of the apoptotic mediator Bcl-x. J Biol Chem. 2005;280:22641–22650. doi: 10.1074/jbc.M501070200. [DOI] [PubMed] [Google Scholar]
- Hua Y, Sahashi K, Hung G, Rigo F, Passini MA, Bennett CF, Krainer AR. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010;24:1634–1644. doi: 10.1101/gad.1941310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachimi A, Benz A, Hartig JS. A comparison of DNA and RNA quadruplex structures and stabilities. Bioorg Med Chem. 2009;17:6811–6815. doi: 10.1016/j.bmc.2009.08.043. [DOI] [PubMed] [Google Scholar]
- Laird FM, Cai H, Savonenko AV, et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConlogue L, Buttini M, Anderson JP, et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J Biol Chem. 2007;282:26326–26334. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- Meredith JE, Jr., Thompson LA, Toyn JH, et al. P-glycoprotein efflux and other factors limit brain amyloid beta reduction by beta-site amyloid precursor protein-cleaving enzyme 1 inhibitors in mice. J Pharmacol Exp Ther. 2008;326:502–513. doi: 10.1124/jpet.108.138974. [DOI] [PubMed] [Google Scholar]
- Modafferi EF, Black DL. A complex intronic splicing enhancer from the c-src pre-mRNA activates inclusion of a heterologous exon. Mol Cell Biol. 1997;17:6537–6545. doi: 10.1128/mcb.17.11.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrer KR, Wolfe MS. Promotion of BACE1 mRNA alternative splicing reduces amyloid beta-peptide production. J Biol Chem. 2008;283:18694–18701. doi: 10.1074/jbc.M801322200. [DOI] [PubMed] [Google Scholar]
- Mowrer KR, Wolfe MS. Identification of a cis-acting element involved in the regulation of BACE1 mRNA alternative splicing. J Neurochem. 2009;109:1008–1016. doi: 10.1111/j.1471-4159.2009.06026.x. [DOI] [PubMed] [Google Scholar]
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J Biomol NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- Satyanarayana M, Kim YA, Rzuczek SG, Pilch DS, Liu AA, Liu LF, Rice JE, LaVoie EJ. Macrocyclic hexaoxazoles: Influence of aminoalkyl substituents on RNA and DNA G-quadruplex stabilization and cytotoxicity. Bioorg Med Chem Lett. 2010;20:3150–3154. doi: 10.1016/j.bmcl.2010.03.086. [DOI] [PubMed] [Google Scholar]
- Scaria V, Hariharan M, Arora A, Maiti S. Quadfinder: server for identification and analysis of quadruplex-forming motifs in nucleotide sequences. Nucleic Acids Res. 2006;34:W683–685. doi: 10.1093/nar/gkl299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MB, Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987;15:7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer O, Marr RA, Rockenstein E, Crews L, Coufal NG, Gage FH, Verma IM, Masliah E. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- Tanahashi H, Tabira T. A novel beta-site amyloid precursor protein cleaving enzyme (BACE) isoform regulated by nonsense-mediated mRNA decay and proteasome-dependent degradation. Neurosci Lett. 2007;428:103–108. doi: 10.1016/j.neulet.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Viglasky V, Bauer L, Tluckova K. Structural features of intra- and intermolecular G-quadruplexes derived from telomeric repeats. Biochemistry. 2010;49:2110–2120. doi: 10.1021/bi902099u. [DOI] [PubMed] [Google Scholar]
- Villemaire J, Dion I, Elela SA, Chabot B. Reprogramming alternative pre-messenger RNA splicing through the use of protein-binding antisense oligonucleotides. J Biol Chem. 2003;278:50031–50039. doi: 10.1074/jbc.M308897200. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Brand NJ, Ying L. G-quadruplexes-novel mediators of gene function. J Cardiovasc Transl Res. 2011;4:256–270. doi: 10.1007/s12265-011-9258-2. [DOI] [PubMed] [Google Scholar]