Abstract
Introduction
Although it has been reported that the work of breathing may be higher in women, inconsistencies among studies leaves this important question unresolved. Also, the association between the oxygen cost of breathing and rating of perceived breathlessness (RPB) during exercise has not been examined between women and men.
Purpose
To measure oxygen cost of breathing during eucapnic voluntary hyperpnea and RPB (Borg 0-10 scale) during 6 minutes of constant work rate cycling at 60 and 90W, respectively, in healthy nonobese women and men.
Methods
Nine women (27yr,21BMI) and ten men (29yr,25BMI) participated. All subjects underwent pulmonary function testing, exercise cycling, and determination of oxygen cost of breathing during eucapnic voluntary hyperpnea. Oxygen cost of breathing was obtained from the slope of the oxygen uptake (ml/min) and ventilation (L/min) relationship. RPB and cardio-respiratory measures were collected during minute 6 of the exercise. Data were analyzed by independent t-test and regression analysis.
Results
Age and pulmonary function were similar between the nonobese women and men. Oxygen cost of breathing was similar between the nonobese women (1.17±0.26ml/L) and men (1.21±0.42ml/L). RPB during exercise was similar between the women (2.1±1.3) and men (2.6±1.2) and was correlated (p<0.05) with relative oxygen uptake (r=0.55), but not the oxygen cost of breathing.
Conclusion
In nonobese women and men, oxygen cost of breathing is not different over the ventilatory ranges studied and RPB is similar at the same relative exercise intensity. In addition, the oxygen cost of breathing was not associated with RPB during constant work rate exercise.
Keywords: Work of breathing, shortness of breath during exercise, breathlessness during exertion, respiratory muscle work
INTRODUCTION
We recently reported that exertional dyspnea in obese women is associated with obesity-related respiratory limitations, specifically an increase in the oxygen cost of breathing during eucapnic voluntary hyperpnea (7). However, we did not find the same relationship between the oxygen cost of breathing and exertional dyspnea in obese men (15). These findings suggest a potential sex difference in the work of breathing. There are many reports suggesting that healthy young adult women may be more susceptible to pulmonary limitations at elevated ventilatory demands than men (19, 20, 24), which could contribute to an increased work of breathing in women. These pulmonary limitations may be associated with women having smaller lungs and airways diameters, lower lung diffusing capacity, and lower maximal expiratory flow rates relative to age and height-matched males (24, 26, 31, 33).
Most of the reports on the work of breathing in normal weight subjects have been conducted in men, using different methodologies, and various ventilatory ranges, which makes it very challenging to make adequate sex comparisons (2, 4, 11, 13, 14, 25, 28). However, two relatively recent studies reported that the work of breathing may be higher in women than men (19, 34). One study measured the mechanical work of breathing at various work rates and extrapolated their findings for comparisons between men and women but did not make simultaneous comparisons at similar ventilatory ranges (19). The other study measured the oxygen cost of breathing using very high levels of dead space to stimulate ventilation, which could increase the work of breathing more for women than men. Furthermore, they analyzed group relationships rather than analyzing individual oxygen cost data (34). Thus, these studies used very different techniques and have methodological limitations, which make it difficult to determine if there is a sex-difference in the oxygen cost of breathing. Our lab has used an oxygen cost of breathing technique that measures oxygen uptake () and minute ventilation () during non-exercising hyperpnea and this technique has been shown to be a good representation of the work of breathing even during exercise by Coast and others (14).
Thus, the purpose of this study was to measure the oxygen cost of breathing with the same technique and ventilatory ranges we used in our obesity studies (7, 15), but in a group of healthy, nonobese women and men. Based upon our earlier work in obese individuals and on the recent articles on sex-differences in the work of breathing (19, 34) we hypothesized that the oxygen cost of breathing would be increased in nonobese women compared with nonobese men.
If there is, in fact, a sex difference in the oxygen cost of breathing, it would be important to document if such differences are associated with an increase in the intensity of respiratory sensations like in obese women (7). Therefore, a secondary aim of this study was to evaluate the ratings of perceived breathlessness (RPB) in healthy nonobese women and men during 6 minutes of constant work rate cycling at 60 and 90W [as done previously in obese subjects (7, 15)], respectively, and to investigate the association between RPB and the oxygen cost of breathing. We hypothesized that RPB would be increased in association with an increased oxygen cost of breathing.
METHODS
Subjects
Nine females and 10 males participated in this study. In accordance with the Institutional Review Board (University of Texas Southwestern Medical Center, approval number 122010-108), all details of the experiments were discussed with the volunteers and informed consent was obtained before participation. All subjects were nonobese (BMI<30) and were recreationally active. All subjects had the same exclusion criteria: history of asthma, cardiovascular disease, musculoskeletal abnormalities, or having participated in regular vigorous exercise for the last 6 months. Subjects with abnormal pulmonary function tests were excluded according to ATS Guidelines (1). Participants were instructed to avoid exercise 24hr prior to study, and food and caffeine for at least 2hr before testing. After screening, the participants underwent pulmonary function testing, exercise testing, and measurement of the oxygen cost of breathing.
Pulmonary Function
All subjects underwent standard spirometry, lung volume, and diffusing capacity determinations (model V62W body plethysmograph, SensorMedics, Yorba Linda, CA) (3). Predicted values for spirometry and lung volumes were based on the norms of Knudson et al. (21, 22), and Goldman and Becklake (17), respectively. Predicted maximal voluntary ventilation (PMVV) was calculated from forced expiratory volume in one second (PMVV=FEV1*37.5).
The Oxygen Cost of Breathing in Combination with Breathing Mechanics
The oxygen cost of breathing was determined from 6-min measurements of and at rest and 4-min measurements of and during eucapnic voluntary hyperpnea at 40L/min and 60L/min (women), or 60L/min and 90L/min (men) as previously described (7). Subjects were seated in an upright position in a chair similar to the position adopted during the cycle exercise test. In order to maintain eucapnia during the voluntary hyperpnea maneuver, subjects breathed from a 1000 L inspiratory reservoir bag containing 4 or 5% CO2 (21% O2 and balance N2) (29). The oxygen cost of breathing was assessed by calculating the slope of the (ml/min) versus (L/min) relationship at rest and during eucapnic voluntary hyperpnea. RPB was obtained during the last 30 seconds of each stage (i.e. rest, 40L/min, 60L/min in women), while physiological data (heart rate, PETCO2, , , etc) were averaged from the 6-min measurements at rest and 4-min measurements during the hyperventilation maneuvers (7). Expiratory and inspiratory flow were measured at rest and continuously during eucapnic voluntary hyperpnea as described previously (5). End-expiratory lung volume (EELV) was estimated from measurement of inspiratory capacity (IC) during the hyperventilation protocol stages and total lung capacity (TLC) during body plethysmography (EELV=TLC-IC) and reported as a percentage of TLC (6, 16).
RPB and Cardio-respiratory Responses During Sub-maximal Exercise
All participants were briefed regarding exercise testing and given detailed written instructions for rating RPB during exercise as described previously (7). Testing began with the subjects seated on the cycle ergometer; after 3min of baseline measurements, the subjects performed a 6-min constant work rate exercise cycling test (cadence was kept between 60 and 70 rpm) at 60W (women), or 90W (men) to assess the intensity of breathlessness during exertion. The exercise workrates (60W for women, and 90W for men) were chosen based on prior studies on obese women and men who reached their first ventilatory threshold at approximately these workrates (6, 16). Briefly, RPB was measured every two minutes of the test and the last value recorded was used for analyses. The intensity of breathlessness was rated using a modified Borg 0-10 scale with verbal expressions of severity anchored to specific numbers (10). Consistent and specific instructions for rating breathlessness were provided to the subjects in written ‘script’ form before testing. The Borg scale for RPB has been demonstrated to be reliable and valid (27). Physiological data (, , etc) were averaged from the 3-min of resting, and last 2-min of the exercise stage. Simple breathing mechanics were measured to characterize differences among the subjects (if any) in tidal flow-volume patterns and lung volume at rest and during exercise.
Data Analyses
Differences between groups were determined by an independent t-test. Relationships among variables were determined with Pearson correlation coefficients. Values are reported as mean ± SD. A p value of < 0.05 was considered significant.
RESULTS
Subjects
Subject physical characteristics are shown in Table 1. Based on the NHLBI clinical guidelines for BMI, all subjects were nonobese (BMI<30). Both women and men had normal spirometry and lung volumes based on predicted values as shown in Table 2.
Table 1.
Subject Characteristics
| Age (yr) |
Ht (cm) |
Wt (kg) |
BMI (kg/m2) |
Wt/Ht (kg/cm) |
|
|---|---|---|---|---|---|
| Women | 27 ± 3 | 164 ± 6 | 58 ± 10 | 21 ± 3 | 0.35 ± 0.05 |
| Men | 29 ± 4 | 179 ± 8 | 79 ± 13 | 25 ± 2 | 0.44 ± 0.05 |
| P value | ns | 0.0003 | 0.0008 | 0.009 | 0.0017 |
Values are means ± SD. Ht, height; Wt, weight; BMI, body mass index; Wt/Ht, ratio of weight to height (kg/cm); ns, non-significant.
Table 2.
Pulmonary Function
| FVC (% pred) |
FEV1 (% pred) |
FEV1/FVC (%) |
PEF (% pred) |
PMVV (L/min) |
TLC (% pred) |
FRC (% TLC) |
RV (% pred) |
RV/TLC (%) |
|
|---|---|---|---|---|---|---|---|---|---|
| Women | 103 ± 15 | 100 ± 12 | 84 ± 6 | 101 ± 19 | 118 ± 19 | 100 ± 11 | 54 ± 7 | 96 ± 20 | 29 ± 6 |
| Men | 103 ± 12 | 104 ± 8 | 84 ± 6 | 113 ± 12 | 176 ± 24 | 102 ± 12 | 48 ± 9 | 91 ± 26 | 22 ± 5 |
| P value | ns | ns | ns | ns | < 0.0001 | ns | ns | ns | ns |
Values are mean ± SD. FVC, forced vital capacity; % pred, percent of predicted; FEV1, forced expiratory volume in one second; PEF, peak expiratory flow; PMVV, predicted maximal voluntary ventilation calculated from FEV1 (PMVV=37.5*FEV1); TLC, total lung capacity; FRC, functional residual capacity (expressed as % of TLC); RV, residual volume; and ns, non-significant. Predicted values for spirometry and lung volumes were based on the norms of Knudson et al. (21, 22), and Goldman and Becklake (17), respectively. ns, non-significant.
Oxygen Cost of Breathing
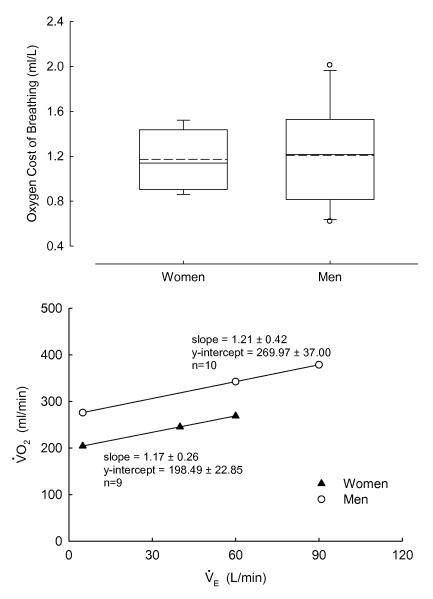
The oxygen cost of breathing during eucapnic voluntary hyperpnea was not different between nonobese women (1.17±0.26ml/L), and men (1.21±0.42ml/L) over the ventilatory ranges studied (Figure 1). Measurements during the eucapnic voluntary hyperpnea bouts of 40 and 60L/min (women), or 60 and 90L/min (men) are presented in Table 3. Note that the relative ventilatory demands (i.e. ratio and heart rate) were remarkably similar between women and men. End-expiratory lung volumes (as a percent of TLC) in women appeared to be slightly increased compared with men, although there was no statistical difference.
Figure 1.
Top panel: Box-plots of the oxygen cost of breathing during eucapnic voluntary hyperpnea (EVH). Boundary of the box closest to zero denotes 25th percentile, solid line within the box denotes median, dashed line denotes mean, and the boundary of the box farthest from zero denotes 75th percentile. Error bars above and below the box denote 10th and 90th percentiles. Outliers are denoted by open circles. Bottom panel: Mean and values during the EVH. There was no difference in the oxygen cost of breathing (i.e. slope) between the nonobese women and men over the ventilatory ranges studied.
Table 3.
Eucapnic Voluntary Hyperpnea in Women at 40 and 60L/min and in Men at 60 and 90L/min
| RPB (0-10) |
(L/min) |
(% pred peak) |
(L/min) |
(L/min) |
VT (% FVC) |
Fb (bpm) |
PETCO2 (torr) |
(%) |
EELV (% TLC) |
HR (% pred peak) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women @ rest |
1 ± 1 | 0.21 ± 0.02 | 10 ± 1 | 0.17 ± 0.02 | 8 ± 1 | 21 ± 7 | 12 ± 4 | 37 ± 3 | 7 ± 2 | 55 ± 7 | 42 ± 8 |
| Men @ rest |
0 ± 0 | 0.28 ± 0.03 | 8 ± 1 | 0.22 ± 0.04 | 10 ± 2 | 15 ± 3 | 12 ± 3 | 42 ± 2 | 6 ± 1 | 50 ± 7 | 38 ± 7 |
|
P value @ rest |
ns | < 0.0001 | 0.0062 | 0.0016 | 0.0262 | 0.0493 | ns | 0.0002 | 0.0159 | ns | ns |
| Women @ 40 |
2 ± 2 | 0.24 ± 0.03 | 11 ± 2 | 0.19 ± 0.03 | 41 ± 2 | 37 ± 8 | 30 ± 0 | 35 ± 1 | 36 ± 7 | 54± 6 | 47 ± 8 |
| Women @ 60 |
4 ± 3 | 0.28 ± 0.03 | 13 ± 2 | 0.18 ± 0.02 | 60 ± 1 | 46 ± 9 | 36 ± 0 | 40 ± 1 | 53 ± 9 | 50 ± 7 | 47 ± 7 |
| Men @ 60 |
3 ± 1 | 0.34 ± 0.05 | 10 ± 1 | 0.24 ± 0.04 | 62 ± 1 | 31 ± 5 | 36 ± 0 | 41 ± 3 | 35 ± 4 | 43 ± 8 | 41 ± 7 |
|
P value @ 60 |
ns | 0.0023 | 0.0028 | 0.0009 | 0.0482 | 0.0004 | ns | ns | < 0.0001 | ns | ns |
| Men @ 90 |
4 ± 1 | 0.38 ± 0.04 | 11 ± 1 | 0.32 ± 0.04 | 93 ± 2 | 43 ± 7 | 40 ± 0 | 40 ± 1 | 54 ± 6 | 44 ± 9 | 45 ± 7 |
Values are mean ± SD. RPB, rating of perceived breathlessness (Borg 0-10 scale); , oxygen uptake (expressed in L/min and as percent of predicted peak based on age and gender); , carbon dioxide production; , minute ventilation; VT, tidal volume (expressed as percent of FVC); Fb, breathing frequency (expressed in breaths per minute, bpm); PETCO2, end-tidal CO2; , ratio of minute ventilation to maximal voluntary ventilation predicted from FEV1 (PMVV=37.5*FEV1); EELV, end-expiratory lung volume (expressed as percent of TLC); HR, heart rate (expressed as percent of predicted peak heart rate based on age and gender); and ns, non-significant.
Ratings of Perceived Breathlessness (RBP) During Exercise
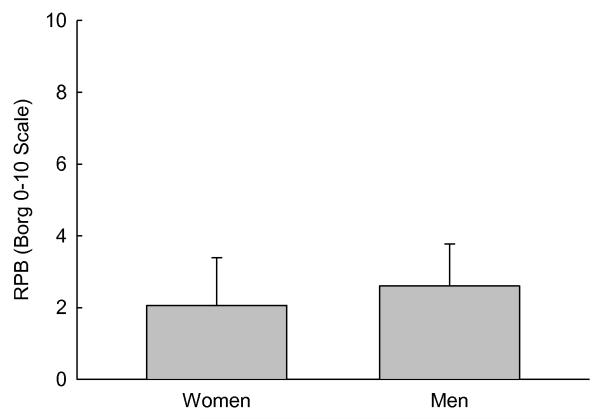
During constant work rate cycling exercise (60W in women, and 90W in men), there were no differences in RPB between women (2.06±1.33) and men (2.60±1.17) (Figure 2). There was no significant relationship between the oxygen cost of breathing and RPB during constant work rate exercise at 60W (women), or 90W (men). RPB during constant work rate exercise was not correlated with any cardio-respiratory measurement, with the exception of relative oxygen uptake (i.e. as a percent of predicted peak ; r=0.76, p=0.018) in women.
Figure 2.
Bar graph showing the rating of perceived breathlessness (RPB) during six minutes of constant work rate cycling. There was no difference between the women and men at 60 and 90W, respectively.
Cardio-respiratory Responses During Sub-maximal Exercise
Cardio-respiratory measures during constant work rate exercise at 60W (women), or 90W (men) are shown in Table 4. Based on ( ratio), (% predicted peak ), and heart rate (%predicted peak HR) the ventilatory demand was low and the relative intensity of exercise were similar in nonobese women and men. In addition, there were no sex differences in the end-expiratory lung volume.
Table 4.
Constant Load Exercise in Women and Men at 60 and 90W, respectively
|
(L/min) |
(% pred peak) | (L/min) |
(L/min) |
VT (% FVC) |
Fb (bpm) |
(%) |
PETCO2 (torr) |
(%) |
EELV (% TLC) |
HR (% pred peak) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women @ rest |
0.23 ± 0.03 | 10 ± 1 | 0.17 ± 0.02 | 8 ± 1 | 20 ± 6 | 13 ± 3 | 49 ± 8 | 38 ± 3 | 7 ± 1 | 56 ± 8 | 40 ± 7 |
| Men @ rest |
0.30 ± 0.06 | 9 ± 1 | 0.26 ± 0.07 | 11 ± 2 | 17 ± 9 | 12 ± 3 | 44 ± 6 | 40 ± 4 | 6 ± 1 | 53 ± 9 | 38 ± 6 |
|
P value rest |
0.0029 | 0.0183 | 0.0046 | 0.0049 | ns | ns | ns | ns | ns | ns | ns |
| Women @ 60W |
1.03 ± 0.08 | 48 ± 7 | 1.06 ± 0.10 | 36 ± 7 | 42 ± 9 | 24 ± 8 | 34 ± 4 | 40 ± 4 | 32 ± 12 | 50 ± 7 | 73 ± 15 |
| Men @ 90W |
1.56 ± 0.07 | 46 ± 6 | 1.52 ± 0.08 | 41 ± 6 | 43 ± 8 | 18 ± 7 | 27 ± 3 | 49 ± 4 | 24 ± 5 | 48 ± 6 | 64 ± 10 |
|
P value exercise |
<0.0001 | ns | <0.0001 | ns | ns | ns | 0.0011 | 0.0002 | 0.0430 | ns | ns |
Values are mean ± SD. , oxygen uptake (expressed in L/min and as percent of predicted peak based on age and gender); , carbon dioxide production; , minute ventilation; VT, tidal volume (expressed as percent of FVC); Fb, breathing frequency (expressed in breaths per minute, bpm);, ventilatory equivalent for CO2; PETCO2, end-tidal CO2; , ratio of minute ventilation to maximal voluntary ventilation predicted from FEV1 (PMVV=37.5*FEV1); EELV, end-expiratory lung volume (expressed as percent of TLC); HR, heart rate (expressed as percent of predicted peak heart rate based on age and gender); and ns, non-significant.
DISCUSSION
There are several important findings from the present research study. First, the oxygen cost of breathing was not different between the nonobese women and men we studied over the ventilatory ranges we tested. Second, the oxygen cost of breathing was not associated with RPB during constant work rate exercise. Finally, RPB during constant work rate exercise was similar between nonobese women and men.
We hypothesized an increased oxygen cost of breathing in the nonobese women compared with the men as suggested by others (18, 19, 34). However, the results from the present study suggest that the oxygen cost of breathing is not higher in nonobese women compared with nonobese men. A higher work of breathing in women at absolute ventilations and for a given exercise work rate relative to body mass ratio has been reported to be due to a higher resistive work of breathing during inspiration and expiration (18). It has also been suggested that total mechanical work of breathing is higher in endurance-trained women compared with men during progressive exercise (19). Another study reported a higher oxygen cost of breathing (i.e., hyperventilation induced by added external dead space) in women compared with men, but the magnitude of the added external dead space and method of analysis limit the interpretation of the data (34). While it is plausible that sex differences in respiratory capacity could influence the work of breathing in women (i.e. higher airway resistance, smaller airway size, lower maximal expiratory flow rates, and increased prevalence of expiratory flow limitation in women compared with men), the comparisons between studies are difficult to interpret because of differences in breathing pattern, breathing mechanics, measurement techniques, and ventilatory ranges, which increases the variability of the results, especially at high ventilatory levels. In a very careful and controlled study, the work of breathing measured during voluntary hyperpnea was not different in the single woman in comparison with seven men (2), which is similar to the findings in our study. Given that we used very similar techniques and ventilatory ranges in the women and men in our study, it is probably safe to suggest that any differences in respiratory function between the women and men were not large enough to make the oxygen cost of breathing different between sexes. The results could be different if measured at higher ventilatory demands but that was not the purpose of this study. While the sample size of this study is not large enough to generalize our results as reference values, they provide a baseline in which to compare our recent data in obese women and men. These data also suggest that sex differences in the oxygen cost of breathing are negligible between women and men at moderate ventilatory levels despite sex-related differences in ventilatory capacities.
In general, the measured oxygen cost of breathing observed in this study was somewhat lower than those reported by some investigators (4, 11, 14), although we had similar values compared with others (2, 12, 13). The ventilatory ranges studied in the present investigation are considerably lower than in publications that reported higher oxygen cost of breathing values. For instance, Bradley & Leith reported an oxygen cost of breathing of approximately 4.5ml/L at an average of 161L/min (11). Another study reported an oxygen cost of breathing of ~2.8ml/L during prolonged (4.5min) maximal hyperventilation (120-240L/min). On the other hand, Aaron et. al. (2) estimated an oxygen cost of breathing of 1.8ml/L at a of 73L/min, which is the closest ventilatory range to that of our study. Their study included data on one woman. In addition, higher levels of are associated with increased variability in the relationship between oxygen cost of breathing and pulmonary ventilation (2, 4, 9, 11). In summary, it is apparent that the studies in which the reported oxygen cost of breathing is substantially higher than our measured values used ventilatory rates that were also much elevated compared with the present investigation. As suggested by Bartlett et al. (9), the measurement of the oxygen cost of breathing at very high (i.e. at or near maximal efforts) results in increased variability, and much less scatter is observed when the effort is sub-maximal. Therefore, a better approach to use the relationship between and (for setting norms and for comparison purposes) would be to perform the maneuvers at lower levels of .
We specifically measured the oxygen cost of breathing during sub-maximal ventilatory ranges (i.e., rather than using maximal ) for several reasons. First, we were interested in measuring the oxygen cost of breathing without additional ventilatory constraints (i.e. expiratory flow limitation) like those observed at high levels of , which would influence the work of breathing (8). Second, we wanted to use similar ventilatory ranges as the ventilations adopted during the 6-min constant work rate exercise so we could investigate potential relationships between the oxygen cost of breathing and breathlessness during moderate exercise. Finally, we wanted these measurements to be comparable with our previous published work in obese adults (7, 15). Therefore, we chose to use lower absolute ventilatory ranges in women (40-60L/min) than in men (60-90L/min) so we could make comparisons between our groups at the same relative ventilatory intensity (i.e. similar ratios between sexes, see Table 3).
Also, we took the values from the current study and compared them with our prior data on obese individuals (7, 15), as the protocols and relative intensities used were very similar. Except for the lack of obesity in the subjects from the current study, this group had similar characteristics as the healthy obese individuals used in earlier projects (7, 15). We expected that the oxygen cost of breathing in obese adults would be higher than in the nonobese subjects from this study, but we wanted to see if the values in the obese were statistically larger. Therefore, we retrospectively compared the oxygen cost of breathing data from the subjects in this investigation with our obese individuals with exertional dyspnea (7, 15). Independent t-test revealed that the oxygen cost of breathing was significantly higher in obese women and men with exertional dyspnea compared with nonobese individuals (3.04 ± 1.08 vs. 1.17 ± 0.26 ml/L, and 2.01 ± 0.75 vs. 1.21 ± 0.42 ml/L, respectively; p < 0.01). This large increase in the work of breathing in obesity is a very important concern, especially during exercise, which is a major component in the prevention and treatment of obesity.
The intensity of respiratory sensations (RPB) were also similar between nonobese women and men. Of the nonobese subjects recruited for this study, only 1 woman (11%) and 2 men (20%) had an RPB ≥ 4 during constant work rate exercise (indicating increased breathlessness with exertion), which is a lower proportion than the reported in obese adults (7, 15, 30, 32, 35, 36). It is important to note that the relative intensity of exercise and ventilatory demand were similar in nonobese women and men (see Table 4). In contrast to our earlier findings in obese women (7), there was no significant relationship between the oxygen cost of breathing and the RPB during constant work rate exercise. However, these observations agree with our findings in obese men (15), since the oxygen cost of breathing is not increased in nonobese women compared with men, there is no reason for RPB to be higher in women (given the exercise intensities were similar).
In summary, this study reports that there is no difference in the oxygen cost of breathing in younger healthy nonobese women compared with men over the ventilatory ranges studied and using the same technique. Therefore, there are no gender differences in the work of breathing in young healthy adults at moderate ventilatory demands. In addition, these values are lower than those previously reported in obese individuals (7, 12, 15, 23). Finally, the rating of perceived breathlessness during 6 minutes of constant work rate cycling is similar in nonobese women and men at the same relative exercise intensity, and the oxygen cost of breathing does not seem to be an important factor in determining RPB in young healthy nonobese women and men.
ACKNOWLEDGEMENTS
The authors appreciate the considerable time and effort of the subjects who participated in this project. This work was supported by the King Charitable Foundation Trust; American Lung Association; American Heart Association; The Research and Education Institute at Texas Health Resources; Cain Foundation; National Institute of Health (HL096782); and Texas Health Presbyterian Hospital Dallas. The authors also wish to express their appreciation for the assistance of Raksa Moran, Todd Bassett, and Sarah Haller on this project.
This work was supported by the King Charitable Foundation Trust; American Lung Association; American Heart Association; The Research and Education Institute at Texas Health Resources; Cain Foundation; National Institute of Health (HL096782); and Texas Health Presbyterian Hospital Dallas.
Footnotes
There are no conflicts of interest.
CONFLICT OF INTEREST: The authors declare no conflicts of interest. The results from the present study do not constitute endorsement by the American College of Sports Medicine.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 2.Aaron EA, Johnson BD, Seow CK, Dempsey JA. Oxygen cost of exercise hyperenea: measurement. J. Appl. Physiol. 1992;72:1810–7. doi: 10.1152/jappl.1992.72.5.1810. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society Standardization of spirometry (1994 update) Am. J. Respir. Crit. Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 4.Anholm JD, Johnson RL, Jr., Ramanathan M. Changes in cardiac output during sustained maximal ventilation in humans. J. Appl. Physiol. 1987;63:181–7. doi: 10.1152/jappl.1987.63.1.181. [DOI] [PubMed] [Google Scholar]
- 5.Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J. Appl. Physiol. 1997;82:746–54. doi: 10.1152/jappl.1997.82.3.746. [DOI] [PubMed] [Google Scholar]
- 6.Babb TG, DeLorey DS, Wyrick BL, Gardner PP. Mild obesity does not limit change in end-expiratory lung volume during cycling in young women. J. Appl. Physiol. 2002;92:2483–90. doi: 10.1152/japplphysiol.00235.2001. [DOI] [PubMed] [Google Scholar]
- 7.Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am. J Respir. Crit Care Med. 2008;178:116–23. doi: 10.1164/rccm.200706-875OC. [DOI] [PubMed] [Google Scholar]
- 8.Babb TG, Rodarte JR. Mechanism of reduced maximal expiratory flow with aging. J. Appl. Physiol. 2000;89:505–11. doi: 10.1152/jappl.2000.89.2.505. [DOI] [PubMed] [Google Scholar]
- 9.BARTLETT RG, Jr., BRUBACH HF, SPECHT H. Oxygen cost of breathing. J. Appl. Physiol. 1958;12:413–24. doi: 10.1152/jappl.1958.12.3.413. [DOI] [PubMed] [Google Scholar]
- 10.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 11.Bradley ME, Leith DE. Ventilatory muscle training and the oxygen cost of sustained hyperpnea. J. Appl. Physiol. 1978;45:885–92. doi: 10.1152/jappl.1978.45.6.885. [DOI] [PubMed] [Google Scholar]
- 12.Cherniack RM. Respiratory effects of obesity. Can Med Assoc J. 1959;80:613–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Cherniack RM. The oxygen consumption and efficiency of the respiratory muscles in health and emphysema. J Clin. Invest. 1959;38:494–9. doi: 10.1172/JCI103826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coast JR, Rasmussen SA, Krause KM, O’Kroy JA, Loy RA, Rhodes J. Ventilatory work and oxygen consumption during exercise and hyperventilation. J. Appl. Physiol. 1993;74:793–8. doi: 10.1152/jappl.1993.74.2.793. [DOI] [PubMed] [Google Scholar]
- 15.Davidoff SL, Rowell CJ, Wood HE, Storms CD, Hollas CN, Ranasinghe KG, Moran RB, Klocko MN, Babb TG. Exertional dyspnea in obese men is associated with low lung volume breathing. Am.J.Respir.Crit.Care Med. 2009;179:A6053. abstract. [Google Scholar]
- 16.DeLorey DS, Wyrick BL, Babb TG. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. Int. J. Obes. 2005;29:1039–47. doi: 10.1038/sj.ijo.0803003. [DOI] [PubMed] [Google Scholar]
- 17.Goldman HI, Becklake MR. Respiratory function tests. Normal values at median altitudes and the prediction of normal results. Am Rev Tuberc. 1959;79:457–67. doi: 10.1164/artpd.1959.79.4.457. [DOI] [PubMed] [Google Scholar]
- 18.Guenette JA, Querido JS, Eves ND, Chua R, Sheel WA. Sex differences in the resistive and elastic work of breathing during exercise in endurance-trained athletes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R166–R175. doi: 10.1152/ajpregu.00078.2009. [DOI] [PubMed] [Google Scholar]
- 19.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol. 2007;581:1309–22. doi: 10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol. 1998;507:619–28. doi: 10.1111/j.1469-7793.1998.619bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knudson RJ, Lebowitz MD, Holberg J, Burrows B. Changes in the Normal Maximal Expiratory Flow-Volume Curve with Growth and Aging. Am Rev Respir Dis. 1983;127:725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 22.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve: Normal standards, variability and effects of age. Am Rev Respir Dis. 1976;113:587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 23.Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing (VO2RESP) at rest. Am. J. Respir. Crit. Care Med. 1999;160:883–6. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 24.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J. Appl. Physiol. 1998;84:1872–81. doi: 10.1152/jappl.1998.84.6.1872. [DOI] [PubMed] [Google Scholar]
- 25.McGregor M, Becklake MR. The relationship of oxygen cost of breathing to respiratory mechanical work and respiratory force. J. Clin. Invest. 1961;40:971–80. doi: 10.1172/JCI104336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am. Rev. Respir. Dis. 1980;121:339–42. doi: 10.1164/arrd.1980.121.2.339. [DOI] [PubMed] [Google Scholar]
- 27.Meek PM, Schwartzstein RM, Adams L, Altose MD, Breslin EH, Carrieri-Kohlman V, Gift A, Hanley MV, Harver A, Jones PW, Killian K, Knebel A, Lareau SC, Mahler DA, O’Donnell DE, Steele B, Stuhlbarg M, Titler M. Dyspnea mechanisms, assessment, and management: a consensus statement. Am. J. Respir. Crit. Care Med. 1999;159:321–40. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 28.Milic-Emili G, Petit JM. Mechanical efficiency of breathing. J. Appl. Physiol. 1960;15:359–62. doi: 10.1152/jappl.1960.15.3.359. [DOI] [PubMed] [Google Scholar]
- 29.Rundell KW, Anderson SD, Spiering BA, Judelson DA. Field exercise vs laboratory eucapnic voluntary hyperventilation to identify airway hyperresponsiveness in elite cold weather athletes. Chest. 2004;125:909–15. doi: 10.1378/chest.125.3.909. [DOI] [PubMed] [Google Scholar]
- 30.Sahebjami H. Dyspnea in obese healthy men. Chest. 1998;114:1373–7. doi: 10.1378/chest.114.5.1373. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz J, Katz SA, Fegley RW, Tockman MS. Sex and race differences in the development of lung fuction. Am. Rev. Respir. Dis. 1988;138:1415–21. doi: 10.1164/ajrccm/138.6.1415. [DOI] [PubMed] [Google Scholar]
- 32.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch. Intern. Med. 2002;162:1477–81. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 33.Thurlbeck WM. Postnatal human lung growth. Thorax. 1982;37:564–71. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topin N, Mucci P, Hayot M, Prefaut C, Ramonatxo M. Gender influence on the oxygen consumption of the respiratory muscles in young and older healthy individuals. Int. J. Sports Med. 2003;24:559–64. doi: 10.1055/s-2003-43267. [DOI] [PubMed] [Google Scholar]
- 35.Weisman IM, Zeballos RJ. Clinical evaluation of unexplained dyspnea. Cardiologia. 1996;41:621–34. [PubMed] [Google Scholar]
- 36.Whipp BJ, Davis JA. The ventilatory stress of exercise in obesity. Am Rev Respir Dis. 1984;129:S90–S92. doi: 10.1164/arrd.1984.129.2P2.S90. [DOI] [PubMed] [Google Scholar]