Abstract
Aberrant sensitivity of incentive neurocircuitry to nondrug rewards has been suggested as either a risk factor for or consequence of drug addiction. Using functional magnetic resonance imaging, we tested whether alcohol‐dependent patients (ADP: n = 29) showed altered recruitment of ventral striatal (VS) incentive neurocircuitry compared to controls (n = 23) by: (1) cues to respond for monetary rewards, (2) post‐response anticipation of rewards, or (3) delivery of rewards. Using an instrumental task with two‐stage presentation of reward‐predictive information, subjects saw cues signaling opportunities to win $0, $1, or $10 for responding to a target. Following this response, subjects were notified whether their success would be indicated by a lexical notification (“Hit?”) or by delivery of a monetary reward (“Win?”). After a variable interval, subjects then viewed the trial outcome. We found no significant group differences in voxelwise activation by task contrasts, or in signal change extracted from VS. Both ADP and controls showed significant VS and other limbic recruitment by pre‐response reward anticipation. In addition, controls also showed VS recruitment by post‐response reward‐anticipation, and ADP had appreciable subthreshold VS activation. Both groups also showed similar mesolimbic responses to reward deliveries. Across all subjects, a questionnaire measure of “hot” impulsivity correlated with VS recruitment by post‐response anticipation of low rewards and with VS recruitment by delivery of low rewards. These findings indicate that incentive‐motivational processing of nondrug rewards is substantially maintained in recovering alcoholics, and that reward‐elicited VS recruitment correlates more with individual differences in trait impulsivity irrespective of addiction. Hum Brain Mapp 33:2174–2188, 2012. Published 2011 Wiley Periodicals, Inc.
Keywords: reward, impulsivity, nucleus accumbens, ventral striatum, alcoholism, substance use disorder, instrumental behavior
INTRODUCTION
Prevailing motivational theories of addiction posit that addicted persons show a bias in behavior allocation toward drugs due to either increased sensitivity of motivational neurocircuitry to drug‐predictive cues, or decreased sensitivity of motivational neurocircuitry to nondrug rewards. A primary account for the former possibility is the incentive salience hypothesis (ISH) [Robinson and Berridge,2001] that attributes compulsive drug use to the repeated chemical perturbation of mesolimbic neurocircuitry that is normally recruited during associative learning. The latter possibility (known primarily as the reward deficiency syndrome (RDS) [Blum et al.,2000] or allostatic hypothesis (AH) [Koob et al.,2004] is that addicted individuals have a deficit in recruitment of dopaminergic‐(DA) motivational circuitry by nondrug rewards, such that drugs are uniquely able to normalize DA levels in the VS to readily motivate drug‐taking behavior. This decrement could either be a premorbid risk factor for addiction, or could be the result of chronic drug use itself [Koob and Le Moal,2005]. Under the AH or “anti‐reward hypothesis,” acute drug intoxication temporarily restores the user's ability to experience pleasure but progressively degrades a homeostatic set‐point of general mood. A third possibility is that addicted subjects would have generally greater mesolimbic response to nondrug rewards as a component of greater premorbid trait impulsivity or internalizing disorder [Bjork et al.,2010a] that increases risk of drug experimentation, and eventual drug abuse.
A handful of studies have now compared non‐drug incentive processing between alcoholics and controls using variants of the monetary incentive delay (MID) task [Knutson et al.,2001a]. In the MID task, cues to quickly respond to a subsequent target for monetary rewards consistently recruit the ventral striatum (VS), including the nucleus accumbens (NAcc), and reward notifications have also frequently activated the VS and ventral mesofrontal cortex (mFC) [Bjork et al.,2004b,2008a,b,2010a,; Knutson et al.,2001a,b,2003]. In accord with the RDS/AH, two of these studies report that alcoholics have decreased reward response‐anticipatory BOLD activation in VS compared to non‐alcoholics, both in terms of absolute signal change, and as a contrast with anticipation of responding for no money [Beck et al.,2009; Wrase et al.,2007]. In contrast, we introduced a variant of the MID task that featured an extended variable interval between reward‐predictive (pre‐response) cues and the actual trial outcomes, to better isolate activation by these two different components [Bjork et al.,2008b]. We found that both alcoholics and controls showed similar behavioral and mesolimbic (VS) responses to potential rewards. Instead, alcoholics showed significantly increased NAcc activation by reward deliveries and more severe deactivation of the NAcc when an expected reward notification was replaced with a demand to repeat the trial. Moreover, across all subjects, Nacc recruitment by small reward (but not large reward) deliveries correlated with a questionnaire measure of emotion‐driven impulsivity. Our finding suggested that at least in some contexts or task demands, pre‐response motivational functioning was essentially intact in alcoholism, or could be sufficiently mobilized for a short time.
We wished to further assess VS recruitment during different phases of instrumental behavior as a function of either addiction or impulsivity. Because of its interconnectivity with both motor and cognitive circuitry of the cortex, the VS has been conceptualized as a nexus between cognitive valuation of a goal‐object, the emotional component of that opportunity, and the motor effectors invoked to attain the goal‐object. Chronic alcoholism may disrupt the relationship between cortical recruitment during valuation of prospective rewards and the strength of motor‐related recruitment of VS, due to the disproportionate effect of alcoholism on cortical morphology compared to the striatum [Cardenas et al.,2007]. Of interest, then, was developing a MID task variant that could isolate reward‐anticipatory activation that is ostensibly unconfounded by motor‐preparatory aspects of the striatal recruitment (which might remain relatively intact in addiction).
The experimenter could theoretically include trials where subjects passively receive rewards and simply watch (but refrain from responding to) targets, but we discovered that cues for passively‐received rewards do not recruit the NAcc in the MID task—at least using constant $1 rewards [Bjork and Hommer,2007]. Therefore, we wished to decouple the motor‐preparatory activity while still retaining an instrumental behavior context. To accomplish this, we developed a MID task variant that featured two‐stage cuing for the potential of a reward delivery, where one cue was delivered before the instrumental response (the typical MID anticipatory magnitude‐signaling cue shape), and a second cue following the motor response that confirmed a potential for a reward delivery. In particular, after a variable delay after the target/response, the subject saw the words “Win?” or “Hit?,” which signaled whether the subject could actually win a reward for hitting a target, or would just receive lexical feedback of whether he or she successfully hit the target. The contrast between BOLD signals elicited by these two word cues can capture the affective component of reward anticipation, since anteceding motor‐anticipatory BOLD responses in the VS would ostensibly be similar.
We hypothesized that in accord with our previous experiment [Bjork et al.,2008b], alcohol‐dependent patients (ADP) would show similar pre‐response reward‐anticipatory recruitment in the VS to controls, but ADP would show increased VS recruitment by reward deliveries. In addition, we hypothesized that the WIN versus HIT contrast in potentially‐rewarded trials (hereafter “post‐response reward anticipation”) would elicit detectable VS activation in controls, but that the ADP would show either reduced VS recruitment by this contrast in accord with the RDS hypothesis, or increased VS recruitment by virtue of greater trait impulsiveness. Finally, we hypothesized that across all subjects, VS recruitment by rewards would correlate with individual differences in trait impulsivity.
METHODS
Recruitment and testing procedures were conducted in accord with the Declaration of Helsinki, and were approved by the Institutional Review Board of the National Institute on Alcohol Abuse and Alcoholism (NIAAA). All subjects provided written informed consent to participate.
Subjects
All subjects were right‐handed. Subjects underwent physical examination, urine drug screen, and a structured clinical interview for DSM‐IV. Exclusion criteria for all study applicants were: current use of psychotropic medication, psychosis, craniofacial or soft‐sign neurological evidence of fetal alcohol spectrum disorder (FASD), history of neurological disorders or serious medical conditions, or head injury resulting in loss of consciousness. Controls (n = 23; age 23–45, mean 30.1 ± 5.9; 12 males) were recruited from community advertisement. In controls, presence of any Axis I disorder was an exclusion criterion, as was a lifetime history of substance use disorder in any first‐degree relative.
Alcohol‐dependent patients (ADP; n = 29; age 20–43, mean 30.9 ± 8.2; 15 males) were inpatients undergoing treatment for alcohol dependence at the National Institutes of Health Clinical Center in Bethesda, Maryland. All ADP had an estimated IQ ≥80 as derived from block design and vocabulary tests to detect mental retardation as part of screening for FASD. All ADP met DSM‐IV criteria for alcohol dependence, and were admitted to the treatment unit based on telephone pre‐screen and clinical assessment at intake that alcohol was the subject's primary drug of abuse. Following admission, any inpatient who exhibited a “cocaine crash” or other evidence that a non‐alcohol drug was his or her primary problem was excluded. Every patient was a regular smoker, and most ADP had some history of non‐alcohol substance use disorder. Comorbid diagnoses and abstinence duration of each ADP is listed in Supporting Information Table S1. Notably, eight ADP had histories at some point in their life that they met criteria for marijuana abuse or dependence, three other ADP met lifetime criteria for cocaine abuse or dependence, and 14 ADP met lifetime abuse or dependence criteria for both cocaine and marijuana. Every ADP was scanned at least 1 week, but not more than 4 weeks of abstinence. All had completed physiological withdrawal (per the Clinical Institute Withdrawal Assessment) by the time of scanning.
Monetary Incentive Delay (MID) Task
Stimuli were viewed on a screen at the foot of the scanner bed using a head coil mirror. Trials (n = 54) were presented pseudorandomly across three scanning runs. Each trial was comprised of four events that were variably spaced (jittered with a uniform distribution), on average, 8 s apart. These were: (1) anticipatory cue presentation, (2) target presentation for the instrumental response, (3) notification whether trial success would be lexically notified or actually rewarded, and (4) trial outcome feedback (see Fig. 1). This version of the MID task thus featured dual‐component reward‐anticipatory cueing, with both the traditional pre‐response reward cue, and here an additional post‐response reward cue. Critically, the post‐response reward anticipation cue occurred, on average, a full hemodynamic response (14–16 s) later than the initial magnitude pre‐response cue. Feedback was then followed by a variable intertrial interval of 0–12 s (mean 6 s).
Figure 1.
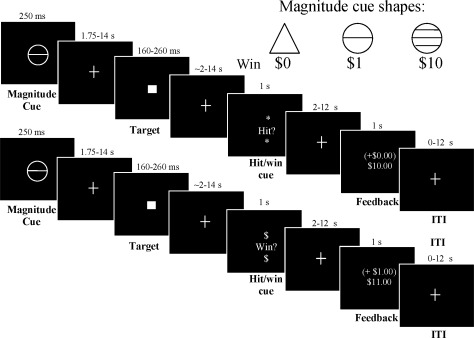
Modified monetary incentive delay (MID) task. Each trial began with presentation of one of three magnitude (response‐anticipatory) cues that signaled the opportunity to either win money (circles) or to respond for no incentive (triangle). Trial success required recording a button press during subsecond presentation of a white square target after a variable interval. After target presentation and the motor response, the subject then waited across a variable delay for a second cue (either “*hit?*” or “Win?$”) that signaled whether a successful response to the target would result in lexical feedback alone (HIT trials, top panel series) or would result in an actual delivery of a reward (WIN trials, bottom panel series). The linear contrast between WIN versus HIT signals across the $1 and $10 magnitude trial types is interpreted here as (affect‐based) post‐response reward anticipation. After another variable interval, the subject was notified of whether he or she hit the target and/or won money. Intratrial intervals between task stimuli and intertrial intervals were pseudorandomly varied in uniform distributions.
Subjects were instructed to respond on a button box while each trial's target was displayed. Subjects could win money for pressing during target presentation. There were three main trial types, as defined by potential reward magnitude (three levels: $0, $1, and $10). Each of these were divided into two subtypes, based on the consequences for hitting the target as revealed to the subject after the target was presented (two levels: lexical feedback of target hits versus actual reward delivery for target hits). First, one of three “magnitude cue” shapes was presented for 250 ms. Circles signaled that if the subject responded during the subsequent target presentation, he or she could win $1 (18 trials) or $10 (18 trials). Cues signaling nonincentive outcomes (18 trials; triangles) were also presented, and subjects were instructed to respond to the target, but that trial outcomes would not alter their winnings (magnitude = $0). Each cue was replaced by a fixation crosshair for 1,750–13,750 ms. Second, a white target square was presented for a variable length of time (180–280 ms) based on each subject's reaction time, and replaced by a crosshair for 1,720–13,820 ms. Third, after the instrumental response to the target, a “hit/win cue” was presented. This cue notified the subject of the two possible consequences for having just hit the target. In half of each of the three magnitude trial types, the word “*Hit?*” was presented for 1 s (HIT subtype trials; n = 9 for each magnitude cue). This indicated that the subject would not actually receive the reward of the prescribed magnitude for that trial (either $0, $1, or $10), but would simply learn whether or not the target was hit by the presence of + or − sign in the forthcoming feedback display. In the other half of each trial magnitude, the word “$Win?$” was presented for 1 s (WIN subtype trials; n = 9 for each magnitude cue). This indicated that a target hit would earn a cash reward of the magnitude ($0, $1, or $10), prescribed for that trial. For a complete factorial design, nonincentive trials were also divided into HIT and WIN subtypes of the $0 magnitude trials, even though there was no money to be won. The hit/win cue was followed by a crosshair for 2–12 s.
Each trial then concluded with feedback (1‐s duration), which notified participants of whether they had hit the target for the trial, whether they had won money during that trial, and their cumulative earnings for that scanning run. A “+” sign prior to the trial reward amount in parentheses indicated that the target was hit, and a “−” sign indicated a miss. In the two rewarded cue conditions, therefore, the reward was dependent on the (cue‐signaled) magnitude of the trial, whether the subject pressed to the target in time, and whether that trial was a WIN‐subtype trial. Target hits on $1 and $10 cue‐magnitude HIT trials were indicated by “+(0.00)”(see Fig. 1 top panel series). For the $0 magnitude trials, the trial result amount was always “0.00,” where the symbol that preceded it indicated if the target had been hit “+(0.00)” or missed “−(0.00).”
Prior to scanning, subjects were shown an envelope containing the cash they could earn in the task. Subjects were read an instruction script that: (1) defined the potential reward magnitudes signaled by the three anticipatory cues, (2) described how to hit the target, (3) explained that HIT or WIN cues would follow each target, and what each meant with regard to potential reward delivery, and (4) clarified to the subject that he or she would actually receive task earnings in cash after the scan. Subjects were then quizzed to ensure understanding before completing a 5‐min off‐line practice session. During practice, reaction times to targets were covertly measured, and a distribution of target presentation durations was set for the scan task such that each participant would likely succeed on ∼66% of trials during the scan. Once in the scanner, each participant engaged in three runs of the MID task (∼10 min each), followed by a structural scan (described below) for anatomical colocalization. Following the scan, subjects rated on four‐point scales of how “excited” and “happy,” they felt when they saw each of the pre‐response magnitude cues, and the HIT and WIN cues. Subjects were then paid their task earnings. Subjects also received a standard $100 compensation for the psychiatric and medical screening visit and $80 compensation for the MRI visit.
FMRI Acquisition
Imaging was performed using a 3 T General Electric MRI scanner (General Electric, Milwaukee, WI) and an 8‐channel head coil. Each MID task run lasted 600 s, and used a T2*‐sensitive echoplanar sequence with a repetition time (TR) = 1,000 ms, echo time (TE) = 40 ms, flip = 90°. In each volume, we collected sixteen 5.0‐mm‐thick contiguous (mid)saggital slices that centered on the intrahemispheric fissure, which encompassed all mesofrontal gray matter, the width of the putamen, and all midbrain structures. This prescription sampled the mesolimbic system once per second to power separable regression of the three different potential striatal responses within the same trial: (1) reward response anticipation‐elicited activation, (2) reward delivery anticipation‐elicited activation, and (3) final feedback‐elicited activation. In‐plane resolution was 3.75 × 3.75 mm2. Structural scans were acquired using a T1‐weighted sequence (TR, 100 ms; TE, 7 ms; flip, 90°), for coregistration of functional data. Each subject's head was restrained with a fabric forehead strap and shaped cushions.
FMRI Analysis
Preprocessing
Blood oxygen‐level dependent (BOLD) signal was analyzed using analysis of functional neuroImages (AFNI) software [Cox,1996]. Time‐series datasets were time‐shifted to compensate for non‐simultaneous slice acquisition, warped into Talairach stereotactic space as 3.75 mm isotropic voxels and corrected for head motion. Brain voxels were spatially smoothed to a uniform 8‐mm full‐width half‐maximum. Processed time series were modeled with canonical gammavariate hemodynamic responses time‐locked to each of: anticipatory magnitude cues, post‐response HIT and WIN cues, and trial outcome notifications. Canonical hemodynamic responses and time‐series datasets were scaled so that beta weights could be interpreted as percent‐signal‐change. Low‐frequency drift in the signal was fitted with extended polynomials for each run. Residual head motion after motion‐correction and target presentations were also modeled as variables of no interest. The regression corrected for temporal autocorrelation of voxel‐wise noise with an ARMA(1,1) model using the AFNI program 3dREMLfit.
Statistical mapping of pre‐ and post‐response reward anticipation
Our statistical mapping centered on two linear contrasts (hereafter “contrasts”). First, reward‐response anticipation was calculated as high and low reward magnitude (circle) cues versus nonincentive (triangle) cues, as conventionally performed using the MID task [Bjork et al.,2004b,2010a,b; Knutson et al.,2001a]. Second, post response reward‐anticipatory activation was calculated by a linear contrast between WIN cues in the combined $1 and $10 magnitude trials versus HIT cues in those trials. We hypothesized that mesolimbic signal change would be differentially elicited by divergent affective responses to the potential for an actual reward versus only lexical feedback of success.
Statistical mapping of reward delivery activation
Reward delivery activation was considered exploratory because the protracted structure of this MID variant was designed to detect post‐response reward anticipation, with only 18 potentially financially‐rewarded outcomes to model. Separate HIT versus WIN trial subtypes, however, enabled isolation of actual reward notification‐elicited activation while masking out activation by notification of successful task performance in itself. This was of interest because alcoholic patients tend to have greater trait anxiety, especially in earlier stages of detoxification [reviewed in Heilig et al.,2010], where this could be manifested in performance anxiety [evident during testing in Bjork et al.,2004a]. This higher order contrast between reward notification and hit notification was calculated as:
To better accommodate potential individual differences in brain responses resulting from alcohol toxicology, groupwise and group‐difference maps were calculated in AFNI using recently‐developed software, 3dMEMA [Chen et al.,2010] (http://afni.nimh.nih.gov/sscc/gangc/MEMA.html). The 3dMEMA utilizes a linear mixed‐effects multilevel model that incorporates both within‐subject and cross‐subjects variability. In particular, individual‐subject contributions to the group‐wise and group‐difference maps are weighted based on the reliability of that subject's effect estimate. Contrast activations in group‐wise maps were assessed across the whole brain, and are reported at the maxima of activated voxel clusters, where voxel‐wise significance was controlled by the false discovery rate (FDR) set to a false‐positive P < 0.05. The FDR correction was utilized because family‐wise error (FWE) cluster‐size based correction axiomatically precludes survival of focal activation in small structures like the NAcc.
Volume‐of‐interest (VOI) analysis of NAcc signal change
Each subject's hemodynamic responses were event‐averaged and passed through masks. To avoid circularity of statistical inference [Kriegeskorte et al.,2009; Vul et al.,2009], these masks were anatomically localized a priori in the NAcc and mFC, which are consistently recruited by the MID task [Bjork et al.,2004b; Knutson et al.,2001a,2005; Scheres et al.,2007]. Each side of the bilateral NAcc mask [also used in Bjork et al.,2010b] was comprised of the 3.75 mm cubic voxel at the activation maxima of previous reports (Talairach ±8, 11, 0) along with its ventral shared‐face neighbor (inset in Fig. 4 and Supporting Information Fig. S3). The Nacc mask was overlaid atop each subject's Talairach‐warped structural image and relocalized as needed‐up to 3.75 mm so as to predominantly reside in gray matter at the junction of caudate and putamen [Breiter et al.,1997]. The mFC mask was comprised of five midsaggital voxels centered at (1, 53, −6) [Bjork et al.,2004b; Knutson et al.,2003] (Supporting Information Fig. S4, inset). Because of the severe signal dropout, mFC VOI data were not collected from one subject.
Figure 4.
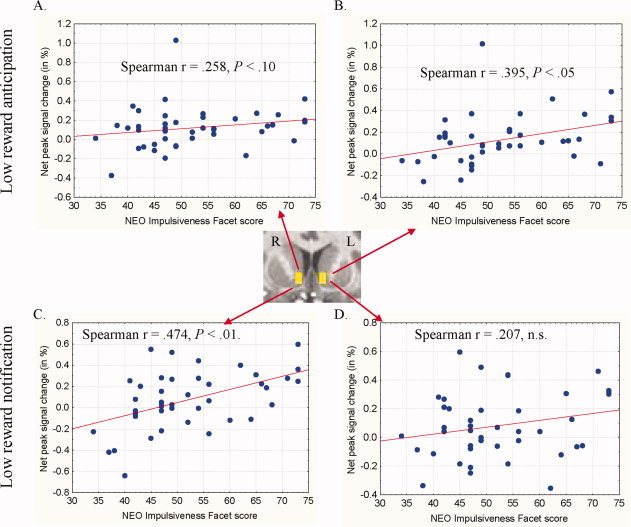
Correlations between NEO Impulsiveness Facet scores and post‐response NAcc activation by anticipation and delivery of low rewards. Signal was averaged across masks drawn in right and left nucleus accumbens (NAcc; inset image, Y = 10). For each of the low ($1) and high ($10) trial types, post‐response reward anticipation was calculated as the net signal change following WIN cues minus signal change following HIT cues for that reward magnitude. Post‐response low reward anticipation correlated with NEO‐IF scores in the left NAcc (B) with a trend in right NAcc (A). Post‐response activation by high reward anticipation, in contrast, did not correlate with NEO‐IF scores. For each of the low ($1) and high ($10) WIN trial subtypes, reward notification activation was calculated as the net signal change following actual wins versus notification of failure to win. NEO‐IF scores correlated with net activation by notification of low rewards in right NAcc (C), but not left NAcc (D). NAcc activation by high reward notification did not correlate with NEO‐IF scores.
Our VOI analysis centered on gammavariate‐modeled peak (∼5 s post‐stimulus lag) hemodynamic responses in order to parallel the core contrast‐based statistical maps. For response‐anticipatory activation, peak modeled signal change was analyzed in a mixed‐model analyses of variance (ANOVA). Incentive magnitude ($0, $1, $10) was the within‐subject factor, and group (ADP and controls) as the between‐subject factor. Side (left, right) was also explored as an additional interaction factor in analysis of NAcc responses. For post‐response reward‐anticipatory activation, incentive magnitude ($0, $1, $10) and outcome modality (HIT, WIN), (and for NACC, side), were within‐subject factors, and group (ADP and controls) as the between‐subject factor. Finally, we analyzed outcome notification‐elicited signal change in the $1 and $10 magnitude trials. In these analyses, success (hit, miss) was added as an additional within‐subject variable. This analysis focused on main or interaction effects of trial outcome.
Behavior Analysis
We performed mixed‐model analyses of variance of affective ratings, hit rates, and reaction times (RT). Incentive magnitude (0, $1, or $10) was the within‐subject factor, and group (ADP and controls) was the between‐subject factor. For the analysis of RT, time (task runs 1–3) was a second within‐subject factor.
Correlation Between Task‐Elicited Striatal Recruitment and Trait Impulsiveness
In our previous alcoholic‐control comparison with a different variant of the MID task [Bjork et al.,2008b], there was a direct correlation across all subjects between NAcc activation by notification of low (but not high) reward deliveries and “hot” impulsivity as indexed by the NEO‐Impulsiveness Factor (NEO‐IF). Specifically, the NEO‐IF captures an “urgency” factor, where “high scorers on urgency are likely to engage in impulsive behaviors in order to alleviate negative emotions despite the long‐term harmful consequences of these actions. [Whiteside and Lynam,2001; p. 685]. This emotion‐driven impulsivity is characteristic of our patients, and the MID task invariably recruits affective (limbic) neurocircuitry. To assess whether the impulsivity and post‐response reward delivery correlation we found previously [Bjork et al.,2008b] would replicate, and possibly extend to post‐response anticipation of reward, the NEO Personality Inventory (NEO‐PI‐R) [Costa and McCrae,1992] was administered to 20 controls and 22 ADP prior to scanning, from which the 8‐item impulsiveness facet score (NEO‐IF) was tabulated.
For this analysis, (1) reward response‐anticipation activation was calculated for each of the $1 and $10 magnitude cues as the net difference from the non‐incentive ($0) magnitude cue, (2) post‐response reward anticipation was calculated as the net difference between WIN versus HIT cue responses for each of the $1 and $10 trial magnitudes, and (3) net reward delivery activation was the difference between responses to actual gains in WIN trials minus nongains (misses) in WIN trials. In addition, a post hoc 3dMEMA analysis generated direct voxelwise correlations between NEO‐IF scores and activation. These correlation maps are uncorrected, but consideration was restricted to limbic structures consistently recruited by the MID task.
RESULTS
Behavior and Affect in the MID Task
No subject moved his or her head more than 3 mm across the whole session or more than 1 mm between successive acquisitions. Affect ratings were not collected from 4 ADP and one control. In repeated‐measures ANOVA of response‐anticipatory affect ratings, there were significant main effects of incentive magnitude (Supporting Information Fig. S1), where participants reported greater happiness (F(2,92) = 49.033, P < 0.000001) and excitement (F(2,92) = 119.173, P < 0.000001) as potential reward amounts signaled by the cue increased from $0 to $10. Similarly, subjects reported greater happiness (F(2,92) = 17.831, P < 0.000001) and excitement (F(2,92) = 80.104, P < 0.000001) upon seeing the WIN cue versus the hit cue (main effects of outcome modality). There were no significant main or interaction effects of subject group on affect ratings.
There was a main effect of incentive magnitude (F(2,100) = 42.277, P < 0.0000001) on RT, where mean RT decreased as incentive magnitudes increased from $0 to $10 (Supporting Information Fig. S1). There was also a trend toward main effect of task run on RT (F(2,100) = 2.649, P = 0.076), where subjects generally responded to targets more slowly as the task progressed from Run 1 to Runs 2 and 3. There were no main or interactive effects of subject group on RT (all P > 0.6). Faster RT to targets of increasing value also resulted in a main effect of incentive magnitude on hit rates (F(2,100) = 55.322, P < 0.0000001; Supporting Information Fig. S1). There was a trend for a group × magnitude interaction effect on hit rates (F(2,100) = 2.467, P = 0.09), which was driven by significantly greater hit rates to non‐incentivized targets in patients.
Statistical Maps
Pre‐response reward anticipation
In both subject groups, the contrast of anticipation of responding for potential reward versus anticipation of responding for no incentive activated voxels throughout the VS (including NAcc, ventral putamen, and anterior caudate) as well as a contiguous band of voxels in posterior mesofrontal cortex extending from supplemental motor area anteroventrally to anterior cingulate cortex. In both groups, there was also bilateral activation of insula, thalamus, occipital cortex, and cerebellum (Table I; Fig. 2). There were no voxels, however, that showed a significant group difference in activation that survived FDR correction.
Table I.
Activation by pre‐response anticipation of reward ($1, $10) versus no incentive ($0)
| Structure | X | Y | Z | t‐statistic | Uncorrected P * |
|---|---|---|---|---|---|
| Controls | |||||
| L Supplementary motor area | −3 | −2 | 53 | 9.141 | <10−11 |
| L Cerebellum | −37 | −48 | −18 | 5.175 | <10−5 |
| R Cerebellum | 30 | −49 | −11 | 9.609 | <10−11 |
| Mesial cerebellum | 0 | −50 | −18 | 5.543 | <10−5 |
| R Superior occipital gyrus | 26 | −64 | 35 | 9.139 | <10−11 |
| L Middle frontal gyrus | −42 | 29 | 23 | 9.003 | <10−10 |
| R Middle frontal gyrus | 30 | 42 | 22 | 5.703 | <10−5 |
| L Thalamus | −4 | −19 | −2 | 8.135 | <10−10 |
| R Thalamus | 6 | −21 | 1 | 8.026 | <10−11 |
| L Nucleus accumbens | −10 | 11 | −1 | 7.219 | <10−8 |
| R Nucleus accumbens | 8 | 11 | −1 | 7.297 | <10−8 |
| L Insula | −36 | 18 | 4 | 6.499 | <10−7 |
| R Insula | 30 | 22 | 7 | 7.647 | <10−8 |
| Anterior cingulate cortex | −2 | 13 | −41 | 8.746 | <10−10 |
| Anterior cingulate cortex | −2 | 29 | 23 | 6.372 | <10−6 |
| R Middle occipital gyrus | 30 | −86 | 5 | 7.082 | <10−8 |
| Mesial cuneus | −2 | −75 | 19 | 4.618 | <10−4 |
| L Precentral gyrus | −41 | −14 | 54 | 5.815 | <10−5 |
| R Precentral gyrus | 35 | −5 | 50 | 6.277 | <10−6 |
| Alcohol‐dependent patients (ADP) | |||||
| R Cerebellum | 28 | −56 | −12 | 9.195 | <10−11 |
| R Superior occipital gyrus | 26 | −59 | 41 | 8.209 | <10−9 |
| L Middle frontal gyrus | −34 | 39 | 27 | 7.541 | <10−8 |
| R Middle frontal gyrus | 36 | 41 | 21 | 6.757 | <10−7 |
| L Thalamus | −8 | −27 | 2 | 9.416 | <10−11 |
| R Thalamus | 7 | −19 | −2 | 8.726 | <10−11 |
| L Nucleus accumbens | −14 | 9 | −5 | 9.129 | <10−11 |
| R Nucleus accumbens | 10 | 11 | −3 | 10.371 | <10−13 |
| L Insula | −33 | 16 | 6 | 7.538 | <10−9 |
| R Insula | 34 | 18 | 4 | 10.550 | <10−13 |
| L Anterior cingulate cortex | −6 | 15 | 42 | 8.943 | <10−10 |
| R Anterior cingulate cortex | 10 | 29 | 27 | 8.988 | <10−11 |
| L Middle occipital gyrus | −37 | −77 | 6 | 7.821 | <10−9 |
| L Cuneus | −8 | −75 | 9 | 9.036 | <10−12 |
| R Cuneus | 10 | −72 | 15 | 9.946 | <10−13 |
| L Precentral gyrus | −39 | −17 | 43 | 8.846 | <10−10 |
All activations in this table represent the maxima of voxel clusters, where each activation survived false discovery rate (FDR) correction to P < 0.05.
Figure 2.

Pre‐response and post‐response activation by anticipatory cues for reward. In these and subsequent statistical maps: (1) all images are right‐left reversed per radiological convention, (2) the underlay is a T1‐weighted structural image from a representative subject, (3) the Talairach coordinate of the image plane is indicated, and (4) illuminated voxels in group‐wise maps survive false discovery rate (FDR) correction to P < 0.05 unless otherwise noted. Both controls (A) and ADP (B) showed significant activation of bilateral ventral striatum, anterior cingulate cortex, and other limbic structures by a contrast between presentations of response‐anticipatory cues that signaled prospective rewards ($1 or $10) versus presentation of cues signaling no potential reward ($0). Following target presentation and motor response, both controls (C) and ADP (D) showed subsequent activation of ventral striatal voxels by the contrast between seeing WIN cues versus HIT cues across the $1 and $10 reward trial series at the voxel‐wise significance threshold indicated. The group map of activation in the ADP (D) did not survive FDR correction, but voxels are illuminated at the same threshold to document noncorrected activation. In either contrast, there were no voxels that indicated a significant difference in activation between controls and ADP that survived FDR correction.
Post‐response reward anticipation
The contrast between seeing the WIN cue versus the HIT cue following the target (in the $1 and $10 trials) activated portions of anteroventral striatum and occipital lobe in both groups at an omnibus voxelwise threshold of P < 0.001 (Table II; Fig. 2), but activations only survived FDR correction in the group map of the controls. Additional suprathreshold activation in controls was present in bilateral insula, thalamus, and mesofrontal cortex. There were no voxels that showed an FDR‐corrected group difference in activation by this contrast.
Table II.
Activation by post‐response anticipation of reward for success (WIN) versus lexical notification (HIT) of success
| Structure | X | Y | Z | t‐statistic | Uncorrected P * |
|---|---|---|---|---|---|
| Controls | |||||
| R Thalamus | 3 | −4 | 4 | 4.865 | <10−4 |
| L Nucleus accumbens | −10 | 9 | −3 | 6.268 | <10−6 |
| R Nucleus accumbens | 8 | 14 | −3 | 5.294 | <10−5 |
| L Insula | −40 | −7 | 11 | 4.242 | <10−3 |
| R Insula | 37 | −3 | −4 | 4.247 | <10−3 |
| L Precuneus | −23 | −47 | 34 | 4.450 | <10−3 |
| R Mesial frontal cortex | 14 | 41 | 0 | 4.514 | <10−4 |
| Alcohol‐dependent patients (ADP) | |||||
| R Putamen | 14 | 9 | −3 | 4.076 | <10−3 |
| L Caudate head | −11 | 10 | 3 | 4.052 | <10−3 |
| L inferior occipital gyrus | −15 | −97 | −7 | 5.202 | <10−5 |
All activations in this table represent the maxima of voxel clusters, where each activation survived false discovery rate (FDR) correction to P < 0.05 in the group‐wise map of the controls. Activation maxima in ADP did not survive FDR correction, but are enumerated to illustrate subthreshold activation.
Activation by reward notifications
The higher‐order contrast of $1 and $10 magnitude trial type outcomes [described in Eq. (1)] revealed activation of the NAcc and bilateral insula in both groups (Table III; Fig. 3). No voxels showed an FDR‐corrected group difference in activation by reward notifications.
Table III.
Activation by notification of reward
| Structure | X | Y | Z | t‐statistic | Uncorrected P * |
|---|---|---|---|---|---|
| Controls | |||||
| L Amygdala | 19 | −3 | −8 | 4.635 | <10−4 |
| L Thalamus | −15 | −32 | 2 | 5.668 | <10−5 |
| L Caudate head | −14 | 13 | 3 | 3.876 | <10−3 |
| R Nucleus accumbens | 8 | 8 | −5 | 5.264 | <10−5 |
| L Insula | −40 | −7 | 11 | 4.121 | <10−3 |
| R Insula | 37 | 12 | 11 | 6.376 | <10−6 |
| L Middle occipital gyrus | −36 | −75 | 15 | 5.790 | <10−5 |
| Alcohol‐dependent patients (ADP) | |||||
| L Nucleus accumbens | −5 | 11 | −3 | 3.552 | <10−3 |
| R Nucleus accumbens | 10 | 10 | −3 | 5.159 | <10−5 |
| L Insula | −31 | 16 | −3 | 5.774 | <10−6 |
| R Insula | 30 | 21 | −7 | 4.058 | <10−3 |
| Anterior cingulate cortex | 0 | 40 | 24 | 4.522 | <10−4 |
| Posterior cingulate cortex | −6 | −27 | 40 | 5.148 | <10−5 |
| L Middle frontal gyrus | −23 | 26 | 40 | 5.505 | <10−5 |
| Ventromesial frontal cortex | −3 | 57 | 24 | 5.422 | <10−5 |
All activations in this table represent the maxima of voxel clusters, where each activation survived false discovery rate (FDR) correction to P < 0.05.
Figure 3.

Activation by notification of winning rewards. A higher‐order contrast calculated activation by notification of having won a reward versus failing to win a monetary reward (in WIN trials), while masking out activation by notification of successful motor performance alone (in HIT trials). This contrast detected bilateral ventral striatum activation and anterior cingulate cortex activation in both controls (A) and in ADP (B). As with reward‐anticipatory activation, there were no voxels that indicated a significant difference in notification activation between controls and ADP that survived FDR correction.
In post hoc MEMA analyses of the ADP, we entered days‐abstinent as a continuous covariate, to determine whether any VS voxels showed MID task activation that correlated with the interval since the patient's last alcohol consumption. There were no abstinence‐correlated VS voxels in any task contrast, or any abstinence correlation in remaining brain voxels that survived multiple‐comparisons correction.
Volume of Interest (VOI) Analyses
Peak modeled BOLD signal change (beta weights) in the NAcc VOI masks is illustrated in Supporting Information Figure S3, and peak modeled BOLD signal change in the mFC mask is illustrated in Supporting Information Figure S4. There were no main or interactive laterality effects in the NAcc in any analysis.
Response‐anticipatory activation
In both the NAcc and the mFC, there was a main effect of incentive magnitude (NAcc: (F(2,100) = 57.522, P < 0.0000001; mFC (F(2,98) = 9.807, P < 0.001) on response‐anticipatory activation, with greater signal change as potential incentive amounts increased. There were no main or interactive effects of subject group.
Post‐response reward‐anticipatory activation
In the NAcc, there were main effects of side (right > left) (F(1,50) = 4.488, P < 0.05), and a main effect of future notification modality (WIN > HIT) (F(1,50) = 17.617, P < 0.001) on signal change, indicating post‐response reward‐anticipatory NAcc recruitment. In the mFC, there was a main effect of group (F(1,50) = 7.769, P < 0.01), with generally lower peak responses in ADP compared to controls across all WIN and HIT cue presentations.
Outcome notification‐elicited activation
In the NAcc, there were significant main effects of trial success (hits > misses; F(1,50) = 10.765, P < 0.01), and notification modality (WIN > HIT; (F(1,50) = 18.100, P < 0.0001). A significant success × notification modality interaction (F(1,50) = 6.310, P < 0.05) indicated that success‐based activation was specific to the WIN trials, with minimal signal differences in HIT trials (i.e., following mere lexical notification of hits versus misses). Accordingly, a trend toward a magnitude × success interaction effect (F(2,100) = 2.377, P = 0.098) indicated that success‐based signal change was sensitive to increasing trial magnitude. Finally, a significant magnitude × success × notification modality effect (F(2,100) = 4.358, P < 0.05) further indicated that the magnitude‐sensitive, success‐elicited signal change was specific to WIN trials. There was no main effect of group on notification elicited signal change in the NAcc. However, there was a significant success × notification modality × group interaction effect (F(1,50) = 4.14, P < 0.05), where controls showed a greater activation following notification of success in both the WIN and HIT trial types, but ADP showed NAcc sensitivity to outcome only in the WIN trials.
In the mFC VOI, there were significant main effects of success (hits > misses; (F(1,49) = 11.913, P < 0.01)), and of magnitude ((F(2,98) = 3.195, P < 0.05), with a trend toward post‐notification signal increases in WIN trials but decreases in HIT trials (F(1,49) = 3.274, P = 0.077). If $0‐magnitude trials were excluded from the notification analysis, the modality × success interaction became significant (F(1,49) = 7.005, P < 0.05). No other main or interaction effects on mFC signal change were significant.
Correlations Between Reward‐Elicited Mesolimbic Recruitment and Impulsivity
NEO‐IF scores were significantly higher in ADP compared to controls (Supporting Information Fig. S2, part A).
VOI analyses
In the NAcc VOI, peak pre‐response reward‐anticipatory signal change (as difference from non‐incentive cues) did not correlate with NEO‐IF scores. Rather, post‐response anticipation of low rewards (but not high rewards), calculated as the difference from anticipating lexical‐only notification, correlated with NEO‐IF scores in left NAcc, with a trend toward a correlation in right NAcc (see Fig. 4). Finally, NEO‐IF scores also correlated with net activation by notification of low (but not high) rewards in the right NAcc. NEO‐IF scores did not correlate with signal change in mFC.
Statistical maps
We reanalyzed the three main contrasts of this report with NEO‐IF scores added to the MEMA as a covariate (where group status was also retained in the model). No voxel showed a direct correlation (across both groups combined) between pre‐response reward‐anticipatory activation (both magnitudes combined) and NEO‐IF scores (Supporting Information Fig. S2). In contrast, voxels in left NAcc and bilateral insula showed a positive correlation between NEO‐IF scores and activation by post‐response reward‐anticipatory activation (i.e. WIN vs HIT cues in reward trials). Finally, voxels in bilateral NAcc showed a positive correlation between NEO‐IF scores and activation by the higher‐order reward notification contrast [c.f., Eq. (1)].
DISCUSSION
Overall Findings
Both controls and ADP showed significant recruitment of VS by the pre‐response reward versus nonreward anticipation contrast of the MID task, in accord with previous experiments [Bjork et al.,2004b,2008a,b,2010a; Knutson et al.,2001a,2003,2005]. Consistent with our initial experiment [Bjork et al.,2008b], there were no significant differences between ADP and controls in pre‐response reward‐anticipatory activation in VS or anywhere else, and there were no behavioral decrements in motivation in ADP as indexed by reaction time to the task targets. Also consistent with our earlier report, VS recruitment following notification of low, but not high rewards correlated with trait impulsivity as indexed by the NEO‐IF scores. Accordingly, a voxelwise correlation analysis of the (higher‐order) reward feedback contrast with NEO‐IF scores also revealed NAcc voxels that showed a direct positive correlation between reward notification‐elicited activation and NEO‐IF scores. Finally, as hypothesized, the newly‐introduced contrast between the second cue that a successful operant response would be rewarded, versus the second cue that success would merely engender lexical feedback, also revealed post‐response reward‐anticipatory activation of the VS. This activation survived FDR correction in controls, but merely survived the same voxelwise illustration threshold (P < 0.001) in the ADP. Activation of the VS by post‐response anticipation of low rewards also correlated with NEO‐IF scores.
Preresponse Reward Anticipation
As with our first experiment, pre‐response reward anticipation activation was robust and substantially similar between ADP and controls in both contrast‐based statistical maps, and in the mesolimbic VOI. We note that all ADP smoked, and Buhler et al. [2010] reported no differences between dependent smokers and controls in VS recruitment by monetary reward‐predictive cues. The substantially intact response to prospective monetary rewards in drug dependence may help explain the success of contingency management therapies for addiction, where non‐drug rewards for maintaining drug abstinence improve outcomes [e.g., Silverman et al.,2007].
We envision some potential reasons why pre‐response reward‐anticipatory activation would show no decrement in ADP in our two experiments. First, it may be that the MID task (or other incentive‐laden tasks) is only partially sensitive to trait‐like decrements in motivation. Putative decrements in dopaminergic motivational functioning in persons with SUD, as inferred from allelic [Comings and Blum,2000] and PET ligand binding [e.g., Volkow et al.,2002] differences may not rise to the level of functional significance due to brain adaptations or other physiological buffering mechanisms that would promote survival. Laboratory incentive tasks would be suboptimal if subjects with generally reduced motivation can at least mobilize attentional and motivational resources: (1) for 30 min, (2) in a novel environment, (3) when appreciable cash reward is contingent on instrumental behavior, and (4) if attentional and cognitive demands of a task are not particularly onerous. The motivational appeal of $10 could have eliminated group differences in striatal motor‐preparatory activation, post‐response reward anticipation, and reward‐notification activation. More naturalistic behavioral tasks, more modest incentives, or more cognitively and attentionally‐demanding tasks may better reveal motivational deficits in ADP. We note too that although cocaine‐dependent subjects show reduced endogenous DA release and “high” from methylphenidate (another stimulant) [Volkow et al.,1997], this decrement need not generalize to reduced motivation or hedonic reactions to a practical non‐drug reward.
Second, early detoxification processes may have sufficiently normalized attentional and motivational neurocircuitry to enable similar activation. Notably, days since last drink did not correlate with any contrast activations. On average, ADP had been detoxified for roughly two weeks, with some subjects having been detoxified for four weeks. Short‐term longitudinal studies of alcohol abstinence indicate onset of normalization of brain volumes [Gazdzinski et al.,2005] and white matter coherence [Gazdzinski et al.,2010] within weeks of sobriety, suggesting a potential for rapid restoration of function, leaving only effects of trait individual differences, such as impulsivity. These data therefore may not extend to actively‐drinking alcoholics or to ADP in acute withdrawal.
Our present findings differ from those of Wrase et al. [2007] and Beck et al. [2009], which conform to the RDS/AH in that they show reduced reward‐anticipatory VS activation in ADP. We suspect that discrepancies between reports may have arisen from differences in task timing between MID tasks (the effects of which have never been systematically investigated within‐subject). Notably, the original MID task used by the Wrase and Beck group was briskly‐paced with brief trials that required constant attention.
Our MID variants featured prolonged (and fewer) trials, with extended variable intervals between stimuli. This might have enabled subjects to allocate attention and effort to the sporadic cues for high‐value targets, which might have also minimized individual differences in VS recruitment. We note too that unlike other reports where regression modeling conflates signal change from targets/motor responses into the anticipatory cue response, our jittering enabled separable modeling of all stimulus events: the magnitude cue, target, HIT/WIN cue, and notification. Finally, differences across studies may arise from differences between American and German inpatient populations of ADP in terms of drinking severity and precise motivations for drinking (e.g., positive versus negative reinforcement) [Victorio‐Estrada et al.,1996].
Postresponse Reward‐Anticipation Activation
Consistent with our hypothesis, the contrast between seeing the WIN cue versus the HIT cue following targets (in trials that began with either the cue for $1 or the cue for $10 magnitude) recruited the NAcc. This contrast was introduced in our experiment to ostensibly reflect instrumental reward anticipation‐related activation of the NAcc, as distinct from motor response mobilization itself. This new contrast activation survived FDR correction in controls, but merely survived a voxelwise omnibus statistical threshold of P < 0.001 in ADP. We mentioned and illustrated this uncorrected activation to avoid the implication that there was no reactivity to this contrast in ADP. As with pre‐response activation, there were no voxelwise differences between groups in either voxelwise contrast‐based maps, or in peak signal change by WIN and HIT cue presentations. Theoretically, all the reasons for groupwise similarity in mesolimbic recruitment by pre‐response reward anticipation discussed above could account for general similarity in post‐response reward anticipation.
Reward Notification‐Elicited Activation
Despite a paucity of trial outcome events, ADP and controls nevertheless showed significant VS recruitment by reward deliveries in the high‐order contrast‐elicited activation. Absolute signal change by reward deliveries in the NAcc VOI analysis was also greater than following notification of missed rewards, where this success‐based contrast was not appreciable in trials with only lexical notification. In the mFC VOI, we found an overall main effect of trial success notification [as in Bjork et al.,2004b]. Not surprisingly, there was a trend for success‐dependent mFC activation to be specific to the WIN trials. Interestingly, a higher‐order‐interaction effect with group indicated that the interactive effect of trial success and notification modality on signal change in the NAcc (in the notification phase of the trial) was more pronounced in the ADP. In contrast, mean NAcc responses to hit versus miss notifications in the HIT trials was more similar in controls.
Unlike our initial experiment [Bjork et al.,2008b], the ADP of this report did not show relatively increased activation by reward notifications compared to controls. We suspect this difference arose from either the use of larger rewards, or due to the difference in framing of rewards in this new MID task variant. Critically, laboratory incentive‐related decision‐making deviates from financially‐optimal choice behavior due to the individual framing his or her options based on a reference point, such as a perceived endowment or status quo [Kahneman,2003; Kahneman and Tversky,1979]. Not surprisingly, VS recruitment by a particular incentive magnitude is dependent on how the reward is framed relative to a reference point, such as other amounts or possibilities in the experimental task [De Martino et al.,2009; Nieuwenhuis et al.,2005]. In this experiment, subjects did not encounter any loss‐avoidance trials (to keep the task duration within ∼30 min), whereas subjects suffered losses in our previous experiment. Moreover, our previous experiment featured trials where an expected reward notification was replaced by a demand to repeat the trial. Therefore, gains in our previous experiment were framed in the context of losses and thwarted gains, and this may have exaggerated the limbic response to the occasional gains.
Group differences in the reference‐dependence of reward deliveries may be evident when one population typically experiences more adversity, or tends to be more affectively reactive. Notably, ADP had greater anterior insula activation by loss notifications in our previous study [Bjork et al.,2008b]. For example, van Hell et al. [2010] reported reduced VS pre‐response reward‐anticipation activation in cannabis users compared to controls in a MID task with reward‐trials only. In contrast, Nestor et al. [2010] reported increased VS recruitment in cannabis users in a MID task that also featured loss‐avoidance trials. Persons with SUD (or persons with proneness to negative affect more generally) may have an increased referential framing effect of gains relative to losses—an interesting open question that could be tested by parametrically changing the framing of an incentive amount within‐subject‐across tasks or task blocks. Alternatively, in light of these data it could be argued that in retrospect, the noteworthy phenomenon in our previous report [Bjork et al.,2008b] was the absence of reward notification‐elicited VS activation in controls. This may have arisen from predominantly cognitive processing of reward deliveries and omissions (such as deducing their respective probabilities) in the controls, versus predominantly affective processing (limbic responses) in the patients.
Correlations Between MID Task Activation and Impulsivity
Interestingly, “hot” impulsivity as indexed by NEO‐IF scores did not correlate with reward anticipation activation of the NAcc before the instrumental response, but instead correlated with reward anticipation activation of the NAcc after the response had been made. NEO‐IF also correlated with VS signal change following low (but not high) reward notifications, consistent with our earlier experiment. This finding differs from that of Beck et al. [2009], who reported that VS recruitment during pre‐response reward anticipation negatively correlated with Barratt Impulsiveness Scale (BIS) [Patton et al.,1995] scores in ADP, with no such correlation in controls. We attribute this difference to how the greater sustained vigilance required by the original, briskly‐paced MID task used by the Beck/Wrase group (discussed above) may have closely related to the greater attention‐related items in the BIS [Patton et al.,1995]. Because of Barratt's conceptualization of impulsivity as a primarily (cold) cognitive trait [discussed in Whiteside and Lynam,2001], BIS questions do not feature explicit emotion, and instead probe general cognitive stability or discipline, such as “I get easily bored when solving thought problems” or “I am restless at the theater or lectures.” It stands to reason that ADP with high BIS scores (and higher range of scores) by virtue of lower attentional capacity would show reduced activation in the VS while attending to an unremitting barrage of cues and targets. Conversely, the NEO‐IF is a narrower set of items that captures impulsivity driven by negative emotional states. Although our MID task variant did not feature losses, the increased insula activation by losses in our previous study [Bjork et al.,2008b] suggests that at least generally, the MID task evokes substantial affect related to trial outcomes in ADP. This discrepancy in impulsivity correlations between reports illustrates a need for future exploration of mesolimbic processing in relation to different facets of impulsivity [Evenden,1999; Swann et al.,2002] using multiple measures.
A key addition of this study is the discovery that impulsivity relates to reward anticipation activation of the VS after motor‐preparatory activity, but not before. Notably, the post‐hoc MEMA reanalysis of time‐series activation contrasts with NEO‐IF scores added as a covariate illustrated direct voxelwise correlations between contrast‐based activations and NEO‐IF scores in the NAcc, while simultaneously accounting for variance related to addiction‐group. This suggests that whereas the requirement to mobilize an operant response for a substantial reinforcer may be so universal as to compress individual differences in pre‐response reward anticipation, brain activity related to the non‐essential affective components of instrumental behavior may remain sensitive to differences in affect‐related impulsivity.
We also replicated our previous finding [Bjork et al.,2008b] that low, but not high reward deliveries correlated with NEO‐IF scores. Trait impulsivity has also correlated with an exaggerated limbic response to immediate reward deliveries in other paradigms [Hariri et al.,2006]. This raises the question of whether this striatal sensitivity to reward deliveries is a result of chronic alcoholism, or reflects a premorbid trait. We suspect the latter, in that unmedicated adolescents with externalizing behavior disorders (who are impulsive and at high risk for alcoholism) also showed an exaggerated VS response to MID task reward deliveries [Bjork et al.,2010a]. We suspect that increased striatal responses to reward deliveries are neurophysiological signatures of the exaggerated impact of reward deliveries on the laboratory decision‐making of both children with externalizing disorders [Fairchild et al.,2009; Fonseca and Yule,1995; Matthys et al.,1998] and in drug‐addicted subjects [Lane and Cherek,2000; Stout et al.,2004; Yechiam et al.,2005].
This study features some shortcomings that warrant caution in data interpretation. Chiefly, the ADP all were regular smokers, and our control group did not select for smokers. Moreover, most ADP had significant life histories of abuse of another drug. Therefore, groupwise differences cannot be confidently attributed to chronic alcohol intoxication, per se. However, our ADP were selected on the basis of alcohol dependence as the subject's primary psychosocial disruption. In addition, as with other cross‐sectional comparisons between addicted patient groups and controls, differences cannot be attributed to the chronic substance exposure versus premorbid traits of impulsivity or emotional reactivity that could portend drug abuse. Finally, recent findings of genotypic differences in therapeutic responses to medications that target certain neurotransmitter systems [Mann and Hermann,2010] suggest that admixture of all ADP may be suboptimal, and that delineation of ADP based on dopamine‐related alleles or medication‐response itself might better identify ADP with broad motivational decrements.
CONCLUSIONS
These data suggest that after detoxification, and under certain environmental or incentive conditions, incentive neurocircuitry in alcoholism is essentially intact, or can at least for a limited period be brought on‐line for both optimal instrumental behavior and concomitant normative neural signatures of affective processing. Our findings suggest then, that either the putative decrement in nondrug reward processing postulated by the RDS and AH is minimal, or can be overcome for short periods. Finally, individual differences in impulsivity are especially sensitive to mesolimbic recruitment by reward‐related events largely uncoupled from mobilization of instrumental motor responses. Data from this report could serve as grist for future investigations of the potential effects of incentive magnitude or framing in addiction and in impulsivity.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Acknowledgements
The authors thank the nurses and social workers of the NIH Clinical Center alcohol inpatient care unit.
This article is a US Government work and is in the public domain in the USA.
REFERENCES
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J ( 2009): Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry 66:734–742. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW ( 2007): Anticipating instrumentally obtained and passively‐received rewards: A factorial fMRI investigation. Behav Brain Res 177: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C ( 2004a) Impulsivity in abstinent alcohol‐dependent patients: Relation to control subjects and type 1‐/type 2‐like traits. Alcohol 34: 133–150. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW ( 2004b) Incentive‐elicited brain activation in adolescents: Similarities and differences from young adults. J Neurosci 24: 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW ( 2008a) Incentive‐elicited striatal activation in adolescent children of alcoholics. Addiction 103: 1308–1319. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW ( 2008b) Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage 42: 1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW ( 2010a) Incentive‐elicited mesolimbic activation and externalizing symptomatology in adolescents. J Child Psychol Psychiatry 51: 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW ( 2010b): Adolescents, adults and rewards: Comparing motivational neurocircuitry recruitment using fMRI. PLoS One 5: e11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE ( 2000): Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 32 ( Suppl: i–iv): 1–112. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE ( 1997): Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591–611. [DOI] [PubMed] [Google Scholar]
- Buhler M, Vollstadt‐Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Buchel C, Smolka MN ( 2010): Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry 67: 745–752. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ ( 2007): Deformation‐based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage 34: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Cox RW ( 2010): Modeling multilevel variance components and outliers in group analysis. 16th Annual Meeting for the Organization of Human Brain Mapping, Barcelona, Spain.
- Comings DE, Blum K ( 2000): Reward deficiency syndrome: Genetic aspects of behavioral disorders. Prog Brain Res 126: 325–341. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR ( 1992): Revised NEO Personality Inventory and NEO Five‐Factor Inventory: Professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Holt B, Dolan RJ ( 2009): The neurobiology of reference‐dependent value computation. J Neurosci 29: 3833–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL ( 1999): Varieties of impulsivity. Psychopharmacology (Berl) 146: 348–361. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Aitken MR, Savage J, Moore SC, Goodyer IM ( 2009): Decision making and executive function in male adolescents with early‐onset or adolescence‐onset conduct disorder and control subjects. Biol Psychiatry 66: 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca AC, Yule W ( 1995): Personality and antisocial behavior in children and adolescents: An enquiry into Eysenck's and Gray's theories. J Abnorm Child Psychol 23: 767–781. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ ( 2005): Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend 78: 263–273. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ ( 2010): Cerebral white matter recovery in abstinent alcoholics—A multimodality magnetic resonance study. Brain 133 ( Part 4): 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB ( 2006): Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci 26: 13213–13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC ( 2010): Acute withdrawal, protracted abstinence and negative affect in alcoholism: Are they linked? Addict Biol 15: 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D ( 2003): A perspective on judgment and choice: Mapping bounded rationality. Am Psychol 58: 697–720. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A ( 1979): Prospect theory: An analysis of decision under risk. Econometrica 47: 263–292. [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D ( 2001a): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D ( 2001b) Dissociation of reward anticipation and outcome with event‐related fMRI. Neuroreport 12: 3683–3687. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D ( 2003): A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event‐related fMRI. Neuroimage 18: 263–272. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G ( 2005): Distributed neural representation of expected value. J Neurosci 25: 4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M ( 2005): Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat Neurosci 8: 1442–1444. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP ( 2004): Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27: 739–749. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI ( 2009): Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci 12: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR ( 2000): Analysis of risk taking in adults with a history of high risk behavior. Drug Alcohol Depend 60: 179–187. [DOI] [PubMed] [Google Scholar]
- Mann K, Hermann D ( 2010): Individualised treatment in alcohol‐dependent patients. Eur Arch Psychiatry Clin Neurosci 260 ( Suppl 2):S 116–S120. [DOI] [PubMed] [Google Scholar]
- Matthys W, van Goozen SH, de Vries H, Cohen‐Kettenis PT, van Engeland H ( 1998): The dominance of behavioral activation over behavioral inhibition in conduct disordered boys with or without attention deficit hyperactivity disorder. J Child Psychol Psychiatry 39: 643–651. [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H ( 2010): Increased ventral striatal BOLD activity during non‐drug reward anticipation in cannabis users. Neuroimage 49: 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Mars RB, Holroyd CB, Yeung N ( 2005): Activity in human reward‐sensitive brain areas is strongly context dependent. Neuroimage 25: 1302–1309. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES ( 1995): Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51: 768–774. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC ( 2001): Incentive‐sensitization and addiction. Addiction 96: 103–114. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX ( 2007): Ventral striatal hyporesponsiveness during reward anticipation in attention‐deficit/hyperactivity disorder. Biol Psychiatry 61: 720–724. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Needham M, Diemer KN, Knealing T, Crone‐Todd D, Fingerhood M, Nuzzo P, Kolodner K ( 2007): A randomized trial of employment‐based reinforcement of cocaine abstinence in injection drug users. J Appl Behav Anal 40: 387–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR ( 2004): Cognitive modeling analysis of decision‐making processes in cocaine abusers. Psychon Bull Rev 11: 742–747. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM ( 2002): Two models of impulsivity: Relationship to personality traits and psychopathology. Biol Psychiatry 51: 988–994. [DOI] [PubMed] [Google Scholar]
- van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF ( 2010): Chronic effects of cannabis use on the human reward system: An fMRI study. Eur Neuropsychopharmacol 20: 153–163. [DOI] [PubMed] [Google Scholar]
- Victorio‐Estrada A, Mucha RF, Stephan ER ( 1996): Excessive drinking situations in German alcoholics: Replication of a three‐factor model used for North Americans. Drug Alcohol Depend 41: 75–79. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N ( 1997): Decreased striatal dopaminergic responsiveness in detoxified cocaine‐dependent subjects. Nature 386: 830–833. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N ( 2002): Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: A preliminary study. Psychiatry Res 116: 163–172. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pasher H ( 2009): Puzzingly high correlations in fMRI studies of Emotion, Personality, and Social Cognition. Perspect Psychol Sci 4: 274–290. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR ( 2001): The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Pers Indiv Diff 30: 669–689. [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. ( 2007): Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35:787–794. [DOI] [PubMed] [Google Scholar]
- Yechiam E, Busemeyer JR, Stout JC, Bechara A ( 2005): Using cognitive models to map relations between neuropsychological disorders and human decision‐making deficits. Psychol Sci 16: 973–978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


