Abstract
Airway surface fluid contains two layers of mucins consisting mainly of 5 different mucin gene products. While the outer layer contains two gel-forming mucins (MUC5AC and MUC5B) that are tightly associated with various biologically active, defensive molecules, the inner layer contains three membrane-tethered mucins (MUC1, MUC4 and MUC16) shed from the apical cell surface. During airway infection, all of these mucins serve as a major protective barrier against pathogens. MUC1 mucin produced by virtually all the surface columnar epithelial cells in the respiratory tract as well as Type II pneumocytes in the alveoli plays an additional, perhaps more critical role during respiratory infection by controlling the resolution of inflammation that is essential to prevent the development of inflammatory lung disease.
Keywords: MUC1 mucin, Pseudomonas, Toll-like receptor, airway infection, inflammation and anti-inflammation
1. Introduction
Airway lining fluid, or mucus, serves as the first line of defense during airway infection. Pathogens trapped in the mucus layer are first removed by the mucociliary clearance mechanism of the underlying airway epithelium as well as macrophages and then by neutrophils recruited into the airways in response to inflammatory mediators released by epithelial cells and macrophages. Mucins are produced by goblet cells of surface epithelium and mucous cells of submucosal glands. The quality and quantity of mucins determine the viscoelastic property of mucus which is critical for the mucociliary clearance. Recent progress in airway mucin research has revealed that the roles of mucins during airway infection are more than just mucociliary clearance. This review will focus on the “unconventional” roles of mucins during airway infection. For the readers who may not be familiar with mucins, I will start with some basic structural information of mucins that should be useful in understanding their functional roles during airway infection. By convention, MUC represents human mucin and Muc for animal mucin. However, these abbreviations will be used somewhat interchangeably in this review just for the purpose of convenience.
2. Structure of mucins
Mucins are high molecular weight glycoproteins with variable numbers of tandem repeats of certain numbers of amino acids that are usually rich in serine, threonine and proline. Both serine and threonine are the sites of glycosylation of the peptide backbone through O-glysosidic linkage with N- acetylgalactosamine of the oligosaccharides, thus called O-linked glycoproteins. More detailed structural information of mucins is summarized in a number of reviews [1–4]. At least 22 mucin genes have been cloned in human [4–7], of which 16 have been identified in the lung (MUC1, MUC2, MUC4, MUC5AC, MUC5B, MUC7, MUC8, MUC11, MUC13, MUC15, MUC16, MUC18, MUC19, MUC20, MUC21, and MUC22) [4,6,6–9]. Deduced amino acid sequences of the cloned mucin genes revealed that there are two types of mucins – secreted mucins and membrane-tethered mucins. Seven mucin gene products (i.e., MUC2, 5AC, 5B, 6, 7, 8 and 19) are characterized as secreted and the remaining 10 mucin gene products are membrane-tethered [3,7]. Most of the transmembrane mucins are expressed on the apical surface of lining mucosal epithelial cells that are in contact with the outside environment. This suggests a defensive role of mucins for the host.
3. Mucins as a scaffold protein
Based on its anatomical location in the body as well as the complex structure of mucins, it was suggested quite some time ago that mucus has multifaceted properties necessary for host defense: anti-microbial, anti-protease, and anti-oxidant activities [10]. Jacquot et al [10] first reported the presence of these properties in airway secretions. Kim et al [11,12] demonstrated that airway mucins are extremely hydrophobic and guanidine hydrochloride (4–6 M), one of the most chaotrophic agents, could not completely dissociate mucins from other macromolecules present in airway secretion. It was postulated that the hydrophobic property may allow for effici1ent packaging of secreted mucins (>106 Dalton) with highly thermodynamic nature into the distinct secretory granules [13]. Recent proteomics analysis of mucins revealed that mucins are tightly associated with various proteins [14,15], including those with anti-microbial, anti-protease, anti-oxidant, or anti-inflammatory properties [15]. Thus, mucins are like a large aircraft carrier carrying a variety of “weapons” to be used against the invading pathogens. It may be possible that association of mucins with the bioactive molecules are formed inside the secretory granules before but not after exocytosis as previously suggested [16], such that they can interact with invading pathogens more effectively and efficiently upon exocytosis. How and when such association takes place in the goblet cell and is packaged into a mucous granule remains to be uncovered.
4. Roles of mucins during airway infection
Although 16 out of 22 mucin genes have been identified in the lung, their functions are largely unknown. A recent review by Sheehan et al [17] describes the roles of five major mucns (MUC5AC, MUC5B, MUC1, MUC4, and MUC16) in protecting and stabilizing the ciliated surface and building the gel in the airway epithelium. Although focused exclusively on intestinal mucins, a recent review by McGuckin et al [18] on the interaction of mucins with intestinal pathogens will greatly help us better understand the role of mucins during respiratory infection. In this review, I will focus mainly on the role of MUC1 mucin during airway infection.
4.1. MUC1 mucin
4.1.1. MUC1 mucin is expressed by lung epithelial cells
MUC1 was the first mucin gene to be cloned [19,20] as a cancer antigen. It is a membrane-tethered mucin that is located on the apical surface of mucosal epithelial cells as well as hematopoietic cells [21]. Although the expression of MUC1 in lymphocytes as well as dendritic cells is well established, its expression in other hematopoietic cells seems less clearly defined. Its expression has also been reported in corneal endothelial cells [22]. Whether other endothelial cells also express MUC1 is totally unknown. Details of the structure of MUC1 as well as its roles in cancer have been reviewed previously [1,23]. The presence of mucin-like glycoproteins on the surface of airway epithelial cells as those releasable by neutrophil elastase was documented in the hamster by Kim et al [24]. Expression of MUC1/Muc1 in the lung tissues as well as airway epithelial cells was later reported by Pemberton et al [25] and Hollingsworth et al [26], respectively. Using a cDNA library developed from primary hamster tracheal epithelial cells, Park et al [27] identified the expression of the Muc1 gene. Using the same cells, Paul et al [28] identified the presence of Muc1 protein in the plasma membrane fraction. Its expression in alveolar type II cells has also been documented [29]. Thus, it seems that MUC1/Muc1 is expressed on the apical surface of the lining epithelial cells of the lung.
4.1.2. MUC1 is a receptor for Pseudomonas aeruginosa
What is the role of this cancer antigen in the normal lung? Lillehoj et al [30,31] first demonstrated that hamster Muc1 expressed on the surface of CHO cells is an adhesion site of Pseudomonas aeruginosa (Pa), binding of which to the extracellular domain of Muc1 in CHO cells results in tyrosine phosphorylation on the cytoplasmic domain (CT) of MUC1 and the subsequent activation of ERK2 [32]. Tyrosine phosphorylation of MUC1 CT was also demonstrated in a chimera protein [33] in which MUC1 CT is covalently linked to the extracellular and plasma membrane domains of CD8 when cells expressing the chimera were treated with anti-CD8 antibody [33,34]. Using the chimera system, Wang et al [35] identified 4 tyrosine moieties on the MUC1 CT that are phosphorylated upon activation with anti-CD8 antibody as Y20, Y35, Y46, and Y60 which constitute consensus sequences for binding of PI3K, Shc, EGFR or Src, and Grb2, respectively [36]. Recently, the specific binding of Pa to the extracellular domain of MUC1 was also confirmed in human lung epithelial cells [37]. Given the importance of airway epithelial cells as a defensive barrier during Pa infection, it was originally speculated that MUC1/Muc1 may play an important role in facilitating Pa clearance during infection.
4.1.3. MUC1 plays an anti-inflammatory role during airway infection
To understand the potential role of Muc1 during airway Pa infection, Muc1−/− mice and their wild type littermates (Muc1+/+) were infected with Pa and the degree of lung inflammation was compared at 4 hours following Pa infection. Muc1−/− mice showed increased KC (mouse ortholog of human IL-8) and TNF-α levels as well as increased numbers of neutrophils in BALF and increased clearance of Pa compared with Muc1+/+ mice indicating that the absence of Muc1 facilitates airway inflammation [38]. Thus, these results suggested that MUC1 plays an anti-inflammatory role during airway Pa infection contrary to the initial prediction. The anti-inflammatory role of Muc1 during airway Pa infection was confirmed [39].
4.1.4. The anti-inflammatory effect of MUC1 is mediated through inhibition of TLR signaling
The study with Pa infection in Muc1−/− mice suggested the inhibitory effect of MUC1 on Pa-induced inflammation [38]. Since Pa-induced inflammation is induced mainly by activation of Toll-like receptor (TLR)5 by its flagellin [40]and because both TLR5 and MUC1 are present on the apical surface of airway epithelial cells, it was postulated that the presence of MUC1 may suppress TLR5 signaling. The inhibitory effect of MUC1 on TLR5 signaling was clearly demonstrated in cultured epithelial cells [41]. In addition, more interestingly, it has been shown, using various cultured cells, that the anti-inflammatory effect of MUC1 is not limited to TLR5 but all the other TLR signaling including TLR2, 3, 4, 7, and 9 [42] suggesting that MUC1 may be an universal negative regulator of TLR signaling. Furthermore, the anti-inflammatory effect of MUC1 did not require the whole molecule but only the cytoplasmic domain of MUC1 [38,42]. Given the importance of inflammation during the early stage of Pa infection and the “universal” anti-inflammatory effect of MUC1, a major speculation has been generated that the expression of Muc1 in the lung might be harmful to the host. This critical question prompted us to investigate the regulation of Muc1 expression in the airways.
4.1.5. MUC1 is upregulated during inflammation by TNF-α and plays a key role in the resolution of inflammation
Since MUC1 is cleaved by neutrophil elastase (NE) produced during inflammation, it was hypothesized that NE can upregulate MUC1. Using A549 cells, Kuwahara et al demonstrated that NE can upregulate MUC1 through a signaling pathway involving PKCδ → Dual Oxidase 1 → Reactive oxygen species → TNF-α converting enzyme → TNF-α → TNFR1 → ERK2 → Sp1 [43–45]. It was interesting that TNF-α, a major proinflammatory mediator during airway infection, has the ability to upregulate MUC1 and is required for NE-induced MUC1 upregulation. To assess the contribution of TNF-α to MUC1 levels during airway infection, animals (Muc1+/+, Muc1−/−, and TNFR−/−) were treated with Pa and both TNF-α and MUC1 levels were monitored over time. The results showed that Muc1 levels of uninfected lungs of Muc1+/+ mice were relatively low and increased steadily following Pa infection reaching a peak at 2–4 days. However. TNFR−/− mice failed to upregulate Muc1 following Pa infection and the inflammatory responses of TNFR−/− mice were very close (only slightly lower) than those of Muc1−/− mice [39]. Both Muc1−/− and TNFR−/− mice failed to resolve Pa-induced inflammation. These results not only supported the previous notion that TNF-α upregulates MUC1 but also indicated that TNF-α production is required for Pa-induced Muc1 upregulation. In addition, the results of this study helped us to answer the critical question as to whether the anti- inflammatory activity of MUC1 is beneficial or harmful during bacterial infection. Thus, the anti-inflammatory role of MUC1 comes into play at the late stage of infection mainly as a result of the increased levels of TNF-α produced at the early stage of infection. This concept is summarized in Fig. 1. The existence of such a feedback loop of inflammation involving TNF-α (pro-inflammatory) and MUC1 (anti-inflammatory) has also been further supported in A549 cells treated with respiratory syncytial virus [46] or nontypeable Hemophilus influenzae [47]. In summary, airway infection results in the production of proinflammatory mediators including TNF-α through activation of appropriate TLRs, which in turn upregulate MUC1 that suppresses TLR signaling, which results in cessation of the production of proinflammatory mediators and ongoing inflammation.
Fig. 1. Anti-inflammatory role of MUC1 during airway infection.
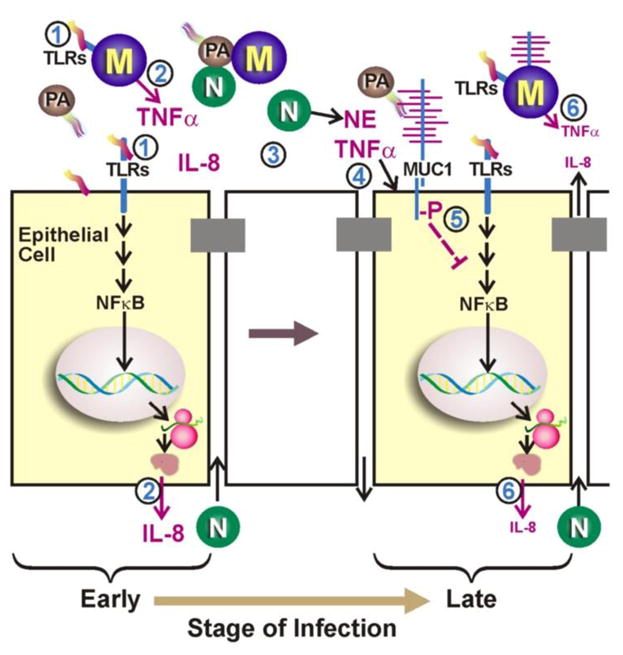
(Step 1) During the early stage of infection by P. aeruginosa (PA), bacterial PAMPs (e.g. flagellin) activate TLRs and NF-κB on epithelial cells and macrophages (M). (2) Activation of NF-κB leads to increased expression of TNF-α and of IL-8, which are subsequently secreted. (3) IL-8 recruits neutrophils (N) across the epithelial barrier that release NE into the lumen of the airways. (4) NE and TNF-α up-regulate MUC1 gene expression resulting in increased expression of MUC1 mucin at the apical surface of lung epithelial cells. (5) During the late stage of infection, tyrosine phosphorylation of MUC1 CT domain leads to inhibition of TLR signaling and (6) down-regulation of inflammation. (Fom Kim and Lillejoj [36])
4.1.6. Mechanism of crosstalk between MUC1 and TLRs
An important question that still remains largely unknown is how the increased levels of MUC1 can suppress TLR signaling during airway infection/inflammation. This study is currently under way in our laboratory. However, our preliminary data (unpublished) with Pa-induced TLR5 signaling suggest that the site of inhibition of TLR5 signaling by MUC1 is at the level of MyD88 recruitment. Briefly, MUC1 binds to TLR5 at its TIR domain thus preventing the recruitment of MyD88 during TLR5 activation by Pa or flagellin. Whether MUC1 phosphorylation is required for the inhibitory effect and, if so, which kinase(s) is responsible for phosphorylation are currently under investigation in our laboratory.
4.1.7. Shedding of MUC1
It has been shown that MUC1, a membrane-tethered glycoprotein, is shed by proteolytic cleavage, both spontaneously and by various stimuli including TNF-α and PMA [48]. Among the proteases responsible for MUC1 shedding are neutrophil elastase [24,43,49], TACE [48], MT1 MMP [50], MMP-14 [51], and gamma-secretase [52]. The functional significance of MUC1 shedding is not fully understood. Given the ability of the MUC1 ectodomain to bind the invading bacteria [30,37,51], it has been postulated that shed MUC1 may serve as a decoy receptor [51] to minimize the direct interaction with the epithelial cell surface and subsequent cell injury. It might also be possible that shed MUC1 may serve as an opsonin to facilitate bacterial clearance during infection.
4.2. Other membrane-tethered mucins (MUC4 and MUC16)
In addition to MUC1, both MUC4 and MUC16 have been shown to be produced and released by airway surface epithelial cells [14]. Sheehan et al [17] demonstrated that shed mucins form a gel in the immediate vicinity of the apical cell surface, likely serving as a protective barrier against invading pathogens and chemicals. How and when these membrane glycoproteins are cleaved remains largely unknown and will be important questions to address in the context with airway infection and inflammation.
4.3. Gel-forming mucins (MUC2, MUC5AC, MUC5B and MUC19)
These are four major gel-forming mucins found in the lung. MUC2 is the major intestinal mucin and Muc2 knockout mice develop colorectal cancer [53]. MUC2 has also been shown to be expressed in the lung [54]. It has been reported that the MUC2 gene is upregulated by Pa LPS in both surface epithelium and submucosal glands of human bronchial explants [55] as well as by both Gram(+) and Gram(−) bacteria in both bronchial explants and cultured epithelial cells [56]. On the other hand, An et al [57] reported that MUC2 gene is downregulated by retinoic acid in primary tracheobronchial epithelial cells of human and nonhuman primates. However, Hovenberg et al [58] as well as Thornton [59] demonstrated that the MUC2 was not detectable in both normal and pathologic human sputum samples based on an immunoassay, suggesting that MUC2 is not a major mucin produced in the lung. A recent proteomics analysis of both cultured epithelial cell secretions and patients’ sputum samples supports this notion [14]. However, the function of MUC2 in the lung remains still uncertain.
MUC5AC and MUC5B are the major gel-forming mucins in the airway and thus believed to contribute to both the defensive barrier function and the rheology of airway mucus. For details of their biochemistry, see a recent review by Thornton [3]. MUC5AC has been shown to be the goblet cell mucin [60] whereas MUC5B the submucosal gland mucin [61]. MUC5AC has been widely used as a marker for goblet cell metaplasia [62]. Recent studies, however, have demonstrated that MUC5B is the major type of mucin produced by goblet cells [14]. Although the exact roles of MUC5AC and MUC5B in the airways remain to be fully elucidated, it has been suggested that MUC5AC expression is inducible during airway inflammation [63], whereas MUC5B expression is constitutive [63,64]. It has also been shown that MUC5AC is more associated with asthma [63,65,66], whereas MUC5B with COPD [67]. Airway disease associated with mucus dysfunction has been recently reviewed [68]. Recently, a MUC5B promoter polymorphism has been associated with pulmonary fibrosis [69]. It is expected that their roles during airway infection will be uncovered very soon with the current availability of their knockout mice.
MUC19, the major salivary glandular mucin [70], has also been identified in tracheolarynx [71]. However, its role in the lung remains unknown.
Although these gel-forming mucins are present mainly in the interciliary space outside the layer of microvilli where membrane-tethered mucins are present [23], the fact that all these mucins form a major protective barrier against the invading pathogens or chemicals seems to suggest the presence of possible interactions between and among these mucins. In fact, mice with a defective CFTR or CF mice have mucus accumulation in the small intestine, however, making these mice Muc1 deficient (CF/Muc1−/− mice) prevented mucus accumulation [72]. Interestingly, the types of intestinal mucins affected by Muc1 were not limited to Muc1 suggesting the modulatory effect of Muc1 on other mucins in this animal model [73]. Interactions among various airway mucins, both structurally and functionally, during physiological and pathological conditions are totally unknown and need active research.
5. Conclusion
Airway surface fluid contains two layers of mucins consisting mainly of 5 different mucin gene products. While the outer layer contains two gel-forming mucins (MUC5AC and MUC5B) that are tightly associated with various biologically active, defensive molecules, the inner layer contains three membrane-tethered mucins (MUC1, MUC4 and MUC16) shed from the apical cell surface. During airway infection, all of these mucins serve as a major protective barrier against pathogens. MUC1 mucin produced by virtually all the surface columnar epithelial cells in the respiratory tract as well as Type II pneumocytes in the alveoli plays an additional, perhaps more critical role during respiratory infection by controlling the resolution of inflammation to prevent the development of inflammatory lung disease.
Acknowledgments
I thank Dr. Erik Lillehoj (Department of Pediatrics, University of Maryland School of Medicine, Baltimore, MD) and Dr. Marla Wolfson (Department of Physiology, Temple University School of Medicine, Philadelphia, PA) for kindly editing the manuscript and Dr. Kosuke Kato for assisting me in literature search. This work was supported by grants from NIH (RO1 HL-47125 and HL-81825).
Abbreviations
- CT
cytoplasmic tail
- NE
neutrophil elastase
- PA
Pseudomonas aeruginosa
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 2.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–86. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 4.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–78. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 5.Yi Y, Kamata-Sakurai M, Denda-Nagai K, Itoh T, Okada K, Ishii-Schrade K, et al. Mucin 21/epiglycanin modulates cell adhesion. J Biol Chem. 2010;285:21233–40. doi: 10.1074/jbc.M109.082875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh Y, Kamata-Sakurai M, Denda-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, et al. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 7.Hijikata M, Matsushita I, Tanaka G, Tsuchiya T, Ito H, Tokunaga K, et al. Molecular cloning of two novel mucin-like genes in the disease-susceptibility locus for diffuse panbronchiolitis. Hum Genet. 2011;129:117–28. doi: 10.1007/s00439-010-0906-4. [DOI] [PubMed] [Google Scholar]
- 8.Davies JR, Kirkham S, Svitacheva N, Thornton DJ, Carlstedt I. MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int J Biochem Cell Biol. 2007;39:1943–54. doi: 10.1016/j.biocel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Simon GC, Martin RJ, Smith S, Thaikoottathil J, Bowler RP, Barenkamp SJ, et al. Up-regulation of MUC18 in airway epithelial cells by IL-13: implications in bacterial adherence. Am J Respir Cell Mol Biol. 2011;44:606–13. doi: 10.1165/rcmb.2010-0384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquot J, Hayem A, Galabert C. Functions of proteins and lipids in airway secretions. Eur Respir J. 1992;5:343–58. [PubMed] [Google Scholar]
- 11.Kim KC, Opaskar-Hincman H, Bhaskar KR. Secretions from primary hamster tracheal surface epithelial cells in culture: mucin-like glycoproteins, proteoglycans, and lipids. Exp Lung Res. 1989;15:299–314. doi: 10.3109/01902148909087860. [DOI] [PubMed] [Google Scholar]
- 12.Kim KC, Singh BN. Hydrophobicity of mucin-like glycoproteins secreted by cultured tracheal epithelial cells: association with lipids. Exp Lung Res. 1990;16:279–92. doi: 10.3109/01902149009108845. [DOI] [PubMed] [Google Scholar]
- 13.Kim KC. Regulation of airway goblet cell mucin secretion. In: Takishima T, Shimura S, editors. Airway Secretion: Physiological Bases for the Control of Mucus Hypersecretion. Lung Biology and Health. Vol. 72. New York: Marcel Dekker, Inc; 1993. pp. 433–449. [Google Scholar]
- 14.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, et al. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296:L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali M, Lillehoj EP, Park Y, Kyo Y, Kim KC. Analysis of the proteome of human airway epithelial secretions. Proteome Sci. 2011;9:4. doi: 10.1186/1477-5956-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KC, Singh BN. Association of lipids with mucins may take place prior to secretion: studies with primary hamster tracheal epithelial cells in culture. Biorheology. 1990;27:491–501. doi: 10.3233/bir-1990-273-428. [DOI] [PubMed] [Google Scholar]
- 17.Sheehan JK, Kesimer M, Pickles R. Innate immunity and mucus structure and function. Novartis Found Symp. 2006;279:155, 66. discussion 167–9, 216–9. [PubMed] [Google Scholar]
- 18.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–78. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 19.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–93. [PubMed] [Google Scholar]
- 20.Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J Biol Chem. 1990;265:15294–9. [PubMed] [Google Scholar]
- 21.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6:339–53. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 22.Jung SE, Seo KY, Kim H, Kim HL, Chung IH, Kim EK. Expression of MUC1 on corneal endothelium of human. Cornea. 2002;21:691–5. doi: 10.1097/00003226-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–57. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 24.Kim KC, Wasano K, Niles RM, Schuster JE, Stone PJ, Brody JS. Human neutrophil elastase releases cell surface mucins from primary cultures of hamster tracheal epithelial cells. Proc Natl Acad Sci U S A. 1987;84:9304–8. doi: 10.1073/pnas.84.24.9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pemberton L, Taylor-Papadimitriou J, Gendler SJ. Antibodies to the cytoplasmic domain of the MUC1 mucin show conservation throughout mammals. Biochem Biophys Res Commun. 1992;185:167–75. doi: 10.1016/s0006-291x(05)80971-4. [DOI] [PubMed] [Google Scholar]
- 26.Hollingsworth MA, Batra SK, Qi WN, Yankaskas JR. MUC1 mucin mRNA expression in cultured human nasal and bronchial epithelial cells. Am J Respir Cell Mol Biol. 1992;6:516–20. doi: 10.1165/ajrcmb/6.5.516. [DOI] [PubMed] [Google Scholar]
- 27.Park H, Hyun SW, Kim KC. Expression of MUC1 mucin gene by hamster tracheal surface epithelial cells in primary culture. Am J Respir Cell Mol Biol. 1996;15:237–44. doi: 10.1165/ajrcmb.15.2.8703480. [DOI] [PubMed] [Google Scholar]
- 28.Paul E, Lee DI, Hyun SW, Gendler S, Kim KC. Identification and characterization of high molecular-mass mucin-like glycoproteins in the plasma membrane of airway epithelial cells. Am J Respir Cell Mol Biol. 1998;19:681–90. doi: 10.1165/ajrcmb.19.4.2908. [DOI] [PubMed] [Google Scholar]
- 29.Jarrard JA, Linnoila RI, Lee H, Steinberg SM, Witschi H, Szabo E. MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer Res. 1998;58:5582–9. [PubMed] [Google Scholar]
- 30.Lillehoj EP, Hyun SW, Kim BT, Zhang XG, Lee DI, Rowland S, et al. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2001;280:L181–7. doi: 10.1152/ajplung.2001.280.1.L181. [DOI] [PubMed] [Google Scholar]
- 31.Lillehoj EP, Kim BT, Kim KC. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am J Physiol Lung Cell Mol Physiol. 2002;282:L751–6. doi: 10.1152/ajplung.00383.2001. [DOI] [PubMed] [Google Scholar]
- 32.Lillehoj EP, Kim H, Chun EY, Kim KC. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L809–15. doi: 10.1152/ajplung.00385.2003. [DOI] [PubMed] [Google Scholar]
- 33.Meerzaman D, Xing PX, Kim KC. Construction and characterization of a chimeric receptor containing the cytoplasmic domain of MUC1 mucin. Am J Physiol Lung Cell Mol Physiol. 2000;278:L625–9. doi: 10.1152/ajplung.2000.278.3.L625. [DOI] [PubMed] [Google Scholar]
- 34.Meerzaman D, Shapiro PS, Kim KC. Involvement of the MAP kinase ERK2 in MUC1 mucin signaling. Am J Physiol Lung Cell Mol Physiol. 2001;281:L86–91. doi: 10.1152/ajplung.2001.281.1.L86. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Lillehoj EP, Kim KC. Identification of four sites of stimulated tyrosine phosphorylation in the MUC1 cytoplasmic tail. Biochem Biophys Res Commun. 2003;310:341–6. doi: 10.1016/j.bbrc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 36.Kim KC, Lillehoj EP. MUC1 mucin: a peacemaker in the lung. Am J Respir Cell Mol Biol. 2008;39:644–7. doi: 10.1165/rcmb.2008-0169TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato K, Lillehoj EP, Kai H, Kim KC. MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front Biosci (Elite Ed) 2010;2:68–77. doi: 10.2741/e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol. 2006;176:3890–4. doi: 10.4049/jimmunol.176.7.3890. [DOI] [PubMed] [Google Scholar]
- 39.Choi S, Park YS, Koga T, Treloar A, Kim KC. TNF-alpha is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 2011;44:255–60. doi: 10.1165/rcmb.2009-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun. 2005;73:7151–60. doi: 10.1128/IAI.73.11.7151-7160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato K, Lu W, Kai H, Kim KC. Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of Toll-like receptor 5 signaling. Am J Physiol Lung Cell Mol Physiol. 2007;293:L686–92. doi: 10.1152/ajplung.00423.2006. [DOI] [PubMed] [Google Scholar]
- 42.Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, et al. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol. 2008;38:263–8. doi: 10.1165/rcmb.2007-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuwahara I, Lillehoj EP, Hisatsune A, Lu W, Isohama Y, Miyata T, et al. Neutrophil elastase stimulates MUC1 gene expression through increased Sp1 binding to the MUC1 promoter. Am J Physiol Lung Cell Mol Physiol. 2005;289:L355–62. doi: 10.1152/ajplung.00040.2005. [DOI] [PubMed] [Google Scholar]
- 44.Kuwahara I, Lillehoj EP, Koga T, Isohama Y, Miyata T, Kim KC. The signaling pathway involved in neutrophil elastase stimulated MUC1 transcription. Am J Respir Cell Mol Biol. 2007;37:691–8. doi: 10.1165/rcmb.2007-0072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y, et al. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol. 2007;293:L693–701. doi: 10.1152/ajplung.00491.2006. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2010;298:L558–63. doi: 10.1152/ajplung.00225.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kyo Y, Kato K, Park YS, Gajhate S, Umehara T, Lillehoj EP, et al. Anti-inflammatory role of MUC1 mucin during nontypeable Haemophilus influenzae infection. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2011-0142OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J Biol Chem. 2003;278:3386–94. doi: 10.1074/jbc.M208326200. [DOI] [PubMed] [Google Scholar]
- 49.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Invest Ophthalmol Vis Sci. 2008;49:1864–71. doi: 10.1167/iovs.07-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thathiah A, Carson DD. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem J. 2004;382:363–73. doi: 10.1042/BJ20040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linden SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin TH, et al. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 2009;5:e1000617. doi: 10.1371/journal.ppat.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Julian J, Dharmaraj N, Carson DD. MUC1 is a substrate for gamma-secretase. J Cell Biochem. 2009;108:802–15. doi: 10.1002/jcb.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–9. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 54.Ohmori H, Dohrman AF, Gallup M, Tsuda T, Kai H, Gum JR, Jr, et al. Molecular cloning of the amino-terminal region of a rat MUC 2 mucin gene homologue. Evidence for expression in both intestine and airway. J Biol Chem. 1994;269:17833–40. [PubMed] [Google Scholar]
- 55.Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A. 1997;94:967–72. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dohrman A, Miyata S, Gallup M, Li JD, Chapelin C, Coste A, et al. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1998;1406:251–9. doi: 10.1016/s0925-4439(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 57.An G, Luo G, Wu R. Expression of MUC2 gene is down-regulated by vitamin A at the transcriptional level in vitro in tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:546–51. doi: 10.1165/ajrcmb.10.5.8179918. [DOI] [PubMed] [Google Scholar]
- 58.Hovenberg HW, Davies JR, Herrmann A, Linden CJ, Carlstedt I. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj J. 1996;13:839–47. doi: 10.1007/BF00702348. [DOI] [PubMed] [Google Scholar]
- 59.Thornton DJ, Carlstedt I, Howard M, Devine PL, Price MR, Sheehan JK. Respiratory mucins: identification of core proteins and glycoforms. Biochem J. 1996;316 ( Pt 3):967–75. doi: 10.1042/bj3160967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hovenberg HW, Davies JR, Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem J. 1996;318 ( Pt 1):319–24. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wickstrom C, Davies JR, Eriksen GV, Veerman EC, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J. 1998;334 ( Pt 3):685–93. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuhdi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol. 2000;22:253–60. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 63.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009;15:4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roy MG, Rahmani M, Hernandez JR, Alexander SN, Ehre C, Ho SB, et al. Mucin Production During Pre- and Post-Natal Mouse Lung Development. Am J Respir Cell Mol Biol. 2011;44:755–760. doi: 10.1165/rcmb.2010-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–23. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 66.Hallstrand TS, Debley JS, Farin FM, Henderson WR., Jr Role of MUC5AC in the pathogenesis of exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2007;119:1092–8. doi: 10.1016/j.jaci.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirkham S, Kolsum U, Rousseau K, Singh D, Vestbo J, Thornton DJ. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:1033–9. doi: 10.1164/rccm.200803-391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–47. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–12. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Zhao YH, Kalaslavadi TB, Hamati E, Nehrke K, Le AD, et al. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2004;30:155–65. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- 71.Das B, Cash MN, Hand AR, Shivazad A, Grieshaber SS, Robinson B, et al. Tissue Distibution of Murine Muc19/Smgc Gene Products. J Histochem Cytochem. 2009 doi: 10.1369/jhc.2009.954891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parmley RR, Gendler SJ. Cystic fibrosis mice lacking Muc1 have reduced amounts of intestinal mucus. J Clin Invest. 1998;102:1798–806. doi: 10.1172/JCI3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malmberg EK, Noaksson KA, Phillipson M, Johansson ME, Hinojosa-Kurtzberg M, Holm L, et al. Increased levels of mucins in the cystic fibrosis mouse small intestine, and modulator effects of the Muc1 mucin expression. Am J Physiol Gastrointest Liver Physiol. 2006;291:G203–10. doi: 10.1152/ajpgi.00491.2005. [DOI] [PubMed] [Google Scholar]


