Abstract
Aims
We tested the hypotheses that vasoconstrictor responses to limb dependency are: 1) greater in the leg than the arm, 2) impaired with age, and 3) not sympathetically mediated.
Methods
Vascular responses to limb dependency (i.e., lowering the limb from heart level to 30 cm below heart level) were determined in 17 young and 17 older adults. Indices of blood flow were obtained in the brachial and popliteal artery (Doppler ultrasound) as well as the cutaneous circulation (forearm and calf using laser Doppler flowmetry). Vasoconstriction was quantified by calculating indices of vascular resistance as height corrected mean arterial pressure/limb blood velocity or skin flux. A second group of subjects repeated the limb dependency trials after acute systemic sympathetic blockade.
Results
Limb dependency increased vascular resistance index in the brachial artery (Δ59±8%; P<0.05) and popliteal artery (Δ99±10%; P<0.05 for change from heart level and brachial vs. popliteal) of young and older adults (Δ60+9% brachial and Δ61±7% popliteal artery; p<0.05 for change from heart level and response in popliteal young vs. older). In contrast, cutaneous vasoconstrictor responses to limb dependency were similar in the forearm (Δ218±29% and Δ200±29% for young and older, respectively) and calf (Δ257±32% and Δ236±29%; all P<0.05 from heart level) of young and older adults. Vasoconstrictor responses to limb dependency were not affected by sympathetic blockade in young or older adults.
Conclusion
These findings indicate that age-, limb-, and tissue-related differences may exist in the vasoconstrictor response to limb dependency in healthy humans, which are not sympathetically mediated.
Keywords: aging, blood flow, adrenergic, venoarteriolar, myogenic
Introduction
Limb dependency elicits a powerful local vasoconstrictor response within skeletal muscle, subcutaneous tissue, and skin of humans (Henriksen et al., 1973a, Henriksen et al., 1973b, Henriksen and Sejrsen, 1977, Henriksen, 1991, Kooijman et al., 2007). This vasoconstrictor response persists after spinal anesthesia (Henriksen and Sejrsen, 1977), proximal sympathetic neural blockade (Henriksen and Sejrsen, 1977, Vissing et al., 1997), and in denervated skin flaps (Zoltie et al., 1989), but is abolished by topical anesthetic (cutaneous circulation) (Crandall et al., 2002, Okazaki et al., 2005, Vissing et al., 1997). Additionally, some (Henriksen, 1976, Henriksen and Sejrsen, 1977, Henriksen, 1977), but not all studies (Kooijman et al., 2009, Crandall et al., 2002) indicate that local administration of α-adrenergic antagonists to various tissues abolishes this vasoconstrictor response. Collectively, these data suggest that vasoconstrictor responses to limb dependency arise from a local, possibly axonal mechanism(s) as a result of venous distention (venoarteriolar response) or as a result of changes in transmural pressure (myogenic response) (Low, 2004, Gaskell and Burton, 1955, Rowell, 1993, Henriksen, 1991).
A locally mediated vasoconstrictor response to limb dependency could be critical during times in which venous pressures are increased, such as during orthostasis. It has been estimated that ~45% of the total vasoconstriction required to avoid a persistent fall in arterial pressure (BP) during orthostasis results from engagement of a local reflex response (Henriksen and Sejrsen, 1977, Henriksen, 1977, Henriksen, 1976). The existence of such a response could help explain how some level of orthostatic tolerance is maintained in spinal cord injured individuals (Theisen et al., 2000, Skagen et al., 1982, Andersen et al., 1986, Kooijman et al., 2007, Kooijman et al., 2009) and those with autonomic failure (Groothuis et al., 2011) despite losses in postganglionic sympathetic function.
As older adults exhibit impaired BP regulation (Monahan, 2007), factors such as age-related alterations in baroreflex function (Monahan, 2007) and local reflex function (Lott et al., 2004) take on an added level of importance. Presently, the effect of aging on the vascular response to limb dependency has not been determined. Moreover, whether or not limb-related differences exist in the vascular response to limb dependency has not been determined. Accordingly, the aim of the present study was to test the hypotheses that vasoconstrictor responses to limb dependency: 1) are augmented in the leg relative to the arm, 2) are impaired with age, and 3) are not sympathetically mediated.
Methods
Subjects
Thirty-four subjects 22-35 (young; n=17) and 58-79 (older; n=17) years of age participated in the protocols associated with this study. All subjects were healthy, as assessed by history and physical examination, normotensive, non-smokers, non-obese (body mass index <30 kg/m2), and unmedicated. No subjects reported taking any vitamin supplements. The experimental protocols conformed to standards set in the latest revision of the Declaration of Helsinki and the Institutional Review Board at the Pennsylvania State University College of Medicine approved them. Informed consent was obtained from all subjects.
Measurements
BP and Heart Rate (HR)
BP and HR at rest were determined at the left arm using a semi-automated device (Dinamap; GE Healthcare) before each trial. Additionally, during each trial BP and HR were determined on a beat-to-beat basis using photoplethysmography (Finapres Medical Systems, Amsterdam, Netherlands) on the third finger of the left hand.
Skin Flux
An integrative index of skin blood flow (i.e., skin flux) was obtained using laser Doppler flowmetry (Moor Instruments, Wilmington, DE). Two laser Doppler probes were inserted in small local heating units affixed to the midpoint of the ventral aspect of the right calf and forearm. Local heating units were set to 34° C, a temperature that does not affect the venoarteriolar response, but assures similar skin surface temperatures between subjects (Davison et al., 2004).
Limb Blood Velocity
Blood velocity in the brachial and popliteal arteries was measured using 4 MHz pulsed wave Doppler probes (Model 500M, Multigon Industries, Yonkers, New York). The Doppler probes were taped in place over the arteries of interest and adjusted until optimal signals were obtained with an insonation angle of 45°. Measurements were obtained in the right arm and leg.
Thoracic Impedance
Impedance cardiography (HIC-2000 Bio-Impedance Technology, Inc.) was used to measure thoracic impedance and provide insight into possible central hypovolemia (baroreceptor unloading) that may have occurred during limb dependency (Ebert et al., 1986, van Lieshout et al., 2005).
Experimental Design
General experimental protocol
Studies were performed on supine subjects in a thermoneutral (20-22° C) laboratory after a minimum 4-hour fast and avoidance of caffeine and alcohol for at least 12 hours. Subjects were asked to void their bladders before the study. The examination table was modified with a pivoted drop out section located under the subject’s right leg. This allowed the whole leg to be lowered solely through passive hip extension into the dependent position. The right arm was positioned (abducted 90° to the torso) on a board extending off the side of the examination table allowing the whole arm to be lowered into the dependent position solely through passive shoulder extension. After initial positioning the subject’s arm and leg were lowered to assure the desired (Δ-30 cm) change in calf and forearm midpoint, measured from the midpoint of the tibia and ulna, relative to the baseline position (heart level; mid-axillary line) could be achieved.
Protocol 1: Age- and limb-related differences in the vasoconstrictor response to limb dependency
Twelve young and 12 older subjects rested quietly in the supine position for at least 30 min prior to data collection. During this protocol the right arm and leg were maintained at heart level at all times except when they were moved into the dependent position. Data collection involved 4 trials (2 arm and 2 leg trials). After measuring BP over the left brachial artery a 3 min baseline period ensued, in which the studied limb was maintained at heart level. After this the limb was passively lowered into the dependent position for 3 min. During each trial BP and HR (Finapres) were measured (left hand) and skin flux and mean blood velocity was measured (final minute of limb dependency). Results of the 2 trials in each limb were averaged.
Protocol 2: Role of sympathetic mechanisms in the age- and limb-related differences in the vasoconstrictor response to limb dependency
After obtaining an initial set of vasoconstrictor responses in the arm and leg to limb dependency (5 young and 5 older adults), as described above in protocol 1, systemic sympathetic blockade was performed. Sympathetic blockade was achieved by intravenous infusion of propranolol (priming dose; 0.25 mg/kg) over 15 min, into the antecubital vein of the left arm, followed by phentolamine (priming dose; 0.18 mg/kg) over 5 min. After the priming doses maintenance doses were infused (propranolol 0.006 mg/kg/min; phentolamine 0.018 mg/kg/min) until study completion (Sielatycki et al., 2011). Vasoconstrictor responses to limb dependency were repeated beginning ~10 min after the start of the maintenance doses. Effectiveness of α-adrenergic blockade was assessed by measuring the pressor response to bolus intravenous infusion of norepinephrine (3 μg) before (pre) and during sympathetic blockade (post) (Esler et al., 1975, Sielatycki et al., 2011). In addition to measures obtained in protocol 1 we also measured thoracic impedance in this protocol.
Data Collection and Analysis
Data were digitally recorded at 400 Hz. Results were quantified over the final minute of the baseline and limb dependent periods. An index of vascular resistance was calculated as mean BP (height corrected)/skin flux or limb mean blood velocity. To account for hydrostatic effects mean BP in the dependent limb was corrected according to the formula: mean BP (height corrected)=mean BP (heart level)+0.766*30, where 30 represent the number of centimeters the limb midpoint was below heart level (Christ et al., 1997). Responses to norepinephrine were quantified as peak increases in mean BP (3-point moving average) from baseline levels after bolus infusion.
Statistical Analysis
Differences in subject characteristics were determined by t-test. Effects of the intervention were determined using 1 and 2 factor repeated-measures ANOVA and Student Newman-Keuls post hoc tests. Statistical significance was accepted at P<0.05.
Results
Subject Characteristics
Subject characteristics are presented in Table 1. Diastolic BP (protocol 1), mean BP (protocol 1), and height (protocol 2) differed between young and older subjects.
Table 1.
Subject characteristics
| Protocol 1 | Protocol 2 | |||
|---|---|---|---|---|
| Young (n=12) |
Older (n=12) |
Young (n=5) |
Older (n=5) |
|
| Sex | 7 male / 5 female | 6 male / 6 female | 3 male / 2 female | 3 male / 2 female |
| Age, years | 25±3 | 66±7* | 25±2 | 69±7* |
| Height, cm | 175.9±10.4 | 173.2±6.9 | 182.9±3.6 | 175.6±4.3* |
| Body mass, kg | 76.6±14.5 | 74.2±12.5 | 87.3±10.3 | 77.0±12.1 |
| BMI, kg/m2 | 24.5±2.4 | 24.6±3.1 | 26.1±2.5 | 24.8±3.1 |
| Systolic BP, mmHg | 116±10 | 120±7 | 123±10 | 126±4 |
| Diastolic BP, mmHg | 66±3 | 73±7* | 71±18 | 75±4 |
| Mean BP, mmHg | 86±3 | 90±3* | 86±4 | 93±4 |
| HR, beats/min | 60±7 | 60±6.9 | 56±10 | 60±7 |
Values are mean±SD
BMI = body mass index; BP = arterial pressure; heart rate = HR
P < 0.05 compared to young
Vasoconstrictor Responses to Limb Dependency in Young Adults (Protocol 1)
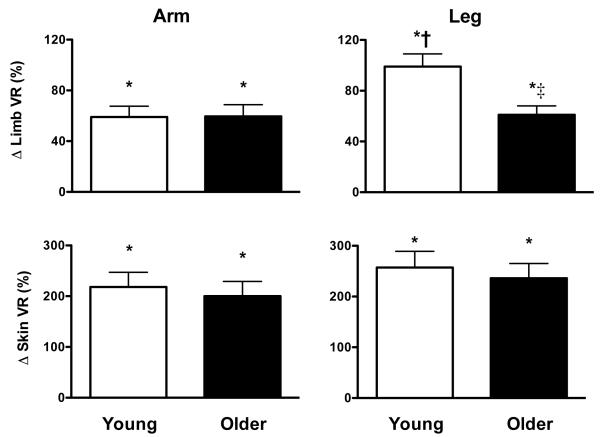
Limb dependency elicited vasoconstriction, as measured by increased brachial and popliteal artery vascular resistance index (Fig. 1 and Table 2), in the lowered arm and leg of young adults. The magnitude of vasoconstriction produced by limb dependency was greater (P<0.05) in the popliteal artery than in the brachial artery of young adults. Limb dependency also produced cutaneous vasoconstriction (increased cutaneous vascular resistance index) in the lowered forearm and calf of young adults (Fig. 2), although the magnitude of this vasoconstriction did not differ between the limbs (P>0.05). HR and BP were unchanged by limb dependency in young adults.
Figure 1.
Percent change (Δ) in limb and skin vascular resistance index (VR) in the arm (left panels) and leg (right panels) of young and older adults in response to limb dependency. VR index was calculated as mean arterial pressure/mean blood velocity (limb) or flux (skin). Responses are presented as the percent change from the baseline position (limb at heart level) to the dependent position (limb lowered 30 cm below heart level). Mean arterial pressure was corrected to account for the hydrostatic pressure gradient created when the limb was studied in the dependent position (mean arterial pressure in control limb at heart level + 0.766 * 30 cm). Limb VR in the arm was assessed in the brachial artery and in the leg in the popliteal artery. Skin VR in was assessed on the ventral aspect of the forearm (arm) and calf (leg). Values are mean±SE.
* P<0.05 from baseline
† P<0.05 from response in arm (same age group)
‡ P<0.05 from response in young (same limb)
Table 2.
Responses to limb dependency (Protocol 1)
| Arm | Leg | |||
|---|---|---|---|---|
| Limb position | Heart level | Limb dependent | Heart level | Limb dependent |
| Young (n=12) | ||||
| HR, beats/min | 61±2 | 61±2 | 63±2 | 61±2* |
| Mean BP, mmHg | 85±1 | 85±2 | 84±1 | 85±1 |
| Mean BP (corrected) | 85±1 | 108±2 | 84±1 | 108±1 |
| Limb MBV, cm/sec | 1.7±0.2 | 1.4±0.2* | 1.3±0.1 | 0.9±0.1* |
| Δ Limb MBV, cm/sec | -- | −0.3±0.1* | -- | −0.4±0.1* |
| Limb VR index, au | 61±10 | 95±15* | 72±8 | 142±15* |
| Δ Limb VR index, au | -- | 34±6* | -- | 70±9*‡ |
| Skin flux, au | 30.1±3.9 | 14.1±2.8* | 25.7±3.8 | 10.0±1.9* |
| Δ Skin flux, au | -- | −16.0±1.8* | -- | −15.7±2.3* |
| Skin VR index, au | 3.3±0.5 | 11.0±1.9* | 4.5±1.0 | 14.7±2.1* |
| Δ Skin VR index, au | -- | 7.7±1.5* | -- | 10.2±1.6* |
| Older (n=12) | ||||
| HR, beats/min | 60±2 | 59±2 | 58±2† | 58±2† |
| Mean BP, mmHg | 89±1† | 90±2† | 92±1† | 93±2† |
| Mean BP (corrected) | 89±1† | 123±2† | 92±1† | 116±2† |
| Limb MBV, cm/sec | 1.7±0.2 | 1.4±0.1* | 1.2±0.10 | 0.9±0.1* |
| Δ Limb MBV, cm/sec | -- | −0.3±0.1* | -- | −0.3±0.1*† |
| Limb VR index, au | 57±5 | 91±10* | 86±9 | 136±13* |
| Δ Limb VR index, au | -- | 34±7* | -- | 50±6*† |
| Skin flux, au | 25.8±4.7 | 11.9±2.8* | 18.4±2.6† | 6.9±0.7* |
| Δ Skin flux, au | -- | −13.9±2.8* | -- | −11.5±2.3* |
| Skin VR index, au | 4.9±0.9 | 14.2±2.6* | 6.1±0.7 | 18.4±1.7* |
| Δ Skin VR index, au | -- | 9.2±2.1* | -- | 12.3±1.2* |
Values are mean±SE; HR=heart rate; BP=arterial pressure; MBV=mean blood velocity; VR=vascular resistance index (mean BP/MBV or flux);
Mean BP (corrected) accounts for hydrostatic pressure effects (mean BP+(0.766*30cm)) when the limb was dependent (i.e., lowered below heart level).
Limb responses were measured in the brachial (arm) and popliteal artery (leg). Skin responses were measured on the ventral aspect of the forearm (arm) and calf (leg).
P<0.05 Δ from heart level
P<0.05 compared to young (same time point)
compared to response in arm (same age group)
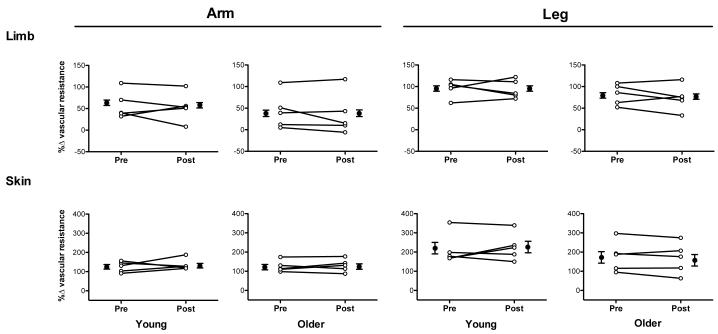
Figure 2.
Percent change (Δ) in vascular resistance index in the arm (left panels) and leg (right panels) of young (n=5) and older adults (n=5) in response to limb dependency. Data obtained before (Pre) and during systemic sympathetic blockade (Post) are presented on both an individual (open symbols) as well as mean basis (closed symbols; mean±SE). Indices of vascular resistance were calculated as mean arterial pressure/mean blood velocity (limb) or flux (skin). Responses are presented as the percent change in vascular resistance index from the baseline (limb at heart level) to the dependent position (limb lowered 30 cm below heart level). Mean arterial pressure was corrected to account for the hydrostatic pressure gradient created when the limb was studied in the dependent position (mean arterial pressure in control limb at heart level + 0.766 * 30 cm). Responses to limb dependency were not altered by sympathetic blockade in young or older adults. Limb VR in the arm was assessed in the brachial artery and in the leg in the popliteal artery. Skin VR in was assessed on the ventral aspect of the forearm (arm) and calf (leg).
Effect of Aging on the Vasoconstrictor Responses to Limb Dependency (Protocol 1)
Limb dependency elicited vasoconstriction, as measured by increased brachial and popliteal artery vascular resistance index (Fig. 1 and Table 2) in older adults. The vasoconstriction produced by limb dependency was reduced with age in the popliteal artery. Similar to responses observed in the skin of young adults limb dependency produced cutaneous vasoconstriction in the forearm and calf of older adults, the magnitude of which did not differ between limbs or when compared to responses in young adults. HR and BP were not altered by limb dependency in older adults.
Effect of Sympathetic Blockade on Vasoconstrictor Responses to Limb Dependency (Protocol 2)
Limb dependency elicited vasoconstriction in young and older adults before (Pre) and during sympathetic blockade (Post) (Fig. 2 and Table 3). The magnitude of vasoconstriction was not influenced by sympathetic blockade in young or older adults. Thoracic impedance, HR, and BP were unchanged by limb dependency (arm and leg) in young and older subjects both Pre and Post blockade.
Table 3.
Responses to limb dependency before (Pre) and during systemic sympathetic blockade (Post) (Protocol 2)
| Pre | Post | |||
|---|---|---|---|---|
| Limb position | Heart level | Limb dependent | Heart level | Limb dependent |
| Young (n=5) | ||||
| Arm | ||||
| Thoracic impedance, Ω | 34.3±4.8 | 33.8±4.7 | 31.8±5.5† | 31.4±5.3 |
| HR, beats/min | 49±3 | 50±3 | 48±3 | 49±3 |
| Mean BP, mmHg | 89±3 | 89±3 | 85±2† | 85±1 |
| Brachial MBV, cm/sec | 1.6±0.4 | 1.3±0.4* | 1.2±0.3† | 1.0±0.2* |
| Brachial VR index, au | 69±15 | 107±21* | 83±18 | 120±19* |
| Skin flux, au | 21.1±3.5 | 12.1±2.3* | 20.1±2.8 | 11.1±1.7* |
| Skin VR index, au | 4.7±0.8 | 10.8±2.1* | 4.6±0.7 | 11.0±2.1* |
| Leg | ||||
| Thoracic impedance, Ω | 32.7±5.5 | 32.8±5.7 | 31.6±5.7† | 31.8±5.8 |
| HR, beats/min | 51±4 | 51±4 | 49±3 | 50±3 |
| Mean BP, mmHg | 88±3 | 89±3 | 84±2* | 84±2 |
| Popliteal MBV, cm/sec | 1.2±0.2 | 0.8±0.1* | 1.2±0.2 | 0.8±0.1* |
| Popliteal VR index, au | 84±13 | 163±26* | 78±12 | 149±20* |
| Skin flux, au | 14.3±3.6 | 5.8±1.5* | 14.4±1.8 | 5.8±0.9* |
| Skin VR index, au | 7.9±1.9 | 23.5±4.6* | 6.1±0.6 | 19.9±2.3* |
| Older (n=5) | ||||
| Arm | ||||
| Thoracic impedance, Ω | 37.9±1.2 | 38.0±1.3 | 36.8±0.5† | 36.9±0.7 |
| HR, beats/min | 58±3 | 58±4 | 55±2† | 56±2* |
| Mean BP, mmHg | 94±3 | 94±2 | 85±3† | 86±3 |
| Brachial MBV, cm/sec | 1.6±0.2 | 1.5±0.3 | 1.3±0.2 | 1.3±0.2 |
| Brachial VR index, au | 67±12 | 95±18* | 69±9 | 90±14* |
| Skin flux, au | 18.6±3.9 | 10.7±2.3* | 20.1±3.5 | 11.4±1.9* |
| Skin VR index, au | 6.8±2.3 | 16.5±6.9* | 4.9±1.0 | 11.5±3.0* |
| Leg | ||||
| Thoracic impedance, Ω | 38.3±1.3 | 38.1±1.4 | 38.3±1.9 | 38.2±1.7 |
| HR, beats/min | 58±3 | 58±3 | 56±2† | 57±2* |
| Mean BP, mmHg | 95±4 | 97±4 | 87±2† | 87±3 |
| Popliteal MBV, cm/sec | 1.1±0.1 | 0.8±0.1* | 1.0±0.1 | 0.7±0.1* |
| Popliteal VR index, au | 90±14 | 162±21* | 91±11 | 154±15* |
| Skin flux, au | 11.2±3.0 | 5.3±1.5* | 11.7±1.8 | 5.8±1.1* |
| Skin VR index, au | 12.9±4.8 | 35.5±14.5* | 8.1±1.2 | 20.8±2.5* |
Values are mean±SE; HR=heart rate; BP=arterial pressure; MBV=mean blood velocity; VR=vascular resistance index (mean BP/MBV or flux); Mean BP was corrected to account for hydrostatic pressure effects (mean BP+(0.766*30cm)) when the limb was dependent.
P<0.05 Δ from heart level
P<0.05 compared to Pre (heart level)
compared to response in arm (same age group)
Pressor responses to norepinephrine (Δ mean BP) were similarly reduced by sympathetic blockade in young (Δ18±2 vs. Δ4±1 mmHg for Pre and Post, respectively; P<0.05) and older (Δ22±7 vs. Δ5±2mmHg for Pre and Post, respectively; P<0.05) adults.
No gender related effects were observed in either Protocol 1 or Protocol 2.
Discussion
This study reports a number of novel findings. First, limb dependency produces a vasoconstrictor response in both the arm and leg (popliteal and brachial artery) of young adults, the magnitude of which is ~2-fold greater in the leg than in the arm. Second, limb vasoconstrictor responses to limb dependency are reduced with age in the leg (popliteal artery). Third, in contrast to those responses observed in the limb (popliteal and brachial artery), vasoconstriction produced by limb dependency in the cutaneous vasculature displays no age- or limb-related differences. Lastly, limb vasoconstrictor responses to limb dependency do not appear to be sympathetically mediated in humans.
Vasoconstrictor responses to limb dependency are greater in the popliteal artery (leg) than in the brachial artery (arm) of young adults and responses in the popliteal artery are decreased with age. The mechanism(s) underlying these effects are unknown. One possibility is that a sympathetic mechanism mediated these effects (Henriksen et al., 1983, Henriksen and Sejrsen, 1977). Consistent with this possibility: 1) α-adrenergic receptor sensitivity is increased in the leg relative to the arm of young adults (Pawelczyk and Levine, 2002) 2) leg α-adrenergic receptor sensitivity is decreased with age (Smith et al., 2007) and 3) enhanced sympathetic tone is present in the leg (Dinenno et al., 2001), but not the arm with age (Dinenno et al., 2002). However, our finding that vasoconstrictor response to limb dependency is unaltered by systemic sympathetic blockade does not support the view that a sympathetic mechanism underlies the observed limb- or age-related effects. A second possibility is that structural differences between the limbs, or associated with aging, contributes to these differences. For instance it is possible that arterial stiffening with age (Hess et al., 2009) or reductions in basal blood flow in the leg (Dinenno et al., 1999) reduces the ability of these arteries to vasoconstrict (i.e., impair vasoconstrictor reserve) (Fu et al., 2004). Additionally, increased arterial diameters at rest could affect vasoconstrictor responsiveness. However, the fact that increases in brachial and popliteal artery diameter with age in men is similar (Green et al., 2010) makes it unlikely that arterial enlargement selectively effects vasoconstrictor responses to limb dependency in the leg, but not the arm.
It is possible that substances, other than norepinephrine, released by sympathetic nerve terminals contributed to the observed age- and limb-related differences in the vasoconstrictor response to limb dependency. For instance it is possible that blunted leg (popliteal artery) vasoconstrictor responses to limb dependency occur as a result of impaired vasoconstrictor responses to sympathetic nerve terminal release of co-transmitters, such as neuropeptide Y or adenosine triphosphate. Based on the present study design we cannot exclude this possibility. However, as both bretylium tosylate (Crandall et al., 2002) and neural blockade (Vissing et al., 1997) do not decrease the vasoconstrictor response to increased venous pressure, despite the fact that these methods should block release of all sympathetic nerve terminal transmitters/co-transmitters, suggests that such effects are unlikely to explain our findings.
Skin vasoconstrictor responses to limb dependency demonstrated no age- or limb-related differences. Consistent with our data a previous study was unable to observe age-related differences in leg cutaneous vasoconstrictor response to limb dependency (Moy et al., 1989). Had a sympathetic mechanism been found to contribute importantly to responses during limb dependency, these responses likely would have been reduced, as aging is associated with decreased cutaneous α-adrenergic responsiveness (Lang et al., 2010, Wilson et al., 2004). Moreover, sympathetic blockade (protocol 2) should have blunted limb cutaneous vasoconstrictor responses to limb dependency in both young and older adults, and it did not. These findings are consistent with the observation that locally applied α-adrenergic antagonists had no effect on cutaneous vasoconstrictor responses to forearm dependency (Crandall et al., 2002). Combined, these results suggest sympathetic mechanisms are not involved in cutaneous vasoconstrictor responses to limb dependency. One reason why early studies may have observed an apparent sympathetic component to the cutaneous vasoconstrictor response to limb dependency may relate to the fact that the locally administered drug concentrations (Henriksen et al., 1983, Henriksen and Sejrsen, 1977, Henriksen, 1976, Henriksen, 1977) were very high, resulting in effects mediated by receptor types beyond that of the α-adrenergic receptors (Johnson, 2002).
It is possible that blood volume translocated from the thorax to the limb, during limb dependency resulting in baroreflex-mediated increases in sympathetic outflow and thus vascular resistance. However, several lines of evidence argue against this possibility. First, we observed no tachycardia when the arm or leg was lowered into the dependent position. Second, we observed no increase in thoracic impedance during any of the limb dependency trials (i.e., during arm or leg dependency before or during sympathetic blockade) in young or older adults. Thus, it is unlikely that the increases in vascular resistance index we observed during the limb dependency trials were mediated by the baroreflexes.
There are a few limitations that deserve comment. First, limb blood flow per se was not measured, but rather limb blood velocity was. As previous studies indicate that arterial diameters in vessels of similar size/composition measured in the present study do not change under numerous physiological conditions/stressors (Lott et al., 2002, Shoemaker et al., 1996) the changes in mean blood velocity we measured should reflect changes in blood flow. Second, vascular responses measured from the brachial and popliteal artery likely involve contributions from tissues beyond skeletal muscle (e.g., skin, subcutaneous, and bone). However, we believe that under the thermoneutral conditions our non-obese subjects were tested under that a large percentage of the measured change refglects responses from skeletal muscle. Third, systemic blockade may not affect the skin to the same extent as other tissues because of lower basal blood flows. The fact that we locally heated the skin at the measurement site should reduce some of this concern. Fourth, it is not possible to directly quantify the stimulus for vasoconstriction other than to verify that all limbs were lowered the same distance from heart level in all subjects. Thus, any observed effects, such as age- or limb-related differences in the vasoconstrictor to limb dependency likely did not result from a difference in input. Measures of intravenous pressure obtained near the sites of vasoconstriction during limb dependency would help to confirm this. Lastly, measuring the vasoconstrictor responses to bolus norepinephrine infusion may have provided better insight into the degree of sympathetic blockade achieved as opposed to measuring the systemic pressor response, which was shown to be largely but not entirely blocked.
In this study we have identified: 1) an augmented vasoconstrictor response to limb dependency in the popliteal artery compared to the brachial artery in young adults, 2) an impaired vasoconstrictor response to limb dependency in the popliteal artery of older compared to young adults, 3) that cutaneous vasoconstrictor responses to limb dependency do not exhibit age- or limb-related differences, and 4) that age- and limb-related differences in the vasoconstrictor response to limb dependency do not appear to be sympathetically mediated. Collectively, these findings suggest that age-, limb-, and possibly tissue-related differences in the vasoconstrictor response to limb dependency exist in healthy humans that are not sympathetically mediated. The functional role of this attenuated popliteal artery vasoconstrictor response to dependency in regards to both BP regulation and orthostatic tolerance in the upright position remains to be determined.
Acknowledgements
Grants from the National Institutes of Health (HL92309, AG24420, M01 RR10732, and C06 RR016499) supported this research.
Footnotes
Conflict of Interest None
References
- Andersen EB, Boesen F, Henriksen O, Sonne M. Blood flow in skeletal muscle of tetraplegic man during postural changes. Clin Sci (Lond) 1986;70:321–5. doi: 10.1042/cs0700321. [DOI] [PubMed] [Google Scholar]
- Christ F, Gamble J, Baschnegger H, Gartside IB. Relationship between venous pressure and tissue volume during venous congestion plethysmography in man. J Physiol. 1997;503(Pt 2):463–7. doi: 10.1111/j.1469-7793.1997.463bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG, Shibasaki M, Yen TC. Evidence that the human cutaneous venoarteriolar response is not mediated by adrenergic mechanisms. J Physiol. 2002;538:599–605. doi: 10.1113/jphysiol.2001.013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison JL, Short DS, Wilson TE. Effect of local heating and vasodilation on the cutaneous venoarteriolar response. Clin Auton Res. 2004;14:385–90. doi: 10.1007/s10286-004-0223-x. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–54. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol. 2001;536:977–83. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert TJ, Smith JJ, Barney JA, Merrill DC, Smith GK. The use of thoracic impedance for determining thoracic blood volume changes in man. Aviat Space Environ Med. 1986;57:49–53. [PubMed] [Google Scholar]
- Esler MD, Julius S, Randall OS, Ellis CN, Kashima T. Relation of renin status to neurogenic vascular resistance in borderline hypertension. Am J Cardiol. 1975;36:708–15. doi: 10.1016/0002-9149(75)90173-3. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004;110:2931–7. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- Gaskell P, Burton AC. Local postural vasomotor reflexes arising from the limb veins. Circ Res. 1955;1:27–39. doi: 10.1161/01.res.1.1.27. [DOI] [PubMed] [Google Scholar]
- Green DJ, Swart A, Exterkate A, Naylor LH, Black MA, Cable NT, Thijssen DH. Impact of age, sex and exercise on brachial and popliteal artery remodelling in humans. Atherosclerosis. 2010;210:525–30. doi: 10.1016/j.atherosclerosis.2010.01.048. [DOI] [PubMed] [Google Scholar]
- Groothuis JT, Thijssen DH, Lenders JW, Deinum J, Hopman MT. Leg vasoconstriction during head-up tilt in patients with autonomic failure is not abolished. J Appl Physiol. 2011;110:416–22. doi: 10.1152/japplphysiol.01098.2010. [DOI] [PubMed] [Google Scholar]
- Henriksen O. Local reflex in microcirculation in human subcutaneous tissue. Acta Physiol Scand. 1976;97:447–56. doi: 10.1111/j.1748-1716.1976.tb10284.x. [DOI] [PubMed] [Google Scholar]
- Henriksen O. Local sympathetic reflex mechanism in regulation of blood flow in human subcutaneous adipose tissue. Acta Physiol Scand Suppl. 1977;450:1–48. [PubMed] [Google Scholar]
- Henriksen O. Sympathetic reflex control of blood flow in human peripheral tissues. Acta Physiol Scand Suppl. 1991;603:33–9. [PubMed] [Google Scholar]
- Henriksen O, Nielsen SL, Paaske WP. Autoregulation of blood flow in human adipose tissue. Acta Physiol Scand. 1973a;89:531–7. doi: 10.1111/j.1748-1716.1973.tb05546.x. [DOI] [PubMed] [Google Scholar]
- Henriksen O, Nielsen SL, Paaske WP, Sejrsen P. Autoregulation of blood flow in human cutaneous tissue. Acta Physiol Scand. 1973b;89:538–43. doi: 10.1111/j.1748-1716.1973.tb05547.x. [DOI] [PubMed] [Google Scholar]
- Henriksen O, Sejrsen P. Local reflex in microcirculation in human skeletal muscle. Acta Physiol Scand. 1977;99:19–26. doi: 10.1111/j.1748-1716.1977.tb10347.x. [DOI] [PubMed] [Google Scholar]
- Henriksen O, Skagen K, Haxholdt O, Dyrberg V. Contribution of local blood flow regulation mechanisms to the maintenance of arterial pressure in upright position during epidural blockade. Acta Physiol Scand. 1983;118:271–80. doi: 10.1111/j.1748-1716.1983.tb07271.x. [DOI] [PubMed] [Google Scholar]
- Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol. 2009;107:1076–1082. doi: 10.1152/japplphysiol.00605.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM. How do veins talk to arteries? J Physiol. 2002;538:341. doi: 10.1113/jphysiol.2001.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman M, de Hoog M, Rongen GA, van Kuppevelt HJ, Smits P, Hopman MT. Local vasoconstriction in spinal cord-injured and able-bodied individuals. J Appl Physiol. 2007;103:1070–7. doi: 10.1152/japplphysiol.00053.2007. [DOI] [PubMed] [Google Scholar]
- Kooijman M, Rongen GA, Smits P, van Kuppevelt HJ, Hopman MT. The role of the alpha-adrenergic receptor in the leg vasoconstrictor response to orthostatic stress. Acta Physiol (Oxf) 2009;195:357–66. doi: 10.1111/j.1748-1716.2008.01904.x. [DOI] [PubMed] [Google Scholar]
- Lang JA, Holowatz LA, Kenney WL. Tetrahydrobiopterin does not affect end-organ responsiveness to norepinephrine-mediated vasoconstriction in aged skin. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1651–5. doi: 10.1152/ajpregu.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott ME, Herr MD, Sinoway LI. Effects of transmural pressure on brachial artery mean blood velocity dynamics in humans. J Appl Physiol. 2002;93:2137–46. doi: 10.1152/japplphysiol.00443.2002. [DOI] [PubMed] [Google Scholar]
- Lott ME, Herr MD, Sinoway LI. Effects of age on brachial artery myogenic responses in humans. Am J Physiol Regul Integr Comp Physiol. 2004;287:R586–91. doi: 10.1152/ajpregu.00612.2003. [DOI] [PubMed] [Google Scholar]
- Low PA. Venoarteriolar reflex. In: ROBERTSON D, editor. Primer on the autonomic nervous system. Elsevier Academic Press; San Diego, CA: 2004. [Google Scholar]
- Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R3–R12. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- Moy S, Opfer-Gehrking TL, Proper CJ, Low PA. The venoarteriolar reflex in diabetic and other neuropathies. Neurology. 1989;39:1490–2. doi: 10.1212/wnl.39.11.1490. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Fu Q, Martini ER, Shook R, Conner C, Zhang R, Crandall CG, Levine BD. Vasoconstriction during venous congestion: effects of venoarteriolar response, myogenic reflexes, and hemodynamics of changing perfusion pressure. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1354–9. doi: 10.1152/ajpregu.00804.2004. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol. 2002;92:2105–13. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. Oxford University Press; New York: 1993. [Google Scholar]
- Shoemaker JK, Pozeg ZI, Hughson RL. Forearm blood flow by Doppler ultrasound during test and exercise: tests of day-to-day repeatability. Med Sci Sports Exerc. 1996;28:1144–9. doi: 10.1097/00005768-199609000-00010. [DOI] [PubMed] [Google Scholar]
- Sielatycki JA, Shamimi-Noori S, Pfeiffer MP, Monahan KD. Adrenergic mechanisms do not contribute to age-related decreases in calf venous compliance. J Appl Physiol. 2011;110:29–34. doi: 10.1152/japplphysiol.00930.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagen K, Jensen K, Henriksen O, Knudsen L. Sympathetic reflex control of subcutaneous blood flow in tetraplegic man during postural changes. Clin Sci (Lond) 1982;62:605–9. doi: 10.1042/cs0620605. [DOI] [PubMed] [Google Scholar]
- Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen D, Vanlandewijck Y, Sturbois X, Francaux M. Blood distribution adaptations in paraplegics during posture changes: peripheral and central reflex responses. Eur J Appl Physiol. 2000;81:463–9. doi: 10.1007/s004210050069. [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, Harms MP, Pott F, Jenstrup M, Secher NH. Stroke volume of the heart and thoracic fluid content during head-up and head-down tilt in humans. Acta Anaesthesiol Scand. 2005;49:1287–92. doi: 10.1111/j.1399-6576.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- Vissing SF, Secher NH, Victor RG. Mechanisms of cutaneous vasoconstriction during upright posture. Acta Physiol Scand. 1997;159:131–8. doi: 10.1046/j.1365-201X.1997.573344000.x. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Monahan KD, Short DS, Ray CA. Effect of age on cutaneous vasoconstrictor responses to norepinephrine in humans. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1230–4. doi: 10.1152/ajpregu.00467.2004. [DOI] [PubMed] [Google Scholar]
- Zoltie N, Young C, Faris I, Tan E. The veno-arteriolar reflex in free skin flaps. Clin Physiol. 1989;9:183–8. doi: 10.1111/j.1475-097x.1989.tb00969.x. [DOI] [PubMed] [Google Scholar]