Abstract
Background
We investigated the etiological nature of comorbid alcohol, tobacco, and cannabis DSM-IV dependence symptoms in late adolescence and young adulthood while accounting for gender differences in the magnitude of genetic and environmental influences.
Methods
Univariate and multivariate twin modeling was used to determine the heritability of each substance and the etiology of multiple drug problems in a sample of 2484 registrants of the Center for Antisocial Drug Dependence who provided data at the second wave of an ongoing longitudinal study. We report on mean and prevalence levels of whole-life DSM-IV dependence symptoms that were assessed with the Composite International Diagnostic Interview-Substance Abuse Module. Biometrical analyses were limited to age-adjusted DSM-IV dependence symptom counts from a subset of twins that reported using alcohol, tobacco, or cannabis in their lifetime.
Results
Male and female alcohol, tobacco, and cannabis DSM-IV symptoms are indicators of a heritable unidimensional latent continuous trait. Additive genetic factors explain more than 60% of the common liability to drug dependence. A larger proportion of the variation in each substance is attributable to substance-specific genetic and environmental factors.
Conclusions
These data suggest that both common and substance-specific genetic and environmental factors contribute to individual differences in the levels of DSM-IV alcohol, tobacco, and cannabis dependence symptoms.
Keywords: Alcohol, Tobacco, Cannabis, Young Adulthood, Dependence, Heritability
1. INTRODUCTION
Substance use and disorders are highly comorbid phenomena. High rates of comorbid substance use and disorders occur more often than expected by chance as evidenced in large national and international epidemiological surveys (Degenhardt et al., 2001; Johnston et al., 2008; Substance Abuse and Mental Health Services Administration (SAMHSA), 2008). For instance, of the 22.3 million persons aged 12 or older with a past year SUD in the 2007 National Survey on Drug Use and Health survey, 3.2 million were dependent on alcohol and an illicit substance. In a recent study using biological markers of substance use, there were strong correlations between tobacco dependence, cannabis use, and alcohol abuse and dependence (Kapusta et al., 2007). This high rate of comorbidity observed among substances suggests a parsimonious mechanism (Vanyukov et al., 2003) of risk for substance use disorders.
Twin studies demonstrate the importance of genetic factors to the liability of alcohol, tobacco, and cannabis dependence and have been used to explore comorbidity among substances (Agrawal and Lynskey, 2008), however, few studies have examined the nature of the liability to endorse Diagnostic and Statistical Manual of Mental Disorders (DSM) dependence symptoms across multiple licit and illicit substances. Bivariate twin studies have suggested largely overlapping genetic influences. True and colleagues (1999) reported a correlation of 0.68 between genetic factors for nicotine and alcohol dependence in a sample of 3300 male twins of the Vietnam Era Twin Registry. Similarly, Sartor and colleagues (2010) identified a genetic correlation of 0.61 using DSM-IV dependence symptoms of alcohol and cannabis in a sample of 6257 individuals from the Australian Twin Registry. Multivariate twin studies have also provided evidence for common and specific factors for substance use disorders. In an examination of several comorbidity models, Rhee and colleagues (2006) compared alternative models of comorbid drug problems and identified a model that suggested that the comorbidity between alcohol and illicit drug problems was best interpreted as alternative manifestations of a common underlying trait. Young and colleagues (2006) used a large subset of community adolescent twins to identify a common genetic liability among alcohol, tobacco, and cannabis problem use (i.e., 1+ symptoms of abuse/dependence). Kendler and colleagues (2007) examined the structure of genetic and environmental risk factors for symptoms of dependence on five licit and illicit psychoactive substances in 4866 adult male and female twins and identified a complex architecture made up of highly intercorrelated genetic factors that differentially loaded unto licit and illicit substances. Using a design very similar to the present study, Xian et al. (2008) computed the contribution of common and specific genetic and environmental factors to nicotine, alcohol, and cannabis dependence in male veterans and concluded that the comorbidity between the three substances was attributable to common and specific genetic factors, as well as unique environmental effects. Overall, despite the knowledge we have gained thus far, additional studies are still necessary to determine whether individual differences in the level of dependence symptoms across multiple substances is the result of same or different genes, especially during young adulthood, when the highest rates of comorbid drug use and dependence are observed.
The twin design is particularly useful for partitioning the liability across multiple substance related outcomes into their genetic and environmental components. The benefit of the extension of the univariate twin model to the multivariate case is that it provides a test of different hypotheses regarding the nature of comorbidity. As seen in the aforementioned studies, researchers may test the hypothesis that there is a single underlying process as opposed to multiple correlated processes. Given the similarity in the neurological response to addictive substances (Goldman and Bergen, 1998; Volkow et al., 2002) and the generalized effect of pharmacological treatments for addiction (Basavarajappa, 2007, Rösner et al., 2010), individual differences in lifetime reports of drug dependence symptoms for multiple drugs could be attributable to common biological (i.e., genetic) factors. In fact, several studies (Vanyukov et al., 2003) have calculated the genetic correlation across dependence on multiple substances and have found them to be in excess of 0.5. However, despite the phenotypic indications of a latent risk, we are unaware of any twin studies that have explicitly tested a model indicating a generalized risk for comorbid DSM-IV drug dependence symptoms during young adulthood (i.e., the period during which the highlest levels of drug use are seen in large epidemiological surveys (SAMHSA, 2008)). Given the common neurological and environmental mechanisms that influence susceptibility to repeated drug exposure, we hypothesized that lifetime assessments of DSM-IV drug dependence symptoms during young adulthood would indicate a heritable common mechanism.
An important consideration in twin and family studies of addiction is the treatment of scores from participants that have never been exposed to a drug, because their scores may bias genetic and environmental estimates. This is because the assessment of DSM-IV (American Psychiatric Association (APA; 1994) symptoms of dependence via structured interviews usually requires a minimum level of exposure to a drug (e.g., using marijuana at least six times). Thus, how we score participants who have not met these criteria can affect estimates of genetic and environmental influences on these behaviors. Scoring individuals (e.g., abstainers) as having zero symptoms makes it difficult to characterize the etiology of dependence symptoms amongst drug users, because the population would also include non-users. Many factors, such as drug availability which determine whether an individual is able to use a substance, may be largely environmental and twin studies report higher estimates of environmental influences for measures related to substance initiation and use compared to measures of drug problems (Hopfer et al., 2003). One approach to overcoming this problem is the implementation of conditional/multi-stage models that conditionally include information of individuals that have not met threshold criteria for each level of involvement via structured missingness (i.e., individuals are included in the analysis as having missing values if they do not meet a certain threshold at each stage of the analysis; for example, only repeat users can report having substance-related problems) (Neale et al., 2006). This conditional approach allows the estimation of influences specific to each level of drug involvement. Several studies have assessed whether the etiology of access to a substance is correlated with those for persistence or dependence (Heath et al., 2002, Heiman et al., 2008; Kendler et al., 1999; Maes et al., 2004)) and have found that there is evidence for common or partially shared genetic and environmental factors for substance initiation/use and problems. For instance, Eaves and Eysenck (1980) suggested a single dimension of risk for initiation and persistent tobacco use. Similarly, Maes and colleagues (2004) found a significant overlap in the liabilities of tobacco initiation, use, and dependence; Gillespie and colleagues (2009) found a strong relationship between the liabilities to use and abuse of cannabis. The current study examined variability in DSM-IV dependence symptoms amongst substance users; non-users were included in our analyses by assigning them a missing value.
In the present study, we utilized a community sample of approximately 1200 twin pairs to examine the etiological nature of comorbid alcohol, tobacco, and cannabis dependence symptoms in late adolescent and young adult males and females; however, unlike its predecessors this study compares two multivariate genetic models that make different assumptions regarding how the risks for these different substances are related.
2. METHODS
2.1 Sample Description
Participants were drawn from a large sample of 2484 (1148 males, 1336 females) respondents between the ages of 17 and 29 who participated in the second wave of a longitudinal study at the NIDA funded Center on Antisocial Drug Dependence at the University of Colorado. Twins were drawn from the Colorado Community Twin Study and Longitudinal Twin Study at the University of Colorado; Rhea and colleagues (2006) provide details regarding the recruitment of each sample. The sample was predominantly Caucasian (87%) and had an equivalent number of male and female participants at each age. The average age of participants in the study was 20 years (standard deviation (SD) = 2.59). There were a total of 681 monozygotic (MZ) female twins (which included 23 incomplete pairs), 415 dizygotic (DZ) females (which included 21 incomplete pairs), 542 MZ males (which included 16 incomplete pairs), 375 DZ males (which included 27 incomplete pairs) and 471 twins from opposite sex (OS) pairs (which included 33 incomplete pairs). Zygosity was determined using a nine-item questionnaire and 11 highly informative DNA markers. Twin number was randomly assigned to each individual within a family unit.
2.2 Assessments
Data on lifetime DSM-IV (American Psychiatric Association, 1994) dependence symptoms were collected using a computerized version of the Composite International Diagnostic Interview – Substance Abuse Module (CIDI-SAM). The CIDI-SAM is regarded as both a reliable and useful diagnostic tool in adolescent and adult samples (Cottler et al., 1989; Crowley et al., 2001; Ustun et al., 1997). Respondents were asked to report whether they experienced one of the following seven DSM-IV dependence symptoms in their lifetime: ‘tolerance’, ‘withdrawal’, ‘substance taken in larger amount and for longer period than intended’, ‘persistent desire or repeated unsuccessful attempt to quit’, ‘much time/activity to obtain, use, recover’, ‘important social, occupational, or recreational activities given up or reduced’, and ‘continued use despite knowledge of adverse consequences’. Participants were assessed for symptoms of alcohol dependence if they reported having consumed alcohol one or more times in their lifetime. Tobacco dependence criteria were assessed in cases where a respondent reported having smoked 20 cigarettes, having smoked a cigar or pipe more than five times, or having used snuff or chewing tobacco more than five times in their lifetime. Cannabis dependence symptoms were assessed if participants reported using marijuana, grass, pot, or hashish six or more times in their lifetime. The dependent variable of interest for this study was the total number of DSM-IV dependence symptoms endorsed by each respondent who reported that they had used alcohol, tobacco, or cannabis in their lifetime. Abstainers were included in the analyses as having missing values. An earlier report using the CIDI-SAM in the CADD has shown that is reasonably reliable (Young et al., 2002).
2.3 Statistical Analyses
Descriptive statistics were obtained using SAS® (2002) and R© (2009). Differences in mean symptom levels between males and females were determined using weighted t-tests and Chi-square test in which the weights were based on the size of the family such that families with a set of twins was weighted 0.5 and families with an incomplete pair of twins was weighted as 1.0. Prior to biometrical modeling, symptom counts were regressed on age, age-squared, and age-cubed within each gender and the residuals scores were standardized within each gender. The logarithm of the scores (log(x+2)) was taken to better approximate a normal distribution. The newly created log-transformed age-adjusted scores were highly correlated (at least 0.97) with the standardized age corrected scores.
Twin studies examine the etiology of a trait by comparing MZ twins who share 100% of their alleles identical by descent and DZ twins who share, on average, 50% of their alleles identical by descent. Using this information, correlations between MZ and DZ twins on a measured trait are compared to estimate (1) heritability/genetic influences on the trait (a2 or A), i.e., effects due to cumulative effects of genetic variants that are shared by family members to differing extents, (2) shared environmental influences (c2 or C), i.e., effects due to environments that family members share in common, and (3) non-shared environmental/residual error influences (e2 or E), i.e., effects due to environments uniquely experienced by family members. The classical twin study is limited in its ability to estimate non-additive genetic (d2 or D) and shared environmental effects simultaneously because both parameters rely on the difference between MZ and DZ twin correlations, making the model unidentified. Given the absence of sibling or adoption data, separate models including shared environmental and non-additive effects were compared using the Akaike Information Criterion (AIC); however, given the lack of evidence for non-additive genetic effects in the literature (Agrawal and Lynskey, 2008) and in our model comparisons, we only report on the results from our ACE models. Further information regarding ADE models are available upon request from the corresponding author.
Extensions of the classical twin model are useful for decomposing the variance and covariance between observed traits into their genetic and environmental components. Typical models used to resolve the covariance of multiple phenotypes are the Independent Pathway model and the Common Pathway model (Neale and Cardon, 1992).
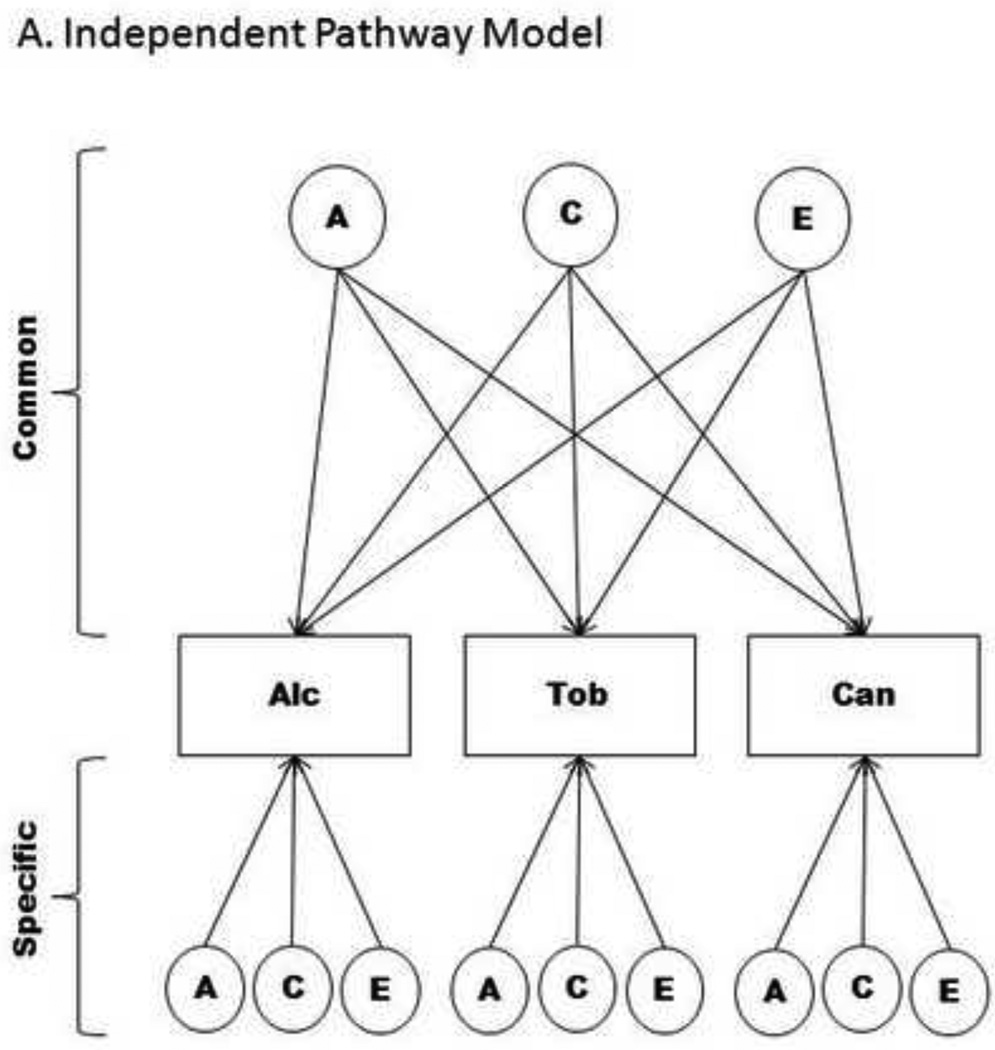
Independent Pathway model
The independent pathway model shown in Figure 1a assumes that single genetic, shared environment, and non-shared environment factors explain the comorbidity among dependence on the three substances. Additionally, the model decomposes the specific variance of each substance into its genetic and environmental sources.
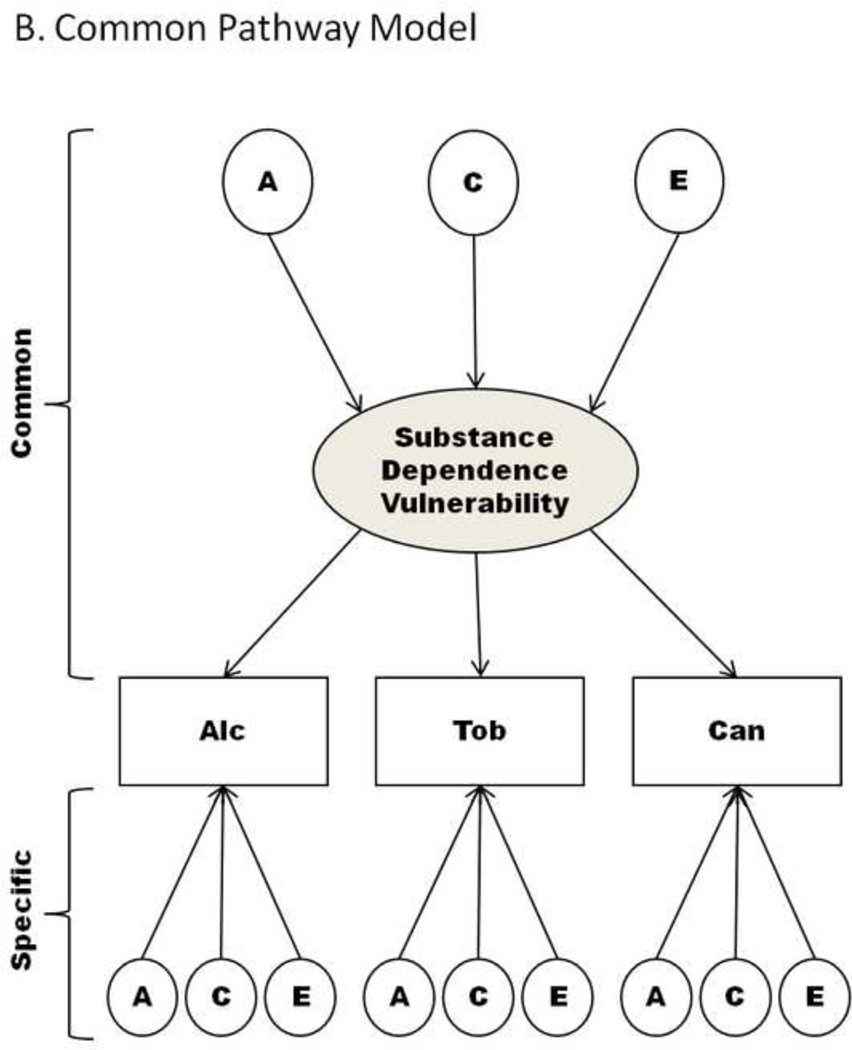
Figure 1. Multivariate biometrical models; Only Twin 1 depicted.
Note: Path diagrams of multivariate behavioral genetic models used to describe multivariate or longitudinal data. Figure 1a describes the Independent Pathway Model that consists of a common genetic and environmental factor that describes the covariance between substances, while the specific factors capture genetic and environmental influences that are unique to each substance. The Common Pathway Model, depicted in Figure 1b, is a restricted form of the Independent Pathway Model created by positing that the covariation between substances arises from a latent factor (referred to as Substance Dependence Vulnerability). Abbreviations: A – additive genetic effects; C – shared environmental effects; Common – identifies the portion of the model which estimates factor loadings common to all phenotypes; E – non-shared environmental effects; Specific – describes the portion of the model that identifies genetic or environmental effects that are unique to a particular phenotype, beyond that which may already be shared; Alc – Alcohol; Tob – Tobacco; Can - Cannabis.
Common Pathway model
The common pathway model, shown in Figure 1b, is a submodel of the independent pathway model that posits that the covariance among dependence on different substances is due to a single latent trait. The variation in the latent trait is partitioned into its genetic and environmental sources. Like the independent pathway model, the variance specific to each substance is partitioned into genetic and environmental factors.
Both multivariate genetic models have the potential to identify shared genetic and environmental mechanisms across alcohol, tobacco and cannabis; however, the Common Pathway model represents a testable hypothesis that there is a single underlying process. Genetic and environmental effects on the latent factor highlight differences in the mechanism that bring about liability to developing dependence symptoms on an array of substances.
Three different forms of sex-limited univariate and multivariate models were fitted to the data to account for the possibility of gender differences in the magnitude of genetic and environmental influences that have been seen in reference to smoking behaviors (Li et al., 2003), and inconsistently observed in large studies of alcohol dependence (Hardie et al., 2008; Heath et al., 1997; Hicks et al., 2007). The general sex-limitation model (Model I) posits that additive genetic effects may be common or specific to each sex. This is achieved by freely estimating the genetic correlation between opposite-sex twins instead of fixing it at 50%. Additionally, the model does not constrain the phenotypic variance or the magnitude of genetic and environmental effects across sexes. Alternatively, we also tested a restricted sub model of the general sex-limitation model, otherwise known as the “Common Effects Sex-Limitation Model” (Neale et al., 2003), that assumes that there are no sex-specific additive genetic factors but that the relative contribution of genetic and environmental factors differs between men and women (Model II). Finally, we tested a model that posits that there are no sex-specific additive genetic factors, and that the magnitude of genetic and environmental effects are the same across sexes (Model III; otherwise known as a “Scalar Effects Sex-Limitation Model” (Neale et al., 2003). All three models were fitted to the data to test for both qualitative and quantitative gender differences. After establishing the best-fitting sex-limitation model, we then proceeded to test specific parameters.
Twin models were fitted using Maximum likelihood estimation procedures operationalized in Mx (Neale, 1999). In addition to parameter estimates and 95% confidence intervals (CI), the program provides Chi-square (χ2) goodness-of-fit statistics that are used to determine how well different models fit the data. Nested models were compared using a Chi-square difference test (i.e., the difference in minus twice the log-likelihood (−2LL) estimates for two nested models is distributed as a Chi-square with degrees of freedom (df) equal to the difference in degrees of freedom between the two models). Nested and non-nested models were also compared using the AIC (Akaike, 1987); lower AIC values indicate a better or more parsimonious model fit. Statistical significance of individual path coefficients were determined by constraining the path to zero and conducting a Chi-square difference test and an examination of the change in the AIC statistic. However, given the limitations of the classical twin design (Keller and Coventry, 2005), we focus primarily on the parameters and parameter space indicated in the best-fitting sex-limitation models, thus providing the best interpretation of our findings.
3. RESULTS
3.1 Descriptive statistics
Table 1 shows the distribution of symptoms for males and females using raw scores and the prevalence of one or more symptoms of dependence (i.e., a dichotomous measure of problem use). Of the 2484 individuals, roughly 80% (938 males, 1029 females) reported use of alcohol, 38% (551 males, 399 females) endorsed use of tobacco, and 34% (457 males, 380 females) reported use of cannabis. For both the continuous and dichotomous measures, males reported experiencing more alcohol dependence symptoms, while females reported experiencing more tobacco dependence symptoms, and there was no gender difference in the level of cannabis dependence symptoms.
Table 1.
Level of lifetime alcohol, tobacco, and cannabis DSM-IV dependence symptoms by gender
| Mean (std) | T-test | Prevalence of Problem Use | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenotype | N | Mean (std) |
Median | Range | t-statistic | p-value | % | χ2 | p- value |
| Alcohol (males) | 938 | 1.32 (1.61) | 1.00 | 7.00 | 0.56 | ||||
| Alcohol (females) | 1029 | 1.02 (1.44) | 0.00 | 7.00 | −4.34 | <0.01 | 0.46 | 11.74 | <0.01 |
| Tobacco (males) | 551 | 2.22 (1.47) | 2.00 | 7.00 | 0.71 | ||||
| Tobacco (females) | 399 | 2.71 (1.44) | 3.00 | 7.00 | 3.74 | <0.01 | 0.82 | 9.51 | <0.01 |
| Cannabis (males) | 457 | 1.14 (1.10) | 1.00 | 7.00 | 0.52 | ||||
| Cannabis (females) | 380 | 1.05 (1.07) | 0.00 | 7.00 | −0.82 | 0.39 | 0.49 | 0.17 | 0.44 |
Note: Table shows the weighted mean-level of symptoms by gender. Weighted t-tests statistics are reported for the Satterthwaite method for the continuous scores. Weighted Chi-square statistics are reported for the dichotomous measure.
Table 2 presents the Pearson correlations between alcohol, tobacco, and cannabis DSM-IV dependence symptom counts. There was a significant association between substances in males and females; correlation values ranged from 0.28 – 0.38. The highest correlation in males was between alcohol and tobacco (0.33) while the highest correlation in females was between alcohol and cannabis (0.38). Overall, the pattern of correlations was similar across males and females.
Table 2.
Phenotypic correlation among substances
| Alcohol | Tobacco | |
|---|---|---|
| Males | ||
| Tobacco | 0.33 (547) a | 1 |
| Cannabis | 0.29 (455) a | 0.29 (371) a |
| Females | ||
| Tobacco | 0.28 (394) a | 1 |
| Cannabis | 0.38 (379) a | 0.33 (271) a |
Note: Table presents the weighted phenotypic correlations between log-transformed DSM-IV dependence symptom counts for each gender. Values presented in the parentheses are the weighted number of individuals in the analysis.
- P<0.001
3.2 Twin correlations
Table 3 presents the pattern of twin correlations within and between substances. In several instances, the MZ twin correlations exceeded DZ twin correlations, which suggested moderate additive genetic influences on variation in dependence symptoms in young adulthood. Notably, in some cases, the MZ twin correlation was more than twice the DZ twin correlation, which suggested the role of non-additive genetic factors; however, formal tests of these parameters yielded results that were consistent with the prior literature (i.e., limited non-additive genetic effects). Shared environmental effects were also evident in instances where the DZ twin correlation was more than one half the MZ twin correlation, such as the cross substance correlation between alcohol and cannabis in females (rMZ=0.29, rDZ=0.22).
Table 3.
Twin correlations and 95% confidence interval for alcohol, tobacco, and cannabis dependence symptom counts
| Alcohol | Tobacco | Cannabis | Alcohol-Tobacco | Alcohol-Cannabis | Tobacco-Cannabis | |
|---|---|---|---|---|---|---|
| MZ males | 0.38 (0.26,0.49) | 0.59 (0.45,0.69) | 0.19 (−0.07,0.40) | 0.23 (0.05,0.39) | 0.05 (−0.16,0.24) | 0.06 (−0.17,0.28) |
| DZ males | 0.15 (−0.03,0.31) | 0.21 (−0.05,0.42) | 0.03 (−022,0.26) | 0.09 (−0.14,0.30) | 0.04 (−0.21,0.28) | 0.09 (−0.20,0.36) |
| MZ females | 0.37 (0.27,0.47) | 0.67 (0.51,0.76) | 0.50 (0.31,0.63) | 0.29 (0.09,0.47) | 0.29 (0.08,0.47) | 0.41 (0.16,0.61) |
| DZ females | 0.40 (0.25,0.53) | 0.24 (−0.14,0.53) | 0.21 (−0.17,0.50) | 0.34 (0.07,0.56) | 0.22 (−0.05,0.46) | 0.12 (−0.28,0.49) |
| OS | 0.07 (−0.07,0.20) | −0.04 (−0.29,0.22) | 0.22 (−0.02,0.42) | 0.03 (−0.21,0.26) | −0.01 (−0.23,0.22) | <−0.01 (−0.28,0.27) |
Note: Table presents the univariate twin correlations derived using log-transformed dependence symptom counts in five zygosity groupings.
Abbreviations: DZ - dizygotic twins; MZ monozygotic twins; OS - opposite sex dizygotic twins
3.3 Univariate twin models
Table 4 presents the model fit statistics for the series of sex-limited univariate models of alcohol, tobacco, and cannabis dependence along with the parameter estimates of the best fitting of the three sex-limitation models. The full/base model (i.e., Model I) for each substance included A, C, and E influences that were estimated separately for each sex. Model I was compared to two reduced forms (i.e. Models II and III) by employing a Chi-Square difference test to determine the best fitting sex-limitation model. In all instances, model comparisons suggested no sex-specific additive genetic effects. Furthermore, for each substance, the Common Effects Sex-Limitation Model (Model II) provided a better fit to the data (based on the AIC statistic) compared to Model III, suggesting that the magnitude of genetic and environmental influences differed between males and females. Based on Model II, the heritability of alcohol, tobacco, and cannabis dependence symptom counts were 37%, 40% and 18% in males, and 0%, 43%, and 49% in females, respectively.
Table 4.
Fit indices and parameter estimates for the ACE univariate sex-limitation models
| Model Fit | Comparison to Model I | Standardized variance components from the best- fitting sex-limitation model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Models | −2LL | df | AIC | Δχ2 | Δdf | P | ΔAIC | Parameter | Males | Females |
| Alcohol | ||||||||||
| Model I | 2164.04 | 1958 | −1751.96 | - | - | - | - | A | 0.37 (0.16,0.48) | 0.00 (0.00,0.28) |
| Model II | 2164.04 | 1959 | −1753.96 | 0.00 | 1 | 1.00 | −2.00 | C | 0.01 (0.00,0.17) | 0.38 (0.12,0.46) |
| Model III | 2190.39 | 1962 | −1733.61 | 26.35 | 4 | <0.001 | 18.35 | E | 0.63 (0.52,0.74) | 0.62 (0.54,0.71) |
| Tobacco | ||||||||||
| Model I | 1492.66 | 941 | −389.34 | - | - | - | - | A | 0.40 (0.06,0.62) | 0.43 (0.01,0.70) |
| Model II | 1495.47 | 942 | −388.53 | 2.81 | 1 | 0.09 | 0.81 | C | 0.14 (0.00,0.42) | 0.21 (0.00,0.55) |
| Model III | 1509.26 | 945 | −380.74 | 16.60 | 4 | <0.01 | 8.60 | E | 0.46 (0.34,0.61) | 0.36 (0.25,0.53) |
| Cannabis | ||||||||||
| Model I | 907.79 | 828 | −748.21 | - | - | - | - | A | 0.18 (0.00,0.37) | 0.49 (0.00,0.63) |
| Model II | 907.79 | 829 | −750.21 | 0.00 | 1 | 1.00 | −2.00 | C | 0.00 (0.00,0.26) | 0.00 (0.00,0.49) |
| Model III | 914.24 | 832 | −749.75 | 6.46 | 4 | 0.17 | −1.54 | E | 0.82 (0.63,1.00) | 0.51 (0.37,0.69) |
Note: Table describes fit statistics across sex-limitation models of drug dependence symptoms. The general sex-limitation model (Model I) represents the full model and estimates different magnitude of genetic and environmental effects for males and females, and freely estimates the opposite sex genetic correlation. Model II is a restricted form of the general sex limitation model that allows for different magnitudes of genetic and environmental effects but constrains the opposite sex genetic correlation at 0.5. Model III is an even more restricted form of the general sex-limitation model that equates the magnitude of genetic and environmental effects across males and females while constraining the opposite genetic correlation at 0.5. Standardized variance components are presented for the best fitting sex-limitation model. Highlighted rows indicate the best fitting sex-limitation model whose parameters are indicated in the right portion of the table.
3.4 Multivariate twin models
To understand the etiology of comorbid drug dependence we derived a series of multivariate models that were based upon the results of the univariate models (i.e., (i) no evidence for gender-specific genetic effects, (ii) slight gender differences in the magnitude of genetic and environmental effects, and (iii) no evidence for non-additive genetic effects. We compared an Independent Pathway model that allowed for gender differences in the magnitude of genetic (A) and environmental (C and E) effects (χ2 = 4327.63, df = 3712, AIC = −3096.37) to a Common Pathway model that allowed for gender differences in the magnitude of genetic and environmental effects, as well as the magnitudes of the factor loadings on the latent trait (χ2 = 4341.47, df = 3720, AIC = −3098.53). This model comparison indicated that of the two models, the Common Pathway model provided a more parsimonious description of data (Δχ2 = 13.84, Δdf = 8, P = 0.09, ΔAIC = −2.16).
Table 5 shows the results of models examining the significance of parameters in the Common Pathway Model. Parameter tests confirmed the presence of the latent trait in males and females, as well as similar magnitudes of effect across genders for (i) the factor structure of the latent trait (further referred to as Substance Dependence Vulnerability (SDV)), and (ii) the magnitude of genetic and environmental effects on SDV. Furthermore, the removal of shared environmental effects on SDV resulted in a more significant improvement in model fit, relative to the removal of additive genetic effect on SDV. It is also important to note that although we had limited power to distinguish between the A and C effects, there were significant familial effects on SDV in males and females. In regards to the substance-specific variance, the parameter tests confirmed the presence of significant familial effects on alcohol and tobacco in males and females, but limited familial effects on cannabis (more so in males (Δχ2 = 1.57, Δdf = 2, P = 0.45, ΔAIC = −2.43) than females (Δχ2 = 4.26, Δdf = 2, P = 0.12, ΔAIC = 0.26)). Model-wide tests of differences in the magnitude of substance-specific genetic and environmental effects between males and females indicated that the genetic and environmental effects specific to tobacco and alcohol could not be equated; however, there was evidence to suggest that cannabis-specific effects could be equated across genders.
Table 5.
Parameter tests of the Common Pathway Model
| Model Fit | Comparison to Full Common Pathway Model |
||||||
|---|---|---|---|---|---|---|---|
| Models | −2LL | df | AIC | Δχ2 | Δdf | P | ΔAIC |
| Full Common Pathway model | 4341.47 | 3720 | −3098.53 | ||||
| Examination of SDV | |||||||
| Drop factor loadings (males) | 4495.92 | 3723 | −2950.08 | 154.46 | 3 | <0.01 | 148.46 |
| Drop factor loadings (females) | 4449.55 | 3723 | −2996.45 | 108.08 | 3 | <0.01 | 102.08 |
| Drop A effect (males) | 4343.07 | 3721 | −3098.93 | 1.61 | 1 | 0.21 | −0.39 |
| Drop C effect (males) | 4340.94 | 3721 | −3101.06 | −0.53 | 1 | 1.00 | −2.53 |
| Drop A & C effects (males) | 4359.77 | 3722 | −3084.23 | 18.30 | 2 | <0.01 | 14.30 |
| Drop A effect (females) | 4342.61 | 3721 | −3099.39 | 1.14 | 1 | 0.29 | −0.86 |
| Drop C effect (females) | 4341.47 | 3721 | −3100.53 | 0.00 | 1 | 1.00 | −2.00 |
| Drop A & C effects (females) | 4371.61 | 3722 | −3072.39 | 30.15 | 2 | <0.00 | 26.14 |
| Examination of alcohol specific effects | |||||||
| Drop A (males) | 4347.78 | 3721 | −3094.22 | 6.31 | 1 | 0.01 | 4.31 |
| Drop C (males) | 4341.47 | 3721 | −3100.00 | 0.00 | 1 | 1.00 | −2.00 |
| Drop A & C effects (males) | 4350.31 | 3722 | −3093.69 | 8.84 | 2 | 0.01 | 4.84 |
| Drop A (females) | 4341.47 | 3721 | −3100.53 | 0.00 | 1 | 1.00 | −2.00 |
| Drop C (females) | 4347.54 | 3721 | −3094.46 | 6.07 | 1 | 0.01 | 4.07 |
| Drop A & C effects (females) | 4351.51 | 3722 | −3092.49 | 10.04 | 2 | <0.01 | 6.04 |
| Examination of tobacco specific effects | |||||||
| Drop A (males) | 4344.05 | 3721 | −3097.95 | 2.58 | 1 | 0.11 | 0.58 |
| Drop C (males) | 4341.47 | 3721 | −3100.53 | 0.00 | 1 | 1.00 | −2.00 |
| Drop A & C effects (males) | 4353.22 | 3722 | −3090.78 | 11.75 | 2 | <0.01 | 7.75 |
| Drop A (females) | 4341.47 | 3721 | −3100.53 | 0.00 | 1 | 1.00 | −2.00 |
| Drop C (females) | 4344.04 | 3721 | −3097.96 | 2.58 | 1 | 0.11 | 0.58 |
| Drop A & C effects (females) | 4355.05 | 3722 | −3088.95 | 13.58 | 2 | <0.01 | 9.58 |
| Examination of cannabis specific effects | |||||||
| Drop A (males) | 4342.45 | 3721 | −3099.56 | 0.98 | 1 | 0.32 | −1.02 |
| Drop C (males) | 4341.47 | 3721 | −3100.53 | 0.00 | 1 | 1.00 | −2.00 |
| Drop A & C effects (males) | 4343.04 | 3722 | −3100.96 | 1.57 | 2 | 0.45 | −2.43 |
| Drop A (females) | 4342.81 | 3721 | −3099.19 | 1.34 | 1 | 0.25 | −0.66 |
| Drop C (females) | 4341.47 | 3721 | −3100.53 | 0.00 | 1 | 1.00 | −2.00 |
| Drop A & C effects (females) | 4345.73 | 3722 | −3098.27 | 4.26 | 2 | 0.12 | 0.26 |
| Gender differences on SDV | |||||||
| Equate factor loadings | 4343.19 | 3723 | −3102.82 | 1.72 | 3 | 0.63 | −4.28 |
| Equate ACE effects | 4342.35 | 3723 | −3103.65 | 0.89 | 3 | 0.83 | −5.12 |
| Gender differences on alcohol residual variance | |||||||
| Equate A | 4347.21 | 3721 | −3094.79 | 5.74 | 1 | 0.02 | 3.74 |
| Equate C | 4347.41 | 3721 | −3094.59 | 5.94 | 1 | 0.02 | 3.94 |
| Equate ACE effects | 4354.10 | 3723 | −3091.90 | 12.63 | 3 | 0.01 | 6.63 |
| Gender differences on tobacco residual variance | |||||||
| Equate A | 4342.94 | 3721 | −3099.06 | 1.47 | 1 | 0.23 | −0.53 |
| Equate C | 4344.05 | 3721 | −3097.95 | 2.58 | 1 | 0.11 | 0.58 |
| Equate ACE effects | 4351.63 | 3723 | −3094.37 | 10.16 | 3 | 0.02 | 4.16 |
| Gender differences on cannabis residual variance | |||||||
| Equate A | 4341.95 | 3721 | −3100.06 | 0.48 | 1 | 0.49 | −1.52 |
| Equate C | 4341.47 | 3721 | −3100.53 | 0.00 | 1 | 1.00 | −2.00 |
| Equate ACE effects | 4346.83 | 3723 | −3099.17 | 5.36 | 3 | 0.15 | −0.64 |
| Model-wide gender differences | |||||||
| Equate SDV loadings and all ACE effects (common and substance-specific) (Model III) | 4384.01 | 3735 | −3085.99 | 42.54 | 15 | 0.00 | 12.54 |
| Equate SDV loadings, ACE (SDV) and ACE (cannabis) | 4348.78 | 3729 | −3109.23 | 7.31 | 9 | 0.61 | −10.69 |
| Best-fitting model | |||||||
| Reduced as described in the text and Table 6 | 4345.25 | 3734 | −3122.75 | 3.78 | 14 | 1.00 | −24.22 |
Note: Table describes fit statistics across parameter tests of the Common Pathway Model. Parameters that were dropped from the model had their values fixed at zero. Differences between males and females were achieved by equating the factor loadings for the respective paths corresponding to the loadings on the latent factor or the genetic and environmental factors. Parameters not mentioned in the bestfitting model were dropped from the model.
Abbreviations: A - additive genetic effect; C - shared environmental effect; E - non0shared environmental effect; SDV - Substance Dependence Vulnerability.
Table 6 describes the standardized parameter loadings from the full Common Pathway Model and the best-fitting Common Pathway Model. Across genders, individual differences in comorbid drug dependence symptom counts were attributable to a single latent trait that accounted for roughly 25%–36% of the variability in a substance with the remaining variance attributable to substance-specific genetic and environmental effects. The best-fitting Common Pathway model consisted of (i) the same magnitude of the loadings from the observed traits to SDV across males and females, (ii) the same magnitude of genetic (A) and non-shared environmental (E) effects on SDV across males and females (shared environmental effects (C) were dropped), (iii) AE substance-specific effects for alcohol and tobacco in males and CE substance-specific effects on alcohol and tobacco in females, and (iv) E-only cannabis-specific effects in males and AE cannabis-specific effects in females. Based on the best-fitting Common Pathway Model, the heritability of SDV was approximately 65% in males and females (i.e., adhering to path tracing rules (i.e., forwards from one variable to another; passing through each variable only once in a chain of variables; and, tracing through no more than one two-way arrow in each chain of paths; h2SDV = 0.81 × 0.81)).
Table 6.
Standardized loadings for the Common Pathway Model (CPM)
| Full CPM Model | Best-fitting Model | |||
|---|---|---|---|---|
| Parameters | Male | Female | Male | Female |
| Etiology of SDV | ||||
| a | 0.64 (0.00,0.91) | 0.85 (0.00,0.96) | 0.81 (0.71,0.90) | |
| c | 0.41 (0.00,0.80) | 0.00 (0.00,0.88) | - | |
| e | 0.64 (0.44,0.82) | 0.52 (0.28,0.74) | 0.58 (0.44,0.71) | |
| Factor loadings on SDV | ||||
| Alcohol | 0.53 (0.44,0.63) | 0.55 (0.43,0.66) | 0.52 (0.44,0.60) | 0.58 (0.50,0.67) |
| Tobacco | 0.57 (0.44,0.69) | 0.52 (0.39,0.64) | 0.57 (0.47,0.66) | 0.50 (0.42,0.59) |
| Cannabis | 0.51 (0.40,0.61) | 0.63 (0.49,0.74) | 0.54 (0.45,0.63) | 0.57 (0.48,0.67) |
| Etiology of residual variance | ||||
| a - alcohol | 0.45 (0.27,0.57) | 0.00 (0.00,0.36) | 0.45 (0.29,0.56) | - |
| c - alcohol | 0.00 (0.00,0.27) | 0.44 (0.24,0.54) | - | 0.42 (0.27,0.53) |
| e - alcohol | 0.72 (0.63,0.80) | 0.71 (0.63,0.78) | 0.72 (0.65,0.80) | 0.69 (0.50,0.73) |
| a - tobacco | 0.58 (0.42,0.69) | 0.00 (0.00,0.72) | 0.58 (0.43,0.68) | - |
| c - tobacco | 0.00 (0.00,0.21) | 0.60 (0.00,0.71) | - | 0.61 (0.45,0.72) |
| e - tobacco | 0.58 (0.47,0.69) | 0.61 (0.47,0.76) | 0.59 (0.48,0.69) | 0.61 (0.50,0.73) |
| a - cannabis | 0.29 (0.00,0.51) | 0.43 (0.00,0.60) | - | 0.47 (0.1 9,0.62) |
| c - cannabis | 0.00 (0.00,0.41) | 0.00 (0.00,0.51) | - | - |
| e - cannabis | 0.81 (0.68,0.90) | 0.65 (0.52,0.78) | 0.84 (0.78,0.89) | 0.67 (0.55,0.79) |
Note: Table describes standardized path loadings (along with their 95% confidence intervals) from the full Common Pathway Model and the best-fitting reduced model that equated (i) the factor structure of the latent trait (Substance Dependence Vulnerability (SDV), (ii) genetic and environmental effects on SDV, and (iii) genetic and environmental effects on variation in cannabis dependence symptomatology that was not accounted for by SDV across genders. Also note that the model does not constrain the variance for each substance to be the same across males and females; thus while the structure of the latent trait is the same across genders, the proportion of variance explained varies from males to females. The proportion of variation in the latent construct and observed traits that is attributable to each factor can be obtained by squaring the path loadings and adhering to path tracing rules. For example, based on the reduced model, the heritability of alcohol dependence symptoms in males was 38% (i.e., (0.81 × 0.53 × 0.53 × 0.81) + (0.45 × 0.45)), and the proportion of variance in alcohol dependence in females that is attributable to shared environmental effects was 17% (i.e., 042 × 0.42))). Dashes indicate parameters that were dropped from the model.
Abbreviations: a (additive genetic path), c (shared environment path), and e (non-shared environment path).
The portion of the variability in each substance that was not explained by SDV could be decomposed into substance-specific genetic and/or environmental effects. In males, while the point estimates from the full model indicated that there was evidence for substance-specific genetic and non-shared environmental effects on alcohol, tobacco, and cannabis, the best-fitting model indicated additive genetic and non-shared environmental effects on alcohol and tobacco, and only non-shared environmental effects on cannabis. In females, the best-fitting Common Pathway model confirmed the pattern of effects observed in full model (i.e., shared and non-shared effects on alcohol and tobacco, and additive genetic and non-shared environmental effects on cannabis). Thus, differences in the magnitude of genetic and environmental effects in males and females that were apparent in the univariate model appear to arise from substance-specific mechanisms.
Based on the best-fitting Common Pathway Model, genetic and environmental effects on each substance originated from common effects that influence SDV, and for certain phenotypes, substance-specific mechanisms. Genetic effects on SDV accounted for 48% of the genetic effects on alcohol dependence symptoms, 39% of the genetic effects on tobacco dependence symptoms, and 72% of the genetic effects on cannabis dependence symptoms in males. In females, genetic effects on SDV accounted for 100% of the genetic effects on alcohol and tobacco dependence symptoms (i.e., there are no substance specific genetic influences that are not accounted for by SDV) and roughly 50% of the genetic effects on cannabis dependence symptoms. While these estimates are quite large, it is important to interpret the overall magnitude of these effects in the context of the twin correlations, specifically how they are affected by our ability to the covariance among dependence on different substances to SDV, especially in the case of alcohol in females. More specifically, the pattern of the cross-twin-cross-trait correlations and the univariate models (Table 4) indicated limited evidence for additive genetic effects on alcohol in females(h2 = 0%); however, in the multivariate model, which provides more power to estimate genetic and environmental effects, genetic effects emerged (h2 = 22%) as a result of being able to equate the phenotypic and etiological structures of SDV across males and females. Heritability estimates derived from the best-fitting CPM were 38%, 55%, and 19% for alcohol, tobacco, and cannabis in males, and 22%, 16%, and 43% for females, respectively.
4. DISCUSSION
The present study adds to our understanding of the genetic etiology of comorbid substance dependence in young adulthood by comparing two multivariate biometrical models to provide an estimation of the genetic, shared environmental, and non-shared environmental influences on vulnerability to comorbid drug dependence. The identification of intermediate phenotypes that describe susceptibility to different substances has become exceedingly important given the difficulties in identifying gene variants that explain some of the genetic vulnerability for substance-related disorders.
4.1 Etiology of the Common Liability to Drug Dependence
The primary research question of this study was whether the liability to dependence across alcohol, tobacco, and cannabis could be explained by either a single common genetic risk factor or a genetic factor acting upon a latent mechanism that brings about vulnerability to developing dependence symptoms on an array of substances. Our results are similar to Xian et al.‘ s (2008) earlier report that suggested a single underlying mechanism for vulnerability to dependence on nicotine, alcohol, and cannabis dependence in adult males, as well as other multivariate twin studies of comorbid illicit drug dependence or problematic use (Tsuang et al., 1998; Kendler et al., 2003). This study confirms the existence of a latent dimension of liability to drug dependence symptoms in males and females, and the presence of substance specific genetic and environmental factors that account for variation in each substance that is unexplained by the latent dimension of risk. Notably, this study utilized structured missingness to exclude dependence information from non-substance users and experimenters, who might have been scored as “non-dependent/asymptomatic” using a diagnostic phenotype, thereby improving our ability to detect genetic and environmental factors specific to the development of DSM-IV dependence symptoms amongst drug users. Comorbid alcohol, tobacco, and cannabis dependence was best characterized as alternate manifestations of an underlying trait, referred to Substance Dependence Vulnerability (SDV). We labeled the latent factor in Figure 1b and Table 6 as Substance Dependence Vulnerability (SDV), to imply that the common mechanism behind endorsing dependence symptoms across these substances was the loss-of-control and repeated use of the substances. This was based upon the biochemical adaptation that takes place in the brain as a result of repeated drug administration and psychosocial problems that comprise the DSM-IV dependence symptom count. Biochemical adaptations in the brain center on the mesolimbic dopamine reward pathway and the changes in the reward cascade that take place with repeated drug exposure, ultimately leading to a downregulated dopamine response (Koob and Le Moal, 2001); thus genetic effects on the latent trait may be indicative of system wide genetic polymorphisms that affect dopamine response. Similarly, substance-specific genetic effects could represent variation in receptors, transporters, or enzymes that specifically bind each drug and ultimately affect dopamine levels.
These findings provide an explanation for the high level of comorbidity observed in national epidemiological surveys (SAMHSA, 2008) and the increased risk for dependence seen in relatives of family members with a family history of drug dependence, compared to relatives of family members without a drug dependence history. Given the data available in this study, we cannot speculate on the role of the role of cannabis as a gateway drug for alcohol or tobacco. However, based on earlier studies using this sample we know that (i) alcohol and tobacco are the primary substances used during adolescence (Young et al., 2002) and (ii) comorbid drug dependence is rare during adolescence and increases with age (Palmer et al., 2009). Furthermore, although comorbid drug dependence is uncommon during adolescence, common genetic and environmental effects explain some of that comorbidity (Young et al., 2006). Thus, reassessing the nature of the lifetime comorbidity in this sample after the respondents have had the time to encounter different substances has increased our ability to detect the latent dimension of risk. The common genetic and environmental influence on dependence vulnerability may be due genetic differences in the neural pathways, such as the mesolimbic dopaminergic system, that are influenced by most drugs of abuse (Chen et al., 2009). Likewise, non-shared or unique environmental contributions to SDV may be partially attributed to environmental circumstances, such as experiencing parental divorce (Thomson et al., 2008)) or childhood trauma (Khoury et al., 2010) that increase the likelihood of drug involvement. Simultaneously, the degree of substance-specific preference and substance-specific liability is also the consequence of one’s experiences and biology. Substance-specific genetic mechanisms most likely reflect differences in biological mechanisms that relay information specific to the presence or absence of a drug, as well as processes related to the metabolism of specific drugs, to name a few. Similarly, unique environmental influences on each drug may be capturing different environmental situations in which the use of these substances occurs.
4.2 Considerations and Limitations
Several limitations of the present study should be considered when interpreting these results. First, we cannot properly distinguish between non-additive and shared environmental effects without the inclusion of extended family data. The incorporation of other family types, such as adoptive sibling pairs and non-twin sibling pairs, would allow us to assess the extent of these possible effects. Second, the low frequency of alcohol, tobacco, and cannabis problem users limited our power to distinguish between additive genetic and shared environmental effects. The study’s low power also influenced our model selection; however, we compensated for this by using both the chi-square difference test and the Akaike Information Criterion to select the best-fitting model. In light of the power limitations, we are still confident that the latent trait exists in males and females and that there are common and specific familial effects operating in each gender. Third, covariates of substance addiction, such as attention deficit hyperactivity disorder (Disney et al., 1999; Elkins et al., 2007), delinquency, conduct disorder, depression, and antisocial tendencies (Fu et al., 2002) were not included in this study. Though this is a limitation to this study, the focus of this paper was to determine the extent to which genetic and environmental risk factors for dependence symptoms in young adulthood are relevant, and the extent to which they are correlated across substances; additional studies are needed to tease apart the comorbidity between substance use disorders and other externalizing problems. Lastly, low prevalence rates and endorsement of symptoms make it difficult to explore these hypotheses using multivariate threshold models without model fit issues. This limitation also affects our ability to compare our results to that of past studies, particularly, the examination of individual symptoms. For example, studies examining the variability of DSM symptoms reveal that for alcohol and tobacco, heritability estimates vary by DSM symptom and phenotype definition (Hardie et al., 2008; Lessov et al., 2004; Slutske et al., 1999). Limitations aside, this community-based study provides evidence for a genetically influenced substance dependence vulnerability underlying DSM-IV tobacco, alcohol, and cannabis dependence symptoms collected using an established structured interview.
4.3 Conclusions and Implications
In this sample of young adult male and female twins, we can conceptualize the lifetime comorbidity among alcohol, tobacco, and cannabis as alternative manifestations of a heritable latent trait. Further, gender differences in the relative influence of genetic and environmental factors on each substance are likely to arise from substance-specific effects. Based on our overall findings, the development of effective prevention and intervention strategies for these three substances will be dependent on identifying and applying biological and environmental strategies that reduce the risk that is both common and specific to these substances.
The existence of an underlying dimension of risk for drug dependence has the potential to be a useful tool for strengthening association studies, largely because it helps to refine the phenotype of interest. Evidence of a common liability for dependence implies that genetic variants involved have pleiotropic effects, which is understandable given that several neurobiological mechanisms and their genetic determinants have been associated with more than one substance. For instance, genetic variants in nicotinic acetylcholine receptor subunits have been associated with both tobacco and alcohol use (Ehringer et al., 2007; Schlaepfer et al., 2008a, 2008b) and the abolition of alcohol preference in mice is achievable through pharmacological targeting of the cannabinoid signaling system (Basavarajappa, 2007).
The implications of an additional source of evidence in support of a common liability for substance use disorders in the realm of gene finding efforts in the context of other comorbid psychiatric disorders is significant for the field. Although externalizing problems were not the focus of this study, others have identified common genetic and environmental factors between externalizing problems, such as conduct disorder, attention deficit hyperactivity disorder, and substance use disorders (Iacono et al., 2008). If it is the case that childhood disruptive disorders and antisocial tendencies are early risk factors for future substance problems, identifying how much of the shared liability across substances overlaps with these disorders will help to further our understanding of addiction processes.
Acknowledgments
This study was funded by MH016880, MH019927, MH063207, HD010333, DA011015, DA021913, and AA007464. MH016880 and MH019927 supported the training of Rohan Palmer, the analysis and interpretation of the data, and the writing of the report. Data collection was supported by DA011015. The development and maintenance of the LTS sample was supported by HD 010333 and MH063207. T.M.M. Button was supported by AA007464. Individual support for the co-authors was provided by DA011015 and DA021913.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
All of the listed authors declare that they have no conflicts of interests.
REFERENCES
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- American Psychiatric Association. Statistical Manual of Mental Disorders. 4th edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Basavarajappa BS. The endocannabinoid signaling system: a potential target for next-generation therapeutics for alcoholism. Mini Rev. Med. Chem. 2007;7:769–779. doi: 10.2174/138955707781387920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung Med. J. 2009;32:148–154. [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The Reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br. J. Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Mikulich SK, Ehlers KM. Whitmore E.A., MacDonald M.J. Validity of structured clinical evaluations in adolescents with conduct and substance problems. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:265–273. doi: 10.1097/00004583-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use and other substance use in the general population. Drug Alcohol Depend. 2001;64:319–327. doi: 10.1016/s0376-8716(01)00130-2. [DOI] [PubMed] [Google Scholar]
- Disney ER, Elkins IJ, McGue M, Iacono WG. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. Am. J. Psychiatry. 1999;156:1515–1521. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Eysenck HJ. New approaches to the analysis of twin data and their application to smoking behavior. In: Eysenck HJ, editor. The Causes and Effects of Smoking. Vol. 1980. London: Maurice Temple Smith; 1980. pp. 140–314. [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch. Gen. Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch. Gen. Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: a multi-stage model from cannabis availability, cannabis initiation and progression to abuse. Addiction. 2009;104:430–438. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Bergen A. Commentary on: general and specific inheritance of substance abuse and alcoholism. Arch. Gen. Psychiatry. 1998;55:964–965. doi: 10.1001/archpsyc.55.11.964. [DOI] [PubMed] [Google Scholar]
- Hardie TL, Moss HB, Lynch KG. Sex differences in the heritability of alcohol problems. Am. J. Addict. 2008;17:319–327. doi: 10.1080/10550490802139010. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Heiman GA, Ogburn E, Gorroochurn P, Keyes KM, Hasin D. Evidence for a two-stage model of dependence using the NESARC and its implications for genetic association studies. Drug Alcohol Depend. 2008;92:258–266. doi: 10.1016/j.drugalcdep.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Bloningen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: a longitudinal twin study. J Abnorm. Psychol. 2007;116:443–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J. Am. Acad. Child Adolesc. Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu. Rev. Clin. Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Bethesda, MD: National Institute on Drug Abuse; 2008. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2007. NIH Publication No. 08-6418. [Google Scholar]
- Kapusta ND, Plener PL, Schmid R, Thau K, Walter H, Lesch OM. Multiple substance use among young males. Pharmacol. Biochem. Behav. 2007;86:306–311. doi: 10.1016/j.pbb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch. Gen. Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan PF, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol. Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Keller MC, Coventry WL. Quantifying and addressing parameter indeterminacy in the classical twin design. Twin Res. Hum. Genet. 2005;8:201–213. doi: 10.1375/1832427054253068. [DOI] [PubMed] [Google Scholar]
- Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ. Substance use, childhood traumatic experience, and posttraumatic stress disorder in an urban civilian population. Depress. Anxiety. 2010;27:1077–1086. doi: 10.1002/da.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, Heath AC, Madden PA. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychol. Med. 2004;34:865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulick CM, Neale MC, Prescott CA, Eaves LJ, Kendler KS. A twin study of genetic and environmental influences in tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004;34:1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th Edition. Richmond, VA: VCU; 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC. MX: statistical modeling. 5th ed. Richmond, VA: VCU; 1999. [Google Scholar]
- Neale MC, Harvey E, Maes HH, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression: applications to substance use and abuse. Behav. Genet. 2006;36:507–524. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behav. Genet. 2006;36:483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP. Colorado Twin Registry. Twin Res. Hum. Genet. 2006;9:941–949. doi: 10.1375/183242706779462895. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Neale MC, Stallings MC. Comorbidity between alcohol dependence and illicit drug dependence in adolescents with antisocial behavior and matched controls. Drug Alcohol Depend. 2006;84:85–92. doi: 10.1016/j.drugalcdep.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Syst. Rev. 2010;8:CD001867. doi: 10.1002/14651858.CD001867.pub3. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Bucholz KK, Madden PA, Heath AC, Agrawal A, Whitfield JB, Statham DJ, Martin NG, Lynskey MT. Common genetic contributions to alcohol and cannabis use and dependence symptomatology. Alcohol. Clin. Exp. Res. 2010;34:545–554. doi: 10.1111/j.1530-0277.2009.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS. SAS/STAT software, Version 9.1 of the SAS System for Windows. Cary, NC, USA: SAS Institute Inc.; 2002–2003. [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol. Psychiatry. 2008a;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Ehringer MA. The genetic components of alcohol and nicotine co-addiction: from genes to behavior. Curr. Drug Abuse Rev. 2008b;1:124–134. doi: 10.2174/1874473710801020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske W, True W, Scherrer J, Heath A, Bucholz K, Goldberg J, Lyons MJ, Tsuang MT. The heritability of alcoholism symptoms: 'indicators of genetic and environmental influence in alcohol-dependent individuals' revisited. Alcohol. Clin. Exp. Res. 1999;23:759–769. doi: 10.1111/j.1530-0277.1999.tb04181.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Office of Applied Studies, NSDUH Series H-34, DHHS Publication No. SMA 08-4343; 2008. Results from the 2007 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Thompson RG, Jr, Lizardi D, Keyes KM, Hasin DS. Childhood or adolescent parental divorce/separation, parental history of alcohol problems, and offspring lifetime alcohol dependence. Drug Alcohol Depend. 2008;98:264–269. doi: 10.1016/j.drugalcdep.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Xiang H, Scherrer JF, Madden PAF, Bucholz KK, Heath AC, Eisen SA, Goldberg J, Lyons MJ, Tsuang MT. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch. Gen. Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch. Gen. Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Üstűn B, Compton W, Mager D, Babor T, Baiyewu O, Chatterji S, Cottler L, Gogus A, Mavreas V, Peters L, Pull C, Saunders J, Smeets R, Stipec MR, Vrasti R, Hasin D, Room R, Van den Brink W, Regier D, Blaine J, Grant BF, Sartorius N. WHO study on the reliability of the alcohol and drug use disorder instruments: overview of methods and results. Drug Alcohol Depend. 1997;47:161–169. doi: 10.1016/s0376-8716(97)00087-2. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. common mechanisms and manifestations. Neurosci. Biobehav. Rev. 2003;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol. Learn. Mem. 2002;78:6610–6624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: prevalence, symptom profiles and correlates. Drug Alcohol Depend. 2002;68:309–322. doi: 10.1016/s0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]
- Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific? Behav. Genet. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Grant JD, Eisen SA, True WR, Jacob T, Bucholz KK. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction. 2008;103:1391–1398. doi: 10.1111/j.1360-0443.2008.02243.x. [DOI] [PubMed] [Google Scholar]