Abstract
The role of TGF-β signaling in tumorigenesis is paradoxical: it can be tumor suppressive or tumor promotional, depending on context. The metastatic regulator, Six1, was recently shown to mediate this switch, providing a novel means to explain this elusive “TGF-β paradox”. Herein, we identify a mechanism by which Six1 activates the tumor promotional arm of TGF-β signaling, via its ability to upregulate the miR-106b-25 microRNA cluster, and further identify a novel function for this cluster of microRNAs. While expression of the miR-106b-25 cluster is known to overcome TGF-β-mediated growth suppression via targeting p21 and BIM, we demonstrate for the first time that this same cluster can additionally target the inhibitory Smad7 protein, resulting in increased levels of the TGF-β type I receptor (TβRI) and downstream activation of TGF-β signaling. We further show that the miR-106b-25 cluster is sufficient to induce an epithelial to mesenchymal transition and a tumor initiating cell phenotype, and that it is required downstream of Six1 to induce these phenotypes. Finally, we demonstrate a significant correlation between miR-106b, Six1, and activated TGF-β signaling in human breast cancers, and further show that high levels of miR-106b and miR-93 in breast tumors significantly predicts shortened time to relapse. These findings expand the spectrum of oncogenic functions of miR-106b-25, and may provide a novel molecular explanation, through the Six1 regulated miR-106b-25 cluster, by which TGF-β signaling shifts from tumor suppressive to tumor promoting.
Keywords: Six1, miRNA, TGF-β, epithelial-to-mesenchymal transition, tumor initiating cell
Introduction
TGF-β signaling plays a critical and dual role in breast tumorigenesis. In normal epithelium as well as in early tumorigenic lesions, TGF-β plays a tumor suppressive role through its ability to induce growth inhibition. As cancer progresses, however, tumor cells become resistant to TGF-β mediated growth inhibition, and instead TGF-β promotes tumor progression and metastasis, likely in part through its promotion of an epithelial-to-mesenchymal transition (EMT) 1,2. The ability of TGF-β to switch cells from tumor suppressive to tumor promotional has been coined the TGF-β paradox 3. The mechanism of this phenomenon is not well understood, but remains an important area of research.
Recently, our laboratory identified the developmental homeotic transcription factor, Six1, as an important mediator of breast cancer progression and metastasis4–7. We have found that Six1 is dependent on upregulation of TGF-β signaling to induce an EMT 7, an increase in tumor-initiating cell (TIC) characteristics 8 (and data not shown), and to induce late stage metastasis 7. Interestingly, using an experimental metastasis model, we demonstrated that Six1 not only upregulates TGF-β signaling, but that it also switches the pathway from tumor suppressive to tumor promotional 7,9. However, until now, the mechanism by which Six1 accomplishes this switch was unknown.
Herein, we have identified a cluster of miRNAs, miR-106b-25, that is upregulated by the homeoprotein Six1. This cluster is highly conserved in vertebrates, and consists of three miRNAs, miR-106b, miR-93, and miR-25, which all reside in the 13th intron of the MCM7 gene (Chr7). Two paralogs of this cluster exist, the miR-17-92 cluster (Chr13, c13ORF25 gene), and the miR-106a-363 cluster (ChrX). The miR-17-92 cluster has received considerable attention as a pro-oncogenic cluster, and was the first cluster of miRNA identified to cooperatively act as an oncogene 10. Interestingly, the miR-106b-25 cluster of miRNA is overexpressed in several cancers 11–15, and like the miR-17-92 paralog, is pro-oncogenic 15. Previous reports have demonstrated the ability of these miRNA to inhibit the growth suppressive functions of TGF-β signaling through repression of downstream mediators p21 and Bim 11. In this study, we show for the first time that this cluster of miRNA can target the TGF-β inhibitor Smad-7 and also activate the TGF-β signaling pathway, providing a novel mechanism by which Six1 overexpression can mediate the switch in TGF-β signaling from tumor suppressive to tumor promotional. We also demonstrate that overexpression of the miR-106b-25 miRNAs is sufficient to induce characteristics of EMT and TICs, and that this cluster is necessary for the ability of Six1 to mediate these phenotypes. Finally, we demonstrate a significant correlation between miR-106b expression and both Six1 and activated TGF-β signaling in human breast cancer tissues, and further show that high expression levels of miR-106b and miR-93 together in early-invasive breast tumors can significantly predict a shorter time to relapse for these patients. Taken together, our results significantly expand the tumorigenic functions of the miR-106b-25 cluster. In addition, we demonstrate a critical role for this cluster in mediating not only the pro-tumorigenic functions of Six1, but also provide a possible mechanism by which Six1 overcomes TGF-β mediated growth suppression while simultaneously activating the pro-metastatic arm of the pathway.
Results
Six1 regulates the miR-106b-25 Cluster of miRNAs
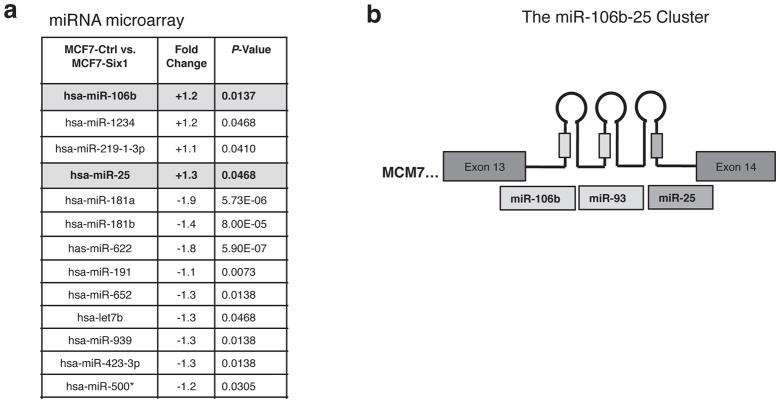
Previous studies have demonstrated substantial cross-talk between miRNAs and homeobox genes 16,17. We therefore asked whether the Six1 homeoprotein might regulate miRNAs to mediate its tumorigenic and metastatic phenotypes. miRNA microarray analysis on RNA isolated from MCF7 breast cancer cells overexpressing Six1 (MCF7-Six1) and control cells (MCF7-Ctrl) led to the identification of several miRNAs that were differentially expressed in a statistically significant manner between the two groups (Figure 1A). Interestingly, we identified two miRNAs, miR-106b and miR-25, that were upregulated in response to Six1 overexpression (Figure 1A), and that belong to a cluster of miRNAs, which also includes miR-93, and reside in the 13th intron of the MCM7 gene (Figure 1B). These miRNA have previously been implicated as a pro-oncogenic cluster of miRNAs 12,15,18. To validate our microarray results, we performed quantitative real-time reverse transcriptase PCR (qRT-PCR) on an independent set of RNA isolated from MCF7-Ctrl and MCF7-Six1 cells, demonstrating that all three miRNA within the cluster are overexpressed 2-3 fold in MCF7-Six1 cells as compared to MCF7-Ctrl cells (Figure 2A). In addition, siRNA knockdown of Six1 in 21PT cells (Supplemental Figure 1), which contain high levels of Six1 endogenously 6, resulted in a clear decrease in all three miRNAs, confirming that endogenous Six1 regulates the miR-106b-25 cluster (Figure 2B). Finally, to examine whether Six1 could regulate the miR-106b-25 cluster in vivo, we analyzed expression of the miRNA cluster in transgenic mice in which Six1 was induced (using doxycycline) in the mammary gland (Six1 + Dox) and in control animals (Ctrl + Dox) 8 (See Supplemental Figure 2 for expression levels of Six1 in the transgenic mammary glands) and found that all three miRNAs, miR-106b, miR-93, and miR-25, are overexpressed in Six1 transgenic mammary glands as compared to control mammary glands (Figure 2C). Importantly, Six1 transgenic mice develop aggressive mammary carcinomas that display multiple histological subtypes as well as an induction of an EMT 8.
Figure 1. A miRNA microarray identifies the miR-106b-25 cluster family members as upregulated by Six1.
(a) miRNAs that are significantly up- or downregulated (P value < 0.05) in MCF7-Six1 vs. MCF7-Ctrl cells as determined by a miRNA profiling array (b) Schematic representation of the miR-106b-25 cluster of miRNA (miR-106b, miR-93, and miR-25) within the 13th intron of the MCM7 gene.
Figure 2. Six1 regulates the miR-106b-25 Cluster.
(a) Stable overexpresson of Six1 in MCF7 cells leads to an increase in miR-106b, miR-93, and miR-25 as determined using qRT-PCR. Data are represented as the mean +/− SEM of three individual MCF7-Six1 and MCF7-Ctrl clones (b) Knockdown of Six1 in 21PT cells using Six1 specific siRNA (siSix1, 50nm and 100nm) leads to a decrease in expression of all 3 miRNA in the miR-106b-25 Cluster when compared to a control knockdown (siNeg). For qRT-PCR analysis, the average of 3 replicates +/− SD is shown. (c) RNA was isolated from the mammary glands of bitransgenic mice in which Six1 was induced with doxycycline (Six1+Dox) versus single transgenic MTB control mice also treated with Dox (Ctrl+Dox), but unable to express Six1. qRT-PCR performed on the isolated RNA for the miR-106b-25 miRNAs demonstrates an increase in expression of all three miRNAs in the Six1+Dox mammary glands, which express high levels of the Six1 transgene (Supplementary Figure 2) as compared to Ctrl+Dox control mammary glands. n=3 mice for each condition, and each miRNA was normalized to U6 RNA. P values represent statistical analysis using a paired t test.
The miR-106b-25 Cluster targets Smad7 for repression
It was previously shown that the miR-106b-25 cluster has the ability to overcome TGF-β mediated growth suppression via repression of the cell cycle inhibitor p21, and the pro-apoptotic factor Bim 11. In addition to the known role of the cluster in TGF-β growth inhibition, using target prediction analysis, we found that the miR-106b-25 cluster might also play a role in activating the TGF-β pathway, providing an attractive mechanism by which Six1 could mediate the switch in TGF-β signaling from tumor suppressive to tumor promoting. Indeed, based on seed sequence alignment, the inhibitory Smad7 (I-Smad7) mRNA is a target for all three miRNAs in the cluster. Smad7 antagonizes TGF-β signaling through multiple mechanisms, including binding to TGF-β type I receptor (TβRI) and interfering with recruitment and downstream phosphorylation and activation of the receptor-Smads (R-Smads), Smad2 and Smad319. Additionally, Smad7 also functions to recruit E3 ubiquitin ligases to TβRI, resulting in its degradation 20. Therefore, repression of Smad7 by the miR-106b-25 miRNAs would be expected to activate the TGF-β signaling pathway, which is known to occur downstream of Six17.
To determine if Smad7 is downregulated in response to Six1, we first performed qRT-PCR on clonal isolates of MCF7-Ctrl and MCF7-Six1 cells and demonstrated that Smad7 expression is indeed reduced in MCF7-Six1 cells, where the miR-106b-25 cluster is overexpressed (Figure 3A, Figure 3D shows protein level differences). To further determine whether the cluster of miRNAs can directly affect Smad7 levels, we generated MCF7 cell lines stably overexpressing the genomic region of the cluster (MCF7-Cluster), or control MCF7 cells expressing either the empty vector (MCF7-EV) or a non-silencing (scrambled control) vector (MCF7-NS). Importantly, stable populations expressing the cluster were chosen to overexpress each miRNA in the cluster only 2 to 3-fold, similar to what is observed with Six1 overexpression (Supplemental Figure 3). Transfection of a Smad7-3′UTR-luciferase construct into these cell lines demonstrates that the miR-106b-25 cluster inhibits the 3′UTR of Smad7 (Figure 3B). Additionally, a decrease in Smad7 protein in MCF7-Cluster cells is observed when compared to MCF7-EV and MCF7-NS cells, demonstrating that a 2–3 fold increase in the miR-106b-25 cluster can downregulate endogenous Smad7 (Figure 3C). Conversely, treatment of MCF7-Six1 cells with transient inhibitors against the individual miRNAs leads to a de-repression of Smad7 protein, with miR-106b and miR-93 being the major mediators of this effect (Figure 3D). Efficacy of the miRNA inhibitors is demonstrated by relative activity of luciferase reporters, containing target sites for each miRNA, in the inhibitor transfected cells (Supplemental Figure 4).
Figure 3. The miR-106b-25 miRNAs repress Smad7.
(a) qRT-PCR reveals a decrease in Smad7 mRNA in MCF7-Six1 cells versus MCF7-Ctrl cells. Data shown are the result of 3 replicate qRT-PCR reactions (+/− SD), normalized to cyclophilin B mRNA levels (b) A renilla luciferase reporter containing the 3′UTR of Smad7 was transfected into MCF7 cells containing an empty vector (MCF7-EV), a non-silencing control (MCF7-NS), or the miR-106b-25 cluster (MCF7-Cluster). Measurement of renilla luciferase normalized to firefly luciferase (present on the same vector but expressed from a different promoter) demonstrates a significant repression of the Smad7 3′UTR in response to miR-106b-25 expression. (c) Western blot analysis demonstrates that MCF7-Cluster cells have decreased Smad7 protein as compared to MCF7-EV and MCF7-NS cells (d) MCF7-Ctrl and MCF7-Six1 cells treated with miRNA inhibitors towards all three miRNA (ALL), miR-106b, and miR-93, show a de-repression of Smad7 protein in MCF7-Six1 cells. P values represent statistical analysis using a paired t test.
The miR-106b-25 Cluster activates TGF-β signaling
Because the miR-106b-25 cluster represses Smad7 in MCF7 cells, and because Six1 overexpression, which leads to increased levels of the miR-106b-25 miRNAs, activates TGF-β signaling 7, we asked whether this cluster of miRNAs, which is known to overcome TGF-β-mediated growth inhibition, is also sufficient to activate the TGF-β pathway. Indeed, with both transient and stable overexpression of the miR-106b-25 cluster, we observed an increase in TβRI protein levels (Figure 4A and Figure 4B), as well as an increase in activated TGF-β signaling as measured by p-Smad3 levels (Figure 4B). To further determine if the miR-106b-25 miRNAs are necessary for the previously observed induction of TGF-β signaling by Six1, we utilized a stable lentiviral miRNA knockdown system (miRZip) to inhibit the miRNAs within the cluster either individually or together (as a control, we used a scrambled sequence, miRZip-SCR). Efficacy of the miRZips was demonstrated by examining their effects on endogenous targets of the miR-106b-25 cluster, p21 and BIM (Supplemental Figure 5). Inhibition of miR-93, as well as the entire cluster in MCF7-Six1 cells reverses the Six1-induced increase in TβRI (Figure 4C), and inhibition of miR-106b, miR-93, as well as the entire cluster reverses the Six1-induced increase in p-Smad3 (Figure 4D).
Figure 4. The miR-106b-25 cluster activates TGFβ signaling.
(a) Transient and (b) stable overexpression of the miR-106b-25 cluster in MCF7 cells leads to increased expression of TβRI protein over controls and an increase in phosphorylated Smad3 (p-Smad3) (c) MCF7-Ctrl and MCF7-Six1 cells expressing stable miRZip inhibitors targeting the miR-106b-25 miRNAs individually and together (miRZip-Cluster) show a reversal of the Six1-induced increase in TβRI protein in miRzip-93 and miRZip-Cluster treated MCF7-Six1 cells as compared to scramble miRZip controls (miRZip-SCR) (d) Introduction of miRZip-106b, miRZip-93, and miRZip-Cluster into MCF7-Six1 cells reverses the Six1-induced increase in p-Smad3 levels, without affecting the Six1-induced increase in total Smad3 levels (e) a real-time PCR array containing TGF-β transcriptional targets shows enrichment for TGF-β target gene expression in MCF7-Cluster cells over MCF7-NS cells. Data is represented as fold change expression of MCF7-Cluster compared to control MCF7-NS from 3 replicate plates of each condition. Genes shown had at least a 2-fold induction in MCF7-Cluster cells as compared to MCF7-NS cells.
Interestingly, previous reports have identified the miR-106b-25 cluster miRNAs as targeting the TGF-β type II receptor (TβRII), resulting in repression of this protein21. Analysis of TβRII protein in our MCF7-Cluster cells did not show a repression of TβRII protein as compared to MCF7-NS cells (Supplemental Figure 6). Similarly, we also did not observe significant downregulation of TβRII protein levels in MCF7-Six1 versus MCF7-Ctrl cells (Supplemental Figure 6). Thus, 2–3 fold overexpression of the miR-106b-25 cluster in MCF7 cells leads primarily to alterations in the TGF-β pathway that would be expected to be activating, as opposed to inactivating.
To analyze global changes in TGF-β signaling, we performed microarray analysis on MCF7-NS versus MCF7-Cluster cells to examine whether the presence of the miR-106b-25 cluster alters the TGF-β response signature (TβRS) 22, similar to what is observed with Six1 overexpression 7. Hierarchical clustering confirmed differential regulation of many of the genes in the TβRS between MCF7-NS and MCF7-Cluster cells, demonstrating that TGF-β signaling is indeed altered in response to miR-106b-25 overexpression (Supplemental Figure 7). In addition, we performed a qRT-PCR array to examine alterations in expression of TGF-β target genes using MCF7-NS and MCF7-Cluster cells, demonstrating that TGF-β signaling is clearly activated downstream of the miR-106b-25 cluster, as numerous TGF-β transcriptional targets are upregulated in MCF7 cells overexpressing the cluster (Figure 4E). Of the 84 genes responsive to TGF-β signaling on the array, 47 were upregulated 1.5 fold or more in MCF7-Cluster cells, 8 genes were downregulated, and the rest remained unchanged (Supplemental Figure 8). Together, these data demonstrate that the miR-106b-25 cluster is capable of activating TGF-β signaling in breast cancer cells, and suggest that this one cluster, which can also overcome the growth suppressive effects of TGF-β, may be responsible for the switch in TGF-β signaling from tumor suppressive to tumor promoting.
The miR-106b-25 cluster induces EMT-like changes
We previously reported that Six1 overexpression leads to an induction of EMT, which is dependent on TGF-β signaling 7. Because the miR-106b-25 cluster is sufficient to activate TGF-β signaling, we asked if this cluster is sufficient to mediate phenotypes associated with EMT. One of the hallmarks of EMT is the loss of membranous E-cadherin from the adherens junctions. We thus analyzed the subcellular localization of E-cadherin from each cell line and demonstrated that E-cadherin is indeed decreased in the insoluble, or membrane bound, fraction of the cell in response to miR-106b-25 overexpression (Figure 5A). β-catenin, which is normally in the membranous adherens junctions with E-cadherin, is also decreased in the insoluble fraction of MCF7-Cluster cells (Figure 5A). Because redistribution of β-catenin away from the membrane may result in its increased nuclear localization and subsequent ability to activate transcription, we measured β-catenin transcriptional activity using the TOP-flash luciferase reporter. Concomitant with the loss of β-catenin from the membrane, MCF7-Cluster cells also exhibit an increase in TOP-flash reporter activity over MCF7-NS cells, similar to the phenotype observed with Six1 overexpression (Figure 5B). To determine if the miR-106b-25 miRNAs are necessary for the Six1-induced increases in β-catenin transcriptional activation, we treated MCF7-Six1 cells with inhibitors toward all three miRNA (miR-106b, miR-93 and miR-25) together and measured TOP-flash activity. A repression of TOP-flash activity in this context demonstrates that Six1 is dependent on the miR-106b-25 miRNAs to induce β-catenin transcriptional activity (Figure 5C). Furthermore, the relocalization of E-cadherin and β atenin away from the membrane in response to miR-106b-25 cluster expression was confirmed using immunofluorescence (Figure 5D).
Figure 5. The miR-106b-25 cluster mediates features of EMT.
(a) miR-106b-25 overexpression results in loss of E-cadherin and β-catenin from the insoluble (cytoskeleton-associated) protein fraction of the cell as determined by western blot analysis. (b) MCF7-Cluster cells show increased activity of the β-catenin responsive-luciferase reporter TOP-Flash, normalized to Renilla luciferase activity. Data are from 3 replicates +/− SD (c) The Six1-induced increase in TOP-Flash activity is reversed with inhibition of the miR-106b-25 cluster using transient hairpin inhibitors (Dharmacon, miRidian) (d) Immunofluorescence to detect E-cadherin and β-catenin in MCF7-NS vs. MCF7-Cluster cells. After incubation with the E-cadherin (left panel) or β-catenin (right panel) antibodies, a FITC conjugated anti-secondary antibody was added to detect the signal. Dapi images merged with FITC are shown along with the FITC images (40x magnification). (e) Expression of genes involved in EMT is enriched in MCF7-Cluster cells versus MCF7-NS cells as shown by a qPCR array analysis. Gene expression is shown by fold change from three replicate plates per group. Genes shown exhibited a 2-fold or greater induction in MCF7-Cluster cells when compared to MCF7-NS cells. (f) Expression of the miR-106b-25 Cluster in MCF7 cells results in decreased adhesion to cell matrix proteins Collagen I, Collagen IV, and Fibronection, similar to what is observed with Six1 overexpression. P values represent statistical analysis using a paired t test (*≤0.05, **≤0.01, ***≤0.001).
To determine whether additional genes associated with EMT are differentially regulated between MCF7-NS and MCF7-Cluster cells, we performed qRT-PCR array analysis on RNA isolated from each cell line. Indeed, miR-106b-25 expression led to a 2-fold or greater upregulation of a number of genes associated with EMT, including Jag1, MMP9, and Vimentin, amongst others (Figure 5E, Supplemental Figure 9 shows all genes regulated 1.5-fold or more). Finally, we observed a decrease in cell-matrix adhesion to Collagen I, Collagen IV, and Fibronectin in the MCF7-Cluster cells as compared to the MCF7-NS and MCF7-EV cells, similar to the decrease in adhesion observed with Six1 overexpression in MCF7 cells (Figure 5F). Together, these data suggest that the miR-106b-25 cluster alone can induce an EMT-like phenotype.
The miR-106b-25 cluster increases tumor initiating cell characteristics
Many genes that induce EMT-like phenotypes also induce tumor initiating cell (TIC) phenotypes23. Indeed, we recently demonstrated that Six1 induces a TIC phenotype in both a transgenic mouse model 8 and when overexpressed in MCF7 cells 24. To determine if the miR-106b-25 miRNAs, which can induce properties of EMT, can also induce TIC characteristics, we performed flow cytometry for the cell surface TIC-associated markers CD24 and CD44 25, and found that miR-106b-25 did indeed increase the percentage of CD24low CD44+ cells, similar to Six1 (Figure 6A). Additionally, secondary tumorsphere assays performed with MCF7-Cluster and MCF7-NS cells demonstrated that, similar to MCF7-Six1 cells, MCF7-Cluster cells could increase tumorsphere formation, a measure of functional TICs within a population (Figure 6B). To further test for functional TIC characteristics using an in vivo assay, we injected cells at limiting dilutions into the mammary fat pad of NOD-SCID mice. MCF7-Cluster cells were able to initiate tumors with a greater frequency then MCF7-NS cells, both when 1000 and 100 cells were injected (Figure 6C). In order to determine if the miR-106b-25 cluster was necessary for Six1-induced increases in TICs in vivo, we utilized our MCF7-Six1-miRZip-Cluster cells (in which all three miRNAs are inhibited), and transplanted these cells into the mammary gland at limiting dilutions, along with miRZip-SCR controls in both MCF7-Ctrl and MCF7-Six1 cells. Inhibition of the miR-106b-25 miRNAs in MCF7-Six1 cells demonstrates a reduction in tumor initiating ability back to similar levels of TIC frequency seen in MCF7-Ctrl cells (Figure 6D). Lastly, we performed a human stem cell qRT-PCR array. Of the 84 genes on this array, 53 were upregulated more than 1.5 fold in MCF7-Cluster cells (Figure 6E and Supplemental Figure 10). Together these data demonstrate for the first time a role for the miR-106b-25 cluster in both EMT and in increased TIC capacity.
Figure 6. The miR-106b-25 cluster increases TIC characteristics.
(a) Overexpression of the miR-106b-25 cluster in MCF7 cells is sufficient to increase the CD24low/CD44+ population, similar to what is observed with Six1 overexpression in MCF7 cells (b) Expression of the miR-106b-25 cluster in MCF7 cells is sufficient to increase tumorsphere formation, a measurement of self-renewal capability, similar to what is observed with Six1 overexpression in MCF7 cells (c) MCF7-Cluster cells transplanted into the 4th mammary fat pad of NOD-SCID mice at limiting dilutions have an increased ability to initiate tumors when compared to MCF7-NS cells (d) Inhibition of the miR-106b-25 cluster in MCF7-Six1 cells (MCF7-Six1-Zip-Cluster) reduces the ability of the cells to initiate tumors, back to levels observed in MCF7-Ctrl cells (MCF7-Ctrl-Zip-SCR). The estimate frequency of tumorigenic cells was calculated by limiting-dilution analysis as described in the methods (e) Genes important for stem cell maintenance, growth, and differentiation are increased in MCF7-Cluster cells as compared to MCF7-NS cells, as determined by a stem cell qPCR array. Data is represented as fold change in gene expression in MCF7-Cluster cells as compared to control MCF7-NS cells from 3 replicate plates of each condition. The Genes shown exhibited at least a 2-fold induction in MCF7-Cluster cells as compared to the MCF7-NS cells.
The miR-106b-25 cluster correlates with Six, activated TGF-β signaling, and shortened time to relapse in human breast cancer
To determine if the Six1/miR-106b-25/activated TGF-β signaling axis is relevant to human breast cancer, we obtained human breast cancer tissue arrays containing 71 cases of invasive ductal carcinoma, with matched cases on which we had previously performed immunohistochemistry (IHC) using an antibody generated against human Six1 and human Smad3 (nuclear Smad3 was scored as an indicator of activated TGF-β signaling) 7. We next performed in situ hybridization (ISH) for miR-106b (as a representative of the miR-106b-25 cluster), to compare expression of this cluster family member with nuclear staining obtained with the Six1 antibody and with activated TGF-β signaling. Importantly, miR-106b and Six1 significantly correlate in breast cancer tissues (p= 0.0028 Spearman R=0.3927) (Figure 7A), as do miR-106b and nuclear Smad3 (p=0.0017, Spearman R=0.3972) (Figure 7B). In addition, the greatest percentage of tumors exhibited activated TGF-β signaling when both miR-106b and Six1 were highly expressed (64.7% show increased nuclear Smad3 when both miR-106b and Six1 are high) (Figure 7C). Of note, the Six1 antibody used in these experiments was generated against a region of Six1 that may allow cross-reactivity with other Six family members, as they are highly conserved. Therefore, we can only confidently say that Six family expression correlates with miR-106b in human tumors. However, examination of the van de vijver public breast cancer gene expression dataset 26 suggests that Six1 is more abundantly expressed than other Six family members in human breast tumors (data not shown). Together, these data strongly suggest a critical role for miR-106b-25 in the Six1-induced activation of TGF-β signaling in human breast cancer.
Figure 7. The miR-106b-25 cluster correlates with Six1 expression, activated TGFβ signaling, and shortened time to relapse in human breast cancers.
Human breast cancer tissue arrays were previously immunostained with an anti-Six1 antibody (Atlas) and a Smad3 antibody (Zymed) as previously described 7 and the staining was scored for nuclear Six1 and Smad3 on a scale of 0–4. A serial section array was also stained for miR-106b expression by in situ hybridization. Expression of miR-106b was scored on a scale of 0-4 and compared to Six1 and nuclear Smad3 scores in the same tissues. (a) Results show that miR-106b and Six1 correlate in human breast cancers (b) as do miR-106b and nuclear Smad3 (c) When the expression of all three molecules are considered, the highest percentage of nuclear Smad3 can be found when both Six1 and miR-106b are highly expressed. P-values obtained using Spearman correlation analysis. (d) In a miRNA expression dataset of 216 early-invasive breast cancers 27, patients whose tumors express both high miR-106b and high miR-93 show a significantly reduced time to relapse. The median value for miR-106b and miR-93 was used to divide the samples into high (above median) and low (below median) miRNA expression. P value was calculated by log-rank analysis.
To explore the prognostic value of these miRNA in human breast cancers, we examined a publicly available dataset comprised of miRNA expression in early-invasive breast cancers27. Figure 7D demonstrates that patients whose tumors express high miR-106b and high miR-93 together have a significantly shortened time to relapse. Analysis of individual miRNA expression in these tumors also demonstrates a significant correlation with high miR-93 (Supplemental Figure 11B), as well as a trend toward shortened time to relapse with high miR-106b and high expression of all three miRNA (Supplemental Figure 11A,D). However, miR-25 expression does NOT demonstrate any difference in patient outcome (Supplemental figure 11C), further suggesting that miR-106b and miR-93 are the primary regulators of this response.
To further explore the role of miR-106b and miR-93 in human breast cancers, we also utilized Gene Set Enrichment Analysis (GSEA) to analyze breast tumors that express high levels of miR-106b and miR-93. Of the enriched gene sets, we found high miR-106b and high miR-93 to significantly correlate with stem cell related gene sets 28 as well as EMT enriched gene sets 29, furthering supporting an important role for these miRNAs in mediating stem cell and EMT-like processes in human breast cancer (Supplemental Figure 12).
Discussion
In the present study, we identify a cluster of miRNA previously shown to overcome TGF-β mediated growth inhibition 11, the miR-106b-25 cluster, as a target of Six1. The data herein not only provide a mechanism for how Six1 may silence TGF-β-mediated growth inhibition in breast cancers, but also for how Six1 activates the TGF-β pathway. This is the first demonstration that the same miRNA cluster that overcomes TGF-β mediated growth suppression can in fact also promote TGF-β signaling.
Indeed, we show that miR-106b-25 miRNAs can target the TGF-β inhibitor Smad7, and that upregulation of miR-106b-25 leads to an increase in TβRI. This increase in TβRI likely occurs, at least in part, due to Smad7 downregulation, since Smad7 is known to mediate degradation of the TβRI protein19. Recently, we demonstrated that upregulation of TβRI protein is necessary and sufficient for TGF-β activation and the induction of EMT downstream of Six1 in MCF7 cells 9. Consistent with our previous data demonstrating that TβRI overexpression is sufficient for TGF-β pathway activation, we also observe an activation of this pathway with overexpression of the miR-106b-25 miRNAs alone as evidenced by an increase in transcriptional targets of the TGF-β signaling pathway (Figure 4E).
Several lines of evidence have demonstrated that the miR-106b-25 cluster and its individual miRNAs have pro-oncogenic functions, including mediating pro-proliferative and anti-apoptotic phenotypes 12,13. Our results in this study expand the oncogenic potential of this miRNA cluster by demonstrating for the first time that these miRNA can also induce properties of EMT and TIC characteristics. The EMT changes induced by these miRNA are consistent with the oncogenic EMT phenotype induced by Six1 in MCF7 cells (Figure 5), suggesting that overexpression of the miR-106b-25 miRNAs may partly contribute to the induction of EMT downstream of Six1.
It is well recognized that the induction of EMT leads to an increase in stem/progenitor cell properties 23. Indeed, Six1 transgenic mice whose mammary tumors display features of EMT, also demonstrate an increase in the stem/progenitor cell population.8. Increased expression of the miR-106b-25 miRNAs in the mammary glands of Six1 transgenic mice suggests a possible role for these miRNA in regulation of the stem/progenitor pool (Figure 2C). Our results show for the first time that the miR-106b-25 miRNAs are sufficient to increase TIC capacity, and that they are required for the ability of Six1 to induce TIC characteristics in vivo. Interestingly, many recent studies have implicated the miR-106b-25 cluster in stem/progenitor cell biology 14,30,31. Of interest, it was recently demonstrated that miR-106b and miR-93, along with iPSC transcription factors, can enhance reprogramming of somatic cells into induced pluripotent stem cells 21. The iPSC phenotype, however, is dependent on downregulation of TGF-β signaling, where miR-106b and miR-93 target TβRII (in a mouse embryonic fibroblast context), while the TIC phenotype is known to be associated with an upregulation of TGF-β signaling 23. In this study, we did not observe a downregulation of TβRII in response to Six1 or miR-106b-25 overexpression in MCF7 cells (Supplemental Figure 6)9. However, interestingly, both the iPSC data and our data suggest a role for the miR-106b-25 cluster in the induction of stem cell properties, possibly through their ability to regulate TGF-β signaling in a context dependent, and seemingly opposite, manner.
Since the increase of TIC capacity is known to be associated with tumor recurrence 32, our data is further strengthened by our analysis of miRNA microarray data which demonstrate that high miR-106b and high miR-93 expression significantly correlate with a shortened time to relapse in human breast cancer patients (Figure 7D). Of note, consistent with miRNA induced iPSC data 21, our data also suggests that miR-106b and miR-93 (which are in the same family of miRNA) are the primary inducers of the TIC phenotype as there is no difference in tumor recurrence when only miR-25 is highly expressed (Supplemental Figure 11). Similarly, in breast tumors where both miR-106b and miR-93 are highly expressed together, an enrichment in stem cell and EMT gene signatures can be observed (Supplemental Figure 12), further strengthening the argument that the miR-106b family of miRNAs may be important regulators of EMT and TIC phenotypes in human breast cancer.
Lastly, our data also demonstrate a significant correlation between miR-106b, Six1, and activated TGF-β signaling (nuclear Smad3) in human breast cancers. Critically, we show that tumors that express both high Six1 and high miR-106b have the highest percentage of activated TGF-β signaling (64.7%) (Figure 7). Of note, our data also show that in the presence of high Six1 and low miR-106b expression, 23.5% of tumors have activated TGF-β signaling, as opposed to 5.9% in tumors with low levels of Six1. These data suggest again that Six1 may activate TGF-β signaling through multiple mechanisms, however the marked increase in TGF-β signaling when Six1 and miR-106b are both highly expressed strongly suggests that the Six1/miR-106b-25 axis is critical for activation of TGF-β signaling in human breast cancer.
In closing, these data have important ramifications for breast cancer treatment. Due to the traditional difficulties in targeting transcription factors such as Six1, and the increasing promise for miRNAs as therapeutic targets 33, the miR-106b-25 cluster could prove to be an effective target in cancers that express high levels of Six1. Furthermore, since TGF-β signaling can be tumor suppressive or tumor promotional, depending on context, one of the greatest concerns surrounding the use of TGF-β inhibitors in cancer is how to predict which patients will benefit in clinical trials. Our studies suggest a mechanism that we hypothesize provides a novel molecular explanation for the TGF-β paradox in breast cancers. Namely, examining breast tumors for Six1 and/or miR-106b-25 expression may ultimately provide a means to distinguish patients likely to benefit from TGF-β inhibitors from those who may actually be harmed by such treatments.
Methods
microRNA microarray and mRNA microarray
Total RNA preparations using TRIzol were sent to Thermo Scientific (Waltham, MA, USA) for miRNA microarray analysis. Three clonal isolates of MCF7-Ctrl and MCF7-Six1 RNA samples were submitted in replicates of four. miRNAs in which the difference between the groups (MCF7-Ctrl and MCF7-Six1) was statistically significant (P-value < 0.05) were chosen for follow-up analysis. For mRNA microarray analysis, RNA was prepared in the same way as above, and submitted to the Genomics and Microarray Core at the University of Colorado Denver. The array was performed using the Affymetrix Human Gene 1.0 ST Array. Analysis of microarray results have been described previously 7.
Cell Culture and Constructs
Generation of MCF7-Ctrl and MCF7-Six1 cell lines was described previously34. The MCF7-Cluster cells were generated by inserting the cloned genomic region of the miR-106b-25 cluster into the miR-Express vector (Open Biosystems). Empty vector (EV) and non-silencing (NS) miR-express constructs were obtained from open biosystems. The miRZip-cluster construct contains all three miRNA inhibitors (miRZip-106b, miRZip-93, miRZip-25) in the same vector. MCF7-Ctrl and MCF7-Six1 cells were infected with miRZip lentivirus (System Biosciences, Mountain View, CA, USA). All MCF7 cells were selected with 2.5 μg/mL puromycin.
Real-time PCR
Total RNA was extracted with the miRNeasy RNA isolation kit (Qiagen, Germantown, MD, USA) following the manufacturer’s protocol. For miRNA quantitative analysis, RNA was reverse transcribed using the miscript system (Qiagen), and qPCR was performed with miscript miRNA primers (Qiagen). All miRNA assays were done using ssoFast Evagreen supermix (Biorad, Hercules, CA, USA). For mRNA qPCR, cDNA synthesis was done with iscript (BioRad). Six1, Smad7, and PPIB primers were part of the gene expression assay collection (Applied Biosystems, Carlsbad, CA, USA). All qPCR was performed with the BioRad CFX96. Real-time PCR arrays were acquired from SABiosciences (RT2 Profiler PCR array, PAHS-235, PAHS-405, and PAHS-090), and performed according to manufacturer instructions.
Western Blot and Immufluorescence
For Western blots, whole cell lysates were isolated using RIPA buffer as previously described34. Antibodies used include: E-cadherin (BD Biosciences, Franklin Lakes, NJ, USA), β-catenin (BD Biosciences), TβRI (SCBT, Santa Cruz, CA), TβRII (Cell Signaling, Danvers, MA, USA), p-Smad3 (Cell Signaling), total Smad3 (Invitrogen), Bim (Cell Signaling), p21 (Cell Signaling), β-Actin (sigma-aldrich, St. Louis, MO, USA), and β-Tubulin (Invitrogen). Cell fractionation was performed as previously described 35. Immunofluorescence was performed as previously described7 with E-cadherin and β-catenin antibodies above.
Cell Adhesion Assay
Cells were plated in 96-well plates coated with Collagen I, Collagen IV, Laminin, or Fibronectin (BD Biocoat, BD Biosciences), and assays were carried out as previously described 7.
In-situ hybridization and Immunohistochemistry
Breast cancer tissue array (BRC711) was purchased from US Biomax Inc. ISH was performed with double DIG labeled miRNA LNA probes (Exiqon) using manufacturer’s one-day protocol36. Modifications include 15ug/mL Proteinase K for 8 minutes, overnight hybridization with 40nM of LNA probe, and a formamide containing hybridization buffer (50% Formamide, 5x SSC, 0.1% Tween, 50 μg/mL Heparin, 500 μg/mL yeast tRNA) at hybridization temperatures 30 degrees below the RNA Tm. Detection was achieved with BM purple (Roche, Basel, Switzerland) solution, and slides were counterstained in Nuclear Fast Red (Poly Scientific). Slides were scored on a 0–4 scale with 4 representing the most intense staining. Scores were assigned independently by 3 individuals in a blinded manner and averaged. Serial sections of tumor arrays were previously stained and scored by a pathologist on a scale of 0–4 for nuclear Six1 (1:100; Atlas antibodies) and nuclear Smad3 (5 μg/mL; Zymed), using IHC protocols previously described 7.
Luciferase assays
The 3′UTR of Smad7 was cloned into the psi-Check2 luciferase reporter (Promega, Madison, WI, USA). For perfect target luciferase assays, the exact complement sequence of each miRNA was also cloned into the psi-Check2 luciferase reporter. Constructs were transfected using Lipofectamine 2000 transfection reagent (Invitrogen), and at 48 hours lysates were prepared and analyzed using the dual luciferase assay following the manufacturer’s protocol (Promega). TOP-flash reporter assays were done as described previously 7 All luciferase assays were analyzed on the Modulus Microplate reader (Turner Biosystems).
TIC assays
Flow cytometry analysis and tumorshpere formation assays were performed as described previously 24 For in vivo tumor initiation assays, cells were counted and serially diluted in 100μl of 1:1 PBS/Matrigel (#354234, BD Biosciences). Diluted cells were injected underneath the nipple of the number 4 mammary fat pad of 6-week old female NOD/SCID mice. Tumor formation was monitored weekly by palpation. The frequency of tumorigenic cells was estimated at a setting of 95% confidence using the ELDA software application at http://bioinf.wehi.edu.au/software/elda/. All animal studies were performed according to protocols reviewed and approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver.
Analysis of miRNA microarray data
miRNA expression and clinical outcome data was acquired from a publically available dataset of 216 early-invasive primary breast cancers27(GSE22220). All samples were median-centered for each miRNA expression, and denoted high expression if above the median and low expression if below the median. Kaplan-Meier curves for tumors expressing both high miR-106b and high miR-93 were generated using WinStat for Excel (R Fitch Software, A-Prompt Corp, Whitehall, PA, USA). GSEA analysis methods are included in the supplementary information.
Supplementary Material
Acknowledgments
Financial support: This work was funded by grants from the National Cancer Institute (2ROI-CA095277) and The American Cancer Society (#RSG-07-183-01-DDC) to H.L.F. A.L.S is funded by a predoctoral fellowship from the Department of Defense Breast Cancer Research Program (W81XWH-10-1-0296). D.S.M. was funded by a predoctoral fellowship from the Department of Defense Breast Cancer Research Program (W81XWH-06-1-0757).
The authors acknowledge the strong support of the University of Colorado Cancer Center for use of their core facilities (Flow Cytometry Core and DNA Sequencing and Analysis Core), as well as to the Genomics and Microarray Core at the University of Colorado. We would also like to thank Dr. Paul Jedlicka for input and critical reading of the manuscript.
Footnotes
Conflict of Interest
The authors declare that no conflict of interest exists
Supplementary information is available at Oncogene’s website.
References
- 1.Massagué J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inman GJ. Switching TGFβ from a tumor suppressor to a tumor promoter. Current Opinion in Genetics & Development. 2011;21:93–99. doi: 10.1016/j.gde.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Tian M, Schiemann WP. The TGF-β paradox in human cancer: an update. Future Oncology. 2009;5:259–271. doi: 10.2217/14796694.5.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc Natl Acad Sci USA. 1998;95:12608–12613. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coletta RD, et al. Six1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer Research. 2008;68:2204–2213. doi: 10.1158/0008-5472.CAN-07-3141. [DOI] [PubMed] [Google Scholar]
- 6.Reichenberger KJ, Coletta RD, Schulte AP, Varella-Garcia M, Ford HL. Gene amplification is a mechanism of Six1 overexpression in breast cancer. Cancer Research. 2005;65:2668–2675. doi: 10.1158/0008-5472.CAN-04-4286. [DOI] [PubMed] [Google Scholar]
- 7.Micalizzi DS, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-β signaling. J Clin Invest. 2009;119:2678–2690. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCoy EL, et al. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J Clin Invest. 2009;119:2663–2677. doi: 10.1172/JCI37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micalizzi DS, Wang CA, Farabaugh SM, Schiemann WP, Ford HL. Homeoprotein Six1 Increases TGF- Type I Receptor and Converts TGF- Signaling from Suppressive to Supportive for Tumor Growth. Cancer Research. 2010;70:10371–10380. doi: 10.1158/0008-5472.CAN-10-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He L, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrocca F, et al. E2F1-Regulated MicroRNAs Impair TGFβ-Dependent Cell-Cycle Arrest and Apoptosis in Gastric Cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, et al. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009;100:1234–1242. doi: 10.1111/j.1349-7006.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- 13.Kan T, et al. The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Gastroenterology. 2009;136:1689–1700. doi: 10.1053/j.gastro.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brett JO, Renault VM, Rafalski VA, Webb AE, Brunet A. The microRNA cluster miR-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging (Albany NY) 2011;3:108–124. doi: 10.18632/aging.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poliseno L, et al. Identification of the miR-106b~25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal. 2010;3:ra29. doi: 10.1126/scisignal.2000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chopra VS, Mishra RK. Mir”acles in hox gene regulation. Bioessays. 2006;28:445–448. doi: 10.1002/bies.20401. [DOI] [PubMed] [Google Scholar]
- 17.Hu YL, Fong S, Largman C, Shen WF. HOXA9 regulates miR-155 in hematopoietic cells. 2010;38:5472–5478. doi: 10.1093/nar/gkq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang L, et al. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-β8. Oncogene. 2011;30:806–821. doi: 10.1038/onc.2010.465. [DOI] [PubMed] [Google Scholar]
- 19.Yan X, Liu Z, Chen Y. Regulation of TGF-β signaling by Smad7. Acta Biochimica et Biophysica Sinica. 2009;41:263–272. doi: 10.1093/abbs/gmp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan X, Chen YG. Smad7: not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochem J. 2011;434:1–10. doi: 10.1042/BJ20101827. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Yang CS, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. EMBO J. 2011;30:823–834. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padua D, et al. TGFβ Primes Breast Tumors for Lung Metastasis Seeding through Angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farabaugh SM, Micalizzi DS, Jedlicka P, Zhao R, Ford HL. Eya2 is required to mediate the pro-metastatic functions of Six1 via the induction of TGF-β signaling, epithelial-mesenchymal transition, and cancer stem cell properties. Oncogene. 2011 doi: 10.1038/onc.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 27.Buffa FM, et al. microRNA-Associated Progression Pathways and Potential Therapeutic Targets Identified by Integrated mRNA and microRNA Expression Profiling in Breast Cancer. Cancer Research. 2011;71:5635–5645. doi: 10.1158/0008-5472.CAN-11-0489. [DOI] [PubMed] [Google Scholar]
- 28.Wong DJ, et al. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarrió D, et al. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Research. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 30.Qian S, et al. MicroRNA expression profile of bronchioalveolar stem cells from mouse lung. Biochem Biophys Res Commun. 2008;377:668–673. doi: 10.1016/j.bbrc.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 31.Ho J, et al. The pro-apoptotic protein Bim is a microRNA target in kidney progenitors. J Am Soc Nephrol. 2011;22:1053–1063. doi: 10.1681/ASN.2010080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nana-Sinkam SP, Croce CM. MicroRNAs as therapeutic targets in cancer. 2011;157:216–225. doi: 10.1016/j.trsl.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Ford HL, et al. Cell cycle-regulated phosphorylation of the human SIX1 homeodomain protein. J Biol Chem. 2000;275:22245–22254. doi: 10.1074/jbc.M002446200. [DOI] [PubMed] [Google Scholar]
- 35.Shtutman M, Levina E, Ohouo P, Baig M, Roninson IB. Cell Adhesion Molecule L1 Disrupts E-Cadherin-Containing Adherens Junctions and Increases Scattering and Motility of MCF7 Breast Carcinoma Cells. Cancer Research. 2006;66:11370–11380. doi: 10.1158/0008-5472.CAN-06-2106. [DOI] [PubMed] [Google Scholar]
- 36.Jørgensen S, Baker A, Møhller S, Nielsen BS. Robust one-day in situ hybridization protocol for detection of microRNAs in paraffin samples using LNA probes. Methods. 2010;52:375–381. doi: 10.1016/j.ymeth.2010.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.