Abstract
Purpose
While the use of quality of life (QoL) assessment has been increasing in clinical oncology, few studies have examined its prognostic significance in prostate cancer. We investigated the association between QoL at presentation and survival in prostate cancer.
Methods
We retrospectively reviewed 673 patients treated at two single-system cancer centers between January 2001 and December 2008. QoL was evaluated using EORTC-QLQ-C30. Patient survival was defined as the time interval between the date of first patient visit and the date of death/date of last contact. Univariate and multivariate Cox regression was performed to evaluate the prognostic significance of QoL.
Results
Mean age at presentation was 63.2 years. Patient stage of disease at diagnosis was I, 4; II, 464; III, 76; IV, 107; and 22 indeterminate. Median overall survival was 89.1 months (95% CI: 46.1–132.0). QoL scales predictive of survival upon univariate analysis were physical, role, emotional, social, fatigue, nausea/vomiting, pain, dyspnea, insomnia, loss of appetite, and constipation (p < 0.01 for all). Multivariate analyses found fatigue (p = 0.02) and constipation (p = 0.01) to be significantly associated with survival.
Conclusions
Baseline QoL provides useful prognostic information in prostate cancer. These findings have important implications for patient stratification in clinical trials and may aid decision making in clinical practice.
Keywords: Quality of life, Prostate cancer, Prognosis
Introduction
Prostate cancer is the most common cancer in men in North America [1–5]. It is also the second leading cause of cancer death among US men after lung cancer [6]. Each year in the USA, approximately 220,000 new cases of prostate cancer are diagnosed, and 30,000 men die of the disease [7]. Despite its high morbidity, the etiology of prostate cancer remains largely unknown [6]. Tumor stage and Gleason score remain the two most powerful prognostic factors in prostate cancer [8]. More recently, quality of life (QoL) has been suggested to be a prognostic indicator in prostate cancer; however, there are only a few published studies reporting upon this association in the medical and scientific literature [9–12].
QoL is a multidimensional construct. A growing consensus among health care providers and researchers is that treatment efficacy should be judged by effects on both quantity and QoL; this has led to the inclusion of QoL assessment as a primary endpoint in cancer clinical trials along with traditional endpoints of tumor response and survival. Most studies measuring QoL in cancer patients compare the effects of different treatments or assess the effect of a single treatment longitudinally. There is general agreement in the medical and scientific research community that patients are the best source of information regarding their QoL. Consequently, the use of self-reported QoL assessment has become a valuable tool for both clinical practice and research. There are extensive data in the literature showing that QoL tools measuring the activities of daily life can predict survival in several different types of cancers independent of the extent of the disease and other clinical prognostic factors [9, 13–30]. These studies have used different combinations of clinical and QoL factors in multivariate models evaluating the prognostic significance on clinical outcomes.
Although there are few studies which demonstrate the relationship between QoL and survival in patients with prostate cancer [9–12], there is no “gold standard” QoL questionnaire available. Rather, the selection of a QoL questionnaire for any particular study is governed by the research goals of the study. The most commonly used instrument for evaluating the QoL of cancer patients is the European Organization for the Research and Treatment of Cancer Core Quality of Life Questionnaire (QLQ-C30), which emphasizes a patient's capacity to function well [31–33]. In the current study, we have employed the QLQ-C30 to investigate its efficacy in predicting survival in prostate cancer patients treated with an integrative model of care combining surgery, radiation, hormonal, and chemotherapy as appropriate, plus complementary therapy consisting primarily of nutritional, psychosocial, and spiritual support, naturopathic supplements, pain management, and physical therapy/rehabilitation.
Materials and methods
Study sample
We retrospectively examined 673 histologically confirmed prostate cancer patients treated at Cancer Treatment Centers of America® at Midwestern (MRMC) and Southwestern (SRMC) Regional Medical Centers between January 2001 and December 2008. None of these patients had received any treatment at our hospitals when contacted to participate in this investigation. The inclusion criteria for participation in this study were a histological diagnosis of prostate cancer and the ability to read English. Patients with all stages of prostate cancer were eligible for the study. Patients were excluded if they were unable to give informed consent or were unable to understand or cooperate with study conditions.
A trained clinical coordinator was responsible for determining eligibility, describing the study, and obtaining written informed consent. Participation in this study was voluntary and all patients were assured that refusal to participate would not affect their future care in any way. Patients were not incentivized to participate in the study. Patients who chose to participate were presented with the questionnaire at their initial visit and instructed to return their completed questionnaires to the clinical coordinator within 24 h; thus, patients completed questionnaires prior to receiving therapy at our facility.
Additional patient data recorded for this study was age at presentation (current age), stage of disease at diagnosis, and prior treatment history. The only follow-up information required was the date of death or the date of last contact/last known to be alive. This study was approved by the Institutional Review Boards at MRMC and SRMC.
QoL assessment
QoL was assessed using the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30), which emphasizes a patient's capacity to fulfill the activities of daily living. The QLQ-C30 is a 30-item cancer-specific questionnaire that incorporates five functioning scales (physical, role, cognition, emotional, and social), nine symptom scales (fatigue, pain, and nausea/vomiting, dyspnea, insomnia, loss of appetite, constipation, diarrhea, financial problems), and a global health status/QoL scale. The raw scores are linearly transformed to give standard scores in the range of 0–100 for each of the functioning and symptom scales. Higher scores in the global and functioning scales and lower scores in the symptom scales indicate better QoL. A difference of 5–10 points in the scores represents a small change, 10–20 points a moderate change, and greater than 20 points a large clinically significant change from the patient's perspective [34]. This instrument has been judged to be reliable and valid as a result of extensive testing in a wide range of clinical cancer populations [35–37].
Prespecified baseline clinical factors
Baseline clinical factors that were assessed for prognostic significance were current age, stage of disease at diagnosis, and prior treatment history. Stage at diagnosis was categorized into two groups consisting of locoregional (stages I–III) and metastatic (stage IV) disease. The prior treatment history variable categorized the patients into those who have received definitive cancer treatment elsewhere before coming to our institution and those who were newly diagnosed at the time of presentation to our institution.
Data analysis and statistical methods
Patient survival was the primary endpoint and defined as the time interval between the date of first patient visit to the hospital and the date of death from any cause or the date of last contact/last known to be alive. Survival data were obtained from the tumor registries of the Midwestern and Southwestern Regional Medical Centers of Cancer Treatment Centers of America.
The overall survival was calculated using the Kaplan–Meier or product-limit method. Clinical and QoL variables were also evaluated using univariate Cox regression analyses to determine which parameters showed individual prognostic value for survival. Multivariate Cox regression analyses were then performed to evaluate the joint prognostic significance of those QoL and clinical factors that were shown to be prognostic in univariate analyses. We used both forward stepwise method as well as the block entry method (all variables entered together at the same time in one block). Forward stepwise method was used because, as is common in QoL data, many of the individual QoL scales are highly correlated. Stepwise regression avoids the problem of multicollinearity because two highly correlated attributes will normally not both be entered in the model.
Cox regression with time-invariant covariates assumes that the ratio of hazards for any two groups remains constant in proportion over time. We checked this assumption by first examining log-minus-log plots for the categorical predictors and then fitting a Cox regression with a time-varying covariate for each predictor in turn. Each QLQ-C30 scale was treated as a continuous variable for the purpose of Cox regression analyses. The effect of QoL parameters on patient survival was expressed as hazard ratios (HRs) with 95% confidence intervals. Pearson's correlation coefficients were used to investigate the association between different QoL variables. An effect was considered to be statistically significant if the p value was less than or equal to 0.05. All data were analyzed using SPSS version 17.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
A total of 767 prostate cancer patients were invited to participate in the study. Of those, 673 responded, resulting in an acceptance rate of 87.7%. Table 1 describes the baseline characteristics of our patient cohort. Mean age at presentation was 63.2 years. Patient stage of disease at diagnosis was I, 4; II, 464; III, 76; IV, 107; and 22 indeterminate. Four hundred thirty-two patients were newly diagnosed at our hospital, while 241 were previously treated elsewhere. At the time of this analysis, 77 patients had expired. Table 2 describes the means, medians, and standard deviations of QLQ-C30 scale scores. Among the QLQ-C30 functioning scales, emotional functioning had the lowest (worst) mean score of 74.9, while the highest (best) mean score of 83.4 was recorded for physical functioning. Among the QLQ-C30 symptom scales, nausea/vomiting had the lowest (best) mean score of 6.9, while the highest (worst) mean score of 28.5 was recorded for insomnia.
Table 1.
Baseline characteristics of 673 prostate cancer patients
| Characteristic | Categories | Number | Percent |
|---|---|---|---|
| Age at presentation (years) | • Mean | 63.2 | |
| • Median | 62.8 | ||
| • Range | 43.7–87.6 | ||
| Tumor stage at diagnosis | • Stage 1 | 4 | 0.6 |
| • Stage 2 | 464 | 68.9 | |
| • Stage 3 | 76 | 11.3 | |
| • Stage 4 | 107 | 15.9 | |
| • Indeterminate | 22 | 3.3 | |
| Vital status | • Expired | 77 | 11.4 |
| • Alive | 596 | 88.6 | |
| Treatment history | • Newly diagnosed (analytic) | 432 | 64.2 |
| • Previously treated (non-analytic) | 241 | 35.8 |
Table 2.
Baseline QoL scores of 673 prostate cancer patients
| QLQ-C30 scale | Mean | Median | SD | Range |
|---|---|---|---|---|
| Global | 67.2 | 75.0 | 26.7 | 0–100 |
| Physical | 83.4 | 93.3 | 22.7 | 0–100 |
| Role | 79.8 | 100 | 30.2 | 0–100 |
| Emotional | 74.9 | 75 | 23.6 | 0–100 |
| Cognitive | 82.3 | 83.3 | 22.2 | 0–100 |
| Social | 79.0 | 100 | 28.7 | 0–100 |
| Fatigue | 27.9 | 22.2 | 26.9 | 0–100 |
| Nausea/vomiting | 6.9 | 0 | 16.6 | 0–100 |
| Pain | 24.7 | 16.7 | 30.6 | 0–100 |
| Dyspnea | 15.1 | 0 | 22.9 | 0–100 |
| Insomnia | 28.5 | 33.3 | 30.2 | 0–100 |
| Appetite loss | 13.5 | 0 | 25.6 | 0–100 |
| Constipation | 13.8 | 0 | 24.7 | 0–100 |
| Diarrhea | 8.5 | 0 | 18.9 | 0–100 |
Table 3 describes the distribution of QoL scores by stage of disease and prior treatment history. Mean global QoL score was significantly higher for patients with stages I–III disease (69.1) than stage IV disease (57.3); p < 0.001. Mean global QoL score was 71.5 and 59.4 for analytic and non-analytic disease, respectively, p < 0.001.
Table 3.
Distribution of QoL scores by stage of disease and prior treatment history
| QLQ-C30 scale | Stage of disease | Treatment history | ||||
|---|---|---|---|---|---|---|
| Locoregional (N = 544) | Metastatic (N = 107) | P value | Analytic (N = 432) | Non-analytic (N = 241) | P value | |
| Global | 69.1 | 57.3 | <0.001 | 71.5 | 59.4 | <0.001 |
| Physical | 84.7 | 77.3 | 0.002 | 86.9 | 77.0 | <0.001 |
| Role | 82.4 | 67.6 | <0.001 | 84.7 | 71.0 | <0.001 |
| Emotional | 75.6 | 70.5 | 0.04 | 76.5 | 72.0 | 0.02 |
| Cognitive | 82.8 | 79.9 | 0.22 | 84.1 | 78.9 | 0.004 |
| Social | 80.9 | 68.1 | <0.001 | 82.8 | 72.3 | <0.001 |
| Fatigue | 25.5 | 40.2 | <0.001 | 23.8 | 35.5 | <0.001 |
| Nausea/vomiting | 6.0 | 11.8 | 0.001 | 4.8 | 10.6 | <0.001 |
| Pain | 21.6 | 39.4 | <0.001 | 19.4 | 34.2 | <0.001 |
| Dyspnea | 14.9 | 14.3 | 0.82 | 13.2 | 18.4 | 0.005 |
| Insomnia | 27.5 | 34.6 | 0.03 | 26.6 | 32.0 | 0.03 |
| Appetite loss | 11.6 | 22.1 | <0.001 | 9.0 | 21.4 | <0.001 |
| Constipation | 12.5 | 20.6 | 0.002 | 10.1 | 20.5 | <0.001 |
| Diarrhea | 8.3 | 8.7 | 0.85 | 7.8 | 9.8 | 0.20 |
Univariate analysis: prognostic factors for overall survival
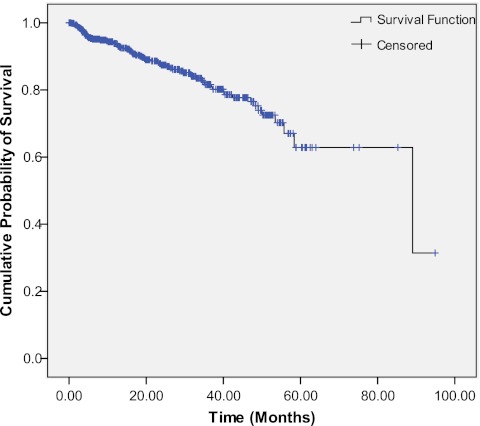
Median overall survival for the entire patient cohort was 89.1 months (95% CI 46.1–132.0 months) as depicted in Fig. 1. The median survival for analytic and non-analytic patients was 81.4 and 45.2 months, respectively, p < 0.001. The median survival for patients with locoregional and metastatic disease was 89.1 and 40.1 months, respectively, p < 0.001.
Fig. 1.
Overall survival curve. Each drop in the probability curve indicates one or more events. Vertical lines indicate censored patients, i.e., those who reached the end of their follow-up without experiencing death
Table 4 describes the results of univariate Cox regression analyses for each QLQ-C30 scale as well as age, stage of disease, and analytic status. The Cox proportional hazard relative risks (RRs) along with their 95% confidence intervals for every 1-U increase in all QLQ-C30 scales are given. On univariate analysis, QoL scales predictive of survival upon univariate analysis were physical, role, emotional, social, fatigue, nausea/vomiting, pain, dyspnea, insomnia, loss of appetite, and constipation (p < 0.01 for all). Age, stage of disease, and prior treatment history were also found to be significant predictors of survival upon univariate analysis (p < 0.01 for all).
Table 4.
Univariate Cox regression analysis for overall survival
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Physical | 0.976 | 0.969–0.984 | <0.001 |
| Role | 0.981 | 0.976–0.987 | <0.001 |
| Emotional | 0.985 | 0.977–0.994 | 0.001 |
| Cognitive | 0.992 | 0.982–1.002 | 0.12 |
| Social | 0.985 | 0.978–0.991 | <0.001 |
| Fatigue | 1.024 | 1.017–1.032 | <0.001 |
| Nausea/vomiting | 1.019 | 1.011–1.028 | <0.001 |
| Pain | 1.018 | 1.012–1.025 | <0.001 |
| Dyspnea | 1.013 | 1.005–1.021 | 0.002 |
| Insomnia | 1.009 | 1.002–1.016 | 0.013 |
| Appetite loss | 1.022 | 1.016–1.028 | <0.001 |
| Constipation | 1.022 | 1.015–1.028 | <0.001 |
| Diarrhea | 1.008 | 0.998–1.018 | 0.12 |
| Age at presentation | 1.053 | 1.024–1.083 | <0.001 |
| Stage at diagnosis (locoregional disease as reference) | 5.8 | 3.6–9.2 | <0.001 |
| Prior treatment history (analytic class as reference) | 7.4 | 4.6–11.9 | <0.001 |
Multivariate analysis: prognostic factors for overall survival
Table 5 describes the results of multivariate Cox regression analyses using the block entry method for those QLQ-C30 scales that were significant upon univariate analysis after controlling for the effects of age, tumor stage, and prior treatment history. Multivariate analyses found fatigue (p = 0.02) and constipation (p = 0.01) to be significantly associated with survival independent of other QoL scales, age, stage, and treatment history such that patients with lower (better) fatigue and constipation scores had better survival. Age, stage of disease, and prior treatment history were also found to be significant predictors in the final multivariate model (p < 0.01 for all). The findings described above were confirmed using the forward stepwise method.
Table 5.
Multivariate Cox regression analysis for overall survival
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Physical | 0.985 | 0.971–1.00 | 0.06 |
| Role | 1.004 | 0.990–1.018 | 0.62 |
| Emotional | 1.003 | 0.990–1.016 | 0.66 |
| Social | 1.005 | 0.994–1.017 | 0.36 |
| Fatigue | 1.017 | 1.001–1.034 | 0.03 |
| Nausea/vomiting | 0.994 | 0.981–1.008 | 0.39 |
| Pain | 0.997 | 0.986–1.008 | 0.62 |
| Dyspnea | 0.996 | 0.985–1.007 | 0.46 |
| Insomnia | 1.004 | 0.994–1.013 | 0.45 |
| Appetite loss | 1.008 | 0.998–1.019 | 0.12 |
| Constipation | 1.012 | 1.003–1.022 | 0.01 |
| Age at presentation | 1.033 | 1.005–1.062 | 0.02 |
| Stage at diagnosis (locoregional disease as reference) | 4.9 | 2.8–8.5 | <0.001 |
| Prior treatment history (analytic class as reference) | 4.3 | 2.5–7.4 | <0.001 |
Discussion
Carcinoma of the prostate continues to be a major health problem in the USA. Newly diagnosed prostate cancers are being detected at an early stage in men presenting with no symptoms with abnormal prostate-specific antigen (PSA) level. As more and more men are being diagnosed with prostate cancer worldwide, knowledge about the etiology and prognosis of this disease is important. Prognostic factors are essential not only in understanding the natural history and the course of the disease but also to predict possible outcomes of different treatments, or perhaps no treatment at all. This is extremely important in a disease such as prostate cancer, in which there is clear evidence that a substantial number of cases discovered by PSA testing are unlikely to ever become clinically significant [38]. In addition to the known prognostic factors, such as tumor stage and Gleason score, QoL has recently emerged as a potential prognostic factor in patients with prostate cancer. The current study was undertaken to investigate whether patient QoL, as measured by the QLQ-C30, could predict survival in prostate cancer.
We chose QLQ-C30 as a valid and a reliable tool to assess patient QoL. The QLQ-C30 developers concentrated on the patients' ability to fulfill the activities of daily life. This emphasis is understandable because it was developed for clinical trials investigating new drugs or novel combinations of agents. Clinical practitioners and clinical investigators need to know what happens to a patient's capacity to fulfill the activities of daily life at work and in the home. Consequently, this instrument has an extensive physical functioning scale and has many questions on clinical symptoms as well. Furthermore, the QLQ-C30 social and role functioning scales provide additional information on an individual's physical functioning.
In this study, we found that fatigue and constipation were predictive of survival independent of the effects of age at presentation, stage of disease, and treatment history. Fatigue is the most frequently reported symptom in cancer patients [39–44]. An estimated 60–96% of cancer patients undergoing treatment experience fatigue, including 60–93% of patients on radiotherapy, and 80–96% of patients on chemotherapy [43, 45]. Several studies have demonstrated the adverse impacts of fatigue on physical, emotional, economic, and social aspects of cancer patients' lives [46–51]. The principal finding of our current study, that fatigue independently predicts patient survival in prostate cancer, substantially augments its importance as a health care measure to evaluate and address.
The association between QoL and subsequent prognosis has been demonstrated in a few studies of patients with prostate cancer. A study by Sullivan PW et al. in metastatic hormone-refractory prostate cancer (HRPC) reported that patients with better baseline health-related quality of life (HRQL) have better predicted survival, time to disease progression, and pain prognosis than those with worse HRQL. Change in HRQL (12 weeks) improved the predictive accuracy for most clinical outcomes [12]. In an analysis of data from three randomized phase III multicenter trials, Halabi S et al. reported statistically significant association between pain and survival in castration-refractory prostate cancer. The median survival times were 17.6 months (95% CI, 16.1–19.1 months) and 10.2 months (95% CI, 8.6–11.3 months; p < 0.001) in men with low (<17) and high (≥17) pain scores, respectively [10]. Another study found that baseline patient satisfaction with health and physical subscale, psychological and spiritual subscale, family subscale, and overall HRQL are predictive of survival in patients with prostate cancer. After adjusting for the effects of treatment history and Gleason score, patient satisfaction with health and physical subscale was found to be significantly associated with survival (p = 0.04) [11]. A pooled analysis of data from three randomized control trials of metastatic HRPC indicated that bone scan result (p < 0.0001), hemoglobin level (p < 0.0001), performance status (p = 0.0322), insomnia (p = 0.002), and appetite loss (p = 0.0015) were independent predictors of survival [9]. While the findings of our study are similar to those reported by the above researchers, direct comparisons between the studies is not possible because of the differences in QoL instruments used, study methodology, and the clinical and demographic factors controlled for in the analyses.
Furthermore, it is important to recognize that the integrative treatment model with its emphasis on aggressive nutritional support, naturopathic therapy, and mind/body medicine in conjunction with conventional prostate cancer therapy which was used to treat the patients investigated in the current study, has the potential for favorably modifying QoL elements. Thus, it is at least conceivable that various parameters such as global performance status and appetite loss are less predictive of prognosis in patients treated with an integrative approach than with conventional therapy only. This issue warrants a great deal more investigation as it holds the potential for demonstrating not only the value of QoL in predicting outcome, but also the elements of QoL most sensitive to modulation by integrative cancer treatment and the effects of such modulation on clinical outcome.
Thus, the results of this study may have important implications for both clinical and research practices in an integrative oncologic care setting. They suggest that health care professionals should evaluate baseline fatigue in all patients and take this into consideration when planning treatment. Fatigue should also be assessed regularly during treatment and appropriate intervention taken to improve fatigue when indicated. The utility of this approach to patient management, based on the findings described in this study, would be validated definitively if interventions that enhance fatigue are shown to enhance survival. This hypothesis should be tested in prospective studies employing the QLQ-C30 subscales in prostate cancer populations.
Although this study presents interesting findings and additional questions for further inquiry, several limitations require acknowledgment. Our study, because of its retrospective nature, relies on data not primarily meant for research. As a result, we could not control for some additional factors in our analyses that could influence survival such as race, medical comorbidities, socioeconomic factors, support system, exercise, and educational level. The patient cohort was limited only to those patients who were English speakers and therefore is not representative of the complete spectrum of cancer patients. The largest cohort of patients had stage II disease at presentation to our hospital, and thus, our findings may not be applicable to all stages of prostate cancer, an issue that needs further investigation. Moreover, this study is not able to reveal causative relationships between any QoL element and patient survival. Rather, QoL was found to act as a surrogate marker for otherwise undetected prognostic factors [15]. We did not control for the multiple comparisons made in this study, but this is acceptable for hypothesis-generating studies [28]. We used a generic QoL questionnaire as opposed to a prostate cancer-specific QoL tool. This is because we have been collecting QoL information at our center as part of our ongoing effort to systematically evaluate and address patients' self-reported QoL. The current study is a retrospective analysis of our routinely collected QoL data on our prostate cancer patients. While patients' self-assessment of their own health status provides a good and strong indicator of their prognosis independent of previously known traditional biomedical parameters, opinions vary as to whether patient self-assessment of QoL is necessary or physician assessment is adequate. There is skepticism about the validity and utility of self-reported QoL measures. Moreover, some clinicians are reluctant to use self-reported QoL measures because they believe that they can obtain all the pertinent information they will need during the clinical assessment of the patient. Also self-assessment of QoL could be challenging for patients who are unable to understand or cooperate with study conditions.
Nevertheless, this study also has several strengths, including complete data for all QLQ-C30 subscales for the entire study sample, high compliance with completion of the questionnaire, a reasonably homogeneous population of patients, most of whom had stage II prostate cancer at presentation to our hospitals, the use of a valid and reliable QoL instrument, comprehensive documented clinical parameters in nearly all patients, and availability of mature and reliable survival data.
Conclusion
This study suggests that baseline QoL provides useful prognostic information in prostate cancer patients subjected to integrative oncologic care. In particular, fatigue and constipation were independently predictive of survival. Given the greatly increased use of nutritional supplements and other types of complementary treatments practiced by prostate cancer patients following their initial diagnosis, these findings illustrate the value of QoL assessments for prognosis, clinical trial design, best clinical practice validation, and optimization of integrative models of care.
Acknowledgments
We thank Norine Oplt and Carol Wages for providing us with reliable and updated survival data. This study was funded by Cancer Treatment Centers of America®.
Conflict of interest
None. The authors have full control of all primary data and they agree to allow the journal to review their data if requested.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Donald P. Braun, Phone: +1-847-8724040, FAX: +1-847-7314134, Email: donald.braun@ctca-hope.com
Digant Gupta, Phone: +1-847-7464322, FAX: +1-847-7464329, Email: gupta_digant@yahoo.com.
References
- 1.http://www.cancer.gov/cancertopics/types/prostate. Retrieved February 17, 2011
- 2.Brawley OW, Barnes S. The epidemiology of prostate cancer in the United States. Semin Oncol Nurs. 2001;17:72–77. doi: 10.1053/sonu.2001.23056. [DOI] [PubMed] [Google Scholar]
- 3.Chodak G. Prostate cancer: epidemiology, screening, and biomarkers. Rev Urol. 2006;8(Suppl 2):S3–S8. [PMC free article] [PubMed] [Google Scholar]
- 4.Damber JE. Prostate cancer: epidemiology and risk factors. Curr Opin Urol. 1998;8:375–380. doi: 10.1097/00042307-199809000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 6.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 7.Wilt TJ. Prostate cancer: epidemiology and screening. Rev Urol. 2003;5(Suppl 6):S3–S9. [PMC free article] [PubMed] [Google Scholar]
- 8.Sartor O. Endpoints in prostate cancer clinical trials. Urology. 2002;60:101–107. doi: 10.1016/S0090-4295(02)01585-6. [DOI] [PubMed] [Google Scholar]
- 9.Collette L, et al. Is baseline quality of life useful for predicting survival with hormone-refractory prostate cancer? A pooled analysis of three studies of the European Organisation for Research and Treatment of Cancer Genitourinary Group. J Clin Oncol. 2004;22:3877–3885. doi: 10.1200/JCO.2004.07.089. [DOI] [PubMed] [Google Scholar]
- 10.Halabi S, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26:2544–2549. doi: 10.1200/JCO.2007.15.0367. [DOI] [PubMed] [Google Scholar]
- 11.Lis CG, Gupta D, Grutsch JF. Patient satisfaction with health-related quality of life: implications for prognosis in prostate cancer. Clin Genitourin Canc. 2008;6:91–96. doi: 10.3816/CGC.2008.n.014. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan PW, Nelson JB, Mulani PM, Sleep D. Quality of life as a potential predictor for morbidity and mortality in patients with metastatic hormone-refractory prostate cancer. Qual Life Res. 2006;15:1297–1306. doi: 10.1007/s11136-006-0003-2. [DOI] [PubMed] [Google Scholar]
- 13.Blazeby JM, Brookes ST, Alderson D. Prognostic value of quality of life scores in patients with oesophageal cancer. Br J Surg. 2000;87:362–373. doi: 10.1046/j.1365-2168.2000.01383-8.x. [DOI] [PubMed] [Google Scholar]
- 14.Coates A, et al. Prognostic value of quality-of-life scores during chemotherapy for advanced breast cancer. Australian New Zealand Breast Cancer Trials Group. J Clin Oncol. 1992;10:1833–1838. doi: 10.1200/JCO.1992.10.12.1833. [DOI] [PubMed] [Google Scholar]
- 15.Coates A, Porzsolt F, Osoba D. Quality of life in oncology practice: prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Canc. 1997;33:1025–1030. doi: 10.1016/S0959-8049(97)00049-X. [DOI] [PubMed] [Google Scholar]
- 16.Coates AS, et al. Quality-of-life scores predict outcome in metastatic but not early breast cancer. International Breast Cancer Study Group. J Clin Oncol. 2000;18:3768–3774. doi: 10.1200/JCO.2000.18.22.3768. [DOI] [PubMed] [Google Scholar]
- 17.Dancey J, et al. Quality of life scores: an independent prognostic variable in a general population of cancer patients receiving chemotherapy. The National Cancer Institute of Canada Clinical Trials Group. Qual Life Res. 1997;6:151–158. doi: 10.1023/A:1026442201191. [DOI] [PubMed] [Google Scholar]
- 18.Dharma-Wardene M, et al. Baseline FACT-G score is a predictor of survival for advanced lung cancer. Qual Life Res. 2004;13:1209–1216. doi: 10.1023/B:QURE.0000037481.36604.eb. [DOI] [PubMed] [Google Scholar]
- 19.Earlam S, Glover C, Fordy C, Burke D, Allen-Mersh TG. Relation between tumor size, quality of life, and survival in patients with colorectal liver metastases. J Clin Oncol. 1996;14:171–175. doi: 10.1200/JCO.1996.14.1.171. [DOI] [PubMed] [Google Scholar]
- 20.Efficace F, et al. Baseline health-related quality-of-life data as prognostic factors in a phase III multicentre study of women with metastatic breast cancer. Eur J Canc. 2004;40:1021–1030. doi: 10.1016/j.ejca.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Fang FM, Liu YT, Tang Y, Wang CJ, Ko SF. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer. 2004;100:425–432. doi: 10.1002/cncr.20010. [DOI] [PubMed] [Google Scholar]
- 22.Fang FM, Tsai WL, Chiu HC, Kuo WR, Hsiung CY. Quality of life as a survival predictor for esophageal squamous cell carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58:1394–1404. doi: 10.1016/j.ijrobp.2003.09.100. [DOI] [PubMed] [Google Scholar]
- 23.Kramer JA, et al. Identification and interpretation of clinical and quality of life prognostic factors for survival and response to treatment in first-line chemotherapy in advanced breast cancer. Eur J Canc. 2000;36:1498–1506. doi: 10.1016/S0959-8049(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 24.Langendijk H, et al. The prognostic impact of quality of life assessed with the EORTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiother Oncol. 2000;55:19–25. doi: 10.1016/S0167-8140(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 25.Luoma ML, et al. Prognostic value of quality of life scores for time to progression (TTP) and overall survival time (OS) in advanced breast cancer. Eur J Canc. 2003;39:1370–1376. doi: 10.1016/S0959-8049(02)00775-X. [DOI] [PubMed] [Google Scholar]
- 26.Maisey NR, et al. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Canc. 2002;38:1351–1357. doi: 10.1016/S0959-8049(02)00098-9. [DOI] [PubMed] [Google Scholar]
- 27.Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. Quality of life in lung cancer patients: as an important prognostic factor. Lung Canc. 2001;31:233–240. doi: 10.1016/S0169-5002(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 28.Roychowdhury DF, Hayden A, Liepa AM. Health-related quality-of-life parameters as independent prognostic factors in advanced or metastatic bladder cancer. J Clin Oncol. 2003;21:673–678. doi: 10.1200/JCO.2003.04.166. [DOI] [PubMed] [Google Scholar]
- 29.Tamburini M, Brunelli C, Rosso S, Ventafridda V. Prognostic value of quality of life scores in terminal cancer patients. J Pain Symptom Manag. 1996;11:32–41. doi: 10.1016/0885-3924(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 30.Herndon JE, et al. Is quality of life predictive of the survival of patients with advanced nonsmall cell lung carcinoma? Cancer. 1999;85:333–340. doi: 10.1002/(SICI)1097-0142(19990115)85:2<333::AID-CNCR10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Blazeby JM, et al. Health-related quality of life measurement in randomized clinical trials in surgical oncology. J Clin Oncol. 2006;24:3178–3186. doi: 10.1200/JCO.2005.05.2951. [DOI] [PubMed] [Google Scholar]
- 32.Davies N. Measuring health-related quality of life in cancer patients. Nurs Stand. 2009;23:42–49. doi: 10.7748/ns2009.04.23.30.42.c6930. [DOI] [PubMed] [Google Scholar]
- 33.Halyard MY, Ferrans CE. Quality-of-life assessment for routine oncology clinical practice. J Support Oncol. 2008;6:221–229. [PubMed] [Google Scholar]
- 34.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 35.Aaronson NK, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Canc Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 36.Groenvold M, Klee MC, Sprangers MA, Aaronson NK. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol. 1997;50:441–450. doi: 10.1016/S0895-4356(96)00428-3. [DOI] [PubMed] [Google Scholar]
- 37.Hjermstad MJ, Fossa SD, Bjordal K, Kaasa S. Test/retest study of the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire. J Clin Oncol. 1995;13:1249–1254. doi: 10.1200/JCO.1995.13.5.1249. [DOI] [PubMed] [Google Scholar]
- 38.Braeckman J, Michielsen D. Prognostic factors in prostate cancer. Recent Results Canc Res. 2007;175:25–32. doi: 10.1007/978-3-540-40901-4_3. [DOI] [PubMed] [Google Scholar]
- 39.Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- 40.Curt GA, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 41.Lesage P, Portenoy RK. Management of fatigue in the cancer patient. Oncology (Williston Park) 2002;16:373–378. [PubMed] [Google Scholar]
- 42.Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist. 1999;4:1–10. [PubMed] [Google Scholar]
- 43.Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98:1786–1801. doi: 10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- 44.Stone P, Richards M, Hardy J. Fatigue in patients with cancer. Eur J Canc. 1998;34:1670–1676. doi: 10.1016/S0959-8049(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 45.Curt GA. The impact of fatigue on patients with cancer: overview of FATIGUE 1 and 2. Oncologist. 2000;5(Suppl 2):9–12. doi: 10.1634/theoncologist.5-suppl_2-9. [DOI] [PubMed] [Google Scholar]
- 46.Ahlberg K, Ekman T, Wallgren A, Gaston-Johansson F. Fatigue, psychological distress, coping and quality of life in patients with uterine cancer. J Adv Nurs. 2004;45:205–213. doi: 10.1046/j.1365-2648.2003.02882.x. [DOI] [PubMed] [Google Scholar]
- 47.Bower JE, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 48.Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- 49.Jacobsen PB, et al. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manag. 1999;18:233–242. doi: 10.1016/S0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancer. J Pain Symptom Manag. 2002;23:417–423. doi: 10.1016/S0885-3924(02)00376-7. [DOI] [PubMed] [Google Scholar]
- 51.Visser MR, Smets EM. Fatigue, depression and quality of life in cancer patients: how are they related? Support Care Canc. 1998;6:101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]