Abstract
The efficacy and safety of ertapenem, 1 g once daily, were compared with that of ceftriaxone, 2 g once daily, for the treatment of adults with acute pyelonephritis (APN) and complicated urinary tract infections (cUTIs) in a prospective, multicenter, double-blinded, randomized study. After ≥ 3 days of parenteral study therapy, patients could be switched to an oral agent. Of 271 patients who were initially stratified by APN (n = 210) or other cUTIs (n = 61), 66 (48.9%) in the ertapenem group and 71 (52.2%) in the ceftriaxone group were microbiologically evaluable. The mean duration of parenteral and total therapy, respectively, was 5.6 and 13.8 days for ertapenem and 5.8 and 13.8 days for ceftriaxone. The most common pathogen was Escherichia coli. At the primary efficacy endpoint 5-9 days after treatment, 58 (87.9%) patients in the ertapenem group and 63 (88.7%) in the ceftriaxone had a favorable microbiological response. When compared by stratum and severity, the outcomes in the two groups were equivalent. The frequency and severity of drug-related adverse events were generally similar in both treatment groups. The results indicate that ertapenem is highly effective and safe for the treatment of APN and cUTIs.
Keywords: Pyelonephritis, Urinary Tract Infections, Ertapenem, Ceftriaxone
INTRODUCTION
Urinary tract infections (UTIs) are considered the most common bacterial infections with an estimated annual global incidence of at least 250 million cases and are costly to both patients and healthcare funding systems (1, 2). Most UTIs are uncomplicated cystitis caused by Escherichia coli in otherwise healthy young women, which are easily managed with short-term oral antibiotic therapy. In contrast, acute pyelonephritis (APN) occurring in this same population requires often hospitalization and prolonged therapy. According to the recent report on APN in the Republic of Korea (ROK) (3), average annual incidence rate was 35.7 per 10,000 population, and approximately one of every seven patients was hospitalized. Treatment of complicated UTIs (cUTIs), including APN requires 10-14 days with an antibiotic agent active against various gram-negative bacilli (4-8). For these infections, a sequential approach with initial parenteral therapy and subsequent adequate oral agent with a more prolonged course could be considered an option as a standard therapy (9). However, clinical trials for UTIs have been relatively limited. Given the importance of these infections, further studies are needed to develop how they should be managed. Furthermore, increasing resistance among E. coli requires continuing reassessment of empirical therapy or more alternative first-line agents (10, 11).
Ertapenem, a long-acting parenteral group 1 carbapenem, has excellent in vitro activity against many gram-positive and gramnegative aerobic and anaerobic pathogens, except for Pseudomonas aeruginosa, Acinetobacter species, methicillin-resistant staphylococci and enterococci (12). Based on several randomized clinical trials performed in other countries, ertapenem administered at 1 g once-a-day is safe and effective in the treatment of complicated intra-abdominal infections, acute pelvic infections, community-acquired pneumonia, skin and soft-tissue infections, and cUTIs (13-16). However, the safety and efficacy of ertapenem in randomized clinical trials has not been evaluated in the Korean population.
The objectives of this study were to compare the efficacy, tolerability, and safety of ertapenem, 1 g once a day, with that of ceftriaxone, 2 g once a day, followed by an optional oral therapy, for the treatment of APN or other cUTIs in a Korean adult population.
MATERIALS AND METHODS
Study design and antimicrobial therapy
The double-blinded, prospective, randomized study was conducted in 9 university hospitals in the ROK from April 2008 to February 2009. The study design was based on the previous clinical trial for the treatment of complicated urinary tract infections (17). Eligible patients were stratified according to the diagnosis of APN (with or without an abnormality of the urinary tract) or other cUTIs. Patients were sequentially randomized to one of two treatment regimens in a 1:1 ratio using a stratified randomization schedule by the pharmacist.
Ertapenem (1 g once daily) or ceftriaxone (2 g once daily) was given by the intravenous (i.v.) route over 30 min. Patients received at least 3 doses of i.v. therapy before being switched to oral therapy. Oral agent was usually ciprofloxacin (500 mg bid). However, cefixime (200 mg bid) were permitted if the patient could not tolerate ciprofloxacin or if the causal pathogen was resistant to ciprofloxacin. The decision to switch from i.v. therapy to oral therapy was made by the investigator, if the patient was afebrile for at least 24 hr; nausea and vomiting were resolved; signs, symptoms and leukocytosis had improved; and urine culture was obtained. The suggested total duration of parenteral drug alone or parenteral plus an oral antibiotics was 7-14 days. The dosages selected for the comparator and oral agents were the usual recommended doses for the cUTIs in Korea.
Patients
Korean patients aged ≥ 18 yr with cUTI or APN who required parenteral antibiotic therapy were eligible for the study. The criteria for APN included fever, flank pain or costovertebral angle tenderness, pyuria (≥ 10 white blood cell per high power field), and followed by positive urine culture (≥ 105 colony forming unit [CFU] of uropathogen/mL) (18). Criteria for other cUTI in males were signs or symptoms of UTI, pyuria, and positive urine culture; female patients were additionally required to have either an indwelling catheter, current bladder catheterization, instrumentation of the urinary tract, or functional or anatomic abnormality of the urinary tract (18).
The exclusion criteria included pregnancy or lactation in women, history of serious allergy or intolerance to either study drug, a baseline pathogen resistant to either study drug, treatment for > 24 hr of effective antimicrobial agents within 72 hr prior to enrolment, complete obstruction of the urinary tract, perinephric or intrarenal abscess, prostatitis, any rapidly progressive or terminal diseases, renal transplantation, requirement for peritoneal dialysis or hemodialysis, creatinine clearance of ≤ 30 mL/min, and absolute neutrophil count < 1,000/µL.
Clinical assessments
Clinical assessments, including detailed description and evaluation of the infectious process, was performed at admission, at the discontinuation of i.v. therapy (DCIV), and at the early follow-up visit (EFU, 5-9 days post-therapy). Clinical responses at the EFU visit were assessed as cure, failure, or indeterminate. Cure was defined as resolution of all or most pre-therapy signs and symptoms and no additional antibiotics required. Failure was defined as persistence or progression of most/all pre-therapy signs and symptoms. Indeterminate was applicable when study data were not available for evaluation of efficacy.
Microbiological assessments
Blood culture and quantitative urine culture were performed at baseline. All isolates were identified at each site laboratory, and pathogens were tested for in vitro susceptibility to ertapenem, ceftriaxone, and ciprofloxacin according to the guidelines of the Clinical Laboratory Standard Institute (19).
Microbiological response for each pathogen present in the admission urine culture was assessed separately at DCIV and EFU visit. Microbiological responses at DCIV and EFU visit were defined as eradication (uropathogen ≥ 105 CFU/mL at entry reduced to < 104 CFU/mL), persistence (urine culture grew ≥ 104 CFU/mL of an original uropathogen), persistence with acquisition of resistance, superinfection (urine culture during therapy grew ≥ 105 CFU/mL of a pathogen other than baseline pathogen), or new infection (urine culture after completion of therapy grew ≥ 105 CFU/mL of a pathogen other than baseline pathogen). Overall microbiological response was determined as "favorable" (eradication) or "unfavorable" (persistence, persistence with acquisition of resistance, superinfection or new infection).
Populations for analysis
The treated population included all randomized patients who received one or more doses of study therapy. The microbiological modified intention-to-treat (MITT) population included treated patients who met the minimal definition of APN or other cUTI, and who had a baseline pathogen isolated in any quantity, and had a follow-up urine culture at or after DCIV. The clinically evaluable population met the following criteria: patients who had clinical evidence of APN or cUTI, had baseline uropathogen present at ≥ 105 CFU/mL, at least one baseline pathogen susceptible to both parenteral study drugs. Microbiologically evaluable patients were those clinically evaluable patients who had a follow-up urine culture at the EFU visit.
Efficacy variables
The primary efficacy endpoint was based on the proportion of patients who had a favorable microbiological response in the microbiologically evaluable patients at the EFU visit. Additional efficacy assessments were the microbiological response rates in the microbiological MITT population and combined microbiological and clinical response rates in evaluable patients at the DCIV and EFU visit.
Safety and tolerability assessment
All patients who received at least one dose of study therapy were evaluated for safety. Patients were monitored for adverse events (AEs) on a daily basis during parenteral therapy and for 14 days after the discontinuation of study antibiotic therapy. The tolerability of each parenteral study drug at the local infusion site was evaluated daily. The primary safety endpoints were the proportion of patients within each treatment group who experience any drug-related AEs leading to discontinuation of parenteral study drug.
Statistical analyses
The study was designed to show non-inferiority in efficacy of the ertapenem compared to ceftriaxone in microbiologically evaluable treatment groups at EFU visit. The definition of non-inferiority is the lower limit of the 95% (two-sided) confidence interval (CI) for the difference in response rates between the two groups does not exceed -20 percentage points and the CI contains zero. The sample size was calculated with the following values: alpha, 0.05; beta, 0.10; the expected response rate for each of the treatment groups, 85%, and the delta was set at -0.20. Assuming 85% response rates for both groups and a significance level of 0.05, a total of 134 evaluable patients (67 patients per group) are needed in order to have 90% probability that the lower limit of 95% (two-sided) CI for the difference in the response rates between the two groups is not less than -20 percentage points.
The strata were accounted for in testing the hypotheses for efficacy. For each endpoint at relevant time points, the difference in response proportion between two groups was displayed with its 95% (two-sided) CI. All CIs were calculated using the normal approximation to the binomial distribution. The CI for the difference between treatment groups was calculated using an adjustment for stratification. The Mantel-Haenszel method was used to calculate the stratified CI. Kaplan-Meier estimate was performed to explore time to discontinuation of parenteral therapy. The SAS software (version 9.1; SAS Institute, Cary, NC, USA) was used for analysis.
Ethics statements
The institutional review board of Korea University Anam Hospital and each study site reviewed and approved the study protocol (approved number of Korea University Anam Hospital: ED07154). Written informed consent was obtained from all participating patients.
RESULTS
Patients and therapy
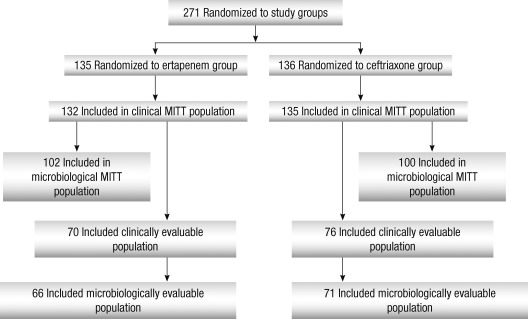
A total of 271 patients were randomized, 135 to the ertapenem group and 136 to the ceftriaxone group, of which 66 (48.9%) and 71 (52.2%) were microbiologically evaluable, respectively (Fig. 1). The most common reason for the microbiologically not evaluable populations was failure to isolate an uropathogen or ≥ 105 CFU/mL at baseline: 38 (28.1%) in the ertapenem group and 43 (31.6%) in the ceftriaxone group.
Fig. 1.
Profile of patient enrollment.
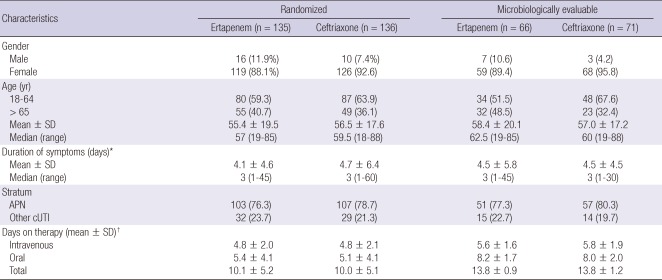
Baseline demographics, clinical characteristics and treatment duration in the randomized and microbiologically evaluable populations were similar across the treatment groups (Table 1). Hypertension and diabetes mellitus were the most frequent comorbid diseases in the randomized population: respectively, 48 patients (35.6%) and 39 (28.9%) in the ertapenem group; 54 (39.7%) and 31 (22.8%) in the ceftriaxone group. In the randomized patients, 10 (7.4%) patients in the ertapenem group and seven (5.1%) in the ceftriaxone group had indwelling catheters or stents: in the microbiologically evaluable population, seven (10.6%) patients in the ertapenem group and one (1.4%) patients in the ceftriaxone group, respectively.
Table 1.
Baseline and clinical characteristics and duration of study therapy in randomized and microbiologically evaluable patients by treatment group
*The duration of symptoms prior to study entry; †In randomized patients: for ertapenem, n = 132; for ceftriaxone, n = 135. SD, standard deviation; APN, acute pyelonephritis; cUTI, complicated urinary tract infection.
The mean duration of parenteral and total study drug therapy, respectively, in the microbiologically evaluable patients was 5.6 days and 13.8 days for those treated with ertapenem, and 5.8 days and 13.8 days for those treated with ceftriaxone. Almost all patients in each treatment group were switched to oral therapy: 51 (77.3%) and 14 (19.7%) in the ertapenem group and 62 (87.3%) and eight (11.3%) in the ceftriaxone group were switched to ciprofloxacin and cefixime, respectively.
Baseline microbiology
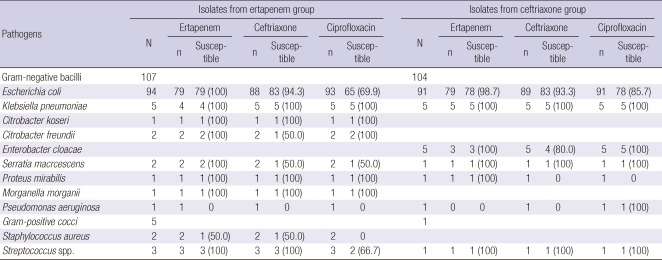
Of the 217 isolates from all randomized patients, E. coli was the most common pathogen (n = 185, 85.3%), followed by Klebsiella pneumoniae (n = 10, 4.6%) (Table 2). Among the E. coli isolates tested, nonsusceptibility rates to ertapenem and ceftriaxone were 0.6% (1/158) and 6.2% (11/177), respectively. Extended-spectrum β-lactamase (ESBL) producing E. coli isolates accounted for 4.3% (7/94) in the ertapenem group and 4.5% (4/88) in the ceftriaxone group. The susceptible rate to ciprofloxacin among the E. coli isolates were 77.7% (143/184): 69.9% (65/93) in ertapenem group and 85.7% (78/91) in ceftriaxone group. In the microbiologically evaluable population, distribution of the pathogens and their susceptibility profiles were comparable in the two treatment groups.
Table 2.
Antibiotic susceptibility of baseline pathogen isolated from all randomized patients
N, number of isolates; n, number of isolates tested.
Forty five patients who received ertapenem and 46 treated with ceftriaxone were bacteremic at baseline. E. coli was the etiologic agent in 83 (91.2%) of these patients. Of those tested, 25.6% (10/39) and 10% (4/40) were resistant to ciprofloxacin in each group. ESBL-producers were 7.5% (3/40) in each group.
Efficacy
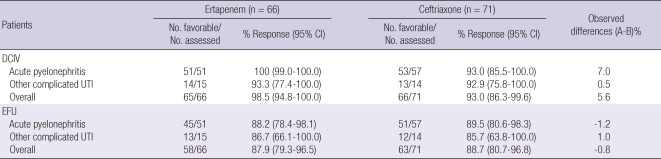
Microbiological response rates by stratum and time of assessment are shown in Table 3. At the primary efficacy endpoint, 87.9% patients in the ertapenem group and 88.7% in the ceftriaxone group had a favorable microbiological response assessment. The difference in the microbiological response rates, adjusting for strata, was -0.8% (95% CI, -11.7 to 10.2), indicating equivalence between the two treatments. Microbiological response rates of other complicated UTIs were lower than that of APN in both groups.
Table 3.
Favorable microbiologic response assessments in microbiologically evaluable patients with APN or other cUTI, by stratum and time of assessment
APN, acute pyelonephritis; cUTI, complicated urinary tract infection; N, number of evaluable patients in each treatment group; CI, confidence interval; DCIV, discontinuation of intravenous (i.v.) therapy includes patients who completed a regimen of i.v. therapy only and patients who discontinued i.v. and then switched to oral therapy; EFU, early follow-up visit, 5-9 days post-therapy.
In the additional microbiological MITT analyses that included 202 (74.5%) of the patients randomized, 91 (89.2%) of 102 in the ertapenem group and 92 (92.0%) of 100 in the ceftriaxone had a favorable microbiological response (95% CI, -11.0 to 5.3), supporting the results of the primary analysis. In the combined clinical and microbiological efficacy assessment at the DCIV, 61 (92.4%) of 66 patients in the ertapenem group and 57 (80.3%) of 71 in the ceftriaxone group had the clinical assessment of cure and a favorable microbiological response (95% CI, -0.4 to 24.2). At the EFU visit, 58 (87.9%) of 66 patients in the ertapenem group and 62 (87.3%) of 71 in the ceftriaxone group had clinical cure and a favorable microbiological response (95% CI, -10.7 to 11.9).
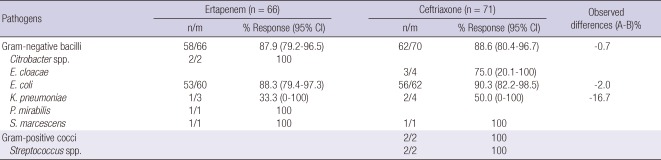
Table 4 depicts the microbiological response rates per pathogen in the evaluable population at the EFU visit. For all of the pathogens listed, the eradication rates were comparable between the two treatment groups. A new infection developed in one patient in the ertapenem group and three patients in the ceftriaxone group. Six patients in the ertapenem group and four patients in the ceftriaxone group had persistence of the original uropathogen. One patient in the ertapenem group and two patients in the ceftriaxone group showed persistence with acquisition of resistance.
Table 4.
Eradication rates at early follow up in microbiologically evaluable patients* with acute pyelonephritis or other complicated UTI by baseline pathogen
*A patient had more than one isolate. UTI, urinary tract infection; CI, confidence interval; n, number of evaluable patients in each treatment group; n/m, number of pathogens with associated favorable assessment/number of patients with an assessment at the early follow-up visit.
Among the bacteremic patients, 17 (81.0%) of 21 patients in the ertapenem group and 19 (82.6%) of 23 patients in the ceftriaxone group had a favorable microbiological response. Unfavorable responses in bacteremic patients were due to persistent bacteriuria.
Safety and tolerability
Of all randomized patients who received at least one dose of parenteral study drug, 14 (10.6%) of 132 patients in the ertapenem group and six (4.4%) of 135 patients in the ceftriaxone group had one or more drug-related clinical AEs during parenteral therapy.
The overall incidence of drug-related clinical AEs was similar between the two groups. The most frequent drug-related clinical AEs were diarrhea and nausea; seven (5.3%) and five (3.8%) in the ertapenem group and three (2.2%) and one (0.7%) in the ceftriaxone group, respectively. No serious drug-related AEs were reported in either treatment group.
The incidence of drug-related laboratory AEs during parenteral therapy was not different between the two treatment groups. Two (1.6%) patients in the ertapenem group and two (1.5%) patients in the ceftriaxone group had elevation of alanine aminotransferase and aspartate aminotransferase levels.
Reactions of moderate to severe intensity (pain and swelling) at the local infusion sites were reported in two (1.5%) patients in the ceftriaxone group, but none in the ertapenem group. Overall, the rates of local reactions of any intensity were low, and were not different between the two treatment groups.
DISCUSSION
In this multicenter, double-blind, randomized study conducted among Korean adults, ertapenem with the option to switch to oral therapy was microbiologically and clinically effective and tolerated well in patients with APN or other cUTIs. The dosing regimen of ertapenem, 1 g once daily, was not therapeutically inferior to ceftriaxone, 2 g once daily, followed by an appropriate oral antimicrobial agent. The overall favorable microbiological response was 87.9% of the patients receiving ertapenem and 88.7% of those receiving ceftriaxone. These results are similar to those of previous studies with a similar protocol conducted in other countries (17, 20, 21).
In the additional microbiological MITT assessment, as well as in the combined microbiological and clinical response assessment at the DCIV and the EFU visit, the response rate to ertapenem therapy was as high as or higher than that to ceftriaxone therapy. Enterobacteriaceae accounted for more than 95% of the baseline isolates, and E. coli was the most common single pathogen. The per-pathogen eradication rates at the EFU visit were comparable between the two treatment groups. Most of the evaluable patients were switched to oral therapy after clinical improvement with an average 5.7 days of parenteral therapy, either with ertapenem or ceftriaxone, which is consistent with current medical practice. These findings support the specific contribution of ertapenem to the total treatment regimen as an initial empirical parenteral therapy.
In this study, overall safety profile and local tolerability of ertapenem was similar to those of ceftriaxone. The most common ertapenem-related AEs reported were mild gastrointestinal symptoms (diarrhea or nausea) and mild to moderate elevation of aminotransferase levels. Local reactions at the infusion site were infrequent. These findings are consistent with a recent review of overall safety and tolerability of ertapenem from 15 clinical trials (17, 20-22).
The resistance patterns of uropathogens have significantly changed over the past few decades worldwide. In the ROK, uropathogenic E. coli are increasingly resistant to trimethoprim-sulfamethoxazole and ciprofloxacin or levofloxacin (23). Furthermore, the proportion of ESBL-producing E. coli isolates has gradually increased to 8.3%-11.8% (24, 25). In this study, ciprofloxacin resistance and ESBL-producing rates of E. coli isolates from the randomized patients were 22.3% (41/184) and 6.0% (11/182), respectively. However, by 2009, the proportion of ESBL-producing E. coli isolates had increased to 33% overall among the isolates (n = 242) from the Asia-Pacific region (26). Therefore, clinicians should choose the right empirical therapy for high-risk patients, on the basis of the type of pathogens responsible for cUTIs and their resistance patterns. Ertapenem, with rare exceptions, has a high degree of in vitro activity against antibiotic-resistant urinary isolates of Enterobacteriaceae, including ESBL-producing K. pneumoniae and E. coli strains (27, 28), and would be considered as the first empirical drug of choice for those situations.
Our study has a limitation. We did not assess long-term outcomes of antimicrobial agents such as relapse and recurrence rates by late follow-up visit at 4 to 6 weeks. However, relapse or recurrence at late follow-up might be mainly due to the underlying urinary tract abnormality or inadequate oral therapy rather than failure of the parenteral antimicrobial agent (17).
In conclusion, our study indicates that ertapenem is not inferior to ceftriaxone in the initial parenteral therapy for cUTIs, including APN, on the basis of its clinical and microbiological efficacy as well as safety profiles, and might be used as an alternative agent.
ACKNOWLEDGMENTS
The authors thank Yong Soo Kim, MD and Hyunjoo Lee at Global Medical Affairs of MSD Korea Ltd. for supporting all administrative processes for the clinical trial.
Footnotes
Min Ja Kim and Kyong Ran Peck report receiving consulting fees from MSD Korea Ltd. but there was no influence on conducting study, interpretation of results, and writing this article. All other authors report no competing interests.
References
- 1.Lichtenberger P, Hooton TM. Complicated urinary tract infections. Curr Infect Dis Rep. 2008;10:499–504. doi: 10.1007/s11908-008-0081-0. [DOI] [PubMed] [Google Scholar]
- 2.Ronald AR, Nicolle LE, Stamm E, Krieger J, Warren J, Schaeffer A, Naber KG, Hooton TM, Johnson J, Chambers S, et al. Urinary tract infection in adults: research priorities and strategies. Int J Antimicrob Agents. 2001;17:343–348. doi: 10.1016/s0924-8579(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 3.Ki M, Park T, Choi B, Foxman B. The epidemiology of acute pyelonephritis in South Korea, 1997-1999. Am J Epidemiol. 2004;160:985–993. doi: 10.1093/aje/kwh308. [DOI] [PubMed] [Google Scholar]
- 4.Nicolle L AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16:349–360. doi: 10.1155/2005/385768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29:745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 6.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 7.Naber KG, Bergman B, Bishop MC, Bjerklund-Johansen TE, Botto H, Lobel B, Jinenez Cruz F, Selvaggi FP Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU) EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU) Eur Urol. 2001;40:576–588. doi: 10.1159/000049840. [DOI] [PubMed] [Google Scholar]
- 8.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 9.The Korean Society of Infectious Diseases; The Korean Society for Chemotherapy; Korean Association of Urogenital Tract Infection and Inflammation; The Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of urinary tract infections: asymptomatic bacteriuria, uncomplicated & complicated urinary tract infections, bacterial prostatitis. Infect Chemother. 2011;43:1–25. [Google Scholar]
- 10.Muratani T, Matsumoto T. Urinary tract infection caused by fluoroquinolone-and cephem-resistant Enterobacteriaceae. Int J Antimicrob Agents. 2006;28:S10–S13. doi: 10.1016/j.ijantimicag.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Caniça MM, Park YJ, Lavigne JP, Pitout J, Johnson JR. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother. 2008;61:273–281. doi: 10.1093/jac/dkm464. [DOI] [PubMed] [Google Scholar]
- 12.Cunha BA. Ertapenem. A review of its microbiologic pharmacokinetic and clinical aspects. Drugs Today (Barc) 2002;38:195–213. doi: 10.1358/dot.2002.38.3.820127. [DOI] [PubMed] [Google Scholar]
- 13.Solomkin JS, Yellin AE, Rotstein OD, Christou NV, Dellinger EP, Tellado JM, Malafaia O, Fernandez A, Choe KA, Carides A, et al. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections: results of a double-blind, randomized comparative phase III trial. Ann Surg. 2003;237:235–245. doi: 10.1097/01.SLA.0000048551.32606.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham DR, Lucasti C, Malafaia O, Nichols RL, Holtom P, Perez NQ, McAdams A, Woods GL, Ceesay TP, Gesser R. Ertapenem once daily versus piperacillin-tazobactam 4 times per day for treatment of complicated skin and skin-structure infections in adults: results of a prospective, randomized, double-blind multicenter study. Clin Infect Dis. 2002;34:1460–1468. doi: 10.1086/340348. [DOI] [PubMed] [Google Scholar]
- 15.Roy S, Higareda I, Angel-Muller E, Ismail M, Hague C, Adeyi B, Woods GL, Teppler H Protocol 023 Study Group. Ertapenem once a day versus piperacillin-tazobactam every 6 hours for treatment of acute pelvic infections: a prospective, multicenter, randomized, double-blind study. Infect Dis Obstet Gynecol. 2003;11:27–37. doi: 10.1155/S1064744903000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortiz-Ruiz G, Caballero-Lopez J, Friedland IR, Woods GL, Carides A Protocol 018 Ertapenem Community-Acquired Pneumonia Study Group. A study evaluating the efficacy, safety, and tolerability of ertapenem versus ceftriaxone for the treatment of community-acquired pneumonia in adults. Clin Infect Dis. 2002;34:1076–1083. doi: 10.1086/339543. [DOI] [PubMed] [Google Scholar]
- 17.Tomera KM, Burdmann EA, Reyna OG, Jiang Q, Wimmer WM, Woods GL, Gesser RM Protocol 014 Study Group. Ertapenem versus ceftriaxone followed by appropriate oral therapy for treatment of complicated urinary tract infections in adults: results of a prospective, randomized, double-blind multicenter study. Antimicrob Agents Chemother. 2002;46:2895–2900. doi: 10.1128/AAC.46.9.2895-2900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidance for industry. Complicated urinary tract infections and pyelonephritis-developing antimicrobial durgs for treatment. Center for Drug Evaluation and Research. [accessed on 17 September 2010]. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070981.pdf.
- 19.Wikler MA Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement [M100-S18] Wayne, PA: CLSI; 2008. [Google Scholar]
- 20.Jimenez-Cruz F, Jasovich A, Cajigas J, Jiang Q, Imbeault D, Woods GL, Gesser RM Protocol 021 Study Group. A prospective, multicenter, randomized, double-blind study comparing ertapenem and ceftriaxone followed by appropriate oral therapy for complicated urinary tract infections in adults. Urology. 2002;60:16–22. doi: 10.1016/s0090-4295(02)01664-3. [DOI] [PubMed] [Google Scholar]
- 21.Wells WG, Woods GL, Jiang Q, Gesser RM. Treatment of complicated urinary tract infection in adults: combined analysis of two randomized, double-blind, multicentre trials comparing ertapenem and ceftriaxone followed by appropriate oral therapy. J Antimicrob Chemother. 2004;53:ii67–ii74. doi: 10.1093/jac/dkh208. [DOI] [PubMed] [Google Scholar]
- 22.Teppler H, Gesser RM, Friedland IR, Woods GL, Meibohm A, Herman G, Mistry G, Isaacs R. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53:ii75–ii81. doi: 10.1093/jac/dkh209. [DOI] [PubMed] [Google Scholar]
- 23.Wie SH, Chang UI, Kim HW, Kim YS, Kim SY, Hur J, Kim SI, Kim YR, Kang MW. Clinical features and antimicrobial resistance among clinical isolates of women with community-acquired acute pyelonephritis in 2001-2006. Infect Chemother. 2007;39:9–16. [Google Scholar]
- 24.Kim ME, Ha US, Cho YH. Prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in female outpatients in South Korea: a multicentre study in 2006. Int J Antimicrob Agents. 2008;31:S15–S18. doi: 10.1016/j.ijantimicag.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 25.Ko KS, Lee MY, Song JH, Lee H, Jung DS, Jung SI, Kim SW, Chang HH, Yeom JS, Kim YS, et al. Prevalence and characterization of extended-spectrum beta-lactamase-producing Enterobacteriaceae isolated in Korean hospitals. Diagn Microbiol Infect Dis. 2008;61:453–459. doi: 10.1016/j.diagmicrobio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Badal R, Bouchillon S, Hawser S, Hoban D, Hackel M, Hsueh PR. Antimicrobial susceptibility of urinary tract infection pathogens in Asia--SMART 2009; 12th Western Pacific Congress on Chemotherapy and Infectious Diseases; 2-5 December 2010; Singapore. p. Abstract no. P026. [Google Scholar]
- 27.Alhambra A, Cuadros JA, Cacho J, Gómez-Garcés JL, Alós JI. In vitro susceptibility of recent antibiotic-resistant urinary pathogens to ertapenem and 12 other antibiotics. J Antimicrob Chemother. 2004;53:1090–1094. doi: 10.1093/jac/dkh218. [DOI] [PubMed] [Google Scholar]
- 28.Livermore DM, Oakton KJ, Carter MW, Warner M. Activity of ertapenem (MK-0826) versus Enterobacteriaceae with potent beta-lactamases. Antimicrob Agents Chemother. 2001;45:2831–2837. doi: 10.1128/AAC.45.10.2831-2837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]