Abstract
Significance: Accumulating evidence shows that hydrogen sulfide may function as a signaling molecule in processes such as neuromodulation in the brain and smooth muscle relaxation in the vascular system. It also has a cytoprotective effect, since it can protect neurons and cardiac muscle from oxidative stress and ischemia-reperfusion injury, respectively. Hydrogen sulfide can also modulate inflammation, insulin release, and angiogenesis. Recent Advances: The regulation of the activity of 3-mercaptopyruvate sulfur transferase (3MST) along with cysteine aminotransferase (CAT), one of the H2S producing pathways, has been demonstrated. The production of H2S by the pathway, which is regulated by Ca2+ and facilitated by thioredoxin and dihydrolipoic acid, is also involved in H2S signaling as well as cytoprotection. Sulfur hydration of proteins by H2S has been proposed to modulate protein functions. H2S-sensitive fluorescent probes, which enable us to measure the localization of H2S in real time, have been developed. Critical Issues: The basal concentrations of H2S have recently been measured and found to be much lower than those initially reported. However, the concentration of H2S reached in stimulated cells, as well as the regulation of H2S producing enzymes is not well understood. It has been proposed that some of the effects of H2S on the regulation of enzymes and receptors might be explained through the properties of sulfane sulfur (S0), another form of active sulfur. Future Directions: The determination of H2S concentrations in activated cells using new methods including H2S-sensitive fluorescent probes, as well as the investigation of the effects of H2S using specific inhibitors, may provide better understanding of the physiological function of this molecule. Clarifying mechanisms of H2S activity may also facilitate the development of new therapeutic compounds. Antioxid. Redox Signal. 17, 45–57.
Introduction
The endogenous concentrations of sulfide in the brains of humans, bovine, and rats are in the range of 50–160 μM, strongly suggesting that endogenous H2S may have significant physiological functions (20, 56, 72). However, the concentrations of sulfide measured in these studies, which contained H2S released from acid-labile sulfur in addition to free H2S, were overestimated (23). Recently, the basal concentrations of H2S in tissues have been measured and found to be much lower than those previously reported (18, 24, 36). During the preparation of this manuscript, H2S-sensitive fluorescence probes have been reported from several laboratories (38, 39, 50, 52, 55). These probes may enable us to detect the sites at which H2S is released in real time and assess the local concentrations of H2S.
We demonstrated that cystathionine β-synthase (CBS), which produces H2S from cysteine or cysteine with homocysteine, is localized to astrocytes, a type of glia, and 3-mercaptopyruvate sulfur transferase (3MST), which produces H2S from 3-mercaptopyruvate, is localized to neurons in the brain (16, 57). The activity of CBS is enhanced by S-adenosyl methionine, and that of 3MST pathway is regulated by Ca2+ (1, 43). Cystathionine γ–lyase (CSE) is expressed little in the brain (1, 26).
H2S facilitates the induction of hippocampal long-term potentiation by enhancing the activity of N-methyl D,L-aspartate (NMDA) receptors (1). In the hypothalamus, H2S suppresses the KCl-stimulated release of corticotropin-releasing hormone (CRH), while it has no effect on the basal release of CRH. S-Adenosyl methionine inhibits the release of glucocorticoid induced by stress without any effect on hypothalamo-pituitary-adrenal function under resting conditions in vivo (13). H2S induces Ca2+ influx in astrocytes that propagates to the surrounding astrocytes as Ca2+ waves (16, 47). There is a reciprocal interaction between neurons and astrocytes: neuronal excitation activates surrounding astrocytes, which in turn modulate synaptic activity. H2S regulates synaptic activity by enhancing the activity of both neurons and astrocytes.
In addition to a possible function as a signaling molecule, we demonstrated a role of H2S as a cytoprotectant in the brain (30–32). H2S protects neurons from oxidative stress by restoring glutathione levels decreased by oxidative stress and suppressing oxidative stress in mitochondria. Philip Moore and colleagues also demonstrated that H2S protects a neuroblastoma cell line from oxidative stress induced by peroxynitrite and hypochlorous acid (76, 77). These oxidants cause the oxidative modification of proteins and lipids seen in vivo in patients with neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease. H2S also protects retinal neurons from light-induced degeneration by suppressing the excessive Ca2+ influx (43). The cytoprotective effect was also found by David Lefer and colleagues in the cardiovascular system. H2S protects cardiac muscle from ischemia-reperfusion injury by preserving mitochondrial function (15). The application of an H2S donor or the regulation of endogenous H2S concentrations by manipulating the CSE gene protected cardiomyocytes from ischemia-reperfusion injury. Based on this study, an H2S donor is being given to the bypass surgery patients in a Phase II clinical trial (51).
We showed that CSE is expressed in the thoracic aorta, portal veins, and ileum, and that H2S relaxes these tissues (22). The smooth muscle relaxant effect of H2S in the thoracic aorta and ileum was further studied by Rui Wang and Philip Moore, and H2S was found to activate adenosine triphosphate (ATP)-dependent K+ (KATP) channels (65, 82). The role of H2S and CSE has also been studied in vivo using CSE-knockout mice (25, 81). The regulation of inflammation, insulin release, and angiogenesis by H2S have also been intensively studied, and this has led to the development of therapeutic agents. This review focuses on the regulation of endogenous H2S and the regulation of its enzymatic production, as well as the methods recently developed to measure it in addition to the function as a signaling molecule and a cytoprotectant.
Permeability of H2S Through the Plasma Membrane
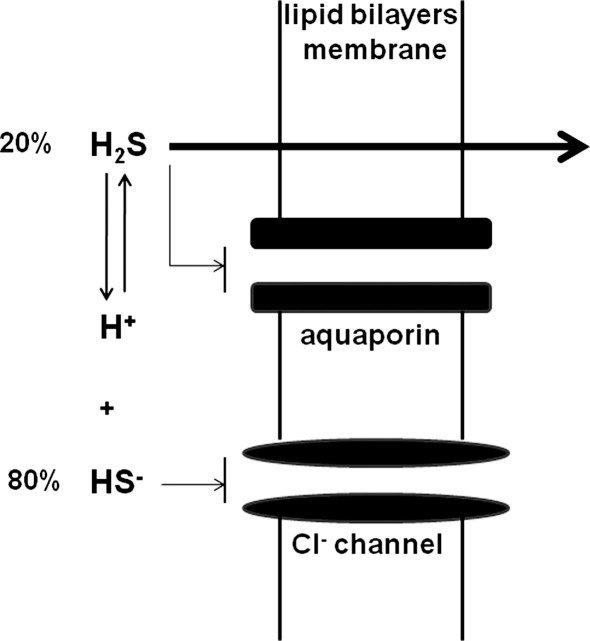
The mechanism of H2S transport through the plasma membrane has recently been demonstrated. Because of the structural similarity of H2S with H2O, it was hypothesized that H2S may pass through aquaporins or water channels (40). However, although reconstituted aquaporin obtained from a sulfide-reducing bacteria, AfAQP, increased the permeability of water in planar lipid bilayers, the permeability of H2S remained unchanged. The membrane resistance to H2S permeation is negligible. Even bilayers containing cholesterol and sphingomyelin, which decrease the diffusiveness of the membrane, had the same H2S permeability. These observations indicate that the membrane is not the limiting factor for H2S transport (40) (Fig. 1). The aquaporin homolog AqpM, which is derived from bacteria that rely on H2S as the terminal electron acceptor for energy production remains a candidate H2S transporter (34).
FIG. 1.
H2S passes through the plasma membrane by diffusion. Aquaporin and anion channels are not required for H2S and HS- respectively to pass through the plasma membrane.
H2S is soluble in water and dissociates into H+ and HS-. In physiologic saline at 37°C and pH 7.4, approximately one-fifth of H2S exists as the undissociated form (H2S), and the remaining four-fifths exists as HS- plus a trace of S2- at equilibrium with H2S (14). However, theoretical calculations indicate that transport of HS- by Cl- channels or other anion channels is unlikely to play a physiological role (40). The form of the molecule passing through the membrane is not necessarily the functional form. It may be difficult to determine which form, H2S or HS-, has a physiological function. In addition, since HS- can be oxidized to persulfide, it is possible that some observed effects with NaHS or Na2S may be even induced by a trace amount of persulfide. As to the possible involvement of other sulfur-containing substances as signaling molecules, see the review by Toohey (68).
H2S Concentrations
In early studies, the concentrations of sulfide in the brain were measured under conditions with high concentrations of acids. Therefore, H2S released from acid-labile sulfur was measured along with free H2S, with a combined concentration of between 50–160 μM in the brain (20, 56, 72). Acid-labile sulfur is derived from sulfur atoms in the iron–sulfur center of mitochondria respiratory chain enzymes. A pH less than 5.4 releases H2S from this source of acid-labile sulfur (24). Since mitochondrial pH is between 7 and 8, H2S may not be released from acid-labile sulfur under physiologic conditions, and therefore, the reported intracellular concentrations for H2S are likely overestimated.
The concentrations of free H2S are much less than those of acid-labile sulfur. Brain homogenates were vigorously mixed in the syringe, and released H2S was measured by gas chromatography. By this method, the concentration of free H2S is 14 nM (0.12 μmol/kg protein), while that released from acid-labile sulfur is 916 μmol/kg protein (18, 36). In another method, free H2S is below detectable levels, while that of H2S released from acid-labile sulfur is 161 nmol/g protein (24). Although 1 μM Na2S efficiently activates vacuolar-type H+ ATPase to suppress Ca2+ influx in the retina, the effects of the basal levels of H2S (10–20 nM) have not been reported. In order to exert its physiological function, μM concentrations of H2S have to be released from the activated enzymes or the intracellular store, but it is enough in a short time of period in a restricted area. After the induction of responses, H2S may be re-stored or degraded to be a steady state to cease the reaction. The basal concentrations of H2S are maintained in low levels in cells to properly respond to H2S. For example, the application of high concentrations of H2S (200 μM NaHS) to astrocytes suppresses Ca2+ influx induced by the second application, suggesting that high concentrations of H2S cause desensitization to H2S (47).
More recent measurements of the basal concentrations of H2S in blood still exhibit significant variation. For example, free H2S in blood measured by gas chromatography, was 0.07 μmol/kg protein (approximately 8 nM) (36). In this method, the efficiency of recovering H2S from a control sodium sulfide solution was estimated as a blank value in the absence of tissue homogenates. However, tissue homogenates efficiently absorb H2S (24), and it is therefore possible that the blank value may be overestimated compared to that without tissues, resulting in the relatively lower estimate of free H2S values in tissue homogenates. Another method mixed blood with monobromobimane, the thiol-specific derivatization agent, and recovered thiol-bound monobromobimane. The concentrations of H2S-bound monobromobimane were specifically measured by mass spectrometry (79). Free H2S in blood with this method was in the range of 0.4–0.9 μM. Depending on the method used, the concentration of free H2S vary up to 100-fold, but a consistent finding is that the basal free H2S in blood is maintained at a low concentration.
A low basal concentration of H2S does not necessarily indicate that these are the biologically functional concentrations of this molecule. Because the physiological stimuli of H2S release have not been identified, it is difficult to estimate the local concentrations of H2S reached when cells are activated. Additionally, the regulation of the activity of H2S-producing enzymes is also not well understood. The production of H2S by CBS is enhanced by S-adenosyl methionine two-fold compared to control samples (1). Activity of the 3MST/CAT pathway is regulated by Ca2+. The activity is highest in the absence of Ca2+ and suppressed by Ca2+ in a concentration-dependent manner. The production of H2S by this pathway is dramatically changed in the range of intracellular Ca2+(43). Although the regulation of CSE by Ca2+/calmodulin was reported, the concentrations of Ca2+ applied in the study were supraphysiological (1–2 mM), as the intracellular concentrations of Ca2+ (100 nM–3 μM) (81).
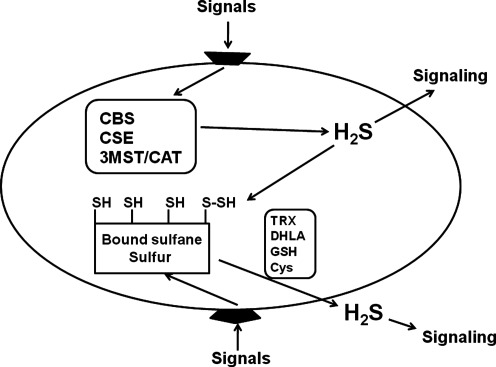
Another source of H2S is bound sulfane sulfur, which may function as an intracellular store of H2S. Cells expressing 3MST and CAT contain twice as much bound sulfane sulfur as control cells. The concentrations of bound sulfane sulfur are low in cells expressing a mutant 3MST that lacks H2S-producing activity (57). Lysates of neurons and astrocytes release H2S in the presence of endogenous levels of glutathione and cysteine at pH 8.4, and brain homogenates release it in the presence of dihydrolipoic acid (DHLA) at pH 8.0 (24, 42). H2S can be released from astrocytes by high K+, which is induced by neuronal excitation (24). The concentration of bound sulfane sulfur in the brain is 1500 nmol/g protein, which is sufficient to release H2S to stimulate target molecules (24). Considering the fact that the Km values of H2S-producing enzymes for substrates are greater than the endogenous concentrations, the endogenous production rate of H2S must be very low. Therefore, it is possible that H2S may be stored after it is produced by enzymes, and released from its intracellular stores when cells are stimulated. The percentage of bound sulfane sulfur that releases H2S in response to cell activation has not been determined (Fig. 2). It is critical that the local concentrations of H2S reached upon cell stimulation are now re-quantified using methods including H2S sensitive probes (38, 39, 50, 52, 55).
FIG. 2.
H2S is released from the producing enzymes and bound sulfane sulfur. H2S produced by enzymes may function as a signaling molecule and may also be stored in bound sulfane sulfur as intracellular stores. When cells are stimulated, H2S can be released from the stores. Although lysates of neurons and astrocytes release H2S in the presence of endogenous reducing substances, H2S release from intact cells with physiological stimulations has not been fully elucidated. Which of the enzymatic production or the intracellular stores mainly contributes to the physiological release of H2S has not been determined.
H2S-Sensitive Probes
The current methods to detect H2S, such as colorimetric assays, gas chromatograpy, and polarographic analysis, require its extraction from cells and tissues. Because the catabolism and extinction of H2S are fast, it has been difficult to measure the concentrations of this molecule accurately. Although a thiol detective imaging probe had been made, it was unable to differentiate sulfide from thiols. Recently, several fluorescent probes specifically detect H2S have been developed (38, 39, 50, 52, 55).
SF1 and SF2
Based on the reaction that azides can be reduced by H2S to amine, the azide-caged rhodamine analogues, SF1 and SF2, which generate highly fluorescent rhodamine products, were prepared (38). In a 30-min reaction with H2S, SF1 and SF2 produce 7- and 9-fold responses, respectively. Although both probes slightly respond to •NO, they do not respond to reactive oxygen species, reactive nitrogen species, and thiols such as glutathione and cysteine. SF1 shows greater responses to H2S in cells compared to SF2, probably due to the higher lipophilicity and cellular retention of SF1 relative to SF2. The improvement in shorter response time may help applying these probes to the studies of physiological and pathological processes.
DNS-Az
Dansyl is well-known for its strong fluorescence and long emission wavelength. DNS-Az is dansyl azide which emits fluorescence when H2S reduces sulfonyl azide into sulfonamide (50). The reaction is similar to that of SF1 and SF2 (38). Twenty five μM H2S induces a 40-fold fluorescence enhancement. Although the detection is based on the reducing properties of H2S, other possible reducing anions such as iodide, bromide, fluoride, bisulfate, and thiosulfate did not induce any responses. Although the selectivity of DNS-Az to H2S compared to the other endogenous thiols, such as glutathione and cysteine, is not shown, its reaction with H2S is fast, saturated in 5 min in 20 mM phosphate buffer in the presence of 0.05% Tween-20.
Probe 1
To selectively detect nucleophilic H2S in the biological system, it is important to differentiate H2S from other nucleophiles, especially thiols such as glutathione and cysteine. H2S, which is a nonsubstituted thiol, can undergo nucleophilic reaction twice, while other thiols such as glutathione and cysteine are monosubstituted thiols that can only undergo nucleophilic reaction once. Probe 1 consists of a fluorophore, which is quenched by an attached structure containing an electrophilic component (39). H2S reacts with the electrophilic component to form a free SH containing intermediate, which reacts with intrastructural ester group and causes cyclization to release the fluorophore.
Probe 1 reacts with 50 μM NaHS and the fluorescence intensity was increased by 55 to 70-fold, and the maximum intensity is reached in 1 h. Although probe 1 did not emit fluorescence in the presence of 50 μM glutathione and cysteine, its response to the endogenous levels of glutathione (mM order) was not shown. COS7 cells pre-incubated probe 1 emits strong fluorescence in the cells after the treatment of 250 μM NaHS.
SFP-1 and SFP-2
SFP-1 and SFP-2 consist of fluorophore and an aromatic framework with acrylate methyl ester and aldehyde ortho to each other (52). The aldehyde group reacts with sulfide to form a hemithioacetal intermediate with an exposed thiol, which reacts with the acrylate to yield a thioacetal. The reaction is similar to that of probe 1 (39). This reaction tunes photo-induced electron transfer of the aromatic structure, leading to the activation of fluorophore.
SFP-1 responds to 10–50 μM Na2S and increases the intensity of fluorescence more than 10 times in 60 min in PBS buffer (pH 7.4). Although it also responds to the most abundant thiols in mammalian cells, glutathione and cysteine, the interference with H2S can be excluded by selecting emission wave length. SFP-2, which has another fluorophore, showed more than 13-fold increase in the fluorescence intensity and more selective to Na2S than to cysteine or glutathione. Although both probes slightly emit fluorescence, even without exogenously applied Na2S in cultured HeLa cells, SFP-1 emits strong fluorescence in the presence of 100 μM Na2S and SFP-1 with 200 μM Na2S.
Hydrogen sulfide imaging probe-1
Azamacrocyclic rings form stable metal complexes with Cu2+ and the paramagnetic Cu2+ center has a pronounced quenching effect on fluorophores. Based on these characteristics of azamacrocyclic rings, hydrogen sulfide imaging probe-1 (HSip-1) was produced (55). H2S binds to the Cu2+ center of HSip-1 to enhance fluorescence, while glutathione does not cause any conformational change in the Cu2+ center or the increase in fluorescence intensity.
The fluorescence intensity is increased by 50-fold by 10 μM H2S in HEPES buffer (pH 7.4) with the maximal intensity at 30 min, while no increase in fluorescence intensity is induced by 10 mM glutathione. HSip-1 has a high selectivity over other thiols such as 1 mM cysteine, homocysteine, and 2-mercaptoethanol, and 100 μM dithiothreitol (DTT), inorganic sulfur compounds, and a reducing compound. It does not respond to reactive oxygen species or reactive nitrogen species. HSip-1 DA, diacetylated HSip-1, is membrane permeable. HeLa cells emit fluorescence upon addition to 200 μM Na2S to DMEM medium.
Production of H2S
CBS
H2S can be produced by three enzymes, CBS, CSE, and 3MST, along with cysteine aminotransferase (CAT), which is identical to aspartate aminotransferase (4, 7, 41). CBS produces H2S from cysteine via a β-elimination reaction, and more efficiently via a β-replacement reaction in which cysteine is condensed with homocysteine (8). The plasma concentrations of homocysteine in Alzheimer's disease, heart attack, and stroke are elevated. The β-replacement reaction provides an explanation for the finding that pharmacological doses of N-acetylcysteine can lower plasma homocysteine concentrations, for N-acetylcysteine passes through plasma membrane to cytoplasm and is converted to cysteine, which is condensed by CBS with homocysteine to produce H2S (8).
In the brain, astrocytes express CBS, which is intensively localized to cerebellar molecular layer and hippocampal dentate gyrus (16, 23) (Fig. 3). The specificity of our affinity-purified antibody against CBS was examined using Western blot analysis and immunohistochemistry by comparing CBS knockout and wild-type mice. The expression of CBS changes during development: it is expressed in neuroepithelial cells in the ventricular zone at early developmental stages and in radial cells and astrocytes in the late embryonic and neonatal periods (16). The level of CBS is increased in reactive astrocytes, which mediate neuronal recovery in the injured brain, and is induced by epidermal growth factor (EGF), transforming growth factor (TGF)-α, and cyclic adenosine monophosphate (cAMP). Its levels are also increased in the hippocampus after kainic acid-induced seizures.
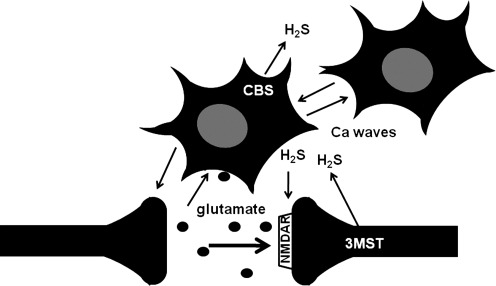
FIG. 3.
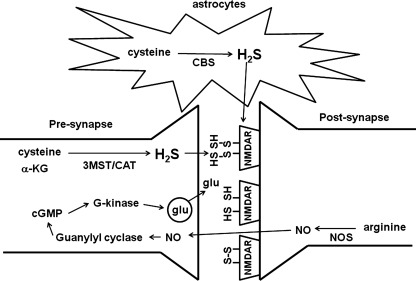
CBS is localized to astrocytes and 3MST to neurons in the brain. There is a reciprocal interaction between neurons and astrocytes. Neuronal excitation induces Ca2+ influx in astrocytes, which in turn regulate synaptic activity. H2S produced by CBS in astrocytes may be involved in the induction of Ca2+ waves, which propagate to surrounding astrocytes and contribute to the signaling between astrocytes. H2S generated by CBS in astrocytes and by 3MST in neurons may be involved in enhancing the activity of NMDA receptors and the synaptic activity.
The cerebellum of CBS-knockout mice is remarkably smaller than that of wild-type animals (16). The abnormalities are not observed at birth but become apparent around postnatal day 8. Although the structural organization is normal, there was a striking reduction in the thickness of the molecular- and granular-layers with stunted Purkinje dendrites.
CBS is localized to human chromosome 21 (80). Because of trisomy 21 in Down syndrome (DS), CBS levels in patients are predicted to be 1.5-fold greater than those in normal individuals. However, the expression of CBS mRNA is 12 times greater in myeloblasts of DS children than in those of normal individuals, and the levels of CBS in DS patients are 2.4–2.9 times greater than those in age-matched normal individuals (23, 64). Individuals with DS almost invariably develop an Alzheimer type of dementia (AD). CBS is localized to astrocytes and the surrounding senile plaques in the brains of DS patients with AD. The overexpression of CBS may cause a developmental abnormality in cognition in DS children and that may lead to AD in DS adults (23). Overproduction of H2S may cause the mental disturbance in DS. Another example of brain damage caused by elevated concentrations of H2S is ethylmalonic encephalopathy, in which the gene that encodes sulfur dioxygenase (ETHE1) is mutated. Defects in sulfur dioxygenase, which normally metabolizes H2S, lead to elevated concentrations of H2S (66).
CSE
CSE can facilitate the production of H2S from cysteine and homocysteine. Under normal conditions, α,β-elimination of cysteine generates H2S. However, with high concentrations of homocysteine because of homocysteinemia, the α,γ-elimination and γ-replacement reactions of homocysteine become dominant for H2S production by CSE (10). We could not detect CSE in the brain, and the activity of CSE is 100 times less than that in the liver (1, 17, 26). Although there are reports that show the existence of CSE activity in the brain, this may be due to the presence of a structurally unrelated protein that nonetheless exhibits CSE activity (72).
CSE is proposed to play a major role in vascular smooth muscle, and is mainly localized to smooth muscle in rodents (22, 82). However, CSE localization was recently reported in mouse, human, and bovine endothelium and cultures of human and bovine endothelium (81), although this finding is controversial (49, 57, 81, 82). The lysates of rat endothelium produce H2S in the presence of cysteine and α-ketoglutarate, but there is no production of H2S in the absence of α-ketoglutarate (57). Since CSE does not require α-ketoglutarate, this suggests that the other two H2S-producing enzymes, 3MST and CAT, produce H2S in rat endothelium. In bovine species, 3MST and CBS are localized to the endothelium, but CSE is not found (49).
Independent studies with CSE-knockout mice show that the involvement of CSE in the regulation of blood pressure is also controversial (25, 81). The observation that blood pressure is unaffected by loss of CSE could be explained by at least two reasons. First, it is possible that 3MST and CAT may themselves be involved in the regulation of blood pressure. Alternatively, increased generation of H2S by 3MST and CAT may compensate for loss of CSE in the knockout mice.
3MST with CAT
The production of H2S from 3-mercaptopyruvate (3MP) by 3MST was initially measured at pH 7.4 (41). However, maximum production was found to occur under alkaline conditions (60, 62). CBS and CSE are localized to the cytoplasm, while 3MST is mainly localized to the mitochondrial matrix, with a pH of approximately 8 (45). 3MST is localized to neurons in the brain and retina (43, 58). Neither CBS nor CSE was found in the retina.
3MP is produced by CAT from cysteine and α-ketoglutarate (11, 70) (Fig. 4). The concentrations of cysteine in the cytosol are 0.15–0.25 mM and those in the mitochondria are 0.7–0.99 mM, which may be enough to produce the basal concentrations of H2S (21, 63). In addition, glutathione, which is an intracellular store of cysteine, is present in 10–100 times greater concentrations in mammalian tissues than is cysteine, and can supply cysteine when the cells need it (63).
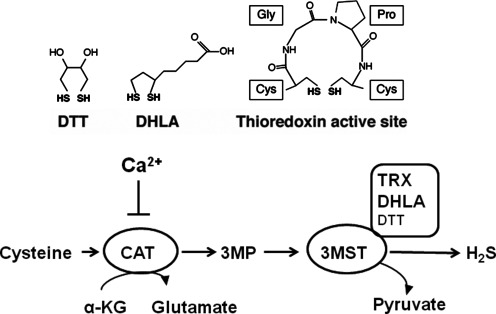
FIG. 4.
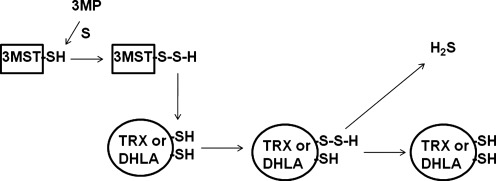
Regulation of H2S production by 3MST. Disulfides, such as thioredoxin (TRX) and dihydrolipoic acid (DHLA), are endogenous reducing substances, which can release H2S from 3MST. The activity of CAT is regulated by Ca2+. In the absence of Ca2+, the production of H2S by 3MST/CAT pathway from cysteine and α–ketoglutarate is the maximum but is suppressed by Ca2+ in a concentration-dependent manner.
A reducing substance such as DTT is required for 3MST to produce H2S. However, an endogenous substance corresponding to DTT has not been identified. We recently found that thioredoxin and DHLA associate with 3MST to release H2S (42) (Fig. 4). Approximately 20 μM thioredoxin exists in mitochondria, and it is 4 times more potent than DTT in enhancing H2S production. The concentration of DHLA is approximately 40 μM in the brain, and DHLA enhances the H2S production as effectively as DTT. The redox potentials of dithiols such as DTT, DHLA, and 2 cysteine residues in the active site of thioredoxin are in the range of −0.26 to −0.33V, while those of monothiols such as glutathione, cysteine, and CoA are from −0.22 to −0.35V (27). Those of nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+) are around −0.32 V. Because DTT, DHLA, and thioredoxin, whose two cysteine residues at the active site may function as dithiol, are able to release H2S from 3MST, the dithiol structure but not the redox potential is a critical factor for H2S release. A possible mechanism is that persulfide, which is produced at the active site cysteine of 3MST by receiving sulfur from 3-MP, is transferred to one of the thiols in thioredoxin or DHLA. The transferred persulfide, in turn, is reduced by another thiol in thioredoxin or DHLA to release H2S (42, 75) (Fig. 5).
FIG. 5.
A mechanism of releasing H2S from 3MST in the presence of thioredoxin or DHLA. Thioredoxin and DHLA receive persulfide from 3MST. Thiol in thioredoxin and DHLA reduces persulfide to release H2S.
We recently found that production of H2S from cysteine by the 3MST/CAT pathway is regulated by Ca2+ (43). The activity of CAT is suppressed by Ca2+ in a concentration-dependent manner. The production of H2S is maximal in the absence of Ca2+, and becomes completely suppressed at 2.9 μM Ca2+. The range of intracellular Ca2+ shifts to the lower concentrations in retinal photoreceptor cells (10 nM–600 nM) compared to other types of cells in which the intracellular concentrations are between 100 nM and 1–2 μM. When retinal photoreceptor cells are exposed to light, the intracellular concentrations of Ca2+ are decreased to 10 nM. Intracellular Ca2+ concentrations are increased to 600 nM in darkness. In this range of Ca2+ concentration, production of H2S by the 3MST/CAT pathway is dramatically changed. Calmodulin or its inhibitor, W-7, does not change the effect of Ca2+ on H2S production, suggesting that calmodulin is not involved in the regulation of H2S production by the 3MST/CAT pathway (43).
Both H2S and DHLA induce nuclear factor erythroid 2-related factor 2 (Nrf 2), which increases the expression of thioredoxin and thioredoxin reductase (6, 59). Since thioredoxin and DHLA enhances the activity of 3MST to produce H2S, both substances may also promote H2S production through Nrf2 induction.
Modification of the Activity by Sulfhydration
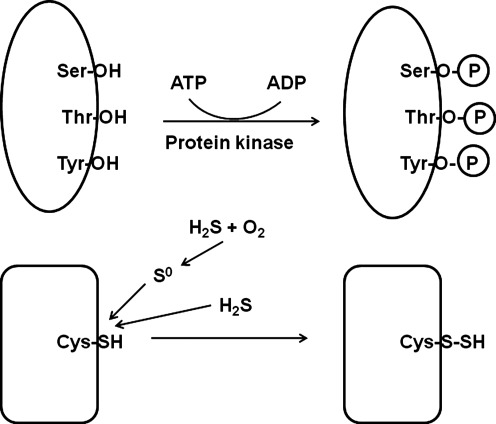
Modification of the activity of enzymes and receptors is brought about by reversible covalent reactions, including phosphorylation and adenosine diphosphate (ADP)-ribosylation. The reversible incorporation of sulfur into proteins has been considered as a further example of regulation by covalent modification (Fig. 6). Kato et al. demonstrated that serine dehydratase was inhibited by CSE in the presence of cysteine (29). The suppression of the enzyme activity is reversible by glutathione or DTT, suggesting the formation of a hydropersulfide group (sulfurhydration) in the enzyme. Enzymes inactivated by the same mechanism include 3-hydroxybutyrate dehydrogenase, alcohol dehydrogenase, and ornithine decarboxylase (67). Solomon Snyder and his colleagues recently demonstrated that H2S induces sulfurhydration to enhance the activity of proteins, including glyceraldehyde 3-phosphate dehydrogenase (GAPDH), β-tubulin, and actin (19, 44). H2S increases the activity of GAPDH by 7-fold, and DTT reverses GAPDH activity induced by H2S. GAPDH is activated in human embryonic kidney (HEK) 293 cells expressing CSE, suggesting that endogenously produced H2S induces sulfurhydration of GAPDH. However, further studies are required to determine the function of sulfurhydrated GAPDH.
FIG. 6.
Modulation of enzyme activity by phosphorylation and sulfurhydration. The reversible incorporation of sulfur into proteins may modulate protein function as phosphorylation does on proteins. H2S may directly induce sulfurhydration. Alternatively, H2S may react with oxygen to form S0 that adds to sulfhydryl groups of proteins.
John Toohey proposed another mechanism for sulfhydration. Some of H2S reacts with oxygen to form S0 that adds to sulfhydryl groups of proteins, forming a persulfide, and to disulfide groups, forming a trisulfide, though it is a generalized chemical mechanism and not site-specific (68).
Signaling Molecule
Synaptic modulation
The victims of H2S poisoning suffer coordination and psychiatric disturbances (53). Based on these observations, the toxic effect of H2S on the nervous system has been intensively studied. H2S increases the levels of neurotransmitters γ-amino butyric acid (GABA), glutamate, serotonin, dopamine, epinephrine, and norepinephrine (33). The levels of serotonin and catecholamine are increased by inhibition of monoamine oxidase (MAO) (74). H2S also enhances the effect of acetylcholine by suppressing the activity of its degradation enzyme, acetylcholinesterase. Excitatory postsynaptic potentials are suppressed by high concentrations of H2S, indicating the suppression of synaptic transmission by H2S (1). The effect may explain the temporary memory loss occurred following H2S exposure.
H2S facilitates the induction of hippocampal long-term potentiation (LTP) similarly to nitric oxide (•NO), albeit by a different mechanism (1, 48) (Fig. 7). •NO, which is produced from arginine by •NO synthetase at postsynapses, diffuses to presynapses to activate guanylyl cyclase. This leads to production of cyclic GMP, which in turn activates G-kinase to increase the release of a neurotransmitter glutamate. Carbon monoxide (CO) facilitates the induction of LTP in a similar fashion to •NO, and both •NO and CO are called retrograde neurotransmitters (48). In contrast H2S does not have any effect on guanylyl cyclase (1). H2S enhances the activity of NMDA receptors, which is known to be required for the induction of LTP. Disulfide bonds play a role in modulating the function of NMDA receptors (2). DTT reduces disulfide bonds to generate thiols in NMDA receptors and facilitates the induction of LTP. H2S further enhances the induction of LTP even after the facilitation induced by DTT (1). These observations suggest that H2S may be involved at two steps in the modulation of NMDA receptor activity. First, as observed for DTT, H2S reduces disulfide bonds to generate thiols and enhance the activity of NMDA receptors. Second, H2S potentiates the activity even further by binding to thiols of receptors and generating persulfide, as suggested by Snyder and Toohey (19, 24, 68).
FIG. 7.
The mechanisms for the induction of LTP. H2S and nitric oxide (•NO) facilitate the induction of hippocampal long-term potentiation (LTP) with different mechanisms. •NO, which is produced from arginine, diffuses to presynapse to activate guanylyl cyclase, leading to the production of cyclic GMP that activates G-kinase to increase the release of a neurotransmitter glutamate. Although H2S does not have any effect on guanylyl cyclase, it enhances the activity of NMDA receptors.
Another possible mechanism for the modulation of synaptic activity observed in the facilitation of LTP induction may involve astrocytes. Astrocytes were thought to be quiescent cells whose primary role was to support neurons. However, they were recently found to have neurotransmitter-responsive receptors themselves. Neuronal excitation activates astrocytes, and these cells in turn modulate synaptic activity (12). In neurons, H2S enhances the responses of NMDA receptors to glutamate, while H2S induces Ca2+ influx in astrocytes, which enhances the synaptic activity (1, 47). The effects of H2S on both neurons and astrocytes may therefore explain its role in the induction of LTP. Astrocyte responses are suppressed by La3+, Gd3+, and ruthenium red, which are known as transient receptor potential (TRP) channel blockers, although the affected channel has not been identified.
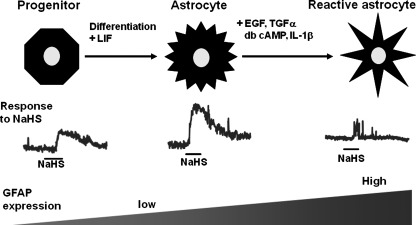
Glial fibrillary acidic protein (GFAP) negative-premature astrocytes do not respond to H2S (69). GFAP positive-mature astrocytes and those induced to mature by treatment with leukemia inhibitory factor (LIF) respond well to H2S. In contrast, reactive astrocytes induced by EGF, TGF-α, cAMP, and interleukin-1β (IL-1β) do not respond to H2S. Reactive astrocytes were induced by brain injuries, including trauma, neurodegenerative diseases, and viral infections. EGF is secreted by microglia and TGF-α is synthesized by injured neurons (28). EGF receptors are upregulated in reactive astrocytes after brain injury, and IL-1β is produced by reactive astrocytes. Astrocytes may exchange signals with neurons under normal conditions, but once reactive astrocytes are induced by brain injury, they may stop responding to H2S to prevent transmitting inappropriate signals (Fig. 8).
FIG. 8.
The sensitivity of astrocytes to H2S depends on the state of maturation and reactivity. GFAP positive-mature astrocytes and those maturation-induced by leukemia inhibitory factor (LIF) respond well to H2S. In contrast, premature astrocytes as well as reactive astrocytes induced by EGF, TGF-α, cAMP, and interleukin-1β do not respond to H2S.
Regulation of the activity of retinal neurons
The center-surround organization of the receptive field is one of the most important characteristics of retinal neurons. The negative feedback from horizontal cells to photoreceptor cells plays a key role in the center-surround organization. The negative feedback has been proposed to occur when Ca2+ channels of photoreceptor cells are suppressed by H+ released by vacuolar-type H+-ATPase present on horizontal cells. When retinal photoreceptor cells are exposed to light, cyclic GMP-gated channels are closed and the intracellular concentrations of Ca2+ are decreased. This results in a decrease in the release of the neurotransmitter glutamate to horizontal cells. Without the activation of glutamate receptors, the intracellular concentration of Ca2+ is maintained at a low level. Low Ca2+ concentrations in both photoreceptor and horizontal cells enhances the activity of 3MST/CAT to produce H2S, which activates vacuolar-type H+-ATPase on horizontal cells (43). H+ suppresses Ca2+ channels on photoreceptor cell membrane. H2S suppresses Ca2+ influx and regulates the negative feedback from horizontal cells to photoreceptor cells.
Cytoprotection
Because H2S is a well-known toxic gas, the concept that it may also have a cytoprotective effect is paradoxical. We and others found that H2S protects neurons from oxidative stress by reinstating glutathione levels decreased by oxidative stress and by scavenging reactive oxygen species (30–32, 76, 77). Primary cultures of immature neurons, which express cystine/cysteine transporters but do not express ionotropic glutamate receptors, are widely used as a model for oxidative stress (61). The model enabled us to study oxidative stress specifically without the involvement of excitotoxicity. Similarly, even 1 mM NaHS is not toxic to a neuroblastoma cell line, SH-SY5Y, which does not express NMDA receptors (76, 77). In contrast, primary cortical neurons, which were cultured for several days and express ionotropic glutamate receptors, were killed by NaHS, and cell death was inhibited by glutamate receptor antagonists, MK801, APV, and CNQX (9). Together, these results suggest the neuroprotective effect of H2S is specific to oxidative stress, but not to excitotoxicity.
Under physiological conditions, H2S dissociates to H+ and HS- and at equilibrium approximately 20% remains as undissociated H2S gas, which can evaporate from the medium. Approximately one-third of total H2S, which includes undissociated H2S gas and dissociated HS-, remains after 15 min of application, and more than 90% is lost from the medium of culture dishes by 30 min (30). After 1 h of application of NaHS, almost all of H2S+HS- has evaporated out of culture dishes. Therefore, the effect of H2S observed in culture dishes following a single application must be rapid and sustainable. It is possible that H2S is bound to proteins as sulfane sulfur and in this manner can regulate their function (24, 68). Under oxidative stress caused by high concentrations of glutamate, neuronal cells started dying 8 h after exposure and only around 10% of cells survived after 16 h. Simultaneous application of glutamate and NaHS delayed the onset of cell death to 12 h and a rate of cell survival is much greater than that without NaHS. Because H2S evaporates in a short time period, this means that the prolonged H2S-dependent resistance to oxidative stress is induced by only a transient exposure.
H2S can also protect cells against oxidative stress when given after the original insult. For example, the application of NaHS 4 h after induction of oxidative stress, still provides significant protection compared to control. This is true despite the initial activation of a cell death cascade. This property of H2S is important for its therapeutic application (51).
Increase in the levels of glutathione
H2S increases the transport of cystine and cysteine by enhancing the activity of XCT- cystine/glutamate antiporters and XA,G- excitatory amino acid transporters to increase the substrate concentrations for glutathione production. H2S also enhances the activity of γ-glutamayl cysteine synthetase, a limiting enzyme for glutathione production. By these integrated effects H2S increases the levels of glutathione in cells, and the effects are more profound when cells are under the conditions of oxidative stress (31, 32).
Various types of proapoptotic signals converge on mitochondria, and mitochondrial dysfunction causes numerous neurodegenerative diseases (37). 3MST and CAT are localized to mitochondria, which are the major organelles implicated in production of reactive oxygen species. H2S produced in mitochondria may directly suppress oxidative stress in this organelle. For example, Neuro2a cells expressing 3MST and CAT show significant resistance to oxidative stress (31).
H2S protects the embryonic brain from ischemia-reperfusion injury (31). Reperfusion after ischemia caused by stopping the blood flow from mother to fetus caused maceration of all the fetal brains, which contained only 24% glutathione of a control, but by the administration of sodium hydrosulfide (NaHS) to mother at the ischemia, 75% of fetal brains were not macerated and contained 90% of the normal glutathione level. The H2S-producing enzymes that are also involved in the increase in the levels of glutathione have not been identified. The involvement of CSE in this process is controversial in two studies with CSE-knockout mice (25, 81).
Suppression of Ca2+ influx
The retina is susceptible to oxidative stress because of its high consumption of oxygen and daily exposure to light. Excessive light exposure leads to photoreceptor degeneration in the retina. Photoreceptor cell death is an irreversible injury that is caused by various factors such as reactive oxygen species (ROS) and elevated intracellular concentrations of Ca2+. H2S suppresses the elevation of Ca2+ in the photoreceptor cells by activating vacuolar-type H+ ATPase, which suppresses L-type Ca2+ channels, and maintains Ca2+ homeostasis (43). Intracellular concentrations of Ca2+ are increased during photoreceptor apoptosis, but the L-type Ca2+ channel blocker diltiazem prevents light-induced photoreceptor cell death. The lack of the vacuolar-type H+ ATPase a3 subunit causes retinal degeneration. The failure of Ca2+ homeostasis due to excessive light may cause retinal cell degeneration. The regulation of Ca2+ and cytoprotective effect of endogenous H2S may fail when photoreceptor cells are exposed to excessive light. Even under such conditions, the administration of H2S protects cells from light-induced degeneration. Many cells in the outer nuclear layer, in which rod inner segments are located, became TUNEL-positive following exposure to light. The administration of 0.4375 μmol NaHS/kg weight of mice decreased the number of TUNEL-positive cells by approximately 80% relative to a control. Light exposure also produces a lot of cells containing 8-hydroxy-2-deoxyguanosine (8-OHdG), which is a product of DNA damaged by ROS, in the outer nuclear layer. In contrast, the number of cells positive to 8-OHdG is decreased in NaHS-treated mice. H2S protects photoreceptor cells from light-induced retinal degeneration and oxidative stress.
Trophic effect
A treatment of primary cultures of neurons with NaHS increases the reduction of tetrazolium salts, which are reduced by cellular activity to form intensely colored formazans and have often been used to quantitate the number of living cells (71). A similar effect is observed in neuroblastoma Neuro2a cells, but it is not observed in other cell types such as primary cultures of astrocytes, COS-7 and NIH3T3 fibroblast cell lines. H2S enhances metabolic activity specifically in neurons and the neuronal cell line. Other reducing agents, glutathione, DTT, α-tocopherol, and 2-mercaptoethanol do not enhance metabolic activity of neurons. The effect is increased in 60 min, and the maximal effect is maintained at least for 24 h after the application of NaHS. The increase of tetrazolium salt reduction in the absence of cells, even without washing out NaHS, was much less than that in the presence of cells with washing out of NaHS. This excludes the possibility that the reduction of tetrazolium salts is directly caused by reducing activity of H2S, and suggests that H2S stimulates a reductive intracellular environment.
It is well-known that H2S inhibits cytochrome c oxidase and suppresses respiratory chain metabolic activity (71). The concentrations of H2S, which reduce tetrazolium salts, do not inhibit cytochrome c oxidase. In contrast, azide, another inhibitor of cytochrome c oxidase, greatly inhibited cytochrome c oxidase but did not enhance the reduction of tetrazolium salts. NaHS increases the metabolic activity without affecting NADH/NAD+ ratio. The effects of NaHS are suppressed by the tyrosine kinase inhibitors, genistein and tyrphostin A23, suggesting that the effect may be mediated by phosphorylation of tyrosine and that H2S may have a trophic effect in the brain. Proliferation is induced by H2S in other cell types, such as in lung cells, endothelial cells, cardiac myocytes, and umbilical vein endothelial cells, while apoptosis is induced in smooth muscle cells, lung primary fibroblast cells, and periodontal ligament cells (3). The induction of proliferation or apoptosis by H2S in these cells is regulated by transcription of genes of kinases and cell cycle proteins.
H2S-Based Therapeutics
A tremendous amount of effort has been devoted into the development of H2S based therapeutics. Na2S is being tested in bypass surgery patients in Phase II clinical trial, and for kidney injury in Phase I clinical trial (51). Since Na2S and NaHS release H2S immediately after they are applied in vivo, compounds slowly release H2S in a physiological manner have been developed. For example, GYY4137 inhibits LPS-induced lung and liver injury by decreasing neutrophil accumulation as well as the levels of the anti-inflammatory chemokine IL-10 (78). GYY4137 also protects human articular chondrocytes and trabecular bone-derived mesenchymal progenitor cells from oxidative stress.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used medications but have side effects, mainly gastric ulceration and bleeding. An H2S-releasing derivative of naproxen (ATB-346) significantly accelerates the ulcer healing process, probably due to increasing the expression of cyclooxygenase (COX)-2 and promoting angiogenesis (5). ATB-346 does not cause gastric damage in rats even at exceedingly high doses. Another H2S-releasing derivative of mesalamine (ATB-429) has greater anti-inflammatory activity in animal model of colitis compared to parental mesalamine. ACS14 and ACS21 are derivatives of aspirin and salicylic acid, respectively. Both compounds reduce hypertension and lower plasma levels of thromboxane B2 and insulin induced by glutathione depletion (54). S-diclofenac (ACS-15) efficiently suppresses LPS-induced inflammation with less gastric toxicity than parental diclofenac. This drug protects the heart from the development of ischemia-reperfusion injury and suppresses progression of vascular injury, leading to the prevention of arterial thrombosis.
Levodopa (L-DOPA) is the most widely used therapy for Parkinson's disease. The H2S-releasing derivatives of L-DOPA (ACS5, ACS48, ACS50, and ACS81) were developed that showed significant anti-inflammatory, antioxidant, and neuroprotective properties (35). H2S released from these compounds suppresses monoamine oxidase and maintains the effective concentrations of dopamine at the synaptic cleft. Released H2S also suppresses the release of neurotoxins such as tumor necrosis factor-α, IL-6, and •NO from stimulated microglia. These compounds may be candidates for future treatment of Parkinson's disease.
Conclusion and Perspective
The basal concentrations of H2S have been found to be much lower than those reported in earlier studies (18, 24, 79). However, the concentrations of H2S achieved following cell stimulation are not well understood. To address this problem, it is necessary to clarify the regulation of the activity of H2S-producing enzymes. For example, the activity of CBS is enhanced by S-adenosyl methionine and the 3MST/CAT pathway is regulated by Ca2+. The physiological stimulations, which regulate the levels of S-adenosyl methionine and Ca2+, may help determine the mechanism underlying the physiological regulation of CBS and 3MST/CAT.
In addition to the release of H2S from the producing enzymes, bound sulfane sulfur, which can be an intracellular store of H2S, releases H2S under physiological conditions. The mechanism for the release of H2S from bound sulfane sulfur may also help understand the endogenous local concentrations of H2S in the activated cells. The release of H2S from activated astrocytes has not been successfully measured in the current method (24). Recently, fluorescent probes for imaging H2S have been developed (38, 39, 50, 52, 55). The probes are able to selectively measure H2S in aqueous environments at physiological pH. Although the sensitivity and intensity of the probes needs further improvement, their responses are selective for H2S and can be applied to live cells for imaging. This represents a significant breakthrough for the detection of H2S and is an improvement over the currently applied methods.
H2S has the potential to be developed into a therapeutic agent (51). Na2S is already in a Phase II clinical trial designed to determine whether it can protect cardiac muscle from ischemia-reperfusion injury following bypass surgery. NSAIDs attached to the H2S-releasing group do not have side effects, such as bleeding or ulcer of gastrointestinal tract, that the parental NSAIDs cause (5). L-Dopa attached to an H2S-releasing group has additional therapeutic effects that L-Dopa does not have (35). It suppresses the release of inflammatory cytokines produced by microglia, a key cause of neurodegeneration observed in Parkinson's disease. These are only a few examples of many H2S-based therapeutic applications. Under physiological conditions, H2S may be locally released to function in a restricted area. In contrast, the administration of H2S systemically increases its concentrations. Monitoring for unexpected side effects will clearly be required. However, once these issues are overcome, the promise of H2S-based therapeutic compounds will be realized.
Abbreviations Used
- AD
Alzheimer type of dementia
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine monophosphate
- CAT
cysteine aminotransferase
- CBS
cystathionine β-synthase
- cGMP
cyclic guanosine monophosphate
- CO
carbon monoxide
- CRH
corticotropin-releasing hormone
- CSE
cystathionine γ-lyase
- DHLA
dihydrolipoic acid
- DS
Down's syndrome
- DTT
dithiothreitol
- EDHF
endothelium-dependent hyperpolarizing factor
- EDRF
endothelium-dependent relaxing factor
- EGF
epidermal growth factor
- GABA
γ-amino butyric acid
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- GFAP
glial fibrillary acidic protein
- HEK
human embryonic kidney
- H2S
hydrogen sulfide
- IL
interleukin
- KATP
ATP-dependent K+
- L-Dopa
levodopa
- LIF
leukemia inhibitory factor
- LTP
long-term potentiation
- MAO
monoamine oxidase
- 3MP
3-mercaptopyruvate
- 3MST
3-mercaptopyruvate sulfurtransferase
- NADH
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NaHS
sodium hydrosulfide
- NMDA
N-methyl D,L-aspartate
- •NO
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor 2
- NSAID
nonsteroidal anti-inflammatory drug
- RNA
ribonucleic acid
- TGF
transforming growth factor
- TRP
transient receptor potential
- TRX
thioredoxin
Acknowledgments
This work was supported by a grant from National Institute of Neuroscience and KAKENHI (23659089) from Grant-in-Aid for Challenging Exploratory Research to HK, KAKENHI (23700434) from Grant-in-Aid for Young Scientists (B) and Health Labour Sciences Research Grant from the Ministry of Health Labor and Welfare to NS, and KAKENHI (22590258) from Grant-in-Aid for Scientific Research (C) to YK.
Author Disclosure Statement
There is no conflict of interest for any of the authors.
References
- 1.Abe K. Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aizenman E. Lipton DA. Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2:1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- 3.Basker R. Bian J. Hydrogen sulfide gas has cell growth regulatory role. Eur J Pharmacol. 2011;656:5–9. doi: 10.1016/j.ejphar.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 4.Braunstein AE. Goryachenkowa EV. Tolosa EA. Willhardt IH. Yefremova LL. Specificity and some other properties of liver serine sulphhydrase: Evidence for its identity with cystathionine β-synthase. Biochim Biophys Acta. 1971;242:247–260. doi: 10.1016/0005-2744(71)90105-7. [DOI] [PubMed] [Google Scholar]
- 5.Caliendo G. Cirino G. Santagada V. Wallace JL. Synthesis and biological effects of hydrogen sulfide (H2S): Development of H2S -releasing drugs as pharmaceuticals. J Med Chem. 2010;53:6275–6286. doi: 10.1021/jm901638j. [DOI] [PubMed] [Google Scholar]
- 6.Calvert JW. Jha S. Gundewar S. Elrod JW. Ramachandran A. Pattillo CB. Kevil CG. Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavallini D. Mondovi B. De Marco C. Scioscia-Santoro A. The mechanism of desulphhydration of cysteine. Enzymologia. 1962;24:253–266. [PubMed] [Google Scholar]
- 8.Chen X. Jhee KH. Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem. 2004;279:52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- 9.Cheung NS. Peng ZF. Chen MJ. Moore PK. Hydrogen sulfide induced neuronal death occurs via glutamate receptor and is associated with calpain activation and lysosomal rupture in mouse primary cortical neurons. Neuropharmacology. 2007;53:505–514. doi: 10.1016/j.neuropharm.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Chiku T. Padovani D. Zhu W. Singh S. Vitvitsky V. Banerjee R. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper AJL. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem. 1983;52:187–222. doi: 10.1146/annurev.bi.52.070183.001155. [DOI] [PubMed] [Google Scholar]
- 12.Dani JW. Chernjavsky A. Smith SJ. Neuronal activity triggers calcium waves in hipp9ocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 13.Dello Russo C. Tringali G. Ragazzoni E. Maggiano N. Menini E. Vairano M. Preziosi P. Navarra P. Evidence that hydrogen sulphide can modulate hypothalamo-pituitary-adrenal axis function: In vitro and in vivo studies in the rat. J Neuroendocrinol. 2000;12:225–233. doi: 10.1046/j.1365-2826.2000.00441.x. [DOI] [PubMed] [Google Scholar]
- 14.Dombkowski RA. Russell MJ. Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol. 2004;286:R678–685. doi: 10.1152/ajpregu.00419.2003. [DOI] [PubMed] [Google Scholar]
- 15.Elrod JW. Calvert JW. Morrison J. Doeller JE. Kraus DW. Tao L. Jiao X. Scalia R. Kiss L. Szabo C, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enokido Y. Suzuki E. Iwasawa K. Namekata K. Okazawa H. Kimura H. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J. 2005;19:1854–1856. doi: 10.1096/fj.05-3724fje. [DOI] [PubMed] [Google Scholar]
- 17.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 18.Furne J. Saeed A. Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1479–R1498. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 19.Gadalla MM. Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin LR. Francom D. Dieken FP. Taylor JD. Warenycia MW. Reiffenstein RJ. Dowling G. Determination of sulfidein brain tissue by gas dialysis/ion chromatography: Postmortem studies and two case reports. J Anal Toxicol. 1989;13:105–109. doi: 10.1093/jat/13.2.105. [DOI] [PubMed] [Google Scholar]
- 21.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 22.Hosoki R. Matsuki N. Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 23.Ichinohe A. Kanaumi T. Takashima S. Enokido Y. Naai Y. Kimura H. Cystathionine beta-synthase is enriched in the brains of Down's patients. Biochem Biophys Res Commun. 2005;338:1547–1550. doi: 10.1016/j.bbrc.2005.10.118. [DOI] [PubMed] [Google Scholar]
- 24.Ishigami M. Hiraki K. Umemura K. Ogasawara Y. Ishii K. Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 25.Ishii I. Akahoshi N. Yamada H. Nakano S. Izumi T. Suematsu M. Cystathionine γ–lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem. 2010;285:26358–26368. doi: 10.1074/jbc.M110.147439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii I. Akahoshi N. Yu X-N. Kobayashi Y. Namekata K. Komaki G. Kimura H. Murine cystathionine γ-lyase: Complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J. 2004;381:113–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jocelyn PC. The standard redox potential of cysteine-cystine form the thiol-disulphide exchange reaction with glutathione and lipoic acid. Eur J Biochem. 1967;2:327–331. doi: 10.1111/j.1432-1033.1967.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 28.Junier MP. Coulpier M. Le Forestier N. Cadusseau J. Suzuki F. Peschanski M. Dreyfus PA. Transforming growth factor alpha expression in degenerating motoneurons of the murine mutant wobbler: A neuronal signal for astrogliosis? J Neurosci. 1994;14:4206–4216. doi: 10.1523/JNEUROSCI.14-07-04206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato A. Ogura M. Suda M. Control mechanism in the rat liver enzyme system converting L-methionine to L-cystine. 3. Noncompetitive inhibition of cystathionine synthetase-serine dehydratase by elemental sulfur and competitive inhibition of cystathionine-homoserine dehydratase by L-cysteine and L-cystine. J Biochem. 1966;59:40–48. doi: 10.1093/oxfordjournals.jbchem.a128256. [DOI] [PubMed] [Google Scholar]
- 30.Kimura Y. Dargusch R. Schubert D. Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 31.Kimura Y. Goto Y-I. Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 32.Kimura Y. Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 33.Kombian SB. Warenycia MW. Mele FG. Reiffenstein RJ. Effects of acute intoxication with hydrogen sulfide on central amino acid transmitter systems. NeuroToxicology. 1988;9:587–596. [PubMed] [Google Scholar]
- 34.Lee JK. Kozono D. Remis J. Kitagawa Y. Agre P. Stroud RM. Structural basis for conductance by the archaeal aquaporin AsqpM at 1.68 A. Proc Natl Acad Sci USA. 2005;102:18932–18937. doi: 10.1073/pnas.0509469102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee M. Tazzari V. Glustarini D. Rossi R. Sparatore A. Soldato PD. McGeer E. McGeer PL. Effects of hydrogen sulfide-releasing L-DOPA derivatives on glial activation. J Biol Chem. 2010;285:17318–17328. doi: 10.1074/jbc.M110.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitt MD. Abdel-Rehim MS. Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: Anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal. 2011;15:373–378. doi: 10.1089/ars.2010.3525. [DOI] [PubMed] [Google Scholar]
- 37.Lin MT. Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 38.Lippert AR. New EJ. Chang CJ. Re-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc. 2010;133:10078–10080. doi: 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- 39.Liu C. Pan J. Li S. Zhao Y. Wu LY. Berkman CE. Whorton AR. Xian M. Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew Chem Int Ed. 2011;50:10327–10329. doi: 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathai JC. Missner A. Kugler P. Saparov SM. Zeidel ML. Lee JK. Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci USA. 2009;106:16633–16638. doi: 10.1073/pnas.0902952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meister A. Fraser PE. Tice SV. Enzymatic desulfuration of β-mercaptopyruvate to pyruvate. J Biol Chem. 1954;206:561–575. [PubMed] [Google Scholar]
- 42.Mikami Y. Shibuya N. Kimura Y. Nagahara N. Ogasawara Y. Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J. 2011;439:479–485. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 43.Mikami Y. Shibuya N. Kimura Y. Nagahara N. Yamada M. Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem. 2011;286:39379–39386. doi: 10.1074/jbc.M111.298208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mustafa AK. Gadalla MM. Sen N. Kim S. Mu W. Gazi SK. Barrow RK. Yang G. Wang R. Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagahara N. Ito T. Kitamura H. Nishino T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: Confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem Cello Biol. 1998;110:243–250. doi: 10.1007/s004180050286. [DOI] [PubMed] [Google Scholar]
- 46.Nagahara N. Okazaki T. Nishino T. Cytosolic mercaptypyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking similarity in active site amino acid sequence and the increase in the mercaptopyruvate sulfurtransferase activity of rhodanese by site-directed mutagenesis. J Biol Chem. 1995;270:16230–16235. doi: 10.1074/jbc.270.27.16230. [DOI] [PubMed] [Google Scholar]
- 47.Nagai Y. Tsugane M. Oka J. Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004;18:557–559. doi: 10.1096/fj.03-1052fje. [DOI] [PubMed] [Google Scholar]
- 48.O'Dell TJ. Hawkins RD. Kandel ER. Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: Evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci USA. 1991;88:11285–1289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olson KR. Whitfield NL. Bearden SE. Leger JS. Nilson E. Gao Y. Maddeen JA. Hypoxic pulmonary vasodilation: A paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regal Integr Comp Physiol. 2010;298:R51–R60. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng H. Cheng Y. Dai C. King AL. Predmore BL. Lefer DJ. Wang B. A fluorescent probe for fast and quantitaqtive detection of hydrogen sulfide in blood. Angew Chem Int Ed. 2011;50:9672–9675. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Predmore BL. Lefer DJ. Development of hydrogen sulfide-based therapeutics for cardiovascular disease. J Cardiovasc Transl Res. 2010;3:487–498. doi: 10.1007/s12265-010-9201-y. [DOI] [PubMed] [Google Scholar]
- 52.Qian Y. Karpus J. KIabil O. Zhang S-Y. Zhu H-L. Banerjee R. Zhao J. He C. Selective fluorescent probes for live-cell monitoring of sulphide. Nat Commun. 2011:2–495. doi: 10.1038/ncomms1506. [DOI] [PubMed] [Google Scholar]
- 53.Reiffenstein RJ. Hulbert WC. Roth SH. Toxicology of hydrogensulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 54.Rossoni G. Manfredi B. Tazzari V. Sparatore A. Trivulzio S. Del Soldato P. Berti F. Activity of a new hydrogen sulfide-releasing aspirin (ACS14) on pathological cardiovascular alterations induced by glutathione depletion in rats. Eur J Pharmacol. 2010;648:139–145. doi: 10.1016/j.ejphar.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 55.Sasakura K. Hanaoka K. Shibuya N. Mikami Y. Kimura Y. Komatsu T. Ueno T. Terai T. Kimura H. Nagano T. Development of a highly selective fluorescence probe for hydrogen sulfide. J Am Chem Soc. 2011;133:18003–18005. doi: 10.1021/ja207851s. [DOI] [PubMed] [Google Scholar]
- 56.Savage JC. Gould DH. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J Chromatogr. 1990;526:540–545. doi: 10.1016/s0378-4347(00)82537-2. [DOI] [PubMed] [Google Scholar]
- 57.Shibuya N. Mikami Y. Kimura Y. Nagahara N. Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 58.Shibuya N. Tanaka M. Yoshida M. Ogasawara Y. Togawa T. Ishii K. Kimura H. 3-Mercaptopyruvate sulfurtransferease produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 59.Suh JH. Shenvi SV. Dixon BM. Liu H. Jaiswal AKI. Liu RM. Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stipanuk MH. Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan S. Schubert D. Maher P. Oxytosis: A novel form of programmed cell death. Cur Top Med Chem. 2001;1:497–506. doi: 10.2174/1568026013394741. [DOI] [PubMed] [Google Scholar]
- 62.Taniguchi T. Kimura T. Role of 3-mercaptopyruvate sulfurtransferase in the formation of the iron-sulfur chromophore of adrenal ferredoxin. Biochim Biophys Acta. 1974;364:284–295. doi: 10.1016/0005-2744(74)90014-x. [DOI] [PubMed] [Google Scholar]
- 63.Tateishi N. Higashi T. Naruse A. Nakashima K. Shiozaki H. Rat liver glutathione: Possible role as a reservoir of cysteine. J Nutr. 1977;107:51–60. doi: 10.1093/jn/107.1.51. [DOI] [PubMed] [Google Scholar]
- 64.Taub JW. Huang X. Matherly LH. Stout ML. Buck SA. Massey GV. Becton DL. Chang MN. Weinstein HJ. Ravindranath Y. Expression of chromosome 21-localized genes in acute myeloid leukemia: Differences between Down syndrome and non-Down syndrome blast cells and relationship to in vitro sensitivity to cytosine arabinoside and daunorubicin. Blood. 1999;94:1393–1400. [PubMed] [Google Scholar]
- 65.Teague B. Asiedu S. Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: Evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tiranti V. Viscomi C. Hildebrandt T. Di Meo I. Mineri R. Tiveron C. Levitt MD. Prelle A. Fagiolari G. Rimoldi M. Zeviani M. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15:200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- 67.Toohey JI. Sulphane sulphur in biological systems: A possible regulatory role. Biochem J. 1989;264:625–632. doi: 10.1042/bj2640625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toohey JI. Sulfur signaling: Is the agent sulfide or sulfane? Anal Biochem. 2011;413:1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 69.Tsugane M. Nagai Y. Kimura Y. Oka J-I. Kimura H. Differentiated astrocytes acquire sensitivity to hydrogen sulfide that is diminished by the transformation into reactive astrocytes. Antioxid Redox Signal. 2007;9:257–269. doi: 10.1089/ars.2007.9.257. [DOI] [PubMed] [Google Scholar]
- 70.Ubuka T. Umemura S. Yuasa S. Kinuta M. Watanabe K. Purification and characterization of mitochondrial cysteine aminotransferase from rat liver. Physiol Chem Physics. 1978;10:483–500. [PubMed] [Google Scholar]
- 71.Umemura K. Kimura H. Hydrogen sulfide enhances reducing activity in neurons: Neurotropic role of H2S in the brain? Antioxid Redox Signal. 2007;9:2035–2041. doi: 10.1089/ars.2007.1802. [DOI] [PubMed] [Google Scholar]
- 72.Vitvitsky V. Tomas M. Ghorpade A. Gendelman HE. Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]
- 73.Warenycia MW. Goodwin LR. Benishin CG. Reiffenstein RJ. Grancom DM. Taylor JD. Dieken FP. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- 74.Warenycia MW. Smith KA. Blashko CS. Kombian SB. Reiffenstein Rj. Monoamine oxidase inhibition as a sequel of hydrogen sulfide intoxication: Increases in brain catecholamine and 5-hydroxytryptamine. Arch Toxicol. 1989;63:131–136. doi: 10.1007/BF00316435. [DOI] [PubMed] [Google Scholar]
- 75.Westrop GD. Georg I. Coombs GH. The mercaptopyruvate sulfurtransferase of Trichomonas vaginalis links cysteine catabolism to the production of thioredoxin persulfide. J Biol Chem. 2009;284:33485–33494. doi: 10.1074/jbc.M109.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whiteman M. Armstrong JS. Chu SH. Jia-Ling S. Wong BS. Hheung NS. Halliwell B. Moore PK. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 77.Whiteman M. Cheung NS. Zhu YZ. Chu SH. Siau JL. Wong BS. Armstrong JS. Moore PK. Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- 78.Whiteman M. Winyard PG. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 79.Wintner EA. Deckwerth TL. Langston W. Bengtsson A. Leviten D. Hill P. Insko MA. Dumpit R. VandenEkart E. Toombs CF. Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol. 2010;160:941–957. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wisniewski KE. Wisniewski HM. Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 81.Yang G. Wu L. Jiang B. Yang W. Qi J. Cao K. Meng Q. Mustafa AK. Mu W. Zhang S, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao W. Zhang J. Lu Y. Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]