Abstract
Significance: Statins (3-hydroxy-3-methylglutarylcoenzyme A reductase inhibitors) are commonly used in the treatment of cardiovascular diseases. Statins reduce plasma low-density lipoproteins, inhibit inflammatory reaction, improve endothelial function, ameliorate oxidative stress, and reduce platelet activity. Consequently, statins markedly decrease the risk of acute cardiovascular events. H2S is synthesized in all layers of the vascular wall, including the endothelium, smooth muscle cells, and perivascular adipose tissue (PVAT). Recent Advances: Recent studies demonstrate that PVAT-derived H2S decreases vascular tone by activating KATP and/or KCNQ potassium channels in smooth muscle cells. Lipophilic atorvastatin, but not hydrophilic pravastatin, increases net H2S production in PVAT by inhibiting its mitochondrial oxidation, and augments the anticontractile effect of PVAT. Inhibition of H2S metabolism results from atorvastatin-induced decrease in coenzyme Q, which is a cofactor of H2S oxidation by sulfide:quinone oxidoreductase. In contrast to H2S, statins do not impair mitochondrial oxidation of organic substrates. Critical Issues: Taking into account antiatherosclerotic and anti-inflammatory effect of H2S, the gas may mediate some of the beneficial effects of statins on the cardiovascular system. In addition, specific statins differ in their ability to enhance H2S signaling. Future Directions: Since both statins and H2S reduce ischemia-reperfusion injury, the possible effect of statins on H2S oxidation in other tissues such as the heart and the kidney needs to be examined. Inhibition of H2S metabolism may be a new therapeutic strategy to improve H2S signaling, especially in the mitochondrial compartment. Antioxid. Redox Signal. 17, 81–94.
Statins: Mechanism of Action and Clinical Application
Statins are competitive inhibitors of 3-hydroxy-3-methylglutarylcoenzyme A (HMG-CoA) reductase—a rate-limiting enzyme in cholesterol synthesis—which converts HMG-CoA to mevalonate (22) (Fig. 1). The first statin, mevastatin, was discovered in the 1970s by the Japanese microbiologist, Akira Endo, in the fermentation broth of Penicillium citrinium during the search of potential new suppressors of lipid metabolism. The first clinically used statin, lovastatin, discovered in Aspergillus terreus in 1978, was approved by Food and Drug Administration for the treatment of hypercholesterolemia in 1987. By inhibiting HMG-CoA reductase in the liver, statins up-regulate the expression of the low-density lipoprotein receptor (LDL-R), thus accelerating the metabolism of LDLs. Statin-induced intracellular cholesterol depletion results in the activation of sterol regulatory element-binding protein-2 (SREBP-2)—the sterol-sensitive transcription factor that up-regulates the expression of LDL-R in maintaining intracellular cholesterol balance. SREBP-2 also up-regulates the expression of HMG-CoA reductase, partially counteracting the hypocholesterolemic effect of statins. Although this effect is negligible in humans, in some mammalian species such as the rat, it is very marked and overcomes statin-induced inhibition of the enzyme; consequently, statins have little or no effect on plasma cholesterol concentration in the rat. Currently used statins may reduce plasma LDL cholesterol by 30%–40% in patients with primary (genetically-determined) and secondary hypercholesterolemia. Since elevated LDL cholesterol is a major risk factor of atherosclerosis, statins effectively reduce the incidence of acute cardiovascular events in both primary and secondary prevention of ischemic heart disease and cerebrovascular diseases (51, 52). Therefore, statins are among the most widely used medications in cardiovascular disorders. Apart from reducing LDL cholesterol, statins exert other beneficial effects on plasma lipid profile such as a decrease in triglyceride concentration, lowering of atherogenic small dense LDL, and an increase in the antiatherogenic high-density lipoproteins (74).
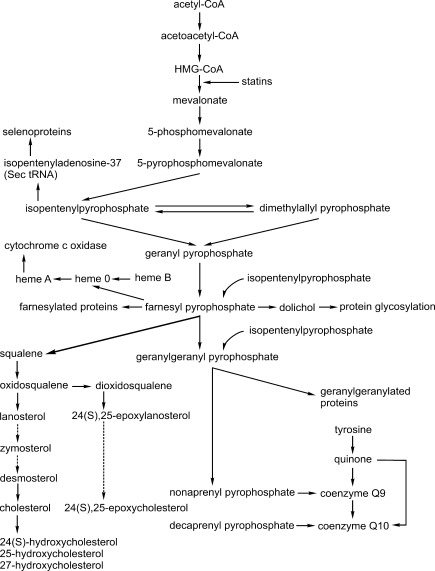
FIG. 1.
Mevalonate cascade. Statins inhibit the rate-limiting enzyme of the mevalonate cascade, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which converts HMG-CoA to mevalonate. Mevalonate is the precursor of many biologically important compounds (see text for details). Broken lines represent the abbreviated multi-step reactions.
However, effects of statins extend far beyond their impact on cholesterol synthesis. Indeed, mevalonate, the product of statin-inhibited reaction, is also the precursor of many other biologically active molecules (Fig. 1). The best known of them, farnesyl- and geranylgeranyl-pyrophosphates, are attached to various proteins in the process referred to as protein isoprenylation, which is catalyzed by protein farnesyl- and geranylgeranyltransferases, respectively. The most important isoprenylated proteins are those belonging to the small GTP-binding (small G protein) family. Among them, Ras proteins, which are farnesylated, are involved in signal transduction by growth factor receptors and activate mitogen-activated protein kinase cascade. In contrast, Rho proteins, which are geranylgeranylated, regulate vesicle transport, assembly, and activation of phagocytic and nonphagocytic NADPH oxidases, cytoskeleton reorganization, and activation of Rho-dependent kinase, which regulates smooth muscle cell contractility and growth. Inhibition of protein farnesylation and/or geranylgeranylation is responsible for lipid-independent or “pleiotropic” effects of statins such as amelioration of oxidative stress, improvement of endothelial nitric oxide (NO) production, inhibition of adhesion protein expression, and leukocyte migration, decreased synthesis of proinflammatory cyto- and chemokines, inhibition of cell hypertrophy/proliferation (including antihypertrophic effect on vascular smooth muscle cells crucial for growth of atherosclerotic plaque), and beneficial modification of coagulation/fibrinolysis balance (6). Pleiotropic effects contribute significantly to antiatherosclerotic properties of statins, which are evident not only in patients with hypercholesterolemia but also in those with normal cholesterol levels (51, 55). Lipid-independent effects of statins are responsible for stabilization of atherosclerotic plaque and mediate rapid improvement of prognosis in statin-treated patients with acute coronary syndromes or cerebral stroke, which is evident before any effect on the lipid profile appears. In addition, due to their pleiotropic effects, statins are beneficial in animal models of and in humans with other pathologies such as ischemia-reperfusion injury, heart failure, osteoporosis, Alzheimer disease, multiple sclerosis, and rheumatoid arthritis (6).
In contrast to cholesterol and isoprenyl pyrophosphates, inhibition of other mevalonate derivatives is rather involved in adverse effects of statins. Coenzyme Q (CoQ) consists of a benzoate ring originating from tyrosine and polyisoprene side chain synthesized from farnesyl pyrophosphate. The predominant form (about 95%) of CoQ in humans is CoQ10 containing 10 isoprene units in the side chain, whereas in the rat, CoQ9 predominates. CoQ exists in either reduced (ubiquinol) or oxidized (ubiquinone) form; transition between them allows it to function as an electron carrier in the mitochondrial respiratory chain. CoQ accepts electrons from mitochondrial complex I (NADH:ubiquinone oxidoreductase) and complex II (succinate:quinone oxidoreductase) and transfers them to complex III (ubiquinone:cytochrome c reductase). In addition, ubuquinol is an important lipid-soluble antioxidant in plasma membranes and plasma lipoproteins. CoQ is the only endogenous lipid-soluble antioxidant in mammals and the only one that on oxidation may be regenerated to its active reduced form by animal enzymes. Many studies have demonstrated that statins decrease plasma and tissue CoQ concentration in experimental animals and humans.
Other important mevalonate derivatives that may be inhibited by statins are oxysterols—endogenous agonists of liver X receptors (LXRs). LXR are ligand-activated transcription factors that stimulate the expression of genes involved in the regulation of cholesterol export from cells, its reverse transport from peripheral tissues to the liver and biliary excretion. LXR are activated by (1) some intermediates of cholesterol synthesis, that is, desmosterol and zymosterol, (2) the product of a “shunt pathway” of cholesterol synthesis, 24(S),25-epoxycholesterol (24,25-EC), (3) oxygenated cholesterol derivatives synthesized from cholesterol by cholesterol hydroxylases: 24(S)-hydroxy-, 25-hydroxy, and 27-hydroxycholesterol (Fig. 1). Several studies have demonstrated that statins decrease plasma and tissue concentrations of at least some oxysterols, in particular, 24,25-EC (5).
In addition, farnesylpyrophosphate is a substrate for dolichol synthesis. Dolichols are polyisoprenoid alcohols consisting of 16–21 isoprene subunits and are essential carriers of oligosaccharides for enzymatic protein glycosylation—an important post-translational modification that determines protein trafficking and function. Until now, statins have been documented to suppress insulin receptor and insulin-like growth factor 1 (IGF-1) receptor glycosylation, resulting in reduced insulin- and IGF-1 stimulated glucose uptake in cultured adipocytes (60), and to inhibit glycosylation of erythropoietin receptor in cultured erythroblastoma cells (19).
Isopentenyl pyrophosphate (Fig. 1) is also attached to adenosine-37 of selenocysteine-tRNA by tRNA isopentenyltransferase. This modification is essential for decoding UGA as a selenocysteine rather that a stop codon, which is crucial for selenoprotein synthesis. It has been demonstrated that lovastatin inhibits selenocysteine-tRNA synthesis and reduces selenoprotein content in cultured Xenopus oocytes (69). Recently, Kromer and Mossmann (29) have demonstrated that atorvastatin, cerivastatin, and lovastatin inhibit de novo synthesis of two selenoproteins, glutathione peroxidase 1 and 4, in cultured hepatoma HepG2 cells, rendering these cells more sensitive to proapoptotic effect of reactive oxygen species. In contrast, statins did not change the level of the other selenoprotein, thioredoxin reductase. It has been suggested that inhibition of selenoprotein synthesis may contribute to some common (i.e., myopathy, hepatotoxicity) and rare (hypothyroidism) side effects of statins, because clinical presentation of statin-induced myopathy closely resembles that of selenium deficiency-induced myopathy, and selenocysteine-containing enzymes, iodothyronine deiodinases, are crucial for thyroid hormone metabolism (42). However, the effect of statins on selenoprotein synthesis in vivo has not been studied so far.
Finally, it has been suggested that statins may inhibit synthesis of heme A—the specific heme molecule being a prosthetic group of cytochrome c oxidase. Heme A is synthesized from heme B—the most common type of heme contained, for example, in hemoglobin—in a two-step reaction. First, heme B is farnesylated to heme O at the C2 position by heme farnesyltransferase (also called Cox10; the 10th subunit of cytochrome c oxidase), and then hydroxyl group at the C8 position of heme O is oxidized to carboxyl group by Cox15. Cytochrome c oxidase contains two heme A molecules, both are bound to the Cox1 subunit (33). Knockout of Cox10 or Cox15 genes results in cytochrome c oxidase deficiency clinically manifesting as severe myopathy, cardiomyopathy, encephalopathy, and lactic acidosis—case reports of such symptoms in statin-treated patients have been described (5). Statins could inhibit synthesis of heme A by depleting farnesylpyrophosphate; however, this possibility has not been studied so far.
Statins may be divided into two groups: hydrophilic (pravastatin and rosuvastatin) and lipophilic (simvastatin, lovastatin, fluvastatin, atorvastatin, and pitavastatin). The structure of statins currently used in clinical practice is demonstrated in Figures 2 and 3, and their solubility in Figure 4. Cerivastatin, withdrawn from the market in 2001 due to many cases of fatal rhabdomyolysis, is also lipophilic. Lovastatin is a natural fungal metabolite, simvastatin and pravastatin are synthesized from lovastatin by chemical modifications, and the remaining statins are completely synthetic compounds. Hydrophilic statins poorly permeate plasma membranes and act primarily in the liver, because they are transported to hepatocytes by organic anion transporters. Since about 50% of LDL is metabolized in the liver, hydrophilic statins effectively reduce plasma LDL cholesterol level but have less pleiotropic extrahepatic effects. In contrast, lipophilic statins easily permeate plasma membranes and are active not only in the liver but also in extrahepatic tissues. Consequently, lipophilic statins have more pleiotropic effects in peripheral tissues as well (59).
FIG. 2.
Structure of hydrophilic statins.
FIG. 3.
Structure of lipophilic statins.
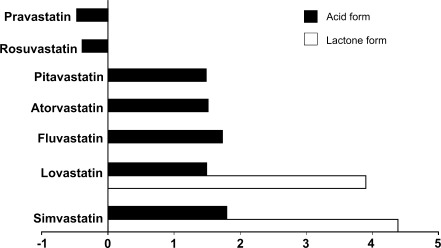
FIG. 4.
Solubility of statins. Logarithms of octanol-to-water distribution coefficients (logD) are presented for each individual drug according to ref. (59). Negative values are characteristic for hydrophilic and positive for lipophilic statins. For lovastatin and simvastatin, used clinically as the inactive precursor lactones, logD values are presented for both lactone (white bars) and acid forms (black bars).
Simvastatin and lovastatin are used as HMG-CoA reductase inactive lactone prodrugs; they are converted in vivo to active open acid forms by paraoxonase 3 (PON3)—an esterase belonging to the three-member PON family. In contrast, other statins are used in active acid forms. Simvastatin, lovastatin, and atorvastatin are metabolized in the liver by cytochrome P450 (CYP) 3A4 isoform. Fluvastatin is not metabolized by CYP3A4 but by CYP2C9. Pravastatin and rosuvastatin are excreted in substantial amounts in the urine in the unchanged form, and rosuvastatin is also partially metabolized by CYP2C9. These pharmacokinetic differences determine the half-life of specific statins and their possible interactions with other CYP-metabolized medications (59).
Hydrogen Sulfide: Synthesis and Function in the Vascular Wall
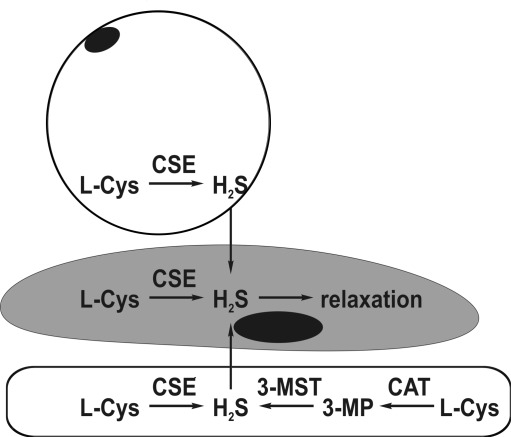
Among three known enzymatic pathways of H2S formation, catalyzed by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and the concerted action of cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3-MST), the latter two are operative in the vascular wall (Fig. 5). Early studies suggested that CSE is expressed exclusively in vascular smooth muscle cells and is the only source of H2S in the cardiovascular system (79). However, more recent studies indicate that the situation is much more complex. First, CSE was found in mice endothelial cells (76), and CSE-mediated H2S production is stimulated by cholinergic agonists, making this gasotransmitter one of the possible endothelium-derived relaxing factors. In addition, both 3-MST and CAT are present in rat aortic endothelial cells, and these cells can generate H2S from cysteine only in the presence of 2-oxoglutarate—an obligatory co-substrate of CAT—suggesting that the 3-MST dependent pathway is the only source of H2S in endothelial cells in this species (57). However, in a recent study (23), expression of CSE in endothelial cells of rat mesenteric arteries was documented by immunohistochemical methods. In addition, endothelial denudation markedly reduced cysteine-induced vasorelaxation, indicating that endothelial CSE may be a predominant source of vascular H2S in small resistance vessels. In bovine pulmonary artery endothelial cells, both CBS and 3-MST but not CSE are expressed (47). H2S concentration in plasma and aortic tissue is markedly reduced in CSE knockout mice, thus demonstrating that CSE is the principal source of H2S in the cardiovascular system (76).
FIG. 5.
Enzymatic synthesis of H2S in the vascular wall. In both smooth muscle cells (middle) and perivascular adipocytes (top), H2S is produced from l-cysteine (l-Cys) by cystathionine γ-lyase (CSE). In endothelial cells (bottom), H2S may be synthesized by either CSE or cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3-MST) with 3-mercaptopyruvate (3-MP) as the intermediate product.
Although in vitro studies suggest that CSE can synthesize H2S also from homocysteine and that the contribution of homocysteine increases in hyperhomocysteinemia (12), it is unclear whether homocysteine serves as a H2S precursor in vivo. Recently, it has been demonstrated that concentration of free H2S (i.e., excluding sulfane sulfur and acid-labile sulfur) in the murine aortic wall is 20–200 times higher than in many other tissues, including the brain, liver, heart, kidney, and striated muscles, thus indicating that H2S plays an important role in vascular homeostasis (34).
The best-characterized role of H2S in the cardiovascular system is the regulation of vascular tone. Currently available data allow concluding that H2S has a complex and concentration-dependent effect of vascular tone (Fig. 6) with vasoconstriction at a lower and vasodilation at a higher concentration (1, 70). However, the precise mechanisms through which H2S regulates vascular tone demonstrated in various studies depends on animal species, vascular bed, and experimental conditions (measurement of isometric or isotonic tension, agonists induced to predilate or preconstrict the vessels before studying the effect of H2S, etc.) [see ref. (4) for review]. H2S-induced vasoconstriction may results from (1) inhibition of l-arginine transport to endothelial cells by cationic aminoacid transporter, (2) attenuation of protein kinase B/Akt-induced phosphorylation of endothelial nitric oxide synthase (eNOS), (3) direct inhibition of eNOS, (4) scavenging of NO to form inactive nitrosothiol, (5) inhibition of adenylate cyclase and decrease in intracellular cyclic AMP in smooth muscle cells, (6) stimulation of anion exchanger-2 (AE-2) in smooth muscle cells leading to import of Cl− ions and export of superoxide anion (O2-·); the latter scavenges NO to form peroxynitrite (37), (7) phospholipase A2-mediated release of arachidonic acid, or its cyclooxygenase-independent metabolites by endothelial cells (14).
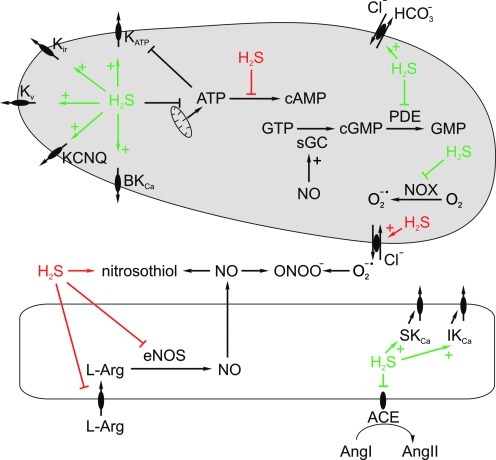
FIG. 6.
Mechanisms of vascular tone regulation by H2S. H2S may dilate blood vessels by activating different types of potassium channels, that is, ATP-inhibited (KATP), inwardly rectifying (Kir), voltage-sensitive (Kv), KCNQ, and large conductance Ca2+-activated (BKCa). By suppressing cytochrome c oxidase, H2S may inhibit ATP synthesis leading to the de-repression of KATP channels. Vasodilation may also be mediated by activation of HCO3− efflux through the HCO3−/Cl− exchanger, inhibition of cGMP-degrading phosphodiesterase (PDE), angiotensin-converting enzyme (ACE), and NADPH oxidase (NOX)—the source of nitric oxide (NO) scavenger, superoxide (O2−•). Finally, by stimulating endothelial cell small- and intermediate-conductance Ca2+-activated potassium channels (SKCa and IKCa), H2S may trigger endothelium-dependent hyperpolarization. On the other hand, H2S may constrict blood vessels by inhibiting adenylate cyclase, scavenging NO to form nitrosothiol, inhibiting l-arginine (l-Arg) transport to endothelial cells and eNOS activity, as well as by stimulating efflux of superoxide, which then binds NO to form peroxynitrite (ONOO−) in the extracellular space. Effects of H2S resulting in vasodilation and vasoconstriction are shown in green and red, respectively. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars). sGC, soluble guanylyl cyclase; eNOS, endothelial nitric oxide synthase.
The main mechanism of H2S-induced vasorelaxation is direct stimulation of ATP-sensitive K+ channels (KATP) in smooth muscle cells. Indeed, vasorelaxant effect of H2S in vitro and hypotensive effect in vivo are markedly attenuated or completely abolished by KATP antagonist, glibenclamide (79), and H2S increases KATP channel's open probability in intact vascular smooth muscle cells (61). H2S stimulates KATP channels by sulfhydrating thiol (–SH) to persulfide (–SSH) groups of extracellularly localized cysteine residues within the channels itself or in the accompanying subunit, sulfonylurea receptor (24). The involvement of other types of K+ channels, that is, voltage-gated (Kv), KCNQ, inwardly rectifying (Kir) and large-conductance Ca2+-activated (BKCa) has also been suggested by some studies (2, 10, 23, 54). Other effects of H2S on vascular smooth muscle cells have been demonstrated, including (1) stimulation of AE-2 resulting in Cl-/HCO3- exchange and cell acidification, (2) reduced affinity of AT1 receptors for angiotensin II, (3) inhibition of ATP synthesis and vasorelaxation induced by energy depletion and/or derepression of KATP channels, (4) inhibition of NADPH oxidase (NOX)—the main source of NO-scavenging superoxide anion in the cardiovascular system, and (5) inhibition of cGMP and/or cAMP-hydrolyzing phosphodiesterases [reviewed in ref. (4)]. Some studies suggest that vasodilating effect of H2S may be partially endothelium dependent and mediated by either NO (78) or endothelial small- and intermediate-conductance calcium-activated potassium channels (SKCa and IKCa); the evidence of the involvement of endothelium-derived hyperpolarizing factor (11, 14). In addition, H2S suppresses renin secretion by renal juxtaglomerular apparatus (39), and decreases angiotensin-converting enzyme activity in endothelial cells (30).
Despite these controversies, there is little doubt that the principal cardiovascular effect of H2S in vivo is vasorelaxation. Indeed, intravenously administered H2S solution or its inorganic (NaHS) or organic (GYY4137) donors decrease blood pressure, whereas CSE inhibitor has the opposite effect (36, 79). Moreover, according to some (76), although not all (21), studies, blood pressure is significantly elevated in CSE−/− mice. Vascular CSE-H2S pathway is suppressed in experimental models of hypertension, such as spontaneously hypertensive rat and hypertension induced by chronic eNOS blockade (4).
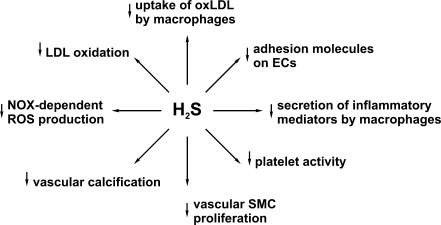
In addition to regulating vascular tone and blood pressure, H2S inhibits atherogenesis by multiple mechanisms (Fig. 7). In particular, H2S suppresses proliferation and stimulates apoptosis of vascular smooth muscle cells, decreases extracellular matrix formation in the vascular wall, and inhibits synthesis of pro-inflammatory cytokines and adhesion proteins (4, 49, 71). In addition, H2S suppresses oxidative modification of LDLs by inhibiting reactive oxygen species (ROS)-generating enzymes, NOX and myeloperoxidase, direct scavenging of ROS, and destruction of already formed lipid hydroperoxides (31, 43, 44). Moreover, H2S protects endothelial cells from oxidative insults induced by various factors (3, 64, 75). Finally, H2S suppresses platelet aggregation (77), inhibits uptake of oxidatively modified lipoproteins by macrophages (80), and up-regulates anti-inflammatory and antiatherogenic heme oxygenase-carbon monoxide system in endothelial cells and macrophages (49).
FIG. 7.
The main mechanisms through which H2S inhibits atherogenesis. ECs, endothelial cells; SMC, smooth muscle cells; NOX, NADPH oxidase; ROS, reactive oxygen species.
Several studies indicate that H2S inhibits atherogenesis in vivo. In particular, plasma H2S concentration and its production in the aortic wall are significantly reduced in apolipoprotein E knockout mice—a well-established experimental model of hyperlipidemia and atherosclerosis (68). Interestingly, aortic CSE expression is up-regulated in these animals, which may be a compensatory response to H2S deficiency. Administration of exogenous NaHS reduces, whereas CSE inhibitor, propargylglycine (PAG), aggravates atherosclerotic lesions in these animals. Similarly, H2S deficiency was observed in rats treated with high doses of vitamin D and nicotine to induce vascular calcifications, and NaHS reduced vascular lesions in this model (35, 73). Down-regulation of CSE-H2S pathway was also demonstrated in balloon-induced injury of rat carotid artery—a model of vascular restenosis—and treatment with NaHS ameliorated neointimal hyperplasia in the injured vessel (41).
H2S in Perivascular Adipose Tissue as a Vasodilator
Most large and small arteries are surrounded by adipose tissue referred to as perivascular adipose tissue (PVAT). PVAT is a part of visceral adipose tissue and consists of both white and brown adipocytes. Brown adipocytes contain large amounts of mitochondria and are highly metabolically active, they also express uncoupling protein-1 at the high level and, thus, may oxidize fatty acids or other substrates in the uncoupled manner, that is, energy is dissipated as heat rather than stored in ATP (50). Although neglected in most studies concerning the regulation of vascular tone, PVAT is an integral part of the vascular wall. It was first demonstrated in 1991 that rat aortic rings with PVAT were less responsive to constricting effect of norepinephrine than aortic rings without PVAT; however, the effect was initially attributed to norepinephrine uptake by adipocytes or sympathetic endings localized in PVAT. In 2002, Löhn et al. (38) demonstrated that vasoconstricting effects of angiotensin II, serotonin and phenylephrine (an α1-adrenergic agonist) were also smaller in aortic rings with PVAT than in those without PVAT, and suggested that periadventitial adipose tissue secretes humoral relaxing factor which they named adipose tissue-derived relaxing factor (ADRF). It was demonstrated that ADRF activity is not accounted for by NO, adenosine, and cyclooxygenase- or cytochrome P450-dependent arachidonate derivatives. Furthermore, vasodilating effect of ADRF was abolished by high, depolarizing extracellular K+ concentrations, suggesting the involvement of potassium channels. In addition, the effect of ADRF was at least partially attenuated by KATP channel blocker, glibenclamide (38). Apart from rat aortic rings, subsequent studies demonstrated the anticontractile effect of periadventitial fat on peripheral arteries that play a more significant role in the regulation of systemic vascular resistance than large conduit vessels (65).
In 2009, Fang et al. (17) demonstrated that incubation of homogenates of rat periaortic adipose tissue (PAAT) with cysteine in the presence of pyridoxal 5′-phosphate resulted in H2S formation. H2S production in PAAT itself was comparable to that in the aortic wall with removed PAAT, and was inhibited by 65%–75% with CSE inhibitors, PAG or β-cyano-l-alanine. These data suggest that CSE is the main source of H2S in PVAT. In addition, the expression of CSE in PAAT was demonstrated by Western blotting, and CSE protein was found in adipocytes by immunohistochemistry (17). H2S concentration measured by sulfur-sensitive electrode was twofold higher in the incubation medium of PAAT+ than of PAAT− rat aortic rings. It should be noted that 3-MST dependent pathway of H2S production could not be detected in that study, because 2-oxoglutarate was not added to the incubation medium. Thus, it cannot be excluded that 3-MST dependent pathway is also operative in the adipose tissue.
Demonstration of H2S synthesis in PAAT led to the hypothesis that it can mediate ADRF activity. Indeed, increase in aortic tension induced by serotonin or phenylephrine was lower in PAAT+ than in PAAT− rat aortic rings, and in PAAT− rings, this anticontractile effect could be mimicked by exogenous H2S. The mixture of l-cysteine and pyridoxal 5′-phosphate augmented, whereas CSE inhibitors abolished the anticontractile effect of PAAT, while having no effect on vascular tone of PAAT− rings. The anticontractile effect of PAAT was not affected by endothelial removal or NO synthase inhibitor, l-NAME, but was abolished by glibenclamide. Moreover, transfer of incubation/culture medium from PAAT+ aortic rings or isolated periadventitial adipocytes to PAAT− rings reduced constricting effect of phenylephrine, serotonin or angiotensin II, and this effect could not be observed if donor PAAT was preincubated with CSE inhibitors before medium collection. Taken together, these results indicate that H2S produced in PAAT by CSE reduces vasoconstriction by activating KATP channels in vascular smooth muscle cells (17).
Subsequently, Schleifenbaum et al. (54) have demonstrated that the presence of PVAT also impairs serotonin-induced contractility of rat mesenteric artery. In contrast to aortic rings, the anticontractile effect of PVAT on the mesenteric artery was not affected by KATP channel antagonist, but was reduced by nonspecific inhibitor of voltage-sensitive K+ channels, 4-aminopyridine, as well as by the specific antagonist of Kv7.x (KCNQ) channels, XE991. In contrast, XE991 had no effect on serotonin-induced contraction of mesenteric artery rings with removed PVAT. Similarly to aorta, anticontractile effect of PVAT on mesenteric artery was abolished by CSE inhibitors. In addition, NaHS relaxed mesenteric artery rings without PVAT, and this effect was inhibited by XE991. Taken together, these data indicate that PVAT-derived H2S reduces vascular tone also in small resistance arteries; however, in contrast to aorta, its effect is not mediated by KATP but rather by KCNQ channels. It was also demonstrated that KCNQ channel activators such as retigabine or VRX0621688 induced more prominent vasorelaxation of PVAT- rings or PVAT+ rings treated with CSE inhibitor in comparison to PVAT+ rings not treated with CSE inhibitors. These results indicate that KCNQ channel-mediated vasorelaxing mechanism is “saturated” by PVAT-derived H2S under physiological conditions. Thus, KCNQ channel activators might be especially useful vasodilators when CSE-H2S pathway in PVAT is impaired.
Given the role of PVAT-derived H2S in the regulation of vascular tone, it is interesting if and how the CSE-H2S pathway is modulated by hemodynamic factors. Fang et al. (17) have demonstrated that phenylephrine, serotonin, and angiotensin II increased H2S production from l-cysteine in isolated PAAT. In contrast, these vasoconstrictors reduced H2S production in aortic rings without PAAT. Since stimulation of H2S release was observed in isolated PAAT without adjacent aortic wall, it could not result from vasoconstriction itself but rather from the direct effect of these mediators on adipose cells.
In experimental hypertension induced in the rat by constriction of the abdominal aorta, H2S synthesis and CSE expression in the aortic wall without PAAT was unchanged in comparison to control normotensive animals; however, H2S production and CSE expression in PAAT increased by 70% and 130%, respectively. Plasma H2S level was also slightly higher in hypertensive animals. Thus, the CSE-H2S system in PAAT could be a back-up vasodilatory mechanism, which is up-regulated in response to both acute effect of vasoconstrictors and chronic hypertension.
Effect of Statins on H2S in PVAT
Taking into account antihypertensive and antiatherogenic properties of H2S, elevating, its level could be a potential novel therapeutic strategy for cardiovascular diseases. However, H2S donors currently used in research are not suitable for pharmacotherapy. Inorganic salts, such as NaHS or Na2S, are converted to H2S in aqueous solutions, but this process results in rapid formation of large supraphysiological amounts of H2S. In addition, these salts easily undergo spontaneous oxidation in solutions, and the fraction of undissociated membrane-permeable H2S is highly pH-dependent. Consequently, it is very difficult to precisely control tissue concentration especially in the in vivo setting. Several H2S-releasing derivatives of anti-inflammatory and other currently used drugs such as diclofenac, naproxen, aspirin, mesalamine, valproic acid, and sildenafil have been synthesized. However, these derivatives retain the activity of a parent compound and, in addition, dithiolethione moiety used as a H2S-releasing group has some H2S-independent effects. Organic H2S-releasing compounds without other activities such as GYY4137 are available (36). However, these agents are now at the early stage of experimental research and their clinical application is at best a matter of future.
Nevertheless, H2S may also be modulated by currently used cardiovascular medications but, until now, very little is known about it. Therefore, we examined the effect of statins—one of the most commonly used drugs in cardiovascular medicine—on H2S formation in the vascular wall (72). We performed this study in healthy normolipidemic rats. Although statins do not reduce plasma cholesterol in the rat, this species is a good model to study cholesterol-independent pleiotropic effects of statins. In addition, in the rat, statins decrease plasma triglycerides, and this effect correlates with the extent of LDL-cholesterol reduction in humans (28). We used two representative statins: hydrophilic pravastatin and lipophilic atorvastatin, and administered them for 3 weeks at doses of 20 and 40 mg/kg/day, respectively; these doses of prava- and atorvastatin exert comparable effects on the lipid profile (reduction of triglycerides by about 30%–35%). Then, we examined H2S formation catalyzed by aortic media and PAAT homogenates under optimal conditions (saturating l-cysteine and pyridoxal 5′-phosphate concentrations). Before homogenization, endothelium was removed to avoid possible interactions between H2S and NO of endothelial origin. Consistently with results obtained by Fang et al. (17), we observed that aortic media and PAAT homogenates were able to synthesize H2S from l-cysteine and this synthesis was almost completely abolished by CSE inhibitor. We found that only atorvastatin, but not pravastatin, increased H2S production in PAAT, whereas neither statin had any effect in the aortic media. Both statins increased H2S production in the liver. Thus, we identified new pleiotropic lipid-independent effect of statins in the vascular wall (72).
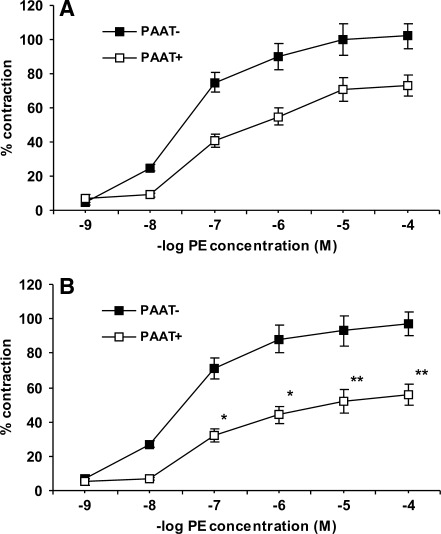
To examine whether the effect of atorvastatin has any implications for vascular tone, we studied phenylephrine-induced contractility of endothelium-denuded aortic rings. We found that although contraction induced by high KCl concentration was independent of the presence of PAAT, and was not modified by statin treatment, phenylephrine-induced contractility was attenuated in rings containing PAAT in comparison to those with removed PAAT (Fig. 8). This anticontractile effect of PAAT was abolished by either PAG or glibenclamide that had no significant effect on the contractility of PAAT− rings. In addition, PAAT+ rings isolated from atorvastatin but not from pravastatin-treated animals exhibited less contractility in response to phenylephrine. In contrast, atorvastatin treatment had no effect on the contractility of PAAT− rings (Fig. 8). Preincubation of PAAT+ aortic rings isolated from atorvastatin-treated rats with either PAG or glibenclamide increased phenylephrine-induced contractility and eliminated the difference between atorvastatin-treated and control rats. Taken together, these results indicate that PAAT-derived H2S decreases vascular tone by activating KATP channels, and atorvastatin treatment augments this effect (72).
FIG. 8.
Contraction of isolated rat aortic rings with intact (PAAT+, white squares) or removed (PAAT−, black squares) periaortic adipose tissue in response to phenylephrine. On the horizontal axis is presented negative logarithm of phenylephrine (PE) concentration and on the vertical axis percent of contraction induced by PE in comparison to maximal contraction induced by 60 mM KCl. Aortic rings were isolated from control rats (top panel, A) and rats treated with atorvastatin for 3 weeks (bottom panel, B). Intact PAAT reduced contractility in both groups. There was no difference in PE-induced contractility of PAAT− rings between groups; however, contractility of PAAT+ rings was smaller in atorvastatin-treated rats. *p<0.05, **p<0.001 in comparison to contractility of control PAAT+ rings induced by the same PE concentration [reproduced with permission from ref. (72)]. PAAT, periaortic adipose tissue.
Mechanism of Statin-Induced Increase in H2S
Recent studies indicate that steady-state tissue H2S level is very low, because the gasotransmitter is rapidly oxidized in mitochondria. Hydrogen sulfide was known to be oxidized by some bacteria; however, its metabolism by eukaryotic mitochondria is a relatively novel finding (8). H2S is the first and the only currently known inorganic substrate for eukaryotic mitochondria that can provide energy for ATP synthesis. H2S is first oxidized to the level of elemental sulfur (sulfane sulfur of protein—SSH groups) by sulfide:quinone oxidoreductase (SQR). The enzyme transfers electrons from H2S to ubiquinone where they enter mitochondrial respiratory chain. Further steps of H2S oxidation are catalyzed by sulfur dioxygenase (ETHE1, enzyme deficient in inherited ethylmalonic encephalopathy) that oxidizes elemental sulfur to sulfite (SO32−), and sulfite oxidase which oxidizes sulfite to sulfate (SO42−). The involvement of additional enzymes (thiosulfate sulfurtransferase and thiosulfate reductase) and thiosulfate (S2O32−) as the intermediate was suggested but is not definitely proved (20).
In our initial experiments just described, H2S formation was measured in postnuclear tissue supernatants that contain both cytosol (where CSE is localized) and mitochondria. Thus, the net H2S production is measured under these conditions and was the difference between gas synthesis and metabolism. To elucidate which of these processes is affected by statins, we measured H2S production separately also in postmitochondrial supernatants. We found that H2S formation in postmitochondrial supernatants of both PAAT and liver was higher than in postnuclear supernatants. In addition, H2S production from l-cysteine in postmitochondrial supernatant was not affected by statins. Statins had no effect on CSE activity toward its primary substrate, l-cystathionine, measured in PAAT and liver (72). The difference in H2S formation between postmitochondrial and postnuclear supernatants is a putative mitochondrial H2S oxidation. Neither statin had any effect on H2S oxidation calculated in this manner in the aortic media, whereas atorvastatin, but not pravastatin, reduced it in PAAT. The calculated H2S oxidation in the liver was higher in both prava- and atorvastatin-treated rats than in control animals (72).
Subsequently, we repeated these experiments in the incubation medium which was deoxygenated by bubbling with the N2 gas. H2S formation in postmitochondrial supernatants measured in deoxygenated buffer tended to be slightly higher (10%–15%) than in normally oxygenated buffer; this difference presumably represents spontaneous H2S oxidation. In contrast, H2S formation in postnuclear supernatants measured under anoxic conditions was much higher than measured under normoxic conditions. The difference between H2S formation between postmitochondrial and postnuclear supernatants measured under anoxic conditions was close to 0 and was not affected by statins either in PAAT or in the liver (72). These results are consistent with previous findings that mitochondrial H2S oxidation is compromised under hypoxic conditions (32).
To further confirm that the difference in H2S formation between postmitochondrial and postnuclear supernatants indeed represents mitochondrial H2S oxidation, we measured this difference in livers obtained from control rats in the presence of inhibitors of mitochondrial complex I (rotenone), complex III (myxothiazole), or complex IV (potassium cyanide). We found that both myxothiazole and KCN, but not rotenone, reduced calculated H2S oxidation by at least 80% (68). These results are consistent with the mechanism of mitochondrial H2S oxidation in which complex I is not involved (8). Taken together, these data indicate that statins increase net H2S formation by inhibiting its mitochondrial metabolism, but have no effect on its cytosolic synthesis. To further confirm this conclusion, we isolated mitochondria from fresh liver samples, incubated them in the presence of 5 μM NaHS, and measured sulfide concentration to calculate the rate of H2S catabolism. Using this approach, we confirmed that H2S was more slowly oxidized by liver mitochondria isolated from both prava- and atorvastatin-treated than from control rats (72).
Mechanism of Statin-Induced Inhibition of Mitochondrial H2S Oxidation
The only currently known factor that may affect mitochondrial H2S oxidation is hypoxia. However, hypoxia could not be responsible for reduced H2S oxidation in statin-treated rats, because we measured it at a fixed level of buffer oxygenation. The next possibility which we considered was that statins reduce the activity of SQR—the first and possibly the rate-limiting enzyme in H2S metabolism. To address this issue, we measured SQR activity in isolated liver mitochondrial membranes in the presence of saturating CoQ concentration. However, neither prava- nor atorvastatin had any effect on SQR activity (72).
Next, we asked which products of the mevalonate cascade are responsible for the effect of statins. To answer this question, we supplemented statin-treated rats with various mevalonate products or the mevalonate itself at doses which, according to previous studies, restored their concentrations to control levels. We found that only mevalonate itself, farnesol, and CoQ9 (the major CoQ species in the rat) normalized mitochondrial H2S oxidation in the liver of atorvastatin or pravastatin-treated rats and in PAAT of atorvastatin-treated rats (13). In contrast, squalene and geranylgeraniol failed to normalize H2S production. Similarly, synthetic LXR agonist, TO901317, also did not restore mitochondrial H2S oxidation in statin-treated rats. These results suggest that statins compromise H2S oxidation by reducing CoQ, because both mevalonate and farnesol, but nor squalene or geranylgeraniol, are CoQ precursors (Fig. 1). In addition, we found that both prava- and atorvastatin decreased CoQ9 concentration in plasma and liver, whereas only atorvastatin did so in PAAT. Administration of exogenous CoQ9 in statin-treated rats also improved NaHS oxidation by isolated liver mitochondria (72). Finally, if isolated liver mitochondria were preincubated with synthetic CoQ9 before placing them in NaHS-containing solution, NaHS oxidation was improved in mitochondria isolated from statin-treated animals but not from control rats (72). Supplementation with CoQ9 had no effect on phenylephrine-induced contraction of PAAT− aortic rings in either control or atorvastatin-treated rats. In addition, CoQ9 did not change phenylephrine-induced contraction of PAAT+ rings isolated from rats not treated with atorvastatin. However, CoQ9 supplementation increased contractility of PAAT+ rings in atorvastatin-treated rats (72). In conclusion, these results indicate that statins compromise H2S oxidation by depleting CoQ.
Inhibition of Mitochondrial H2S Oxidation by Statins: Specific Effect or Generalized Mitochondrial/Dysfunction?
Since statins reduced mitochondrial H2S oxidation, it is important if this effect is specific for H2S or results from generalized impairment of mitochondrial function. In theory, statins could impair mitochondrial function by (1) decreasing CoQ and reducing the activity of CoQ-dependent respiratory complexes I, II, and/or III, (2) depleting heme A—a prosthetic group of cytochrome c oxidase, (3) reducing the amount of mitochondria in the cell.
Impairment of mitochondrial function is commonly observed in inherited CoQ deficiency (40). However, in these disorders, CoQ concentration in plasma and tissues is extremely low, usually below 10% of normal level. Decrease in CoQ after statin treatment, if observed, is much more moderate, rarely exceeding 30%–40%. If statins impair mitochondrial function by depleting CoQ remains highly controversial. In some studies, statin-induced mitochondrial dysfunction was observed, but this effect was usually associated with the development of side effects such as severe myopathy or hepatotoxicity, or was observed in vitro when high concentrations of statins were used. For example, Päivä et al. (48) observed that high-dose simvastatin treatment reduced combined activity of complex II and complex III (i.e., succinate: cytochrome c oxidoreductase) in skeletal muscle biopsies of patients with hypercholesterolemia. Combined activity of complex II and III or complex I and III is a reliable marker of CoQ availability because this activity relies on endogenous ubiquinone, in contrast to the measurement of individual activities of these complexes when exogenous CoQ is supplied at the saturating concentrations. However, in that study, (48) individual activities of complex I and IV were also reduced, and citrate synthase activity (a marker of mitochondria density in the cell) was lower in statin-treated patients, suggesting that simvastatin decreased mitochondrial density rather than impaired mitochondrial function. On the other hand, Duncan et al. (16) examined mitochondrial enzymes in two patients with symptomatic simvastatin-induced myopathy. In both of them, total activity of complex II and III was normal despite significant reduction of ubiquinone level (16). Similarly, lovastatin reduced CoQ concentration in cultured rat astrocytes by 50% but had no effect on complex I or complex II+III activities (16). Nakahara et al. found that, despite 70% reduction of CoQ in skeletal muscles of simvastatin- or pravastatin-treated rabbits, combined activities of complex I+III and II+III as well as individual activity of cytochrome c oxidase was normal (45). On the other hand, Tavintharan et al. (63) have demonstrated that simvastatin reduced CoQ in cultured HepG2 hepatocytes by 90%, which was associated with the decrease in ATP synthesis by as much as 80%; this effect was abolished by adding exogenous CoQ to the incubation medium. Decrease in ATP production induced by atorvastatin was also observed in cadriomyocyte mitochondria of guinea-pigs (15), and in myoblasts cultured in the presence of simvastatin, lovastatin, or fluvastatin, but not pravastatin or rosuvastatin (66). Thus, it is controversial whether statins can impair mitochondrial respiratory chain by suppressing CoQ synthesis. In addition, some studies suggest that statins may alter the amount of mitochondrial in the cell. For example, Schick et al. (53) have demonstrated that treatment with high doses of simvastatin or atorvastatin reduced mitochondrial DNA copy number in skeletal muscles of hypercholesterolemic patients. In contrast, fluvastatin increased citrate synthase activity—a marker of mitochondria density—in human HepG2 cells as well as in freshly isolated rat hepatocytes (67).
To examine whether statins specifically inhibit H2S oxidation or have a general detrimental effect on mitochondrial function, we isolated mitochondria from the liver of statin-treated rats and measured oxidation of NaHS as well as of organic substrate of complex II, succinate. We measured two markers of mitochondrial function: ATP production and mitochondrial membrane potential (Δψm). Δψm is potential difference across inner mitochondrial membrane, between mitochondrial matrix and mitochondrial intermembrane space (negative potential in the matrix). During electron transport through the mitochondrial respiratory chain, protons (H+) are also transferred from the matrix to the intermembrane space. The resulting H+ gradient provides energy for ATP synthesis and makes mitochondria the most negatively charged organelles in the cell with a Δψm from −150 to −180 mV. Thus, Δψm is a global marker of electron transport efficacy. We measured Δψm in suspended liver mitochondria by lipophilic cationic fluorescent probe, JC-1 (13). This probe accumulates in negatively charged space of mitochondrial matrix and changes not only the intensity but also the character of fluorescence in a concentration-dependent manner. In diluted solutions, JC-1 exists as monomers, which, when excited with the wavelength of 488 nm, emit green light at 535 nm. When JC-1 concentration increases, aggregates are formed that exhibit maximal emission within the orange range (595 nm). The ratio between intensity of orange-to-green fluorescence increases very sharply with increasing JC-1 concentration, which, inside mitochondria, is proportional to Δψm. To measure Δψm, we incubated suspended liver mitochondria with 1 μM JC-1 in the presence of either succinate or NaHS, and then measured fluorescence at both wavelengths to calculate this ratio. We found that Δψm measured in the presence of succinate was similar in control and statin-treated rats. In contrast, Δψm measured in the presence of NaHS was significantly lower in statin-treated in comparison to the control group (13). In addition, the highly significant correlation between Δψm in the individual samples and the rate of NaHS oxidation was observed. Similar results were obtained when ATP synthesis by isolated mitochondria was assessed. These results indicate that statins specifically reduce H2S oxidation while having no effect on oxidation of organic substrates. We suggest that this specificity may be accounted for by different Km values of SQR versus complex I/complex II for CoQ; however, this hypothesis requires further research. Since under physiological conditions H2S constitutes only a minor fraction of mitochondrial substrates, this effect of statins is unlikely to impair ATP production and cell energy status but is related only to H2S signaling. In addition, statins did not reduce either citrate synthase activity or cytochrome c content in liver or PAAT (13), indicating that reduced H2S oxidation did not result from the decrease in mitochondria density per cell.
Statins, H2S, and Regulation of Insulin Sensitivity
Enzymatic H2S synthesis is not confined to PVAT. Indeed, H2S is synthesized from l-cysteine also by epidydimal, perirenal, and brown adipose tissue in the rat (18). Both CSE expression and H2S synthesis were also observed in cultured rat epidydimal adipocytes and preadipocytes, and H2S production from cysteine was by 30% higher in mature fat cells than in preadipocytes (18).
In freshly isolated rat epidydimal adipocytes, H2S in solution (10–1000 μM) reduced basal and insulin-stimulated uptake of glucose as well as of nonmetabolizable 2-deoxyglucose in a time- and concentration-dependent manner (18). Although H2S concentration used in that study was relatively high, the effect seems to be physiologically relevant, because it was reproduced when adipocytes were incubated in the presence of cysteine and pyridoxal 5′-phosphate to increase endogenous H2S formation. Moreover, either PAG or β-cyano-l-alanine not only abolished cysteine+pyridoxal phosphate-induced reduction of glucose uptake, but also reduced baseline H2S production in adipocytes and stimulated glucose uptake either in the absence or in the presence of insulin. These data indicate that H2S produced under physiological conditions regulates glucose uptake and insulin sensitivity of adipocytes.
In primary culture of epidydimal rat adipocytes, high concentrations of glucose reduced H2S production in a time- and concentration-dependent manner (18). Thus, negative feedback regulatory mechanism between glucose and H2S may exist in the adipose tissue, with H2S inhibiting glucose uptake, and glucose inhibiting the CSE-H2S pathway. Furthermore, CSE expression and H2S production in adipose tissue was up-regulated in rats fed high fructose diet for 12 weeks, which is a widely used experimental model of insulin resistance (18). In addition, the significant negative correlation between H2S production and insulin-stimulated glucose uptake in the adipose tissue was observed (18). These observations suggest that CSE-H2S system in adipose tissue may contribute to insulin resistance in the metabolic syndrome. In addition, H2S inhibits insulin secretion by activating KATP channels in pancreatic β-cells (62).
Several recent studies have demonstrated that statins modulate insulin sensitivity in a drug-specific manner. Although the results are controversial, most studies indicate that hydrophilic pravastatin may improve insulin sensitivity and reduce the incidence of type 2 diabetes, whereas lipophilic statins have the opposite detrimental effects [for review, see refs. (26, 27)]. Effect of statins on insulin sensitivity and carbohydrate metabolism is of high clinical significance. Insulin resistance is an important risk factor of cardiovascular diseases, and diabetes mellitus is one the most common causes of hyperlipidemia; thus, many statin-treated patients suffer from impaired insulin sensitivity and glucose intolerance.
Recently, we have demonstrated that pravastatin increased, whereas atorvastatin reduced insulin sensitivity measured by hyperinsulinemic euglycemic clamp in the rat (Beltowski et al., manuscript submitted for publication). In addition, pravastatin reduced fasting plasma nonesterified fatty acids and glycerol concentrations, which are the markers of adipose tissue lipolysis in vivo, whereas atorvastatin had the opposite effect. Since inhibition of lipolysis is one of the principal effects of insulin, these results indicate that pravastatin improves whereas atorvastatin impairs insulin sensitivity of the adipose tissue.
The mechanism(s) through which atorvastatin impairs insulin sensitivity of adipocytes is currently unclear. In vitro, lipophilic statins have been demonstrated to suppress differentiation of preadipocytes to more insulin sensitive mature adipocytes, decrease glycosylation of insulin receptors and their translocation to the plasma membrane, and to suppress insulin-induced trafficking of glucose transporter GLUT4 from intracellular stores to the plasma membrane (5). However, all these effects were observed at high statin concentrations, far exceeding those found in the blood of statin-treated patients. It is unclear whether atorvastatin (and possibly other lipophilic statins) increases H2S level in other adipose tissue depots in the similar manner as it does in PVAT. If this is the case, detrimental effect of lipophilic statins on insulin sensitivity could be mediated by H2S. However, it should be noted that other adipose tissue depots contain less or no brown adipocytes, and white adipocytes contain less mitochondria than brown fat cells. Thus, the rate of mitochondrial oxidation may have a less significant effect on H2S availability in subcutaneous or in non-PVAT visceral adipose tissue depots. The possible role of H2S in detrimental effect of atorvastatin on insulin sensitivity is currently under research.
Inhibition of H2S Oxidation: A New Target for Pharmacotherapy?
Currently, several possibilities are considered to augment the beneficial effects of H2S on the cardiovascular system: (1) stimulation of H2S synthesizing enzymes, (2) supplying more substrate (l-cysteine) for H2S synthesis, and (3) administration of exogenous H2S or its donors. Each of these strategies has well-known limitations. Statins are the first and are currently the only drugs shown to increase H2S level by inhibiting its metabolism. However, other potential strategies to pharmacologically inhibit H2S breakdown are possible, such as administration of SQR inhibitors (56). Inhibiting H2S oxidation may be an interesting variant of H2S-directed pharmacotherapy. First, as exemplified by our results, statins affect H2S metabolism in a drug- and tissue-specific manner, determined by physical properties (and possibly pharmacokinetics) of individual drugs. In addition, inhibiting H2S oxidation is expected to stimulate H2S-mediated signaling most effectively in tissues with highest SQR activity and, in general, high oxidative metabolism such as the heart, while having less or no effect on tissues with low SQR level such as the brain (32). In contrast, H2S or its donors are expected to increase H2S level in a less tissue-dependent manner, and rather should be more effective in those with low SQR activity. In addition, after in vivo administration, a large fraction of H2S released from the donors may bind to hemoglobin or other hemoproteins, limiting its intracellular availability. H2S donors are also more likely to impair mitochondrial oxidation of organic substrates, because SQR may compete with complexes I and II for a common pool of CoQ. Indeed, in the presence of high H2S concentration, complexes I and II may even operate in the reverse manner, that is, reducing rather than oxidizing their substrates (8). Such a possibility is unlikely if SQR is inhibited. Moreover, intracellular compartmentalization may be the advantage, that is, inhibiting H2S oxidation will increase its concentration in mitochondria more markedly than in other intracellular compartments. That H2S has some specific roles in mitochondria is supported by several observations. First, H2S is synthesized in mitochondria in a 3-MST-dependent pathway, and in some tissues such as the brain, this is the main source of the gas (58). Second, H2S increases reduced glutathione concentration in mitochondria more than in other organelles (25). Third, stimulation of mitochondrial KATP channels may be the important mechanism of H2S-mediated inhibition of ischemia-reperfusion injury of the heart and other organs, for example, the kidney (9). Fourth, moderate inhibition of cytochrome c oxidase by intramitochondrial H2S may also be a significant mechanism of cytoprotection (7). Finally, hypoxia increases H2S level first of all in mitochondria, and oxygen sensing is now considered one of the principal roles of H2S (46). Combining H2S donors with agents suppressing its H2S oxidation may be the other therapeutic option. Such a combination could allow potentiating the H2S-elevating effect of each individual component in the synergistic manner and to reduce their doses and avoid undesirable side effects. It should be noted that H2S, in contrast to NO and CO, is the only gasotransmitter which is enzymatically metabolized giving us this additional opportunity to control its level. However, it should be kept in mind that inhibiting H2S oxidation is a potential hazard for tissues such as colonic mucosa where effective metabolic gas clearance is essential to avoid the toxicity of H2S generated by the commensal bacteria.
Abbreviations Used
- 3-MST
3-mercaptopyruvate sulfurtransferase
- 24,25-EC
24(S),25-epoxycholesterol
- ADRF
adipose tissue-derived relaxing factor
- AE-2
anion exchanger-2
- CAT
cysteine aminotransferase
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- CoQ
coenzyme Q
- CYP
cytochrome P450
- eNOS
endothelial nitric oxide synthase
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- LDL
low-density lipoprotein
- LDL-R
low-density lipoprotein receptor
- LXR
liver X receptor
- NO
nitric oxide
- NOX
NADPH oxidase
- ONOO−
peroxynitrite
- PAAT
periaortic adipose tissue
- PAG
propargylglycine
- PVAT
perivascular adipose tissue
- PON
paraoxonase
- ROS
reactive oxygen species
- SQR
sulfide:quinone oxidoreductase
- SREBP-2
sterol regulatory element-binding protein-2
Acknowledgments
The authors' original studies quoted in this article (13, 68) were supported by grant DS 476 from Medical University, Lublin, Poland, as well as by EU Project “The equipment of innovative laboratories doing research on new medicines used in the therapy of civilization and neoplastic diseases” within the Operational Program Development of Eastern Poland 2007–2013, Priority Axis I Modern Economy, Operations I.3 Innovation Promotion.
References
- 1.Ali MY. Ping CY. Mok YY. Ling L. Whiteman M. Bhatia M. Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulfide? Br J Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Magableh MR. Hart JL. Mechanism of vasorelaxation and role of endogenous hydrogen sulfide production in mouse aorta. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:403–413. doi: 10.1007/s00210-011-0608-z. [DOI] [PubMed] [Google Scholar]
- 3.Bearden SE. Beard RS. Pfau JC. Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. Am J Physiol Heart Circ Physiol. 2010;299:H1568–H1576. doi: 10.1152/ajpheart.00555.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bełtowski J. Jamroz-Wiśniewska A. Tokarzewska D. Hydrogen sulfide and its modulation in arterial hypertension and atherosclerosis. Cardiovasc Hematol Agents Med Chem. 2010;8:173–186. doi: 10.2174/187152510792481207. [DOI] [PubMed] [Google Scholar]
- 5.Bełtowski J. Wójcicka G. Jamroz-Wiśniewska A. Adverse effects of statins—mechanisms and consequences. Curr Drug Saf. 2009;4:209–228. doi: 10.2174/157488609789006949. [DOI] [PubMed] [Google Scholar]
- 6.Blum A. Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203:325–330. doi: 10.1016/j.atherosclerosis.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Bos EM. Leuvenink HG. Snijder PM. Kloosterhuis NJ. Hillebrands JL. Leemans JC. Florquin S. van Goor H. Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J Am Soc Nephrol. 2009;20:1901–1905. doi: 10.1681/ASN.2008121269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouillaud F. Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxid Redox Signal. 2011;15:379–391. doi: 10.1089/ars.2010.3678. [DOI] [PubMed] [Google Scholar]
- 9.Calvert JW. Coetzee WA. Lefer DJ. Novel insights into hydrogen sulfide—mediated cytoprotection. Antioxid Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheang WS. Wong WT. Shen B. Lau CW. Tian XY. Tsang SY. Yao X. Chen ZY. Huang Y. 4-aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul Pharmacol. 2010;53:94–98. doi: 10.1016/j.vph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y. Ndisang JF. Tang G. Cao K. Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physio. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 12.Chiku T. Padovani D. Zhu W. Singh S. Vitvitsky V. Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chylińska B. Effect of pravastatin and atorvastatin on synthesis and metabolism of endogenous hydrogen sulfide in the vascular wall. Ph.D. Dissertation. Medical University; Lublin: 2011. [Google Scholar]
- 14.d'Emmanuele di Villa Bianca R. Sorrentino R. Coletta C. Mitidieri E. Rossi A. Vellecco V. Pinto A. Cirino G. Sorrentino R. Hydrogen sulfide-induced dual vascular effect involves arachidonic acid cascade in rat mesenteric arterial bed. J Pharmacol Exp Ther. 2011;337:59–64. doi: 10.1124/jpet.110.176016. [DOI] [PubMed] [Google Scholar]
- 15.Diebold BA. Bhagavan NV. Guillory RJ. Influences of lovastatin administration on the respiratory burst of leukocytes and the phosphorylation potential of mitochondria in guinea pigs. Biochim Biophys Acta. 1994;1200:100–108. doi: 10.1016/0304-4165(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 16.Duncan AJ. Hargreaves IP. Damian MS. Land JM. Heales SJ. Decreased ubiquinone availability and impaired mitochondrial cytochrome oxidase activity associated with statin treatment. Toxicol Mech Methods. 2009;19:44–50. doi: 10.1080/15376510802305047. [DOI] [PubMed] [Google Scholar]
- 17.Fang L. Zhao J. Chen Y. Ma T. Xu G. Tang C. Liu X. Geng B. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J Hypertens. 2009;27:2174–2185. doi: 10.1097/HJH.0b013e328330a900. [DOI] [PubMed] [Google Scholar]
- 18.Feng X. Chen Y. Zhao J. Tang C. Jiang Z. Geng B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem Biophys Res Commun. 2009;380:153–159. doi: 10.1016/j.bbrc.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 19.Hamadmad SN. Hohl RJ. Lovastatin suppresses erythropoietin receptor surface expression through dual inhibition of glycosylation and geranylgeranylation. Biochem Pharmacol. 2007;74:590–600. doi: 10.1016/j.bcp.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrandt TM. Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishii I. Akahoshi N. Yamada H. Nakano S. Izumi T. Suematsu M. Cystathionine γ-lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem. 2010;285:26358–26368. doi: 10.1074/jbc.M110.147439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Istvan ES. Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 23.Jackson-Weaver O. Paredes DA. Bosc LV. Walker BR. Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca2+-activated potassium channels. Circ Res. 2011;108:1439–1447. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang B. Tang G. Cao K. Wu L. Wang R. Molecular mechanism for H2S-induced activation of KATP channels. Antioxid Redox Signal. 2010;12:1167–1178. doi: 10.1089/ars.2009.2894. [DOI] [PubMed] [Google Scholar]
- 25.Kimura Y. Goto Y. Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 26.Koh KK. Sakuma I. Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis. 2010;215:1–8. doi: 10.1016/j.atherosclerosis.2010.10.036. , [DOI] [PubMed] [Google Scholar]
- 27.Kostapanos MS. Liamis GL. Milionis HJ. Elisaf MS. Do statins beneficially or adversely affect glucose homeostasis? Curr Vasc Pharmacol. 2010;8:612–631. doi: 10.2174/157016110792006879. [DOI] [PubMed] [Google Scholar]
- 28.Krause BR. Newton RS. Lipid-lowering activity of atorvastatin and lovastatin in rodent species: triglyceride-lowering in rats correlates with efficacy in LDL animal models. Atherosclerosis. 1995;117:237–244. doi: 10.1016/0021-9150(95)05576-i. [DOI] [PubMed] [Google Scholar]
- 29.Kromer A. Moosmann B. Statin-induced liver injury involves cross-talk between cholesterol and selenoprotein biosynthetic pathways. Mol Pharmacol. 2009;75:1421–1429. doi: 10.1124/mol.108.053678. [DOI] [PubMed] [Google Scholar]
- 30.Laggner H. Hermann M. Esterbauer H. Muellner MK. Exner M. Gmeiner BM. Kapiotis S. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J Hypertens. 2007;25:2100–2104. doi: 10.1097/HJH.0b013e32829b8fd0. [DOI] [PubMed] [Google Scholar]
- 31.Laggner H. Muellner MK. Schreier S. Sturm B. Hermann M. Exner M. Gmeiner BM. Kapiotis S. Hydrogen sulfide: a novel physiological inhibitor of LDL atherogenic modification by HOCl. Free Radic Res. 2007;41:741–747. doi: 10.1080/10715760701263265. [DOI] [PubMed] [Google Scholar]
- 32.Lagoutte E. Mimoun S. Andriamihaja M. Chaumontet C. Blachier F. Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797:1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Lenaz G. Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal. 2010;12:961–1008. doi: 10.1089/ars.2009.2704. [DOI] [PubMed] [Google Scholar]
- 34.Levitt MD. Abdel-Rehim MS. Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal. 2011;15:373–378. doi: 10.1089/ars.2010.3525. [DOI] [PubMed] [Google Scholar]
- 35.Li W. Tang C. Jin H. Du J. Regulatory effects of sulfur dioxide on the development of atherosclerotic lesions and vascular hydrogen sulfide in atherosclerotic rats. Atherosclerosis. 2011;215:323–330. doi: 10.1016/j.atherosclerosis.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Li L. Whiteman M. Guan YY. Neo KL. Cheng Y. Lee SW. Zhao Y. Baskar R. Tan CH. Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 37.Liu YH. Bian JS. Bicarbonate-dependent effect of hydrogen sulfide on vascular contractility in rat aortic rings. Am J Physiol Cell Physiol. 2010;299:C866–C872. doi: 10.1152/ajpcell.00105.2010. [DOI] [PubMed] [Google Scholar]
- 38.Löhn M. Dubrovska G. Lauterbach B. Luft FC. Gollasch M. Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 39.Lu M. Liu YH. Goh HS. Wang JJ. Yong QC. Wang R. Bian JS. Hydrogen sulfide inhibits plasma renin activity. J Am Soc Nephrol. 2010;21:993–1002. doi: 10.1681/ASN.2009090949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mancuso M. Orsucci D. Volpi L. Calsolaro V. Siciliano G. Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr Drug Targets. 2010;11:111–121. doi: 10.2174/138945010790031018. [DOI] [PubMed] [Google Scholar]
- 41.Meng QH. Yang G. Yang W. Jiang B. Wu L. Wang R. Protective effect of hydrogen sulfide on balloon injury-induced neointima hyperplasia in rat carotid arteries. Am J Pathol. 2007;170:1406–1414. doi: 10.2353/ajpath.2007.060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moosmann B. Behl C. Selenoproteins, cholesterol-lowering drugs, and the consequences: revisiting of the mevalonate pathway. Trends Cardiovasc Med. 2004;14:273–281. doi: 10.1016/j.tcm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Muellner MK. Schreier SM. Laggner H. Hermann M. Esterbauer H. Exner M. Gmeiner BM. Kapiotis S. Hydrogen sulfide destroys lipid hydroperoxides in oxidized LDL. Biochem J. 2009;420:277–281. doi: 10.1042/BJ20082421. [DOI] [PubMed] [Google Scholar]
- 44.Muzaffar S. Shukla N. Bond M. Newby AC. Angelini GD. Sparatore A. Del Soldato P. Jeremy JY. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res. 2008;45:521–528. doi: 10.1159/000129686. [DOI] [PubMed] [Google Scholar]
- 45.Nakahara K. Kuriyama M. Sonoda Y. Yoshidome H. Nakagawa H. Fujiyama J. Higuchi I. Osame M. Myopathy induced by HMG-CoA reductase inhibitors in rabbits: a pathological, electrophysiological, and biochemical study. Toxicol Appl Pharmacol. 1998;152:99–106. doi: 10.1006/taap.1998.8491. [DOI] [PubMed] [Google Scholar]
- 46.Olson KR. Whitfield NL. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid Redox Signal. 2010;12:1219–1234. doi: 10.1089/ars.2009.2921. [DOI] [PubMed] [Google Scholar]
- 47.Olson KR. Whitfield NL. Bearden SE. St Leger J. Nilson E. Gao Y. Madden JA. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol. 2010;298:R51–R60. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Päivä H. Thelen KM. Van Coster R. Smet J. De Paepe B. Mattila KM. Laakso J. Lehtimäki T. von Bergmann K. Lütjohann D. Laaksonen R. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78:60–68. doi: 10.1016/j.clpt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Pan LL. Liu XH. Gong QH. Wu D. Zhu YZ. Hydrogen sulfide attenuated tumor necrosis factor-α-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS One. 2011;6:e19766. doi: 10.1371/journal.pone.0019766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Rajsheker S. Manka D. Blomkalns AL. Chatterjee TK. Stoll LL. Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ridker PM. Rifai N. Clearfield M. Downs JR. Weis SE. Miles JS. Gotto AM. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 52.Sacks FM. Pfeffer MA. Moye LA. Rouleau JL. Rutherford JD. Cole TG. Brown L. Warnica JW. Arnold JM. Wun CC. Davis BR. Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 53.Schick BA. Laaksonen R. Frohlich JJ. Päivä H. Lehtimäki T. Humphries KH. Côté HC. Decreased skeletal muscle mitochondrial DNA in patients treated with high-dose simvastatin. Clin Pharmacol Ther. 2007;81:650–653. doi: 10.1038/sj.clpt.6100124. [DOI] [PubMed] [Google Scholar]
- 54.Schleifenbaum J. Köhn C. Voblova N. Dubrovska G. Zavarirskaya O. Gloe T. Crean CS. Luft FC. Huang Y. Schubert R. Gollasch M. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- 55.Sever PS. Dahlöf B. Poulter NR. Wedel H. Beevers G. Caulfield M. Collins R. Kjeldsen SE. Kristinsson A. McInnes GT. Mehlsen J. Nieminen M. O'Brien E. Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 56.Shahak Y. Arieli B. Padan E. Hauska G. Sulfide quinone reductase (SQR) activity in Chlorobium. FEBS Lett. 1992;299:127–130. doi: 10.1016/0014-5793(92)80230-e. [DOI] [PubMed] [Google Scholar]
- 57.Shibuya N. Mikami Y. Kimura Y. Nagahara N. Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 58.Shibuya N. Tanaka M. Yoshida M. Ogasawara Y. Togawa T. Ishii K. Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 59.Shitara Y. Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Siddals KW. Marshman E. Westwood M. Gibson JM. Abrogation of insulin-like growth factor-I (IGF-I) and insulin action by mevalonic acid depletion: synergy between protein prenylation and receptor glycosylation pathways. J Biol Chem. 2004;279:38353–38359. doi: 10.1074/jbc.M404838200. [DOI] [PubMed] [Google Scholar]
- 61.Tang G. Wu L. Liang W. Wang R. Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol. 2005;68:1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- 62.Taniguchi S. Niki I. Significance of hydrogen sulfide production in the pancreatic β-cell. J Pharmacol Sci. 2011;116:1–5. doi: 10.1254/jphs.11r01cp. [DOI] [PubMed] [Google Scholar]
- 63.Tavintharan S. Ong CN. Jeyaseelan K. Sivakumar M. Lim SC. Sum CF. Reduced mitochondrial coenzyme Q10 levels in HepG2 cells treated with high-dose simvastatin: a possible role in statin-induced hepatotoxicity? Toxicol Appl Pharmacol. 2007;223:173–179. doi: 10.1016/j.taap.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Tyagi N. Moshal KS. Sen U. Vacek TP. Kumar M. Hughes WM., Jr. Kundu S. Tyagi SC. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal. 2009;11:25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verlohren S. Dubrovska G. Tsang SY. Essin K. Luft FC. Huang Y. Gollasch M. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- 66.Wagner BK. Kitami T. Gilbert TJ. Peck D. Ramanathan A. Schreiber SL. Golub TR. Mootha VK. Large-scale chemical dissection of mitochondrial function. Nat Biotechnol. 2008;26:343–351. doi: 10.1038/nbt1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang W. Wong CW. Statins enhance peroxisome proliferator-activated receptor gamma coactivator-1α activity to regulate energy metabolism. J Mol Med. 2010;88:309–317. doi: 10.1007/s00109-009-0561-1. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y. Zhao X. Jin H. Wei H. Li W. Bu D. Tang X. Ren Y. Tang C. Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 69.Warner GJ. Berry MJ. Moustafa ME. Carlson BA. Hatfield DL. Faust JR. Inhibition of selenoprotein synthesis by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J Biol Chem. 2000;275:28110–28119. doi: 10.1074/jbc.M001280200. [DOI] [PubMed] [Google Scholar]
- 70.Webb GD. Lim LH. Oh VM. Yeo SB. Cheong YP. Ali MY. El Oakley R. Lee CN. Wong PS. Caleb MG. Salto-Tellez M. Bhatia M. Chan ES. Taylor EA. Moore PK. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J Pharmacol Exp Ther. 2008;324:876–882. doi: 10.1124/jpet.107.133538. [DOI] [PubMed] [Google Scholar]
- 71.Whiteman M. Li L. Rose P. Tan CH. Parkinson DB. Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wójcicka G. Jamroz-Wiśniewska A. Atanassova P. Chaldakov GN. Chylińska-Kula B. Bełtowski J. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol Res. 2011;63:68–76. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Wu SY. Pan CS. Geng B. Zhao J. Yu F. Pang YZ. Tang CS. Qi YF. Hydrogen sulfide ameliorates vascular calcification induced by vitamin D3 plus nicotine in rats. Acta Pharmacol Sin. 2006;27:299–306. doi: 10.1111/j.1745-7254.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 74.Yamashita S. Tsubakio-Yamamoto K. Ohama T. Nakagawa-Toyama Y. Nishida M. Molecular mechanisms of HDL-cholesterol elevation by statins and its effects on HDL functions. J Atheroscler Thromb. 2010;17:436–451. doi: 10.5551/jat.5405. [DOI] [PubMed] [Google Scholar]
- 75.Yan SK. Chang T. Wang H. Wu L. Wang R. Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;351:485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 76.Yang G. Wu L. Jiang B. Yang W. Qi J. Cao K. Meng Q. Mustafa AK. Mu W. Zhang S. Snyder SH. Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zagli G. Patacchini R. Trevisani M. Abbate R. Cinotti S. Gensini GF. Masotti G. Geppetti P. Hydrogen sulfide inhibits human platelet aggregation. Eur J Pharmacol. 2007;559:65–68. doi: 10.1016/j.ejphar.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 78.Zhao W. Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 79.Zhao W. Zhang J. Lu Y. Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao ZZ. Wang Z. Li GH. Wang R. Tan JM. Cao X. Suo R. Jiang ZS. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med. 2011;236:169–176. doi: 10.1258/ebm.2010.010308. [DOI] [PubMed] [Google Scholar]