Abstract
Short bowel syndrome results from surgical resection, congenital defect or disease-associated loss of absorption. Parenteral support (PS) is lifesaving in patients with short bowel syndrome and intestinal failure who are unable to compensate for their malabsorption by metabolic or pharmacologic adaptation. Together, the symptoms of short bowel syndrome and the inconvenience and complications in relation to PS (e.g. catheter-related blood steam infections, central thrombosis and intestinal failure associated liver disease) may impair the quality of life of patients. The aim of treatment is to maximize intestinal absorption, minimize the inconvenience of diarrhea, and avoid, reduce or eliminate the need for PS to achieve the best possible quality of life for the patient. Conventional treatments include dietary manipulations, oral rehydration solutions, and antidiarrheal and antisecretory treatments. However, the evidence base for these interventions is limited and treatments that improve the structural and functional integrity of the remaining intestine are needed. Teduglutide, an analog of glucagon-like peptide 2, improves intestinal rehabilitation by promoting mucosal growth and possibly by restoring gastric emptying and secretion, thereby reducing intestinal losses and promoting intestinal absorption. In a 3-week, phase II balance study, teduglutide reduced diarrhea by around 700 g/day and fecal energy losses by around 0.8 MJ/day. In two randomized, placebo-controlled, 24-week, phase III studies, similar findings were obtained when evaluating the fluid composite effect, which is the sum of the beneficial effects of teduglutide – reduction in the need for PS, increase in urine production and reduction in oral fluid intake. The fluid composite effect reflects the increase in intestinal fluid absorption (and the concomitant reduction in diarrhea) and may be used in studies in which metabolic balance assessments are not performed. In studies of up to 24 weeks’ duration, teduglutide appears to be safe and well tolerated. Treatment with teduglutide was associated with enhancement or restoration of the structural and functional integrity of the remaining intestine with significant intestinotrophic and proabsorptive effects, facilitating a reduction in diarrhea and an equivalent reduction in the need for PS in patients with short bowel syndrome and intestinal failure.
Keywords: home parenteral nutrition, short bowel syndrome, teduglutide
Introduction
Short bowel syndrome (SBS) results from surgical resection, congenital defects or disease-associated loss of absorption. In adults, intestinal resections leading to SBS are mainly due to inflammatory bowel disease (IBD), mesenteric vascular disease, complications of surgery (ileus, volvulus, leakage of anastomoses etc.) and complications arising from treatment of cancers. SBS is highly heterogeneous – patients with intestinal insufficiency are able to compensate for their malabsorption by increasing oral intake (hyperphagia) and adapt metabolically or pharmacologically [Messing et al., 1991; Jeppesen and Mortensen, 2000], whereas patients with intestinal failure (SBS-IF) require parenteral support (PS, intravenous fluids and nutrition) to maintain fluid, electrolyte, trace element, vitamin and nutrient balances [Fleming and Remington, 1981; O’Keefe et al. 2006; Buchman et al. 2003]. Much of this heterogeneity is explained by differences in remnant bowel anatomy (bowel length and, in particular, the absorptive capacity), differences in the ability to compensate for malabsorption by hyperphagia, in the ability to adapt metabolically and the willingness of the patient to adhere to pharmacological treatment [Nightingale and Lennard Jones, 1993; Messing et al. 1999].
The large variance in the reports of the incidence and prevalence of SBS-IF reflects the fact that access to treatment varies among countries. In countries with a centralized referral system and a long tradition of PS treatment, incidence and prevalence rates are much higher than in countries where the treatment of SBS-IF is less established. In Denmark, where there is a long tradition of centralized referral and established PS treatment, the current annual incidence of adult SBS-IF patients is around 8 per million and the prevalence is around 30 per million (unpublished data). Medical companies have estimated that the number of patients with SBS-IF in Europe is approximately 10,000–15,000, with an equal number being present in the USA (http://www.drugs.com/clinical_trials/gattex-teduglutide-shownreduce-parenteral-support-volumepatients-adult-short-bowel-syndrome-11724.html; http://www.nycomed.com/media/news-releases/2011/nycomed-submits-european-marketing-authorisation-application-for-teduglutide—revestive/).
Although frequently lifesaving in patients with SBS-IF, the parenteral administration of fluids, electrolytes, trace elements, vitamins and nutrients (i.e. PS) has been associated with potentially life-threatening complications. Poor catheter care technique, insertion site-, tunnel- and catheter-related bloodstream infections may lead to bacteremia and even septicemia, and the presence of a central catheter may lead to central venous thrombosis and, on occasion, embolism [Buchman et al. 2003]. In addition, constituents of the parenteral solutions and chronic dehydration may contribute to progressive intestinal failure associated liver and renal disease, and eventually organ failure [Goulet et al. 2009; Lauverjat et al. 2006]. Together, the symptoms of SBS-IF, that is, diarrhea, large stomal output, stomal problems, fear of fecal incontinence, meteorism, abdominal pain, and the inconvenience and complications related to PS may cause potential restrictions in the lifestyle of patients and may lead to significant impairment of their quality of life [Jeppesen et al. 1999b; Baxter et al. 2006].
Therefore, treatments aim to maximize remnant intestinal absorptive capacity, to minimize the symptoms of malabsorption and to avoid, eliminate or minimize the need for PS. The current evidence base for treatment strategies in the management of patients with SBS-IF is weak and relies mainly on small, open-label, pilot studies. The most common medical treatment strategies for the management of SBS are presented in Figure 1.
Figure 1.
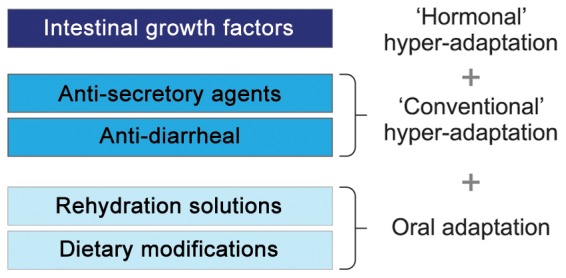
The most common medical strategies in patients with short bowel syndrome.
In general, patients are encouraged to compensate for malabsorption by adjusting their diet and by promoting hyperphagia. Historically, treatment strategies have included changes in dietary composition, for example, high-carbohydrate, low-fat diets for patients with a preserved colon [Nordgaard et al. 1994]. Enteral hyperalimentation has been suggested to improve macronutrient absorption [Joly et al. 2009], but it may be accompanied by increased fecal, fluid and electrolyte losses, which could aggravate abdominal discomfort and diarrhea and accentuate the need for parenteral fluids and electrolytes. Oral rehydration solutions with a high sodium concentration are unpalatable, precluding their long-term use, and their effects on intestinal fluid and electrolyte absorption remain to be established in balance studies in patients with SBS and more severe intestinal failure [Nightingale et al. 1992]. Agents such as codeine, loperamine and tincture of opium reduce intestinal motility but, again, their effects in patients with SBS and severe malabsorption remain to be established [Newton, 1978; Tytgat and Huibregtse, 1975; King et al. 1982]. Antisecretory drugs, that is, H2-receptor antagonists [Cortot et al. 1979; Jacobsen et al. 1986], proton-pump inhibitors [Jeppesen et al. 1998] or somatostatin analogs, reduce gastric acid secretions, jejunostomy wet weight output and diarrhea in patients with a preserved colon, but no effects on macronutrient absorption have been established [Cooper et al. 1986; Rodrigues et al. 1989; Ladefoged et al. 1989; O’Keefe et al. 1994]. Thus, a high unmet need for medical treatment exists in patients with SBS and intestinal failure. In the last two decades, a hormonal treatment paradigm focusing on intestinal rehabilitation by promoting intestinal hyperadaptation has been proposed [Jeppesen, 2007]. In this respect, glucagon-like peptide 2 (GLP-2) and the GLP-2 analog, teduglutide, have received particular attention. Here, the effects of treatment with the GLP-2 analog, teduglutide, in patients with SBS-IF are reviewed. The approaches for evaluating the effects of treatment on intestinal absorption are also discussed.
Measurements of intestinal function in patients with short bowel syndrome
The gold standard when evaluating intestinal absorption in patients with SBS is the 72 h metabolic balance study, during which feces and urine are analyzed, with each study repeated using a duplicate of the oral intake [Jeppesen et al. 1998]. Traditionally, the effects of treatment interventions are evaluated by undertaking repeated balance studies before (at baseline) and subsequent to the interventions. However, such metabolic balance studies are routinely performed in only a few centers worldwide. The best centers have skilled staff to ensure high patient compliance regarding correct oral intake and a dietician to prepare the duplicate meals. The staff must also educate patients to achieve accurate fecal and urine collections. The timing, quantity and quality of the oral intake of food and beverages should be kept constant during the repeated balance studies to ensure that the effect of a given treatment is not caused by changes in oral intake. If patients require PS, the quantity, quality and timing of infusions should also be documented to ensure that infusions are identical during the subsequent repeat balance study. As a consequence, admissions for balance study assessments are staff labor intensive, costly and inconvenient for the patients but, given the right analytical procedures, they provide high-quality data on intestinal absorption of energy, macronutrients, fluid and electrolytes.
In the more advanced centers, repeated admissions permit more elaborate, standardized evaluations of body composition, function and metabolism to document the consequences of improved intestinal absorption [Jeppesen et al. 2009].
The required standardization of the oral intake and the PS precludes the evaluation of the effect of a given treatment on spontaneous oral intake. Between admissions, it is also difficult to enforce standardized conditions, including oral intake and physical activity. To circumvent these barriers, balance studies have also been performed in a setting where a spontaneous oral intake is permitted – possibly reflecting a more ‘habitual’ patient situation [Jeppesen and Mortensen, 2000]. Regardless of the setup, it is important to document as many as possible of the parameters reflecting the metabolic balance situation of the patient during the balance study admissions.
In the ideal situation, where oral intake and PS are kept constant throughout balance studies in patients with SBS, an increase in intestinal fluid and electrolyte absorption would be reflected in an equivalent increase in urine excretion. However, the human physiological responses are often more complex – the increase in fluid and electrolyte absorption may also lead to accumulation within body fluids or cause changes in the ability to perspire. Whereas the increase in urine excretion is easily measured, it is more difficult to measure changes in body fluids and changes in perspiration. A rapid increase in body weight and the occurrence of edema are clinical signs of excess body fluid accumulation. Ideally, an increase in intestinal energy absorption would be reflected as an increase in body weight. However, increased intestinal absorption may also increase basal metabolic rate or allow for increased physical activity. These parameters are not easily measured and require even more elaborate laboratory facilities.
Due to the complexity of metabolic balance studies, the ability to wean from PS has been suggested as a surrogate marker of improved intestinal absorption [Jeppesen et al. 2011]. However, it is important to emphasize that although most patients with SBS-IF can be partially weaned from PS, regulatory mechanisms in human physiology will try to compensate for these changes in the metabolic balance. A reduction in parenteral fluid and electrolyte support will lead to diminished urine excretion and possibly less perspiration. If these conditions persist, dehydration, electrolyte disturbances and renal impairment may occur. Frequently, patients sense increased thirst and, consequently, they may try to compensate for dehydration and electrolyte deficiencies by increasing oral intake. A reduction in parenteral energy support may lead to body weight loss, a reduced level of physical activity and, possibly, a reduced basal metabolic rate. Again, patients may try to compensate by increasing their oral macronutrient intake. Thus, when evaluating the ability to wean PS following treatment interventions that are perceived to improve intestinal absorption, it is pertinent to ensure that oral intake is, in fact, kept constant, and that urine excretion, body weight, composition and function are maintained. However, it is also important to note that improved intestinal absorption may lead to a compensatory reduction in oral intake if PS is not reduced accordingly.
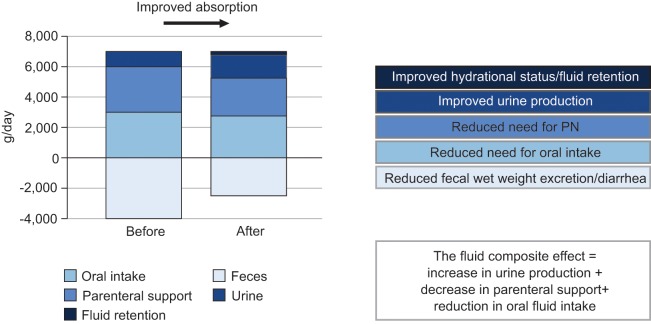
To overcome the reticence to perform balance studies and the collection and analysis of feces, the concept of the ‘fluid composite effect’ has been introduced [Jeppesen et al. 2011]. Here, it is assumed that improvement in intestinal fluid absorption (reflected as a reduction in diarrhea) may have the following positive outcomes, provided that perspiration is constant: improved hydration status (if in excess, fluid retention); increased urine production; reduction in the need for parenteral fluid support; and reduction in the need for oral fluid compensation.
Assuming a constant body weight, the fluid composite effect represents the change in intestinal fluid absorption and is calculated as the sum of the increase in urine production, the reduction in parenteral fluid support and the reduction in oral fluid intake (Figure 2).
Figure 2.
The fluid composite effect. PN, parenteral nutrition.
Based on this knowledge about measurements of intestinal function, the effects of teduglutide on intestinal absorption may be evaluated. In the phase II, ‘proof of concept’ study of teduglutide, conventional balance studies were performed [Jeppesen et al. 2005]. In the two subsequent phase III studies of teduglutide, the ability to wean patients from PS was evaluated [Jeppesen et al. 2011]. It was expected that improved intestinal absorption would directly translate into increased urine production and that this would lead to equivalent reductions in parenteral fluid support. However, as will be described later, study protocol restrictions made it relevant to analyze the data using fluid composite effect considerations.
Surrogate markers of intestinal function have been suggested. In this respect plasma citrulline has received special attention [Crenn et al. 2000]. Citrulline is synthesized by enterocytes of intestinal mucosa and is regarded as a biomarker of remnant functional absorptive metabolic mass of enterocytes. A close correlation between citrullinemia and remnant functional small bowel length has been described, but the correlation between citrullinemia and remnant absorptive capacity is less well established [Peters et al. 2007; Luo et al. 2007]. Thus, future studies should investigate the role of plasma citrulline as a pharmaco-dynamic indicator of the effect of teduglutide.
Glucagon-like peptide 2 and teduglutide
GLP-2 is a single-chain polypeptide of 33 amino acid residues, which is produced by a tissue-specific post-translational processing of the 160-amino-acid proglucagon molecule in enteroendocrine L cells. These cells are distributed throughout the gastrointestinal (GI) tract with the highest density being in the terminal ileum and the colon. GLP-2 is secreted from the intestinal L cells following ingestion of food. Repeated administration of GLP-2 promotes the expansion of the intestinal mucosa, via the stimulation of crypt cell growth and the reduction of enterocyte apoptosis [Drucker et al. 1996]. Exogenous GLP-2 administration inhibits gastric acid secretion and gastric emptying [Wojdemann et al. 1998, 1999], stimulates intestinal blood flow [Guan et al. 2006; Bremholm et al. 2009, 2011], increases intestinal barrier function [Benjamin et al. 2000; Cani et al. 2009] and enhances nutrient and fluid absorption in both preclinical and clinical models [Brubaker et al. 1997; Jeppesen et al. 2001, 2009]. However, a paradoxical resistance to exogenous GLP-2-induced adaptation was reported in piglets [Pereira-Fantini et al. 2008]. GLP-2 has also been suggested to have anti-inflammatory effects [Ivory et al. 2008; Sigalet et al. 2007]. In addition, GLP-2 may decrease bone resorption [Henriksen et al. 2009]. These effects are mediated via GLP-2 receptors (GLP-2Rs), which are G-protein coupled receptors belonging to the class B glucagon–secretin receptor family [Munroe et al. 1999]. GLP-2R expression is found primarily in the GI tract and the central nervous system, with limited expression in lung, cervix and vagal afferents [Dube and Brubaker, 2007]. Within the GI tract, the most abundant expression of GLP-2R is found in the jejunum, followed by the ileum, colon and stomach. Different studies have identified expression of GLP-2R in enteroendocrine cells [Yusta et al. 2000], enteric neurons [Bjerknes and Cheng, 2001] and subepithelial myofibroblasts [Orskov et al. 2005]. However, neither crypt epithelial cells nor enterocytes express the GLP-2R. This finding has led to the hypothesis that GLP-2 requires an indirect signal – perhaps functioning through a paracrine mechanism – to induce its effects on intestinal growth. It is currently unknown if GLP-2Rs are present on intrahepatic and extrahepatic bile ducts, the gallbladder and pancreatic ducts. GLP-2R activation results in the release of several growth factors, such as insulin-like growth factor 1, epidermal growth factor and keratinocyte growth factor [Bahrami et al. 2010].
The biologically active GLP-21–33 is broken down at the alanine residue in position 2 from the N-terminus, catalyzed by the proteolytic enzyme dipeptidyl peptidase-4 (DPP-4), and it is thereby transformed into the biologically less active metabolite GLP-23–33. Teduglutide is a GLP-2 analog, in which a substitution of alanine with glycine at position 2 results in a peptide resistant to degradation by DPP-4 and, therefore, has a longer half life than native GLP-2 [Marier et al. 2008, 2010]. Following subcutaneous injection, this corresponds to a biological half life for teduglutide of 2–3 h compared with a half life of 7 min for GLP-2. Bioavailability after subcutaneous administration of teduglutide is 87 ± 14%. It is suggested that these pharmacokinetic characteristics translate to an enhanced biological activity of teduglutide, relative to native GLP-2. Teduglutide is likely to be metabolized by high-capacity endogenous pathways. The kidneys have been suggested to have a key role in its clearance. Hence, it is believed that teduglutide is removed from the circulation via mechanisms involving glomerular filtration and tubular metabolism [Hartmann et al. 2000].
The clinical efficacy of teduglutide
The clinical efficacy of teduglutide in SBS has been evaluated in an open-label, phase II, metabolic balance pilot study, published in 2005 [Jeppesen et al. 2005] and in a multicenter, multinational, randomized, placebo-controlled, double-blind, phase III study, published in 2011 [Jeppesen et al. 2011]. A second phase III study has been conducted, but the results are not published yet. In the phase II, metabolic balance study, 16 patients with SBS received three different doses of teduglutide for 21 days. Patients were divided into three subgroups, based on remnant functional anatomy: end-jejunostomy (n = 10), less than 50% colon in continuity (n = 1) or more than 50% colon in continuity (n = 5). Patients with end-jejunostomy received once daily teduglutide 0.03 mg/kg/day (n = 2), teduglutide 0.10 mg/kg/day (n = 5) or teduglutide 0.15 mg/kg/day (n = 3). The patient with less than 50% colon in continuity received teduglutide 0.03 mg/kg/day and all of the patients with more than 50% colon in continuity received teduglutide 0.10 mg/kg/day. The doses were chosen to examine dose–response in patients with SBS over a range of teduglutide doses that were expected to provide clinical benefit. It was intended that dietary intake would be fixed during the balance studies and the study did not seek to evaluate the effects of teduglutide on spontaneous dietary intake. Three 72 h balance studies were performed: at baseline, during teduglutide treatment at days 18–21 and after terminating teduglutide treatment at days 39–42. During balance studies, all oral intake, fecal/stomal output and urine were collected, weighed and analyzed for energy, nitrogen, fat, and sodium and potassium content. Likewise, intestinal mucosa biopsies were performed at baseline, day 21 and day 42. Patients took their usual medications, such as proton-pump inhibitors, codeine or loperamide, and oral and parenteral supplements were kept constant throughout the study.
Compared with baseline, 21 days of treatment with teduglutide increased absolute intestinal wet weight absorption in 15 of the 16 patients. The average increase in wet weight absorption was 743 ± 477 g/day (p < 0.001), an increase of 22 ± 16% (p < 0.001). The magnitude of the relative wet weight absorption was similar for patients with end-jejunostomy (20 ± 18%, p = 0.007) and those with more than 50% colon in continuity (26 ± 16%, p = 0.023). Alongside the increase in wet weight absorption, fecal wet weight decreased significantly compared with baseline in the entire group of patients (711 ± 734 g/day, p = 0.001). Since oral intake and PS were kept constant in this study, the benefits on intestinal wet weight absorption and hydration translated into an increase in urine production in 14 of the 16 patients – urine output increased by 555 ± 485 g/day (p < 0.001). Furthermore, a significant decrease in fecal energy excretion was observed in the entire population (group 1: 808 ± 1453 kJ/day, p = 0.042), in the subgroup of patients with colon in continuity (group 3: 1343 ± 916 kJ/day, p = 0.031) and in patients with high dietary compliance (group 4: 1060 ± 1083 kJ/day, p = 0.013). This reduction in energy excretion translated well into the observed significant effects on improved absolute energy absorption (group 3: 1037 ± 798 kJ/day, p=0.045; group 4: 963 ± 1290 kJ/day, p = 0.043) and relative energy absorption (group 3: 10 ± 7%, p = 0.030; group 4: 8 ± 11%, p = 0.040). However, the results did not translate into an overall significant effect with respect to absolute or relative energy absorption, possibly due to variability in dietary intake among the different groups during the study periods. In this context, it is worth mentioning that a small, but significant, decrease in fecal energy excretion (1060 ± 1083 kJ/day, p = 0.01) was seen in group 4 (the subgroup with high dietary compliance), which translated into a significant increase in absolute (963 ± 1290 kJ/day, p = 0.04) and relative (10 ± 7%, p = 0.03) intestinal energy absorption. In addition to the metabolic balance studies, the phase II study examined the histological changes in bowel biopsies obtained from the patients. In the jejunum biopsies (obtained from eight patients with end-jejunostomy), significant histological changes were seen in seven of eight patients. More specifically, an increase in villus height (38 ± 45%, p = 0.030), an increase in crypt depth (22 ± 18%, p = 0.010) and an increase in mitotic index (115 ± 108%, p = 0.010) were observed. Small intestinal biopsies were not obtained from the patients with colon in continuity. Intestinal biopsies measuring the colonic crypt depth were obtained from all of the group 3 patients. An increase in crypt depth was shown in four of five sets of biopsies; however, the mean increase in crypt depth (13 ± 22%, p = 0.330) was not statistically significant, nor was the increase in mitotic index (76 ± 112%, p = 0.170). Most of the changes in intestinal absorption and histology observed during teduglutide treatment had reversed at follow up 3 weeks after treatment had been stopped.
Thus, in the pilot study, teduglutide showed intestinotrophic properties in humans, possibly as a result of increasing the absorptive surface area of the intestine. The general observation, at follow up 3 weeks after termination of treatment, was that the positive effects of teduglutide had reversed. This suggests that long-term treatment with teduglutide will be necessary and also that teduglutide treatment does not lead to irreversible structural changes.
In addition to its trophic effects on the intestinal mucosa, the positive effects of teduglutide on intestinal absorption may be related to other physiological mechanisms. Patients with SBS and end-jejunostomy, who have little or no endogenous, meal-stimulated GLP-2 secretion [Jeppesen et al. 1999a], often suffer from gastric hypersecretion and rapid gastric emptying. GLP-2 has previously been shown to diminish gastric acid secretion in sham-fed healthy humans [Wojdemann et al. 1999] and to prolong gastric emptying in patients with SBS [Jeppesen et al. 2001]. Thus, pharmacological replacement of GLP-2 (in the form of teduglutide) may restore the so-called ‘ileal brake’ mechanism. However, in the pilot study, the observed effects of teduglutide were similar across the patient subgroups – even in patients with colon in continuity, most of whom have increased endogenous GLP-2 concentrations [Jeppesen et al. 2000]. In general, these patients do not suffer from gastric hypersecretion or rapid gastric emptying and so the reason why an effect of teduglutide is still observed in this subgroup of patients remains speculative. However, regardless of the mechanism, the positive results suggest that pharmacological doses of teduglutide may have effects that differ from those of endogenously produced native GLP-2. It is possible that local high concentrations of teduglutide positively affect gastric secretion and emptying, and induce mucosal bowel growth. It is noteworthy that the results reported in the phase II teduglutide study were seen in steady-state patients who were optimally treated with conventional antidiarrheal and antisecretory medications.
In the phase II study, the effects of teduglutide on wet weight absorption were of similar magnitude in the 0.10 and 0.15 mg/kg/day dosing groups, suggesting that a plateau of the effect may have been reached at 0.10 mg/kg/day or lower. Based on this finding, the doses of teduglutide chosen for the phase III studies were 0.10 and 0.05 mg/kg/day.
Based on the results of the phase II study described, it was speculated that, by reducing diarrhea and increasing intestinal absorption, teduglutide treatment could reduce the need for PS. Such an effect would be expected to improve the quality of life for patients with SBS-IF. However, it was also demonstrated that patients with intestinal insufficiency benefited from teduglutide treatment. Since these patients often suffer from repeated episodes of dehydration, renal insufficiency and kidney stones, increasing wet weight absorption could be expected to diminish these complications. Balance studies were not deemed possible in large-scale, multinational, multicenter studies and, therefore, reduction in PS volume was selected as the primary efficacy endpoint for the teduglutide phase III clinical development program. PS volume would be expected to be reduced as a result of increased urine volume following increased intestinal wet weight absorption observed during teduglutide treatment.
Based on an assumption that patients with SBS-IF received PS on average 5 days per week, it was agreed that a 1-day reduction or a 20% reduction in PS volume would be an appropriate endpoint for the teduglutide phase III studies. Therefore, a randomized, placebo-controlled trial of teduglutide measuring the reduction in parenteral nutrition and/or intravenous requirements in patients with SBS was performed. This first phase III study of teduglutide was the largest randomized, placebo-controlled trial ever performed in patients with SBS-IF. Of the 139 patients screened, 84 were eligible for randomization and the results from 83 patients were eligible for analysis. The remaining 55 patients were not enrolled due to one or more of the following reasons: did not meet inclusion criteria (n = 21) or had one or more exclusion criteria (n = 7), had screening failures due to the decision of the investigator (n = 4), withdrawal of consent (n = 14) or other reasons (n = 12).
The primary objective of the study was to determine whether teduglutide could reduce the burden of parenteral nutrition and intravenous fluid requirements. In addition, secondary objectives were to explore if the patients could obtain additional days off PS or eliminate the need for PS altogether. Before randomization the patients went through a period of PS optimization for a maximum of 8 weeks, followed by a 4–8-week period of PS stabilization. During the PS optimization period, the goal was to establish a baseline minimal tolerated PS volume that resulted in a urine output of 1.0–2.0 liters/day. Patients were instructed on how to perform home collections of their 48 h urine output and were asked to try to keep the timing, quantity and quality of beverages as constant as possible during the 48 h collection period. Adjustment of the baseline parenteral volume was performed when urine volume fell below 1.0 liters/day or exceeded 2.0 liters/day. Patients were excluded from the study if PS optimization was not achieved after 8 weeks. Following PS optimization, patients were maintained for 4–8 weeks on the stabilized tolerated PS volume. Patients were eligible for randomization if they had a urine volume of 1.0–2.0 liters/day, while also maintaining constant oral beverages. Eligible patients were randomized to receive teduglutide at doses of 0.05 or 0.10 mg/kg/day or placebo (2:2:1) given subcutaneously in the morning for a period of 24 weeks.
A strict parenteral weaning algorithm allowed for no more than a 10% reduction in parenteral volumes at 4-week intervals. Weaning was performed if the 48 h urinary volumes exceeded the baseline values by more than 10%, regardless of the absolute amount, and a maximum of five reductions in PS were allowed from baseline to week 24.
The primary efficacy variable was initially the responder rate – that is, the percentage of patients who had a reduction from baseline in parenteral volume of 20–100% at week 20 of treatment and again at week 24. An expanded primary endpoint was later introduced to compare the patients treated with teduglutide versus placebo with respect to a graded response score (GRS) criterion. This endpoint accounted for intensity and duration of response at the end of the 24-week period (from 20–100% reduction in weekly parenteral volume and the responses from weeks 16–20 and weeks 20 and 24) and, thus, took into account higher levels of response and earlier onset of response coupled with a longer duration of response.
Secondary efficacy endpoints included the number and percentage of patients who responded (defined as a parenteral volume reduction of 20–100% from baseline to week 20 and maintained at week 24); the absolute reduction from baseline in parenteral volume and parenteral kilojoules; achievement of at least a 1-day reduction in the weekly parenteral administration or total weaning from PS.
With respect to the primary efficacy endpoint – the GRS – the difference between the teduglutide 0.10 mg/kg/day and placebo groups was not statistically significant. However, the group of patients receiving teduglutide 0.05 mg/kg/day showed a statistically significant improvement compared with placebo on the GRS (p = 0.007).
Similarly, for the secondary efficacy endpoint, the responder rate for the teduglutide 0.10 mg/kg/day group was not significantly different from the placebo group, whereas the responder rate for the teduglutide 0.05 mg/kg/day dose group was significantly higher compared with placebo − 46% (16 of 35) versus 6% (1 of 16), p = 0.005.
Three patients were completely weaned from PS. Two patients in the teduglutide 0.05 mg/kg/day group became completely independent of PS, although they had previously received this treatment for 25 and 6.5 years, receiving 5.4 and 3.5 liters/week at baseline respectively. One patient in the teduglutide 0.10 mg/kg/day group also became independent of PS at the end of week 24, after receiving 4.5 liters/week PS for a period of 3.7 years.
Neither of the active treatment arms produced a significant reduction in the number of days on PS, which could be explained by the fact that the algorithm for weaning PS did not specify for conversion of accumulated effects into days off PS and many investigators probably found it easier to simply reduce daily parenteral volumes.
In the teduglutide 0.05 mg/kg/day group, patients produced significantly more urine at all time points, in spite of maintaining a constant oral fluid intake and having parenteral volume significantly decreased. Since urine output increased steadily during the study, this was in contrast to the objective of the study protocol, which was to maintain constant urine output by progressively reducing the PS volume. Thus, the absolute effect of the teduglutide 0.05 mg/kg/day dose on the reduction in PS volume appeared to be underestimated. As mentioned previously, the fluid composite effect reflects the sum of the reduction in oral fluid intake, the increase in urine volume and reductions in daily PS volume. Thus, the fluid composite effect endpoint was used as a measure of the overall effect of teduglutide treatment. Teduglutide 0.05 mg/kg/day significantly increased the fluid composite effect by 816 ± 982 ml/day compared with placebo (p = 0.03) at week 20. Teduglutide 0.10 mg/kg/day also significantly increased the fluid composite effect by 489 ± 619, 700 ± 723 and 953 ± 830 ml/day at weeks 12, 16 and 20, respectively (all p < 0.05 compared with placebo). Urine volume increased by around 350 ml/day and the PS volume was decreased by around 350 ml/day in the teduglutide 0.05 mg/kg/day group, whereas the oral fluid intake decreased by around 350 ml/day and the PS volume decreased by around 350 ml/day in the teduglutide 0.10 mg/kg/day group. The true effect of either teduglutide dose on intestinal wet weight absorption can, therefore, be estimated to be approximately 700 ml/day (4.9 liters/week), closely reflecting the effects demonstrated in the phase II study.
Reductions in parenteral energy were not significant when comparing the teduglutide groups and the placebo group (p = 0.11) but, unlike the phase II study, the oral energy intake and fecal excretions were not measured.
Histological effects were evaluated in a subset of patients who were willing to undergo small bowel biopsies. Analysis showed a significant increase in small bowel villus height in both teduglutide treatment groups compared with baseline and placebo. Furthermore, a significant increase in fasting plasma citrulline (a biomarker of enterocyte mass in patients with SBS) was seen when comparing the teduglutide groups with baseline and placebo.
It is suggested that the lack of statistical significance of the effects of the teduglutide 0.10 mg/kg/day dose versus placebo could be explained by limitations imposed by the study protocol, such as the inability to start reduction of PS until 4 weeks after initiating teduglutide treatment, the maximally allowed reductions in parenteral support of 10% per month and a trend towards larger baseline parenteral volume requirements in the teduglutide 0.10 mg/kg/day group.
Based on these findings a confirmatory phase III trial for the dose of 0.05 mg/kg/day of teduglutide has been performed and met the primary endpoint. These results have not yet been published.
Safety and tolerability of teduglutide
In the phase II pilot study, no deaths and no withdrawals due to adverse events (AEs) were reported. Four of the patients reported five serious AEs, which included dehydration, sepsis and catheter-related infection. None of the serious AEs were judged to be related to the study drug and the incidence of AEs was similar between groups. The most commonly reported AEs were edema of the lower limbs (7 of 16 patients, 44%) and localized swelling of the jejunostomy nipple (7 of 10 patients, 70%). Other AEs included headache (4 of 16 patients, 25%) and abdominal pain (3 of 16 patients, 19%). No clinically significant abnormal laboratory values were identified in relation to teduglutide treatment.
In the first phase III study, both doses of teduglutide were well tolerated and safe throughout the 24 weeks of treatment. Of the 83 patients included in this study, 79 (95%) experienced at least one AE. The most frequent AEs in the teduglutide treatment groups were abdominal pain (24%), headache (24%), nausea (22%), nasopharyngitis (16%) and vomiting (15%). The most frequently reported serious AEs were catheter-related complications (catheter sepsis, catheter site infection), small intestinal obstruction and fever. No deaths were reported during the active treatment phase of the study. One patient died from a bleeding ulcer during the screening phase of the study. With this exception, there were no major differences in hematology tests or in laboratory values in the patients receiving teduglutide compared with those receiving placebo. Histopathological evaluation of the 390 intestinal tissue samples confirmed no dysplasic transformation in any of the intestinal biopsies. Considering the intestinotrophic effect of teduglutide, reflected in increases in plasma citrulline associated with teduglutide treatment and based on preclinical findings, it cannot be excluded that teduglutide may have a tumor-promoting effect in patients with SBS-IF undergoing long-term treatment with teduglutide [Thulesen et al. 2004]. Therefore, cancer surveillance colonoscopies may be relevant in patients with SBS-IF with a preserved colon. Future studies should address whether GLP-2Rs are present on intrahepatic and extrahepatic bile ducts, the gallbladder and pancreatic ducts. Postmarketing, prospective, long-term observational cohort studies should survey the occurrence of cancer, cholecystolithiasis, gallbladder complications and cholestatic liver dysfunction, but since patients with SBS-IF receiving PS are already at an increased risk of these conditions, selecting a proper matched control group will be important.
Regulatory status of teduglutide
Teduglutide has received orphan drug designation for the treatment of SBS from the US Food and Drug Administration (FDA) in 2000 and the European Medicines Agency in 2001.
In 2007, NPS Pharmaceuticals, Inc. (Bedminster, NJ, USA), a specialty pharmaceutical company developing innovative therapeutics for rare GI and endocrine disorders, granted Nycomed; a Takeda Company (Nycomed GmbH, Konstanz, Germany), the rights to develop and commercialize teduglutide outside the USA, Canada and Mexico. NPS retains all rights to teduglutide in North America.
Nycomed has recently announced the submission of a marketing authorisation application to the European Medicines Agency for teduglutide (Revestive) as a once-daily subcutaneous treatment for SBS (http://www.nycomed.com/media/news-releases/2011/nycomed-submits-european-marketing-authorisation-application-for-teduglutide—revestive/). As reported in the public domain, NPS Pharmaceuticals, Inc. expects to file for FDA approval of teduglutide (GATTEX) in the second half of 2011 as a first-in-class treatment for SBS (http://www.drugs.com/clinical_trials/gattex-teduglutide-shown-reduce-parenteral-support-volume-patients-adult-short-bowel-syndrome-11724.html).
Conclusion
Based on the results from the two published studies, it can be concluded that teduglutide appears to be safe and well tolerated, and has demonstrated restoration of structural and functional integrity of the remaining intestine with significant intestinotrophic and proabsorptive effects facilitating reduction in PS in patients with SBS-IF. Thus, teduglutide has the potential to reduce malabsorption-related consequences of intestinal failure, PS-related inconvenience and potentially life-threatening complications. The limited number of patients in the studies makes firm conclusions on the safety evaluations difficult. Postmarketing, prospective, observational cohort studies may be required to adequately address potential adverse events.
In conclusion, a substantial unmet medical need exists for patients with SBS-IF. At present it seems that teduglutide will provide clinically meaningful benefits in a condition with a limited treatment armamentarium, but future studies and postmarketing surveillance will further address the benefit–risk ratio of teduglutide in this rare and debilitating condition. Due to a large heterogeneity in the SBS-IF population with respect to effects, AEs and perceived risks, it is likely that teduglutide dosing in future clinical use will be titrated accordingly on a patient-by-patient basis.
Future studies should address whether such an approach could positively affect the quality of life of patients with SBS, which is the ultimate treatment aim. Future studies should also address whether a combination of the so-called ‘ileal-brake hormones’, that is, GLP-1, oxyntomodulin or peptide YY, could add to the effect of GLP-2 and teduglutide.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Palle Bekker Jeppesen has served as a consultant for Nycomed and NPS Pharmaceuticals.
References
- Bahrami J., Yusta B., Drucker D.J. (2010) ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology 138: 2447–2456 [DOI] [PubMed] [Google Scholar]
- Baxter J.P., Fayers P.M., McKinlay A.W. (2006) A review of the quality of life of adult patients treated with long-term parenteral nutrition. Clin Nutr 25: 543–553 [DOI] [PubMed] [Google Scholar]
- Benjamin M.A., McKay D.M., Yang P.C., Cameron H., Perdue M.H. (2000) Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut 47: 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M., Cheng H. (2001) Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci U S A 98: 12497–12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremholm L., Hornum M., Andersen U.B., Hartmann B., Holst J.J., Jeppesen P.B. (2011) The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul Pept 168: 32–38 [DOI] [PubMed] [Google Scholar]
- Bremholm L., Hornum M., Henriksen B.M., Larsen S., Holst J.J. (2009) Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand J Gastroenterol 44: 314–319 [DOI] [PubMed] [Google Scholar]
- Brubaker P.L., Izzo A., Hill M., Drucker D.J. (1997) Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol 272: E1050–E1058 [DOI] [PubMed] [Google Scholar]
- Buchman A.L., Scolapio J., Fryer J. (2003) AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 124: 1111–1134 [DOI] [PubMed] [Google Scholar]
- Cani P.D., Possemiers S., Van de Wiele T., Guiot Y., Everard A., Rottier O., et al. (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J.C., Williams N.S., King R.F., Barker M.C. (1986) Effects of a long-acting somatostatin analogue in patients with severe ileostomy diarrhoea. Br J Surg 73: 128–131 [DOI] [PubMed] [Google Scholar]
- Cortot A., Fleming C.R., Malagelada J.R. (1979) Improved nutrient absorption after cimetidine in short-bowel syndrome with gastric hypersecretion. N Engl J Med 300: 79–80 [DOI] [PubMed] [Google Scholar]
- Crenn P., Coudray-Lucas C., Thuillier F., Cynober L., Messing B. (2000) Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 119: 1496–1505 [DOI] [PubMed] [Google Scholar]
- Drucker D.J., Erlich P., Asa S.L., Brubaker P.L. (1996) Induction of intestinal epithelial proliferation by glucagon- like peptide 2. Proc Natl Acad Sci U S A 93: 7911–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube P.E., Brubaker P.L. (2007) Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am J Physiol Endocrinol Metab 293: E460–E465 [DOI] [PubMed] [Google Scholar]
- Fleming C.R., Remington M. (1981) Intestinal failure. In: Hill G.L. (ed.), Nutrition and the Surgical Patient. New York: Churchill Livingstone, pp; 219–235 [Google Scholar]
- Goulet O., Joly F., Corriol O., Colomb-Jung V. (2009) Some new insights in intestinal failure-associated liver disease. Curr Opin Organ Transplant 14: 256–261 [DOI] [PubMed] [Google Scholar]
- Guan X., Karpen H.E., Stephens J., Bukowski J.T., Niu S., Zhang G., et al. (2006) GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130: 150–164 [DOI] [PubMed] [Google Scholar]
- Hartmann B., Harr M.B., Jeppesen P.B., Wojdemann M., Deacon C.F., Mortensen P.B., et al. (2000) In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab 85: 2884–2888 [DOI] [PubMed] [Google Scholar]
- Henriksen D.B., Alexandersen P., Hartmann B., Adrian C.L., Byrjalsen I., Bone H.G., et al. (2009) Four-month treatment with GLP-2 significantly increases hip BMD: a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone 45: 833–842 [DOI] [PubMed] [Google Scholar]
- Ivory C.P., Wallace L.E., McCafferty D.M., Sigalet D.L. (2008) Interleukin-10-independent anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 295: G1202–G1210 [DOI] [PubMed] [Google Scholar]
- Jacobsen O., Ladefoged K., Stage J.G., Jarnum S. (1986) Effects of cimetidine on jejunostomy effluents in patients with severe short-bowel syndrome. Scand J Gastroenterol 21: 824–828 [DOI] [PubMed] [Google Scholar]
- Jeppesen P.B. (2007) Growth factors in short-bowel syndrome patients. Gastroenterol Clin North Am 36: 109–21, vii; [DOI] [PubMed] [Google Scholar]
- Jeppesen P.B., Gilroy R., Pertkiewicz M., Allard J.P., Messing B., O’Keefe S.J. (2011) Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 60: 902–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P.B., Hartmann B., Hansen B.S., Thulesen J., Holst J.J., Mortensen P.B. (1999a) Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut 45: 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P.B., Hartmann B., Thulesen J., Graff J., Lohmann J., Hansen B.S., et al. (2001) Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120: 806–815 [DOI] [PubMed] [Google Scholar]
- Jeppesen P.B., Hartmann B., Thulesen J., Hansen B.S., Holst J.J., Poulsen S.S., et al. (2000) Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut 47: 370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P.B., Langholz E., Mortensen P.B. (1999b) Quality of life in patients receiving home parenteral nutrition. Gut 44: 844–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P.B., Lund P., Gottschalck I.B., Nielsen H.B., Holst J.J., Mortensen J., et al. (2009) Short bowel patients treated for two years with glucagon-like peptide 2: effects on intestinal morphology and absorption, renal function, bone and body composition, and muscle function. Gastroenterol Res Pract 2009: 616054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P.B., Mortensen P.B. (2000) Intestinal failure defined by measurements of intestinal energy and wet weight absorption. Gut 46: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P.B., Sanguinetti E.L., Buchman A., Howard L., Scolapio J.S., Ziegler T.R., et al. (2005) Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 54: 1224–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P.B., Staun M., Tjellesen L., Mortensen P.B. (1998) Effect of intravenous ranitidine and omeprazole on intestinal absorption of water, sodium, and macronutrients in patients with intestinal resection. Gut 43: 763–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly F., Dray X., Corcos O., Barbot L., Kapel N., Messing B. (2009) Tube feeding improves intestinal absorption in short bowel syndrome patients. Gastroenterology 136: 824–831 [DOI] [PubMed] [Google Scholar]
- King R.F., Norton T., Hill G.L. (1982) A double-blind crossover study of the effect of loperamide hydrochloride and codeine phosphate on ileostomy output. Aust N Z J Surg 52: 121–124 [DOI] [PubMed] [Google Scholar]
- Ladefoged K., Christensen K.C., Hegnhoj J., Jarnum S. (1989) Effect of a long acting somatostatin analogue SMS 201-995 on jejunostomy effluents in patients with severe short bowel syndrome [see comments]. Gut 30: 943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauverjat M., Hadj A.A., Vanhems P., Bouletreau P., Fouque D., Chambrier C. (2006) Chronic dehydration may impair renal function in patients with chronic intestinal failure on long-term parenteral nutrition. Clin Nutr 25: 75–81 [DOI] [PubMed] [Google Scholar]
- Luo M., Fernández-Estívariz C., Manatunga A.K., Bazargan N., Gu L.H., Jones D.P., et al. (2007) Are plasma citrulline and glutamine biomarkers of intestinal absorptive function in patients with short bowel syndrome?, JPEN J Parenter Enteral Nutr 33: 1–7 [DOI] [PubMed] [Google Scholar]
- Marier J.F., Beliveau M., Mouksassi M.S., Shaw P., Cyran J., Kesavan J., et al. (2008) Pharmacokinetics, safety, and tolerability of teduglutide, a glucagon-like peptide-2 (GLP-2) analog, following multiple ascending subcutaneous administrations in healthy subjects. J Clin Pharmacol 48: 1289–1299 [DOI] [PubMed] [Google Scholar]
- Marier J.F., Mouksassi M.S., Gosselin N.H., Beliveau M., Cyran J., Wallens J. (2010) Population pharmacokinetics of teduglutide following repeated subcutaneous administrations in healthy participants and in patients with short bowel syndrome and Crohn’s disease. J Clin Pharmacol 50: 36–49 [DOI] [PubMed] [Google Scholar]
- Messing B., Crenn P., Beau P., Boutron-Ruault M.C., Rambaud J.C., Matuchansky C. (1999) Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 117: 1043–1050 [DOI] [PubMed] [Google Scholar]
- Messing B., Pigot F., Rongier M., Morin M.C., Ndeindoum U., Rambaud J.C. (1991) Intestinal absorption of free oral hyperalimentation in the very short bowel syndrome. Gastroenterology 100: 1502–1508 [DOI] [PubMed] [Google Scholar]
- Munroe D.G., Gupta A.K., Kooshesh F., Vyas T.B., Rizkalla G., Wang H., et al. (1999) Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci U S A 96: 1569–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C.R. (1978) Effect of codeine phosphate, Lomotil, and Isogel on ileostomy function. Gut 19: 377–383 [PubMed] [Google Scholar]
- Nightingale J.M., Lennard Jones J.E. (1993) The short bowel syndrome: what’s new and old? Dig Dis 11: 12–31 [DOI] [PubMed] [Google Scholar]
- Nightingale J.M., Lennard Jones J.E., Walker E.R., Farthing M.J. (1992) Oral salt supplements to compensate for jejunostomy losses: comparison of sodium chloride capsules, glucose electrolyte solution, and glucose polymer electrolyte solution. Gut 33: 759–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgaard I., Hansen B.S., Mortensen P.B. (1994) Colon as a digestive organ in patients with short bowel [see comments]. Lancet 343: 373–376 [DOI] [PubMed] [Google Scholar]
- O’Keefe S.J., Buchman A.L., Fishbein T.M., Jeejeebhoy K.N., Jeppesen P.B., Shaffer J. (2006) Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 4: 6–10 [DOI] [PubMed] [Google Scholar]
- O’Keefe S.J., Peterson M.E., Fleming C.R. (1994) Octreotide as an adjunct to home parenteral nutrition in the management of permanent end-jejunostomy syndrome. JPEN J Parenter Enteral Nutr 18: 26–34 [DOI] [PubMed] [Google Scholar]
- Orskov C., Hartmann B., Poulsen S.S., Thulesen J., Hare K.J., Holst J.J. (2005) GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept 124: 105–112 [DOI] [PubMed] [Google Scholar]
- Pereira-Fantini P.M., Nagy E.S., Thomas S.L., Taylor R.G., Sourial M., Paris M.C., et al. (2008) GLP-2 administration results in increased proliferation but paradoxically an adverse outcome in a juvenile piglet model of short bowel syndrome. J Pediatr Gastroenterol Nutr 46: 20–28 [DOI] [PubMed] [Google Scholar]
- Peters J.H., Wierdsma N.J., Teerlink T., van Leeuwen P.A., Mulder C.J., van Bodegraven A.A. (2007) Poor diagnostic accuracy of a single fasting plasma citrulline concentration to assess intestinal energy absorption capacity. Am J Gastroenterol 102: 2814–2819 [DOI] [PubMed] [Google Scholar]
- Rodrigues C.A., Lennard Jones J.E., Thompson D.G., Farthing M.J. (1989) The effects of octreotide, soy polysaccharide, codeine and loperamide on nutrient, fluid and electrolyte absorption in the short-bowel syndrome. Aliment Pharmacol Ther 3: 159–169 [DOI] [PubMed] [Google Scholar]
- Sigalet D.L., Wallace L.E., Holst J.J., Martin G.R., Kaji T., Tanaka H., et al. (2007) Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 293: G211–G221 [DOI] [PubMed] [Google Scholar]
- Thulesen J., Hartmann B., Hare K.J., Kissow H., Orskov C., Holst J.J., et al. (2004) Glucagon-like peptide 2 (GLP-2) accelerates the growth of colonic neoplasms in mice. Gut 53: 1145–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat G.N., Huibregtse K. (1975) Loperamide and ileostomy output – placebo-controled double-blind crossover study. Br Med J 2: 667–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdemann M., Wettergren A., Hartmann B., Hilsted L., Holst J.J. (1999) Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J Clin Endocrinol Metab 84: 2513–2517 [DOI] [PubMed] [Google Scholar]
- Wojdemann M., Wettergren A., Hartmann B., Holst J.J. (1998) Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol 33: 828–832 [DOI] [PubMed] [Google Scholar]
- Yusta B., Huang L., Munroe D., Wolff G., Fantaske R., Sharma S., et al. (2000) Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 119: 744–755 [DOI] [PubMed] [Google Scholar]