Abstract
Ever since Rudolf Virchow in 1858 publicly announced his apprehension of neuroglia being a true connective substance, this concept has been evolving to encompass a heterogeneous population of cells with various forms and functions. We briefly compare the 19th–20th century perspectives on neuroglia with the up-to-date view of these cells as an integral, and possibly integrating, component of brain metabolism and signalling in heath and disease. We conclude that the unifying property of otherwise diverse functions of various neuroglial cell sub-types is to maintain brain homoeostasis at different levels, from whole organ to molecular.
Keywords: Alzheimer's disease, astrocyte, disease, health, neuron, white matter
Abbreviations: AD, Alzheimer's disease; CNS, central nervous system; GFAP, glial fibrillary acidic protein; NCX, Na+/Ca2+ exchanger
INTRODUCTION
‘Ich habe bis jetzt, meine Herren, bei der Betrachtung des Nervenapparatus immer nur der eigentlich nervösen Theile gedacht. Wenn man aber das Nervensystem in seinem wirklichen Verhalten im Körper studiren will, so ist es ausserordentlich wichtig, auch diejenige Masse zu kennen, welche zwischen den eigentlichen Nerventheilen vorhanden ist, welche sie zusammenhäund dem Ganzen mehr oder weniger seine Form gibt.’
(‘Hitherto, gentlemen, in considering the nervous system, I have only spoken of the really nervous parts of it. But if we would study the nervous system in its real relations in the body, it is extremely important to have a knowledge of that substance also which lies between the proper nervous parts, holds them together and gives the whole its form in a greater or less degree’; translated from German) – Rudolf Virchow, Die Cellularpathologie, p. 246 (Virchow, 1858).
With these words, Rudolf Virchow publicly announced the concept of neuroglia when delivering a lecture to medical students of Charite Hospital in Berlin on 3 April 1858. The actual term neuroglia appeared 2 years earlier in Virchow's commentary to his own paper; here Virchow contemplated the existence of ‘…connective substance, which forms in the brain, in the spinal cord, and in the higher sensory nerves a sort of Nervenkitt (neuroglia), in which the nervous system elements are embedded’ (Virchow, 1856).
For Virchow, the neuroglia was a true connective tissue (the Zwichenmasse – ‘in between tissue’ of mesodermal origin), which most likely did not have a cellular origin, but was represented by fibres and intercellular masses. Although the first histological images of neuroglial cells were drawn by Heinrich Müller, Max Schulze (retinal Müller cells; Müller, 1851), Karl Bergmann (cerebellar Bergmann glia; Bergmann, 1857) and Otto Deiters [stellate astrocytes (Deiters, 1865); see also Kettenmann and Verkhratsky (2008) for an historic account], the first in-depth investigation of neuroglia was performed by Camillo Golgi in early 1870s (Golgi, 1873, 1903). It was also Golgi who, after discovering glial-vascular contacts or end feet (Figure 1A), introduced the first grand theory of glial function; this theory postulated that, in the CNS (central nervous system), glial cells provide for the bridge between vasculature and the organ parenchyma and are therefore responsible for metabolic support and substance exchange. These CNS glial cells were soon named ‘astrocytes’ by Michael von Lenhossék (Lenhossék, 1891); they were further classified into protoplasmic (grey matter) and fibrous (white matter) astrocytes (Andriezen, 1893).
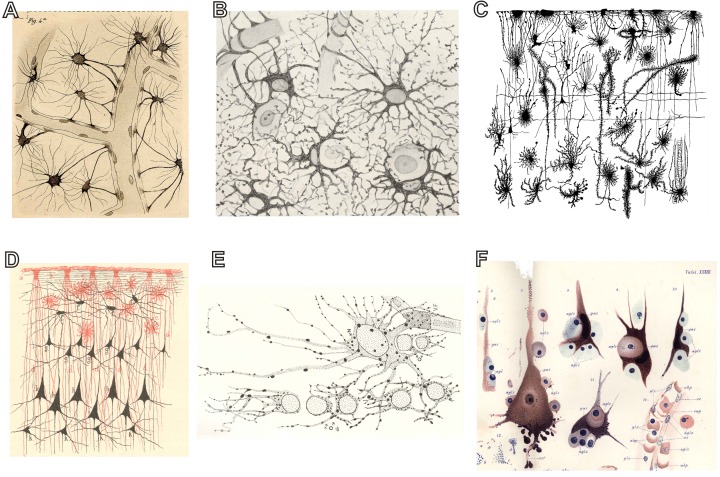
Figure 1. Early 20th century survey of glial biology in medicine: form and function.
(A) Camillo Golgi's drawings of astrocytes contacting blood vessels (from Golgi, 1903). (B) The close envelopment of neurons by the processes of neuroglial cells as seen by Santiago Ramón y Cajal (redrawn from Cajal's original plate by De Castro, from Glees, 1955). (C) Morphological diversity of neuroglia in human fetal cortex (Retzius, 1894–1916, Vol. 6, Plate II, Figure 5). (D) Close interactions between neuroglial (red) and neuronal (black) networks (from Schleich, 1894). (E) Oligodendroglia of the white matter and one astrocyte with a vascular endfoot showing the distribution of darkly stained granules or gliosomes in their processes (from Penfield, 1924), the image is taken from Glees (1955). (F) Pathological potential of neuroglia. The drawing by Alois Alzheimer (Alzheimer, 1910) shows association of glial cells (glz) with pathologically modified neurons (gaz or ganglion cells).
Research into neuroglia was very much advanced by Santiago Ramón y Cajal and his pupils. Cajal developed a gold chloride-sublimate staining technique (Ramón y Cajal, 1913) that was specific for both protoplasmic and fibrous astrocytes [as we know now this technique labels the intermediate filament GFAP (glial fibrillary acidic protein), which is widely used today as an astrocytic marker]. Cajal found very close appositions between astroglia and neurons (Figure 1B) and thought that astrocytes could work as insulators ascertaining spatial specificity of information flow in the neural circuits. By the turn of the 20th century the notion of the extraordinary morphological heterogeneity of the CNS glia was also firmly established (Figure 1C).
Cajal's pupil Pío del Río-Hortega identified two other principal classes of glial cells, initially considered by Cajal as the ‘third element’ (a group of adendritic cells) and what represented the oligodendrocytes and the microglia (Del Río-Hortega, 1921, 1932). Incidentally, oligodendrocytes were first observed (with a specific platinum stain technique) and described by William Ford Robertson (Robertson, 1899) who, however, did not understood the role of these cells and identified them as mesoglia.
The idea of active neuronal–glial interactions as a substrate for brain function was first introduced in 1894 by Carl Ludwig Schleich (Schleich, 1894) (Figure 1D). According to Schleich, glial cells represented the general inhibitory mechanism in the brain; swelling/shrinking of glial perisynaptic processes controlled information flow through synapses. Maximal swelling of the glial cells fully inhibited synaptic transmission and plunged the brain into a coma, which, for example, underlies general anaesthesia. Similar ideas were developed by Cajal, who suggested that swelling of perineuronal glia may terminate transmission and even considered this to be a mechanism of sleep (Ramón y Cajal, 1925). Another of Cajal's pupils, Fernando De Castro, suggested that neuroglial cells may release neuroactive substances and directly participate in neural transmission (De Castro, 1951).
Neuroglial secretory activity was proposed by H. Held (Held, 1909) and Jean Nageottte (Nageotte, 1910; see also Glees, 1955 for review). Using his molybdenum haematoxylin stain, Held discovered darkly stained granular inclusions (granules) in processes of specialized astrocytes, marginal (subpial) glial cells. This stain also allowed him to determine that glial fibres were actually cellular extensions (but not interstitial mass as per Virchow) forming an elaborate three-dimensional intercellular (astrocytic) network that interacts with vascular endothelium. These two findings led him to hypothesize that granular inclusions he observed, and referred to as ‘gliosomes’ by Alzheimer (Alzheimer, 1910), represent evidence for glial secretion or metabolic proviso for neurons, since glial cells reside at the interface between the blood vessels and brain parenchyma. As Penfield (Penfield, 1924) improved his staining methods to better disclose oligodendrocytic processes, it became apparent that gliosomes can be seen in astrocytes and oligodendrocytes alike (Figure 1E). It was Nageotte, however, who provided the first description of secretion in neuroglia using the Altmann aniline-acid fuchsin staining method (see the English translation of this report in Verkhratsky et al., 2011). He observed various secretory grains, which he thought represented successive transformation steps from mitochondria into grains. Gliosomes in the cytoplasm of glial cells were, under various terms, also reported by other microanatomists of that era: Eisath (1906), Fieandt (1910), Cajal (1913), Achucarro (1913) and Hortega (1916) (Glees, 1955, and references therein). It should be noted that the early 20th century term gliosomes should neither be confused with nor matched to its recent usage (Stigliani et al., 2006) for glial subcellular re-sealed particles (Nakamura et al., 1993), which are secretion competent pinched off astrocytic processes containing transmitter-laden vesicles (Stigliani et al., 2006). Besides secretory capabilities, in the first decade of the 20th century, glial function to metabolize and take up transmitters was speculated by Ernesto Lugaro (Lugaro, 1907).
Finally, neuroglial cells were implicated in various pathological processes. Nissl and Alzheimer considered the pathological role of neuroglia (Figure 1F) in a variety of neurological diseases (Alzheimer, 1910), and astrocytes were considered to ‘exhibit a morbid hypertrophy in pathological conditions’ by Andriezen (Andriezen, 1893). The role of astrocytes in scar formation was perceived by Cajal, Hortega and Penfield in the early 1920s (see Glees, 1955).
The above-outlined hypotheses with regard to glial function were unexplored for about three-quarters of the century as glial research was pushed aside due to a combination of (i) the victory of the neuronal doctrine which considers neurons as the sole determinant of brain signalling and (ii) the technical inability to study the function of glial cells since they lack traditional electrical excitability in the form of action potentials. Following this neglect, however, it took about three decades to provide data in support of neuroglia as an integral (and possibly integrating) component of the brain action. As a consequence we have guest edited a special online collection for ASN NEURO entitled ‘Neuroglia–more than a nervenkitt’, which encompasses a collection of review articles along with original research papers (http://www.asnneuro.org/an/online_collection.htm). By no means can we claim that this collection represents an all-inclusive coverage of the rapidly expanding fields of glial biology and bidirectional glial–neuronal interactions. Rather, the goal of this collection is to integrate the body of information that has accumulated during these recent years in a selected subset of topics to illustrate the active role of glia in (patho)physiological processes in the CNS.
Astrocytes display excitability based on variations of their cytosolic Ca2+ levels (Cornell Bell et al., 1990). A prominent output of this process is evident in the Ca2+-dependent exocytotic/vesicular release of chemical transmitters, most notably ATP and glutamate, from astrocytes, which is the subject reviewed by Zorec et al. (2012). This essay describes Ca2+ sources and sinks that provide for astrocytic intracellular Ca2+ dynamics, which are necessary and sufficient for regulation of the exocytotic release of transmitters. It also discusses the secretory machinery, which supports this release mechanism, together with the description of secretory vesicles in astrocytes. It is the traffic of these transmitter-storing vesicles that in due course results in setting transmitter(s) free into the extracellular space. Transmitter(s) released from astrocytes affect neuronal excitability and the sleep homoeostat behaviour. Besides such physiological signalling, this route can also play a role in pathophysiology, and as such there is a concise discussion on its contribution to epilepsy. This review article is accompanied by the original research paper by Reyes et al. (2012), which reports on the role of the plasmalemmal NCX (Na+/Ca2+ exchanger) in exocytotic glutamate release from astrocytes. It is proposed that this exchanger, by operating in reverse mode, i.e. delivering Ca2+ from the extracellular space into the cytosol, could be involved in fast and spatially restricted exocytosis in astrocytes. Such a pathway would operate in parallel to a more accepted and widely distributed, albeit slower, exocytotic pathway in astrocytes, which predominately depends on Ca2+ drawn from the ER (endoplasmic reticulum) store upon, for example, activation of metabotropic receptors. Since NCX-associated Ca2+ dynamics are inherently coupled to astrocytic cytosolic Na+ control, this could also have significance to astrocytic metabolism. Namely, astrocytic cytosolic Na+ increases can lead to lactate production, which upon release from astrocytes can be taken up and utilized by nearby neurons, a process referred to as lactate shuttle (Pellerin and Magistretti, 2011). The issue of lactate against glucose utilization for energy consumption of needy neurons and the brain in general has been a matter of an ongoing debate (Dienel, 2011).
At this juncture, we transition to the neuroenergetics area. Kreft et al. (2012) provide a review of astrocytic glucose metabolism at the interface of intra- and inter-cellular signalling. The emphasis has been placed on the effects that the two major signalling molecules, ATP and glutamate (along with this amino acid homoeostasis), as well as adrenergic receptor activation have on astrocytic metabolism with an outlook on the contribution of the third, astrocytic, partner in the tripartite synapse. A contentious notion that metabolic events can be compartmentalized at the level of a single astrocyte is then discussed. Similarly the original research article by Bak et al. (2012) follows up on the compartmentalization theme of glucose and lactate metabolism in neuronal parts contributing to the tripartite synapse. It appears that pre- and post-synaptic compartments metabolize glucose, while the postsynaptic compartment utilizes both glucose and lactate. However, the postsynaptic usage of glucose, unlike that of lactate, positively correlates with Ca2+ signalling in glutamatergic neurons. This raises the notion that Ca2+ signalling may represent a router for utilization of glucose against lactate as a preferred metabolic substrate at the postsynaptic terminals.
Neuroglial cells in their capacity as homoeostatic cells (see below) of the CNS are involved essentially in all types of neurological and psychiatric disorders. Astrocytes respond to the nervous system insult by a complex defensive reaction known as reactive astrogliosis, which manifests as cellular hypertrophy and GFAP overexpression. Interestingly, the mutations of the gene encoding for GFAP result in the human disorder named Alexander disease. Verkhratsky et al. (2012) review this topic to provide up-to-date information of the astrocytic contribution to Alexander disease and AD (Alzheimer disease), ischaemia, stroke and epilepsy. The review calls for development of comprehensive therapeutic approaches, which in addition to presently used agents, all acting upon neurons, would also utilize agents targeting glia to ameliorate brain disorders. The original research paper in this area provided by Yeh et al. (2011) deals with astrocytic morphofunctional changes in AD; there is an astrocytic atrophy (in contrast with hypertrophy as per Andriezen) in the entorhinal cortex of a transgenic animal model of this neurodegenerative disease. Thus astrocytic atrophy could be considered as a target for future therapeutic intervention in AD. Finally, Matute and Ransom (2012) review the intercellular signalling molecules, ATP and glutamate, that underlie signalling in heath and disease within the white matter, greatly composed of neuroglia and axons; stroke, multiple sclerosis and AD are discussed.
Our understanding of the role and place of neuroglia in the brain remains scattered and superficial. Even the definition of neuroglia is not agreed upon by the field, as generally neuroglial cells are defined as all cells in the brain which are not neurons. This loose and by exclusion definition hampers the fact of profound heterogeneity of glial cell forms and functions. Indeed neuroglia embraces a host of cells with very different origin, morphology and function. The primary neuroglial cells in the CNS are of ectoderm in origin and represented by astrocytes, oligodendrocytes and NG-2-positive cells; the astrocytes in addition include several types of radial glia, numerous specialized astrocytes (e.g. velate, interlaminar or polarized astrocytes), choroid plexus cells and ependymocytes. The microglia is altogether different, being of mesoderm in origin, a myeloid descent, and an invading alien to the brain. There is, however, one unifying property of all these cells – their primary function is to maintain brain homoeostasis at different levels, from whole organ (control of blood–brain barrier and defence) to cellular (neurogenesis, synaptogenesis and synapse maintenance) and molecular (ion and neurotransmitter homoeostasis). Therefore neuroglia can be broadly defined as homoeostatic cells of the nervous system.
REFERENCES
- Alzheimer A. Beiträge zur Kenntnis der pathologischen Neuroglia und ihrer Beziehungen zu den Abbauvorgängen im Nervengewebe. In: Nissl F, Alzheimer A, editors. In: Histologische und Histopathologische Arbeiten über die Grosshirnrinde mit besonderer Berücksichtigung der pathologischen Anatomie der Geisteskrankheiten. Jena: Verlag von Gustav Fischer; 1910. pp. 401–562. [Google Scholar]
- Andriezen WL. The neuroglia elements of the brain. Br Med J. 1893;2:227–230. doi: 10.1136/bmj.2.1700.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Obel LF, Walls AB, Schousboe A, Faek SA, Jajo FS, Waagepetersen HS. Novel model of neuronal bioenergetics: post-synaptic utilization of glucose but not lactate correlates positively with Ca2+ signaling in cultured mouse glutamatergic neurons. ASN NEURO. 2012;4((3)):art:e00083. doi: 10.1042/AN20120004. doi:10.1042/AN20120004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann K. Notiz über einige Strukturverhältnisse des Cerebellums und Rükenmarks. Z Med. 1857;8:360–363. [Google Scholar]
- Cornell Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- De Castro F. Anatomical aspects of the ganglionic synaptic transmission in mammalians. Arch Int Physiol. 1951;59:479–525. doi: 10.3109/13813455109150845. [DOI] [PubMed] [Google Scholar]
- Deiters O. Untersuchungen über Gehirn und Rückenmark des Menschen und der Säugethiere. Vieweg, Braunschweig. 1865 [Google Scholar]
- Del Río-Hortega P. Estudios sobre la neuroglia. La glia de escasas radiaciones oligodendroglia. Biol Soc Esp Biol. 1921;21:64–92. [Google Scholar]
- Del Río-Hortega P. Microglia. In: Penfield W, editor. In: Cytology and Cellular Pathology of the Nervous System. New York: Hoeber; 1932. pp. 482–534. [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.175. doi:10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glees P. Oxford: Blackwell; 1955. Neuroglia Morphology and Function. [Google Scholar]
- Golgi C. Vol. 33. Lombardia: Gazzetta Medica Italiana; 1873. Suella struttura della sostanza grigia del cervello (comunicazione preventiva). pp. 244–246. [Google Scholar]
- Golgi C. Milano: Hoepli; 1903. Opera Omnia. [Google Scholar]
- Held H. Über die Neuroglia marginalis der menschlichen Grosshirnrinde. Monatschr f Psychol u Neurol. 1909;26:360–416. [Google Scholar]
- Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends Neurosci. 2008;31:653–659. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kreft M, Bak LL, Waagepetersen HS, Schousboe A. Aspects of astrocyte energy metabolism, amino acid neurotransmitter homeostasis and metabolic compartmentation. ASN NEURO. 2012;4((3)):art:e00086. doi: 10.1042/AN20120007. doi:10.1042/AN20120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhossék Mv. Zur Kenntnis der Neuroglia des menschlichen Ruckenmarkes. Verh Anat Ges. 1891;5:193–221. [Google Scholar]
- Lugaro E. Sulle funzioni della nevroglia. Riv Patol Nerv Ment. 1907;12:225–233. [Google Scholar]
- Matute C, Ransom BR. Roles of white matter in CNS patho-physiologies. ASN NEURO. 2012;4((2)):art:e00079. doi: 10.1042/AN20110060. doi:10.1042/AN20110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. Zur Histologie der Netzhaut. Z Wissenschaft Zool. 1851;3:234–237. [Google Scholar]
- Nageotte J. Phenomenes de secretion dans le protoplasma des cellules nevrogliques de la substance grise. C R Soc Biol (Paris) 1910;68:1068–1069. [Google Scholar]
- Nakamura Y, Iga K, Shibata T, Shudo M, Kataoka K. Glial plasmalemmal vesicles: a subcellular fraction from rat hippocampal homogenate distinct from synaptosomes. Glia. 1993;9:48–56. doi: 10.1002/glia.440090107. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.149. doi:10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W. Oligodendroglia and its relation to classical neuroglia. Brain. 1924;47:430–452. [Google Scholar]
- Ramón y Cajal S. Un nuevo proceder para la impregnación de la neuroglía. Bol Soc Esp Biol. 1913;II:104–108. [Google Scholar]
- Ramón y Cajal S. Contribution a la connaissance de la nevroglia cerebrale et cerebeleuse dans la paralyse generale progressive. Trab Lab Invest Biol Univ Madrid. 1925;23:157–216. [Google Scholar]
- Retzius G. Biol Untersuchungen. Die neuroglia des Gehirns beim Menschen und bei Saeugethieren. 1894–1916 [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN NEURO. 2012;4((1)):art:e00075. doi: 10.1042/AN20110059. doi:10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson W. On a new method of obtaining a black reaction in certain tissue-elements of the central nervous system (platinum method). Scottish Med Surg J. 1899;4:23. [Google Scholar]
- Schleich CL. Psychophysik des natürlichen und künstlichen Schlafes. Berlin: Julius Springer; 1894. Schmerzlose Operationen: Örtliche Betäubung mit indiffrenten Flüssigkeiten. [Google Scholar]
- Stigliani S, Zappettini S, Raiteri L, Passalacqua M, Melloni E, Venturi C, Tacchetti C, Diaspro A, Usai C, Bonanno G. Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. J Neurochem. 2006;96:656–668. doi: 10.1111/j.1471-4159.2005.03631.x. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V, Rodriguez JJ. Where the thoughts dwell: the physiology of neuronal-glial ‘diffuse neural net’. Brain Res Rev. 2011;66:133–151. doi: 10.1016/j.brainresrev.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Sofroniew MV, Messing A, Delanerolle NC, Rempe D, Rodriguez Arellano JJ, Nedergaard M. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN NEURO. 2012;4((3)):art:e00082. doi: 10.1042/AN20120010. doi:10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R. Frankfurt a.M: Verlag von Meidinger Sohn & Comp; 1856. Gesammelte Abhandlungen zyr wissenschaftlischen Medizin. [Google Scholar]
- Virchow R. Zwanzig Vorlesungen gehalten während der Monate Februar, März und April 1858 im pathologischen Institut zu Berlin. Berlin: First edition. August Hirschwald; 1858. Die Cellularpathologie in ihrer Begründung auf physiologische and pathologische Gewebelehre. [Google Scholar]
- Yeh CY, Vadhwana B, Verkhratsky A, Rodriguez JJ. Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer's disease. ASN NEURO. 2011;3((5)):art:e00071. doi: 10.1042/AN20110025. doi:10.1042/AN20110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signaling route. ASN NEURO. 2012;4((2)):art:e00080. doi: 10.1042/AN20110061. doi:10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]