Abstract
AIM: To investigate the anti-oxidative and anti-fibrotic effects of aloe vera in patients with liver fibrosis.
METHODS: Aloe vera high molecular weight fractions (AHM) were processed by patented hyper-dry system in combination of freeze-dry technique with microwave and far infrared-ray radiation. Fifteen healthy volunteers as the control group and 40 patients were included. The patients were randomly subdivided into two equal groups: the conventional group was treated with placebo (starch), and AHM group was treated with 0.15 gm/d AHM, both for 12 consecutive weeks. The patients were investigated before and after treatment. Serum activity of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), hyaluronic acid (HA), transforming growth factor-β (TGF-β) and matrixmetalloproteinase-2 (MMP-2) were determined. The reduced glutathione (GSH) and malondialdehyde (MDA) levels in liver were assayed and the expression of hepatic α-smooth muscle actin (α-SMA) was identified by immunohistochemistry.
RESULTS: At the start of the study, the hematoxylin and eosin staining revealed fibro-proliferated bile ductules, thick fibrous septa and dense inflammatory cellular infiltration in the patients before treatment. The use of AHM for 12 wk significantly ameliorated the fibrosis, inhibited the inflammation, and resulted in minimal infiltration and minimal fibrosis compared to the conventional group. The enzyme activities of the liver (ALT, AST and ALP) were attenuated after treatment in both groups, and the decrease in the AHM group was more significant as compared with the conventional group. Similar to the AST, the MDA levels were significantly higher before treatment, and were attenuated after treatment in both groups. In contrast, the hepatic glutathione content in the patients were decreased significantly in the AHM group compared to the controls. The serum levels of the fibrosis markers (HA, TGF-β and MMP-2) were also reduced significantly after treatment. The expression of α-SMA was modified in patients before and after treatment as compared with the normal controls. In the conventional group, there was only thin and incomplete parenchymal α-SMA positive septum joining the thickened centrilobular veins, while in the AHM group, few α-SMA positive cells were present in sinusoid and lobule after treatment.
CONCLUSION: Oral supplementation with AHM could be helpful in alleviating the fibrosis and inflammation of hepatic fibrosis patients.
Keywords: Hepatic fine periportal fibrosis, Aloe vera, α-smooth muscle actin, Transforming growth factor-β, Hyaluronic acid
INTRODUCTION
Hepatic fine periportal fibrosis is a common response to liver injury caused by viral hepatitis, hepatitis B virus (HBV), and hepatitis C virus (HCV) infection, and other factors. The pathophysiological events leading to periportal fibrosis provoke excessive hepatocytes apoptosis and necrosis[1,2]. The damaged hepatocytes are the activators of Kupffer cells. The activated Kupffer cells release a number of soluble agents, including cytokines, reactive oxygen species (ROS), and other factors. These factors act on the hepatic stellate cells (HSCs), which undergo morphological transition to myofibroblast-like cells and proliferate. This transition is characterized by an accelerated production of large amounts of extracellular matrix (ECM) involving molecular and histological re-arrangement of various types of collagens, proteoglycans, structural glycoprotein and hyaloronic acid[3].
Oxidative stress has been recognized as a fundamental factor in the pathological changes observed in various liver diseases[4,5]. It can cause excessive damage to hepatocytes through lipid peroxidation and protein alkylation[6].
Acute and chronic liver diseases constitute a global concern, and treatment for these diseases is difficult and have limited efficacy. Therefore, considerable efforts are being made to obtain useful herbal medicine from documented medicinal plants for a wide variety of clinical conditions. Developing therapeutically effective agents from natural products may reduce the risk of toxicity when the drug is used clinically.
Aloe vera is a cactus-like plant that grows in hot, dry climates. Two distinct preparations of aloe plants are most frequently used. The leaf exudate (aloe) is used as a laxative, and the mucilaginous gel (aloe vera) extracted from the leaf parenchyma is used as a remedy against a variety of skin disorders. Aloe vera gel has been demonstrated to have liver protective effect in rats[7-9], and many toxicity studies have been conducted to determine the LD50 of aloe vera[10-13]. However, the antifibrotic effect of aloe vera on liver fibrosis has not yet been reported. The aim of this study was to investigate the anti-oxidative, and anti-fibrotic effects of aloe vera in patients with acute liver fibrosis.
MATERIALS AND METHODS
Extraction and preparation
Aloe vera high molecular weight fractions (AHM) were obtained from water-washed gel of aloe vera leaves cultivated in Okinawa, Japan. Voucher specimens of aloe vera collected in Okinawa, were compared and determined to be aloe vera L. plant (syn. Aloe barbadensis Miller) (Herbarium number 54-3 in Medicinal garden, Fukuyama University by Emeritus Prof. A Yagi). AHMs were processed by patented hyper-dry system in combination of freeze-dry technique with microwave and far infrared-ray radiation. AHM contains the following chemical and physical properties: molecular weight (MW): 1119500 D by high performance liquid chromatography (HPLC) analysis; TSK gel GMPW column: two columns in series, 10 μm, 7.8 mm × 30 cm; eluent 0.2 mol NaNO3; flow rate: 1.0 mL/min; temperature: 40 °C; and use of refractive index detector.
Sample treatment
AHM sample (1 g) was homogenized in 2 mL of 0.2 mol NaNO3. Homogenate was centrifuged at 2000 × g for 1 min. Upper solution was introduced as 200 μL aliquots to size-exclusion-chromatography. Aloin content was less than 10 ppm by HPLC analysis[14], water content: 3% ± 0.5%, colony formulating unit: less than 300/g, Na: 430 mg/100 g, Ca: 2100 mg/100 g. AHM contained the neutral polysaccharides with MW of about 1000 kDa, and 90% carbohydrate and 7% protein. Glycoprotein and verectin composed of carbohydrate and protein in a ratio of 10.7% and 82.0%, respectively, with MW of 29 kDa[15], was obtained in a ratio of 20% by immunochemical assay in AHM. Chemical shifts of AHM were determined in D2O with a JOEL JNM α-400 and 100 MHz for proton and carbon, respectively. The infrared spectra were determined with a FTIR-8600PC, Shimadzu, Japan.
Patients
The subjects in this study were selected from the Internal Medicine Department, Tanta University Hospitals. They included 15 healthy volunteers as the control group and 40 patients (32 men and 8 women, ranged 25-56 years). Among the 40 patients, 15 had HCV, 24 had HBV and 1 had bilharziasis. Patients were included in the study if they were positive for serum hepatitis B surface antigen or C antibodies and had persistently elevated serum aminotransferase concentrations 1.5 times higher than the upper limit of the reference range for at least 6 mo. All the patients were diagnosed according to the International Autoimmune Hepatitis Group Report protocol[16].
For assessment of liver fibrosis scores, all patients underwent liver biopsy as part of the normal diagnostic procedure and were sub-classified according to the score for the histological activity index (HAI). Patients with a history of gastrointestinal bleeding and chronic liver disease (Wilson's disease, hemochromatosis, α 1-antitrypsin deficiency, or hepatocellular carcinoma), active intravenous drug abuse, and liver transplantation were excluded.
All the patients were subjected to full history taking, thorough clinical examination, biopsy and histological examinations, and laboratory investigations (Table 1).
Table 1.
Characteristics of the study populations (mean ± SD)
| Parameter | Control group (n = 15) | Conventional group1 (n = 20) | AHM group2 (n = 20) |
| Age (yr) | 40.2 ± 10.4 | 41.6 ± 15.4 | 40.5 ± 13.9 |
| Sex (M/F) | 15/0 | 15/5 | 17/3 |
| HBV | - | 12 | 12 |
| HCV | - | 8 | 7 |
| Bilharziasis | - | 0 | 1 |
| Fibrosis Stage | |||
| F1 | 0 | 3 | 3 |
| F2 | 0 | 9 | 10 |
| F3 | 0 | 8 | 7 |
| F4 | 0 | 0 | 0 |
| Total bilirubin (mg/dL) | 0.55 ± 0.20 | 1.19 ± 0.20 | 1.18 ± 0.19 |
| ALT (IU/L) | 19.4 ± 8.2 | 87.4 ± 8.4 | 85.9 ± 9.1 |
| AST (IU/L) | 25.0 ± 4.3 | 50.8 ± 6.7 | 51.0 ± 4.2 |
| ALP (IU/L) | 55.0 ± 14.3 | 225.0 ± 85.6 | 223.0 ± 74.5 |
| Albumin (g/dL) | 4.4 ± 0.1 | 4.2 ± 0.2 | 4.3 ± 0.1 |
| INR | 0.88 ± 0.22 | 1.47 ± 0.3 | 1.51 ± 0.2 |
Patients treated with the conventional treatment with placebo (starch) for 12 consecutive weeks;
Patients treated with the conventional treatment with 0.15 g/d AHM for 12 consecutive weeks. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; INR: International normalization ratio; HBV: Hepatitis B virus; HCV: Hepatitis C virus; AHM: Aloe vera high molecular weight fractions.
Informed consent was obtained from all the participants. The protocol of the study was approved by the Ethical Committee of the University.
Treatment was initiated if they met the inclusion criteria. Treatment of each patient was according to a standard protocol. Hepatitis C patients were treated with pegylated interferon (180 μg/wk) + ribavirin (800-1200 mg/d). Hepatitis B patients were treated with adefovir (10 mg/d) or lamivudin (100 mg/d).
The patients were randomly subdivided into two equal groups: the conventional group treated with the conventional treatment with placebo (starch) for 12 consecutive weeks, and the AHM group treated with the conventional treatment with 0.15 g/d AHM (0.05 g three times daily) for 12 consecutive weeks. The dosage was calculated according to Williams et al[10]. The AHM preparation was provided in sachets, which contained powder to be dissolved in 50 mL fresh water. Liver and blood samples were collected at the start and at the end of the study period for assessment.
Histological assessment
Liver biopsy fragments were fixed in 10% neutralized formaldehyde, embedded in paraffin, and then stained with hematoxylin and eosin. Liver biopsy samples were examined in a double-blinded fashion using a METAVIR scoring system. Blood samples were withdrawn; serum was separated and utilized for biochemical analysis of aspartate aminotransferase (AST), alanine aminotransferase (ALT) alkaline phosphatase (ALP), hyaluronic acid (HA), transforming growth factor-β (TGF-β) and matrix metalloproteinase-2 (MMP-2). Liver sample was dried, cut into portions and kept frozen at -80 °C until used for analysis. Liver tissues were weighed and homogenized in solution containing ice-cold isotonic saline[17]. The homogenates were centrifuged at 4500 rpm for 15 min at 4 °C and the supernatants were taken for determination of reduced glutathione (GSH), and malondialdehyde (MDA). The protein content of the tissue aliquots was determined by the Lowry method[18].
Biochemical assays
Measurement of liver enzyme activities: The serum enzyme activities of ALT and AST were measured colorimetrically according to the method of Reitman and Frankel[19], using Boehringer Mannheim Kit. The optical density was read at 546 nm using UV-160A Shimadzu Spectrophotometer. Serum total alkaline phosphatase activity was estimated by commercially available kits (BioMerieux, France) according to the method of Kind and King[20].
Measurement of fibrosis markers: Serum hyaluronic acid was measured using a kit provided by Corgenix Inc. (Colorado, United States, under license of Chugai Diagnostic Science Co.). It was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. Serum TGF-β1 and MMP-2 levels were evaluated using a commercially available human TGF-β1 and MMP-2 ELISA kit according to the manufacturer’s instructions (TGF-β1: R and D System, Abingdon, United Kingdom; MMP-: Amersham, Bucks, United Kingdom).
Measurement of liver oxidative markers: Hepatic GSH concentration was determined by the method of Richardson and Murphy[21], using Ellman’s reagent (5, 5’-dithiobis-2-nitrobenzoic acid; DTNB) and the absorbance was measured at 412 nm. The results were calculated as μmol GSH/g tissue. Lipid peroxidation was assessed by measuring MDA using thiobarbituric acid according to the method of Yoshioka et al[22]. Thiobarbituric acid reactive substances was measured in μmol/g of tissue according to the absorbance at 532 nm.
Immunohistochemical analysis
For immunohistochemical analysis, sections were incubated with anti-α-smooth muscle actin (α-SMA) (1/1000; Dako North America, Inc., Carpenteria, CA) for 30 min. Staining was visualized using the horseradish peroxidase-conjugated Dako staining system (Dako In-Vision; Dako North America, Inc.).
Statistical analysis
Data were statistically analyzed by 2-way analysis of variance (ANOVA) (repeated measure type) to compare the results before (baseline) and after treatment within the same group, and unpaired Student’s t test was used to compare the means of different groups. Significant differences between the groups were statistically analyzed using one-way ANOVA using the computer program SPSS for Windows version 10 (Chicago, IL, United States). All results were expressed as mean ± SD. The level of significance was set at P < 0.05.
RESULTS
Macroscopic presentation and histological evaluation
The histological findings of liver tissues are presented in Figure 1. There were thick fibrous septa and dense inflammatory cellular infiltration in the patients before treatment. However, the control group showed normal lobular architecture and cell structure. The conventional group showed moderate fibrosis with inflammatory infiltration and slight ballooning of liver cells after treatment. AHM treatment could inhibit the inflammation, and showed minimal infiltration and minimal fibrosis compared to the conventional group. The histopathological evaluation of the two groups before and after treatment is shown in Table 2.
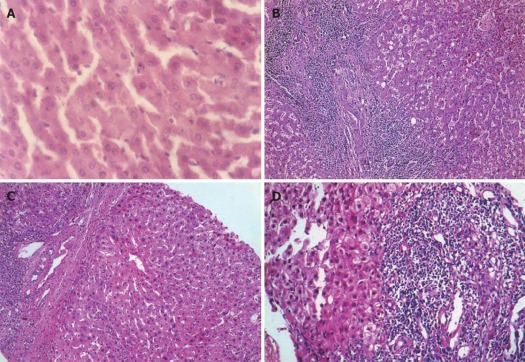
Figure 1.
Hematoxylin and eosin staining of liver tissues (HE, × 200). A: Control group showed normal lobular architecture and cell structure; B: Patients before treatment showed fibro-proliferated bile ductules, thick fibrous septa and dense inflammatory cellular infiltration; C: The conventional group showed moderate fibrosis with inflammatory infiltration and slight ballooning of liver cells; D: Aloe vera high molecular weight fractions group showed minimal infiltration and minimal fibrosis.
Table 2.
Histopathological evaluation of the patients before and after treatment n (%)
|
Conventional group |
AHM group |
|||||
| Before | After | χ2/P | Before | After | χ2/P | |
| Grade | ||||||
| 0 | 0 (0) | 3 (15) | 4.390/ | 0 (0) | 3 (15) | 9.390/ |
| 1 | 3 (15) | 5 (25) | 0.223 | 3 (15) | 9 (45) | 0.024a |
| 2 | 9 (45) | 6 (30) | 10 (50) | 4 (20) | ||
| 3 | 8 (40) | 6 (30) | 7 (35) | 4 (20) | ||
| Stage | ||||||
| 0 | 0 (0) | 0 (0) | 0.000/ | 0 (0) | 2 (10) | 6.170/ |
| 1 | 3 (15) | 3 (15) | 1.000 | 3 (15) | 7 (35) | 0.103 |
| 2 | 9 (45) | 9 (45) | 10 (50) | 4 (20) | ||
| 3 | 8 (40) | 8 (40) | 7 (35) | 7 (35) | ||
P < 0.05 vs conventional group. AHM: Aloe vera high molecular weight fractions.
Determination of liver enzyme activities
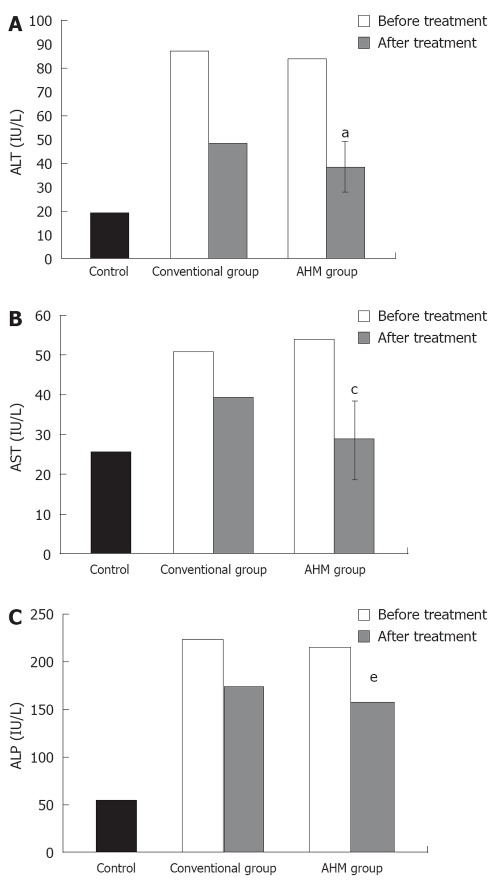
The serum ALT, AST and ALP activities were significantly higher in the patients at the beginning of the study as compared with the control group. These increases were attenuated after treatment in both the conventional group and the AHM group, and the decrease in the AHM group was more significant than in the conventional group (P < 0.05, Figure 2). There was no significant difference in the liver enzyme activities between HBV-induced fibrosis and HCV after treatment (P < 0.05, Table 3).
Figure 2.
Serum activity of alanine aminotransferase, alkaline phosphatase and aspartate aminotransferase of hepatic fibrosis patients and controls. A: Serum activity of alanine aminotransferase (IU/L) of hepatic fibrosis patients and controls; B: Serum activity of aspartate aminotransferase (IU/L) of hepatic fibrosis patients and controls; C: Serum activity of alkaline phosphatase (IU/L) of hepatic fibrosis patients and controls. aP < 0.05, cP < 0.05, eP < 0.05 vs before treatment and conventional group; Data are presented as mean ± SD. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; AHM: Aloe vera high molecular weight fractions.
Table 3.
Biochemical parameters of hepatitis B virus-induced fibrosis and hepatitis C patients after treatment
|
Conventional group |
AHM group |
|||
| Parameter | HBV (n = 12 ) | HCV (n = 8 ) | HBV (n = 12 ) | HCV (n = 7 ) |
| ALT (IU/L) | 50.1 | 47.2 | 50.1 | 37.3 |
| AST (IU/L) | 40.4 | 38.3 | 30.3 | 28.1 |
| ALP (IU/L) | 173.3 | 177.8 | 156.3 | 160.4 |
| MDA (μmol/g) | 596.1 | 607.4 | 571.0 | 565.0 |
| GSH (μg/g) | 22.9 | 20.4 | 23.7 | 27.6 |
| TGF-β (pg/mL) | 38.2 | 41.1 | 34.6 | 38.4 |
| HA (ng/mL) | 63.3 | 59.7 | 58.1 | 52.1 |
| MMP-2 (ng/mL) | 259.1 | 277.8 | 238.8 | 256.7 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; MDA: Malondialdhyde; GSH: Reduced glutathione; TGF-β: Transforming growth factor-β; HA: Hyaluronic acid; MMP-2: Matrix metalloproteinase-2; HBV: Hepatitis B virus; HCV: Hepatitis C virus.
Determination of liver oxidative markers
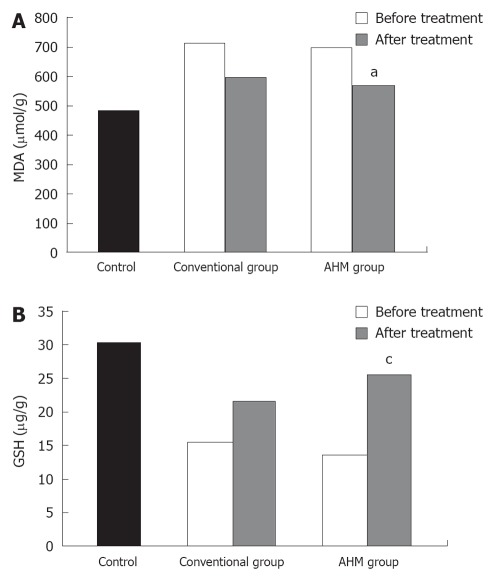
Similar to the aminotransferase activities, the MDA levels were significantly higher in the patients before treatment, and were attenuated after treatment in both groups (P < 0.05, Figure 3). In contrast, the hepatic glutathione content in the patients was decreased to about 49% of the control. Twelve weeks of treatment could increase the concentration of reduced glutathione. The increase was more significant in the AHM group (P < 0.05, Figure 3). There was no significant difference in the liver oxidative markers between HBV-induced fibrosis and HCV after treatment (P < 0.05, Table 3).
Figure 3.
Hepatic malondialdhyde and glutathione of hepatic fibrosis patients and controls. A: Hepatic malondialdhyde (μmol/g) of hepatic fibrosis patients and controls; B: Hepatic reduced glutathione (μg/g) of hepatic fibrosis patients and controls. aP < 0.05, cP < 0.05 vs before treatment and conventional group. Data are presented as mean ± SD. MDA: Malondialdehyde; GSH: Glutathione; AHM: Aloe vera high molecular weight fractions.
Determination of fibrosis markers
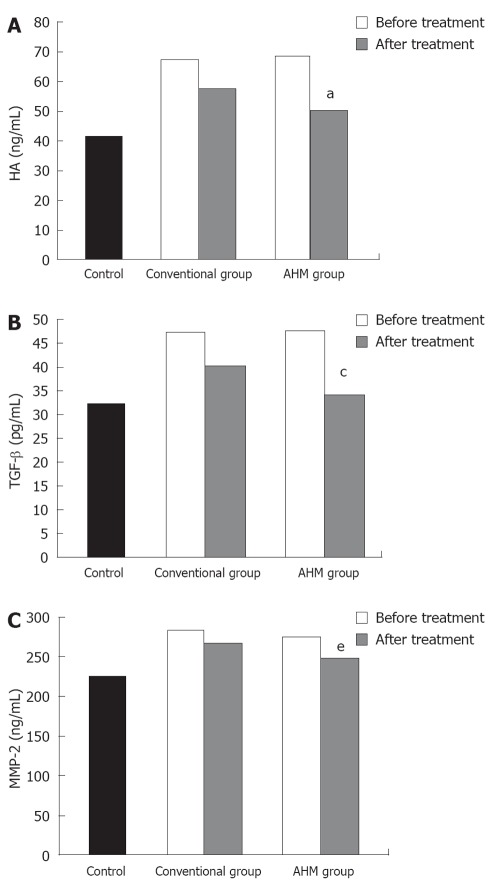
As shown in Figure 4, a significant increase in the serum level of TGF-β1, HA and MMP-2 was observed in the patients before treatment. Both the conventional and the AHM groups showed significant decrease in the levels after treatment (P < 0.05). There was no significant difference of fibrosis markers between HBV-induced fibrosis and HCV after treatment (P < 0.05, Table 3).
Figure 4.
Serum hyaluronic acid, transforming growth factor-β and matrix metalloproteinase-2 of hepatic fibrosis patients and controls. A: Serum hyaluronic acid (ng/mL) of hepatic fibrosis patients and controls; B: Serum transforming growth factor-β (pg/mL) of hepatic fibrosis patients and controls; C: Serum matrix metalloproteinase-2 (ng/mL) of hepatic fibrosis patients and controls. aP < 0.05, cP < 0.05, eP < 0.05 vs before treatment and conventional group. Data are presented as mean ± SD. HA: Hyaluronic acid; TGF-β: Transforming growth factor-β; MMP-2: Matrixmetalloproteinase-2; AHM: Aloe vera high molecular weight fractions.
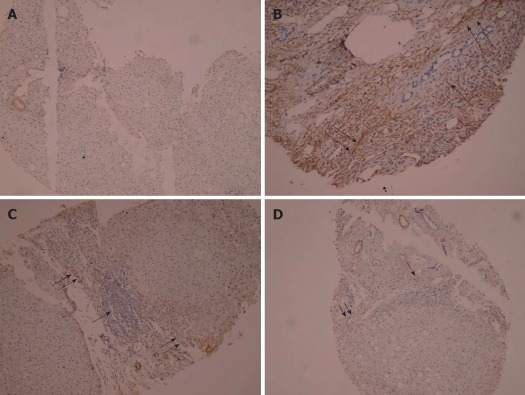
The expression of α-SMA was modified in patients before and after treatment as compared with the normal control. Before treatment, α-SMA positive cells were detected in portal space, sinusoid, lobule and areas where fibrotic septum appeared. After treatment, activation of HSC appeared to be strikingly decreased. After 12 wk of conventional treatment, there were only thin and incomplete parenchymal α-SMA positive septum joining thickened centrilobular veins. α-SMA positive cells were mainly found in portal space and areas around fibrotic septum. In AHM group, few α-SMA positive cells were present in sinusoid and lobule (Figure 5).
Figure 5.
Immunohistochemical staining of α-smooth muscle actin. A: Section of liver from controls showing negative staining (PAP × 125); B: Section of liver from liver fibrosis patients before treatment showing dense fibrotic reaction with strong positivity for α-smooth muscle actin (α-SMA) ↑↑ and dense mononuclear cellular infiltration ↑ (PAP × 225); C: Section of liver from conventional group after treatment showing dense mononuclear inflammatory infiltration ↑ and moderate positivity for α-SMA in fibrotic lesion↑↑ (PAP × 125); D: Section of liver from AHM group after treatment showing moderate mononuclear cellular infiltration ↑ surrounded by minimal fibrotic reaction stained mildly with α-SMA ↑↑ (PAP × 125).
DISCUSSION
The present study evaluates the anti-inflammatory, anti-oxidative and anti-fibrotic effect of aloe vera in hepatic fine periportal fibrosis. AHM treatment for the hepatic fibrosis patients markedly attenuated the release of ALT, AST and ALP as compared with the control group and the conventional group. Histological findings of liver samples strongly supported the release of aminotransferases by damaged hepatocytes and the protective effect of AHM.
GSH constitutes the first line of defense against free radicals and is a critical determinant of tissue susceptibility to oxidative damage. This is evident in this study by the significant increase in hepatic content of MDA and depletion of GSH in the patients before treatment. AHM exhibited hepato-protective effects by impairing oxidative stress through decreased production of free radical derivatives, as evidenced by the decreased MDA level. Furthermore, it attenuated hepatic glutathione depletion. This increase in the hepatic glutathione level could result from either its effect on the de novo synthesis of glutathione and its regeneration, or both. These results suggest that the antioxidant properties may be one mechanism by which AHM protects against liver damage.
Previous findings are in agreement with the finding by Anilakumar et al[23] who showed that aloe vera gel extract is able to reduce azoxymethane (AOM) induced-oxidative stress and toxicity in rat liver. Rajasekaran et al[24] also revealed that aloe vera leaf extract has a modulatory effect on oxidative stress in rats treated with streptozotocin by decreasing the thiobarbituric acid reactive substances, and improving reduced glutathione in the pancreas of STZ-induced diabetic rats. However, Yang et al[25] showed that oral aloe supplementation caused increase in liver enzymes and subsequent acute liver injury.
TGF-β, a multifunctional growth factor, is the most potent fibrogenic cytokine[26]. It is involved in regulation of liver growth and induction of hepatocyte apoptosis. TGF-β can promote the development of liver fibrosis by inducing the synthesis of ECM proteins and down-regulating the expression of matrix[27]. The effect of oral administration of aloe vera gel on the simulation of TGF-β was studied by Atiba et al[28]. The present study showed that TGF-β1 increased in the serum of fibrotic patients and decreased after treatment. These results suggest that TGF-β1 is closely correlated with hepatic fibrosis and that the improvement of hepatic fibrosis is related to the decreasing expression of TGF-β1. The serum level of TGF-β1 was low in the AHM group compared with the conventional group and this decrease was not significantly different as compared with the control group, suggesting that AHM can inhibit the expression of TGF-β1. These results coincide with that of Kim et al[7] who showed that ACTIValoe®N-931 complex decreased the TGF-β1 level and the hepatic hydroxyproline content in CCl-4-induced hepatotoxicity rats.
HA is mostly synthesized by the hepatic stellate cells and degraded by the sinusoidal endothelial cells[29]. Some investigators[30,31] have shown that there is good correlation between HA and the degree fibrosis. In the present study, 12 wk co-treatment of AHM and the conventional treatment decreased the serum HA by 25% as compared before treatment. It showed a trend toward greater improvement in the AHM group, however due to the short duration of treatment in our study, this was statistically insignificant as compared with control group.
Matrix metalloproteinases (MMPs) comprise a family of zinc-dependent enzymes that degrade extracellular matrix components and act as a marker of HSCs activation[32]. It was recently shown that it promoted HSCs apoptosis by cleavaging N-cadherin as an essential HSCs survival factor[33]. During fibrogenesis, the expression of MMP-2 increased and decreased significantly after treatment, suggesting that the treatment favored a collagenolytic activity.
The present study showed no significant difference in the serum liver fibrosis markers between HBV and HCV patients which is in agreement with Elmetwally et al[34], who revealed that serum HA concentrations did not differ significantly among chronic hepatitis subtypes (HBV vs HCV), while its level correlates with the degree of fibrosis. This suggested that progression of liver fibrosis (and inflammation) was accompanied by impairment in the liver endothelial cell function and reduced degradation of this hetero-polysaccharide, eventually resulting in elevation of serum HA concentrations.
α-SMA is a reliable marker of hepatic stellate cell activation which precedes fibrous tissue deposition, and it can be used for identification of the earliest stage of hepatic fibrosis and for monitoring the efficacy of the therapy[35]. In the present study, the expression of α-SMA was detected by immunohistochemistry; it was activated in the fibrotic patients, and the lowest level was observed in the AHM group. These data were consistent with Dechene et al[36] who revealed significant increase in serum tissue inhibitors of metalloproteinases and MMP as well as histological evidence of collagen formation and α-SMA expression in acute liver failure patients. They demonstrated an ongoing profibrotic process together with an increased HSC activity. It is possible that a collagen matrix is synthesized and deposited as a structural framework to preserve the liver architecture. Acute liver fibrosis may serve as a part of beneficial wound healing process by transiently conserve the organ’s structure until defective tissue areas are replaced by functional hepatocytes.
In conclusion, AHM has antifibrotic effects which could be attributed to its ability to attenuate oxidative stress, and enhance the collagenolytic activity. This study provides evidences that AHM could be used as adjunct treatment to prevent or treat hepatocellular damage in hepatic fine periportal fibrosis.
ACKNOWLEDGMENTS
The authors express their deep thanks to Mr. Yagi, S., Ellie Corporation, Shizuoka, Japan for providing aloe vera high molecular weight fractions and to Mr. Kaku, T., CEO, Japan Bioproducts Co., Ltd. for encouragement of the study. The authors fully acknowledge the valuable contributions of Prof. Karima EL-Desoky, Professor of Pathology, Faculty of Medicine, Tanta University, as well as Dr. Amr El-Bakry, lecturer of radiology, Faculty of Medicine, Tanta University.
COMMENTS
Background
Acute and chronic liver diseases constitute a global concern, but medical treatment up to now has limited efficacy. The therapeutically effective herbal medicine from documented medicinal plants may reduce the risk of drug toxicity. Aloe vera gel has been demonstrated to have liver protective effect in rats, and many toxicity studies have been conducted to determine the LD50 of aloe vera. This study investigated the antifibrotic effects of aloe vera for patients with acute liver fibrosis.
Research frontiers
Various in vitro studies have been conducted in an attempt to restore the integrity of damaged hepatocytes and reveal the hepatoprotective role of aloe vera in hepatic fibrosis. This study was undertaken to evaluate the antifibrotic effect of aloe vera in patients with hepatic fine periportal fibrosis and its mechanism of action.
Innovations and breakthroughs
The antifibrotic effect of aloe vera high molecular weight fractions (AHM) is attributed to its ability to attenuate oxidative stress, and enhance the collagenolytic activity. In the light of the potential of aloe vera plant extract, the discoveries of novel low-cost drug of natural non-toxic origin are promising for developing countries.
Applications
This study provides evidences that AHM could be used as adjunct treatment to prevent or treat hepatocellular damage in hepatic fine periportal fibrosis.
Peer review
The study is interesting and goes along with previous studies that showed hepatoprotective effect of aloe vera extracts in experimental models.
Footnotes
Peer reviewer: Ali Reza Mani, Department of Physiology, Faculty of Medical Sciences, Tarbiat Modares University, Jalal-Ale-Ahmad Highway, Tehran 14115, Iran
S- Editor Gou SX L- Editor Ma JY E- Editor Zheng XM
References
- 1.Singhal S, Jain S, Kohaar I, Singla M, Gondal R, Kar P. Apoptotic mechanisms in fulminant hepatic failure: potential therapeutic target. Appl Immunohistochem Mol Morphol. 2009;17:282–285. doi: 10.1097/PAI.0b013e3181906f6d. [DOI] [PubMed] [Google Scholar]
- 2.Bechmann LP, Marquitan G, Jochum C, Saner F, Gerken G, Canbay A. Apoptosis versus necrosis rate as a predictor in acute liver failure following acetaminophen intoxication compared with acute-on-chronic liver failure. Liver Int. 2008;28:713–716. doi: 10.1111/j.1478-3231.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 3.Rutherford A, Chung RT. Acute liver failure: mechanisms of hepatocyte injury and regeneration. Semin Liver Dis. 2008;28:167–174. doi: 10.1055/s-2008-1073116. [DOI] [PubMed] [Google Scholar]
- 4.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 5.Lai MM. Hepatitis C virus proteins: direct link to hepatic oxidative stress, steatosis, carcinogenesis and more. Gastroenterology. 2002;122:568–571. doi: 10.1053/gast.2002.31474. [DOI] [PubMed] [Google Scholar]
- 6.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Cheon HJ, Yun N, Oh ST, Shin E, Shim KS, Lee SM. Protective effect of a mixture of Aloe vera and Silybum marianum against carbon tetrachloride-induced acute hepatotoxicity and liver fibrosis. J Pharmacol Sci. 2009;109:119–127. doi: 10.1254/jphs.08189fp. [DOI] [PubMed] [Google Scholar]
- 8.Gbadegesin MA, Odunola OA, Akinwumi KA, Osifeso OO. Comparative hepatotoxicity and clastogenicity of sodium arsenite and three petroleum products in experimental Swiss Albino Mice: the modulatory effects of Aloe vera gel. Food Chem Toxicol. 2009;47:2454–2457. doi: 10.1016/j.fct.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Chandan BK, Saxena AK, Shukla S, Sharma N, Gupta DK, Suri KA, Suri J, Bhadauria M, Singh B. Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacol. 2007;111:560–566. doi: 10.1016/j.jep.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Williams LD, Burdock GA, Shin E, Kim S, Jo TH, Jones KN, Matulka RA. Safety studies conducted on a proprietary high-purity aloe vera inner leaf fillet preparation, Qmatrix. Regul Toxicol Pharmacol. 2010;57:90–98. doi: 10.1016/j.yrtph.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Final report on the safety assessment of AloeAndongensis Extract, Aloe Andongensis Leaf Juice, aloe Arborescens Leaf Extract, Aloe Arborescens Leaf Juice, Aloe Arborescens Leaf Protoplasts, Aloe Barbadensis Flower Extract, Aloe Barbadensis Leaf, Aloe Barbadensis Leaf Extract, Aloe Barbadensis Leaf Juice,aloe Barbadensis Leaf Polysaccharides, Aloe Barbadensis Leaf Water, Aloe Ferox Leaf Extract, Aloe Ferox Leaf Juice, and Aloe Ferox Leaf Juice Extract. Int J Toxicol. 2007;26 Suppl 2:1–50. doi: 10.1080/10915810701351186. [DOI] [PubMed] [Google Scholar]
- 12.Fogleman RW, Chapdelaine JM, Carpenter RH, McAnalley BH. Toxicologic evaluation of injectable acemannan in the mouse, rat and dog. Vet Hum Toxicol. 1992;34:201–205. [PubMed] [Google Scholar]
- 13.Logarto Parra A, Silva Yhebra R, Guerra Sardiñas I, Iglesias Buela L. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8:395–400. doi: 10.1078/0944-7113-00044. [DOI] [PubMed] [Google Scholar]
- 14.Okamura N, Asai M, Hine N, Yagi A. High-performance liquid chromatographic determination of phenolic compounds in Aloe species. J Chromatogr A. 1996;746:225–231. [Google Scholar]
- 15.Yagi A, Egusa T, Arase M, Tanabe M, Tsuji H. Isolation and characterization of the glycoprotein fraction with a proliferation-promoting activity on human and hamster cells in vitro from Aloe vera gel. Planta Med. 1997;63:18–21. doi: 10.1055/s-2006-957595. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM, Farrag MM. The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2008;243:261–270. doi: 10.1016/j.tox.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Kind PR, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954;7:322–326. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson RJ, Murphy SD. Effect of glutathione depletion on tissue deposition of methylmercury in rats. Toxicol Appl Pharmacol. 1975;31:505–519. doi: 10.1016/0041-008x(75)90274-4. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135:372–376. doi: 10.1016/0002-9378(79)90708-7. [DOI] [PubMed] [Google Scholar]
- 23.Anilakumar KR, Sudarshanakrishna KR, Chandramohan G, Ilaiyaraja N, Khanum F, Bawa AS. Effect of Aloe vera gel extract on antioxidant enzymes and azoxymethane-induced oxidative stress in rats. Indian J Exp Biol. 2010;48:837–842. [PubMed] [Google Scholar]
- 24.Rajasekaran S, Sivagnanam K, Subramanian S. Modulatory effects of Aloe vera leaf gel extract on oxidative stress in rats treated with streptozotocin. J Pharm Pharmacol. 2005;57:241–246. doi: 10.1211/0022357055416. [DOI] [PubMed] [Google Scholar]
- 25.Yang HN, Kim DJ, Kim YM, Kim BH, Sohn KM, Choi MJ, Choi YH. Aloe-induced toxic hepatitis. J Korean Med Sci. 2010;25:492–495. doi: 10.3346/jkms.2010.25.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta. 2006;364:33–60. doi: 10.1016/j.cca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Xu XB, He ZP, Liang ZQ, Leng XS. [Obstruction of TGF-beta1 signal transduction by anti-Smad4 gene can therapy experimental liver fibrosis in the rat] Zhonghua Gan Zang Bing Zazhi. 2004;12:263–266. [PubMed] [Google Scholar]
- 28.Atiba A, Nishimura M, Kakinuma S, Hiraoka T, Goryo M, Shimada Y, Ueno H, Uzuka Y. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-β and fibroblast growth factor production. Am J Surg. 2011;201:809–818. doi: 10.1016/j.amjsurg.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Afdhal NH. Diagnosing fibrosis in hepatitis C: is the pendulum swinging from biopsy to blood tests? Hepatology. 2003;37:972–974. doi: 10.1053/jhep.2003.50223. [DOI] [PubMed] [Google Scholar]
- 30.Fontana RJ, Goodman ZD, Dienstag JL, Bonkovsky HL, Naishadham D, Sterling RK, Su GL, Ghosh M, Wright EC. Relationship of serum fibrosis markers with liver fibrosis stage and collagen content in patients with advanced chronic hepatitis C. Hepatology. 2008;47:789–798. doi: 10.1002/hep.22099. [DOI] [PubMed] [Google Scholar]
- 31.Esmat G, Metwally M, Zalata KR, Gadalla S, Abdel-Hamid M, Abouzied A, Shaheen AA, El-Raziky M, Khatab H, El-Kafrawy S, et al. Evaluation of serum biomarkers of fibrosis and injury in Egyptian patients with chronic hepatitis C. J Hepatol. 2007;46:620–627. doi: 10.1016/j.jhep.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Das SK, Vasudevan DM. Genesis of hepatic fibrosis and its biochemical markers. Scand J Clin Lab Invest. 2008;68:260–269. doi: 10.1080/00365510701668516. [DOI] [PubMed] [Google Scholar]
- 33.Hartland SN, Murphy F, Aucott RL, Abergel A, Zhou X, Waung J, Patel N, Bradshaw C, Collins J, Mann D, et al. Active matrix metalloproteinase-2 promotes apoptosis of hepatic stellate cells via the cleavage of cellular N-cadherin. Liver Int. 2009;29:966–978. doi: 10.1111/j.1478-3231.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 34.Elmetwally IM, Elmahalaway AM, Abuhashem SH, Ahmed AM. Determination of serum fibrosis index in patients with chronic hepatitis and its relationship to histological activity index. Saudi Med J. 2009;30:638–646. [PubMed] [Google Scholar]
- 35.Carpino G, Morini S, Ginanni Corradini S, Franchitto A, Merli M, Siciliano M, Gentili F, Onetti Muda A, Berloco P, Rossi M, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37:349–356. doi: 10.1016/j.dld.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Dechêne A, Sowa JP, Gieseler RK, Jochum C, Bechmann LP, El Fouly A, Schlattjan M, Saner F, Baba HA, Paul A, et al. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology. 2010;52:1008–1016. doi: 10.1002/hep.23754. [DOI] [PubMed] [Google Scholar]