Abstract
AIM: To investigate the methylation status of secreted protein acidic and rich in cysteine (SPARC) in human hepatocellular carcinoma (HCC) and evaluate its clinical implication.
METHODS: The methylation status of SPARC was analyzed in one HCC cell line (SMMC-7721) and 60 pairs of HCC and corresponding nontumorous tissues by methylation-specific polymerase chain reaction and bisulfite sequencing. The expression of SPARC mRNA and protein were examined by reverse transcription polymerase chain reaction and immunohistochemistry, respectively. The correlations between the methylation status and the gene expression, the clinicopathological parameters, as well as the prognosis after surgery were analyzed.
RESULTS: In the SMMC-7721 cell line, the loss of SPARC expression was correlated with the aberrant methylation and could be reactivated by the demethylating agent 5-aza-2’-deoxycytidine. Methylation frequency of SPARC in HCC was significantly higher than that in the corresponding nontumorous tissues (45/60 vs 7/60, P < 0.001), and it was correlated with the pathological classification (P = 0.019). The downregulation of the SPARC mRNA expression in HCC was correlated with the SPARC methylation (P = 0.040). The patients with methylated SPARC had a poorer overall survival than those without methylated SPARC (28.0 mo vs 41.0 mo, P = 0.043).
CONCLUSION: Aberrant methylation is an important mechanism for SPARC inactivation in HCC and SPARC methylation may be a promising biomarker for the diagnosis and prognosis of HCC.
Keywords: Biomarker, Diagnosis, Hepatocellular carcinoma, Methylation, Prognosis, Tumor suppressor gene
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the third leading cause of cancer death in the world[1,2]. To date, surgical resection is still considered the most important treatment for patients with resectable HCC[3]. Unfortunately, most patients are at inoperable stages when the tumor is diagnosed[4]. In addition, the high incidence of tumor recurrence after curative resection also leads to poor clinical outcomes[5,6]. Therefore, the development of biomarkers for early diagnosis and accurate prognosis of HCC is valuable for improving patients’ survival.
Although the detailed molecular mechanisms of hepatocarcinogenesis remain largely unclear, the accumulating evidences have shown that aberrant methylation of promoter CpG islands causes inactivation of tumor suppressor genes, which is involved in the occurrence and development of HCC[7-10]. Detections of such an aberrant DNA methylation of tumor suppressor genes could be used as a diagnostic or a prognostic marker for HCC.
Secreted protein acidic and rich in cysteine (SPARC) is a matricellular glycoprotein involved in some biological processes, including tissue remodeling, angiogenesis, extracellular matrix production and so on[11-13]. It has been reported that SPARC has tumor suppressing properties to various cancers, such as ovarian cancer and pancreatic cancer[14-16]. Moreover, SPARC is epigenetically silenced through promoter hypermethylation in these cancers, and the demethylating agent 5-aza-2’-deoxycytidine (5-Aza-CdR) can rescue SPARC expression[17-20]. The SPARC promoter methylation is an important factor in the carcinogenesis of these cancers and may be a promising epigenetic marker for them. However, up to date, there have been few reports about the methylation status in HCC.
In this study, in order to explore the status of SPARC methylation in HCC, we examined the methylation and expression of SPARC in HCC cell line and tissues. We correlated the methylation status with clinicopathologic features and evaluated whether the methylation of SPARC can serve as a potentially diagnostic or prognostic biomarker for HCC.
MATERIALS AND METHODS
Cell line and patient samples
The SMMC-7721 cell line used in this study was obtained from the Shanghai Institute of Cell Biology (Shanghai, China). HCCs and their corresponding nontumorous tissues were obtained from 60 patients who were diagnosed and treated at the Department of Hepatobiliary Surgery, Tianjin Third Central Hospital in China from October 2003 to June 2008. This study protocol was approved by the Clinical Research Ethics Committee of our institution and the informed consent was obtained from each of these patients. After surgical resection, samples were immediately stored in the liquid nitrogen for later analysis. For the gene expression analysis, the hematoxylin-eosin-stained samples from each tumor block were examined microscopically to confirm the presence of more than 80% tumor cells. The nontumorous samples from each patient were also microscopically confirmed.
Cell culture and 5-Aza-CdR treatment
SMMC-7721 cells were grown in DMEM supplemented with 100 g/L fetal bovine serum and incubated in 37 °C and 50 mL/L CO2. For the 5-Aza-CdR (Sigma, St Louis, MO, United States) treatment, cells were split to 5 × 105 per 75-cm2 culture bottle and incubated overnight in the growth media. The normal growth media was replaced with the growth media supplemented with 5-Aza-CdR (10 μmol as a final concentration) for 6 d with the media change on day 4. Cells cultured with vehicle alone served as 5-Aza-CdR negative control. After the culture, cells were harvested for the extraction of genomic DNA and total RNA. In order to detect the SPARC protein in different groups by immunocytochemical staining, SMMC-7721 cells were also seeded onto 6-well plates containing coverslips to induce cells to spread and adhere to the glass.
DNA extraction and bisulfite treatment
The genomic DNA was extracted from the cell line and tissue samples by digesting with sodium dodecyl sulfate/proteinase K in Tris ethylenediamine tetraacetic acid (TE) buffer followed by a standard phenol/chloroform extraction. The extracted DNA was subjected to the bisulfite treatment as previously described[21-23]. Briefly, 1-2 μg genomic DNA was denatured with 0.3 mol/L NaOH at 37 °C for 20 min, and incubated in 3.0 mol/L sodium bisulfite and 10 mmol/L hydroquinone at 55 °C for 16 h. The DNA was desalted with a QIAquick gel extraction kit (Qiagen, Valencia, CA, United States) and dissolved in 50 μL of 10 mmol/L TE buffer (pH 8.0). Then, 5.5 μL of 3.0 mol/L NaOH was added and incubated at 37 °C for 20 min to desulfonate it. The modified DNA was neutralized with 30 μL of 10 mol/L ammonium acetate, precipitated using 2 volumes of ethanol, and resuspended in 40 μL of 1.0 mmol/L TE buffer (pH 7.6).
Methylation specific polymerase chain reaction and sequencing
Methylation specific polymerase chain reaction (MSP) was performed to examine the methylation status at CpG island of SPARC promoter in both SMMC-7721 cells and tissue samples. The primers used in this study for polymerase chain reaction (PCR) are shown in Table 1. A PCR mixture contained 1 × PCR buffer (10 mmol/L Tris, 50 mmol/L KCl, 1.5 mmol/L MgCl2 and 10 mmol/L β-mercaptoethanol), deoxynucleotide triphosphates (each at 0.2 mmol/L), primers (10 pmol each), bisulfite-modified DNA templates (2 μL) and 1 U of Taq polymerase, and the final volume was 25 μL. The PCR conditions were as follows: 94 °C for 2 min; then 40 cycles of 94 °C for 30 s, at optimum annealing temperature for 30 s and 72 °C for 30 s; and final extension for 5 min at 72 °C. The normal leukocyte DNA methylated in vitro with SssI methyltransferase (New England Biolabs Inc., Beverly, MA, United States) was used as the positive control of methylation, and the normal leukocyte DNA was used as the negative control. The distilled water without template DNA was used as a blank control for all tests. Five microliters of PCR products underwent electrophoresis on 25 g/L agarose gel, and was visualized under ultraviolet illumination with the ethidium bromide staining. To verify the accuracy of MSP, the PCR products of both methylation and unmethylation were randomly chosen and cloned into the pMD-18-T vector (TaKaRa, Dalian, China) followed by a sequencing analysis.
Table 1.
Primer sequences for polymerase chain reaction
| Gene | Primer sequences (forward/reverse 5’-3’) | Accession No. | Location to transcription start | Product size (bp) | Annealing temperature (°C) |
| SPARC methylation | GAGAGCGCGTTTTGTTTGTC | NM_003118.2 | +52 to +71 | 112 | 54 |
| AACGACGTAAACGAAAATATCG | +142 to +163 | ||||
| SPARC unmethylation | TTTTTTAGATTGTTTGGAGAGTG | NM_003118.2 | +36 to +58 | 132 | 59 |
| AACTAACAACATAAACAAAAATATC | +143 to +167 | ||||
| SPARC BS | GATAGAGATAGTTTTGGTTATGGGA | NM_003118.2 | -119 to -95 | 401 | 55 |
| CCACCTTCTAAAAAACA ACAAAC | +260 to +282 | ||||
| SPARC mRNA | CGCATGCGGGACTGGCTCAA | NM_003118.2 | +601 to +620 | 148 | 60 |
| GCTCCACGGGG TGGTC TCCT | +729 to +748 | ||||
| GAPDH mRNA | GGGCATCCTGGGCTACACTGA | NM_002046.3 | +915 to +935 | 143 | 58 |
| CAAATTCGTTGTCATACCAGGAAATG | +1032 to +1057 |
SPARC: Secreted protein acidic and rich in cysteine; BS: Bisulfite sequencing; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
To investigate the status of CpG sites in the region of SPARC promoter of SMMC-7721 cells, bisulfite sequencing analysis was performed for the bisulfite-treated DNA. The PCR products were cloned into a pMD-18-T vector and 8 individual clones of each group were sequenced.
RNA preparation and reverse transcription-PCR
RNA was extracted from the cell line and tissues using the Trizol (Tiangen, Beijing, China) according to the manufacturer’s instructions. The total mRNA was digested with the DNase I(Ambion, Austin, TX, United States) to remove the genomic DNA contamination and then subjected to reverse transcription using the reverse transcription system (Promega, Madison, WI, United States). SPARC expression of SMMC-7721 cells and tissues were tested by reverse transcription (RT)-PCR and quantitative RT-PCR, respectively. Real-time quantitative RT-PCR was done on the ABI Prism 7000 sequence detection system in combination with the SYBR green real-time PCR master mix (Toyobo, Shanghai, China). The PCR amplification was carried out for 2 min at 94 °C for the initial denaturation, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. Melting curve analyses following amplification were performed to assure the product specificity. The relative expression of SPARC mRNA was normalized to the housekeeping gene Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in the same cDNA using the comparative CT method. For the quantification of gene expression, the target gene (SPARC) value normalized to the expression of GAPDH was designated as ΔCT [ΔCT = CT (SPARC) - CT (GAPDH)]. The ΔCT for the nontumorous samples was then subtracted from the ΔCT for the tumorous samples to generate ΔΔCT [ΔΔCT = ΔCT (tumor) - ΔCT (nontumorous sample)]. The ΔΔCT measurement was used to calculate the relative expression (2-ΔΔCT).
Immunohistochemistry
The protein expression of SPARC was examined in 23 primary HCCs and the correspording nontumorous tissues by immunohistochemistry. Sections (5 μm) from the tumor and nontumorous tissues were cut onto coated slides and deparaffinized by the routine techniques. The antigen retrieval was performed in 10 mmol sodium citrate buffer (pH 6.0), and heated at 95 °C for 10 min. After endogenous peroxidase activity was blocked with 30 g/L H2O2 for 5 min, the sections were incubated with an anti-SPARC monoclonal antibody at a 1:100 dilution (Santa Cruz Biotechology, United States) overnight. Labeling was detected with the PV-9000 Kit (Zhongshan, Beijing, China), following the protocol afforded by the manufacturer, and all sections were counterstained with hematoxylin. Cytoplasm staining of more than 90% parenchyma cells (tumor cells or liver cells) was regarded as positive for SPARC.
Similarly, SPARC protein was also tested in SMMC-7721 cells growing on the coverslips by immunocytochemistry.
Analysis for clinicopathological data and statistics
The gene methylation status in HCC was evaluated in the correlation with the clinicopathological parameters of patients, including age, gender, tumor size, virus infection, liver function, tumor number, vascular infiltration, pathology class and the level of alpha-fetal protein (AFP). The Pearson χ2 test or the Fisher’s exact test was used to analyze associations between methylation frequencies and categorical variables. Disease free or overall survival was calculated from the date of the operation until tumor recurrence or death or the date of the last follow-up (censored). Survival was analysed by the Kaplan-Meier method, and differences in their distribution were evaluated by the log-rank test. A multivariate Cox’s proportional-hazard model was developed to evaluate the covariates’ joint effects. All P values were two-sided, and P value less than 0.05 was defined as being statistically significant. Analyses were performed with SPSS V 13.0 software for Windows (SPSS, Chicago, United States).
RESULTS
Methylation status and expression of SPARC in SMMC-7721 cells
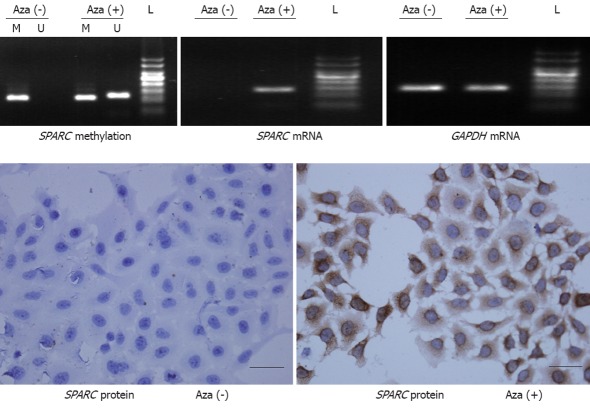
We used MSP to measure both methylated and unmethylated segments in the SPARC promoter region. The results demonstrated that only the methylated segment was detected in SMMC-7721 cells of the control group. However, both methylated and unmethylated segments were found in the cells after treated with 5-Aza-CdR. These results indicated that SPARC was homologously methylated in SMMC-7721 cells and 5-Aza-CdR could convert the methylation status of SPARC. RT-PCR revealed that the SPARC mRNA expression was absent in the cells without the 5-Aza-CdR treatment, however, the cells treated with the 5-Aza-CdR restored the SPARC mRNA expression. Consistently, the immunocytochemical analysis of the cultured cells displayed that the SPARC protein expression was restored in the cells previously lacking of the SPARC expression. The concordance between the loss of gene expression and the aberrant methylation suggested that the DNA methylation played a causal role in the loss of the SPARC expression in SMMC-7721 cells. The representative results are shown in Figure 1.
Figure 1.
Secreted protein acidic and rich in cysteine methylation and expression in SMMC-7721 cell line. SPARC: Secreted protein acidic and rich in cysteine; Aza: 5-aza-2’-deoxycytidine; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; M: Methylation; U: Unmethylation; L: 50 bp ladder; Scale bar: 50 μm.
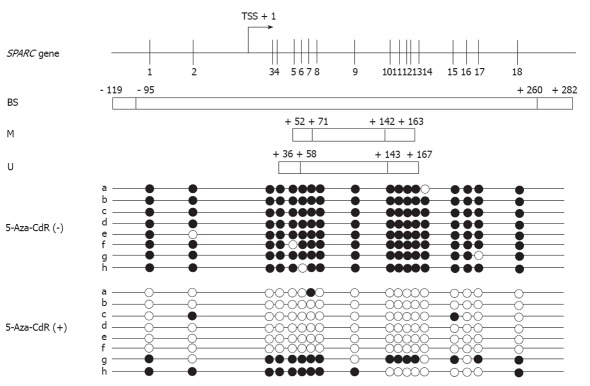
The bisulfite sequencing displayed that the control cells were methylated at almost all the 18 CpG sites in the 8 clones. On the contrary, most of CpG sites were unmethylated in the cells treated with 5-Aza-CdR. Figure 2 shows the methylation pattern of the SPARC promoter in SMMC-7721 cells.
Figure 2.
Bisulfite sequencing of secreted protein acidic and rich in cysteine in SMMC-7721 cell line. SPARC: Secreted protein acidic and rich in cysteine; TSS: Transcription start site; BS: Bisulfite sequencing; M: Methylation; U: Unmethylation; 5-Aza-CdR: 5-aza-2’-deoxycytidine; 1-18: CpG sites; - 119 to - 95, + 260 to + 282, + 52 to + 71, + 142 to + 163, + 36 to + 58, + 143 to + 167: Polymerase chain reaction primers position; Black dots: Methylation; Blank rings: Unmethylation.
Frequent SPARC hypermethylation in human HCC
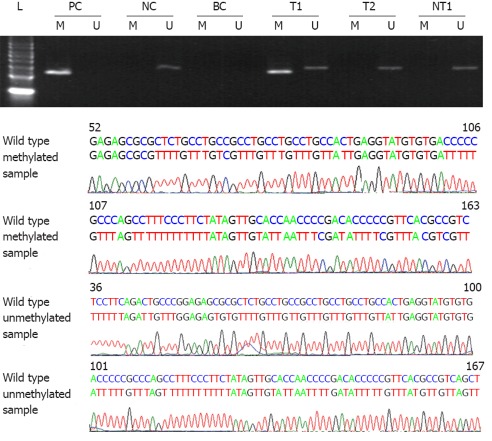
We used MSP to evaluate the SPARC methylation status of the CpG island in 60 pairs of tissues. Methylation alleles in 75.00% (45/60) of HCC samples were detected, however, only 11.67% (7/60) methylated alleles could be found in the correspording nontumorous tissues. The methylation frequence of SPARC in HCC was significantly higher than that in noncancerous liver tissues (Table 2). If methylation was used as an indicator for distincting HCC from nontumorous tissues, the sensitivity, specificity and accuracy were 86.54%, 77.94% and 81.67%, respectively. To validate the accuracy of MSP, we randomly chose the PCR products of methylation or unmethylation for sequencing. The results were according to the PCR aim segments. The representative results of PCR and sequencing are demonstrated in Figure 3.
Table 2.
Methylation frequencies of secreted protein acidic and rich in cysteine in 60 cases
| SPARC methylation status | |||
| Tissue | Methylated (%) | Unmethylated (%) | P value |
| Tumorous | 45 (75.00) | 15 (25.00) | < 0.001 |
| Nontumorous | 7 (11.67) | 53 (88.33) | |
SPARC: Secreted protein acidic and rich in cysteine.
Figure 3.
Representative results of methylation specific polymerase chain reaction analysis and sequencing in tissues. L: 50 bp ladder; PC: Positive control; NC: Negative control; BC: Blank control; M: Methylation; U: Unmethylation; T: Hepatocellular carcinoma tissue; NT: Nontumorous tissue.
Correlation between SPARC methylation and mRNA expression
The expression of SPARC mRNA was examined in 60 pairs of HCC and nontumorous tissues by quantitative RT-PCR. Most of primary HCC tissues (65.00%, 39/60) showed a lower expression level when compared with their corresponding nontumorous livers (Figure 4A). Moreover, the median of relative expression was statistically different between the methylated and unmethylated SPARC samples of HCC (P = 0.040) (Figure 4B). The methylated samples had a lower median of expression.
Figure 4.
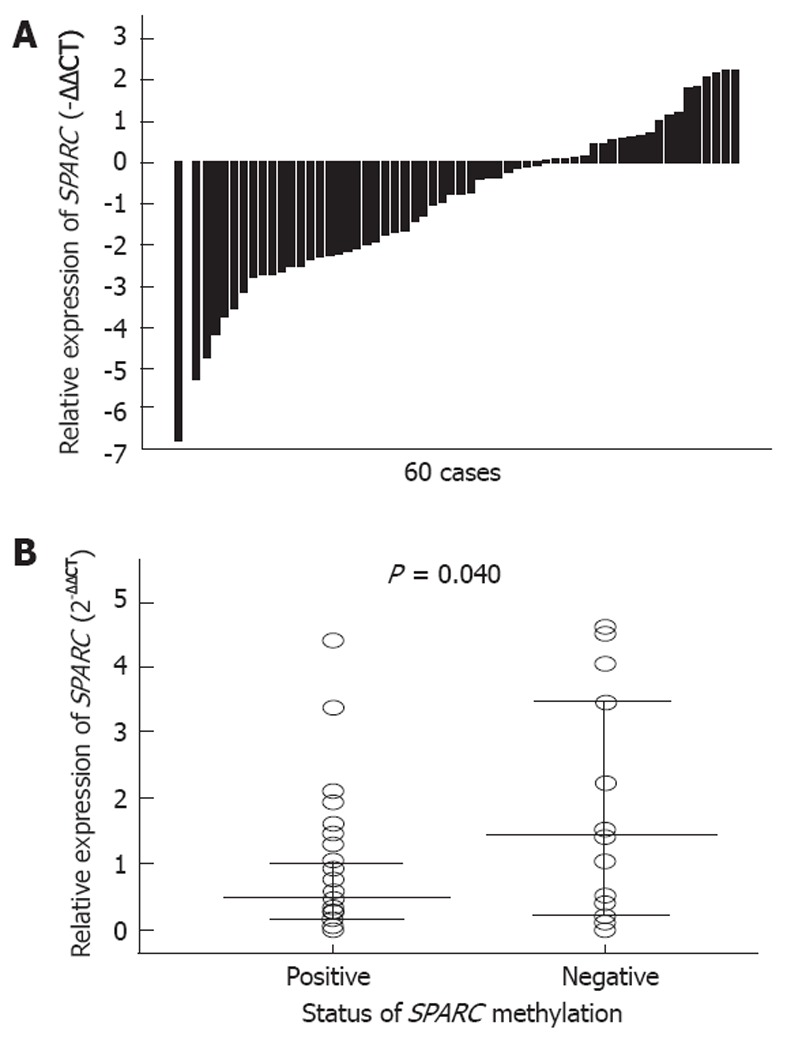
Expression of secreted protein acidic and rich in cysteine mRNA in hepatocellular carcinoma. Horizontal lines represent the median, and range indicates a 25%-75% quartile. SPARC: Secreted protein acidic and rich in cysteine.
Methylation and protein expression
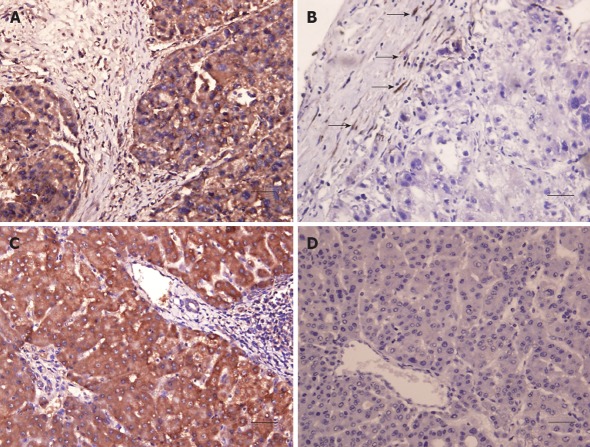
The protein expression of SPARC was examined in 23 pairs of HCC and nontumorous tissues by immunostaining. The positive frequency of tumor cells in HCC was relatively lower than that of liver cells in nontumorous tissues, but there was no statistical significance between two groups (Table 3). We divided all 46 samples into methylation and unmethylation groups (14 vs 32). There was no statistical correlation between the methylation and the protein expression (Table 4). In some HCC samples, stromal cells around tumor cells showed a positive signal even if the parenchyma cells had no expression of SPARC. The representative immunohistochemical staining is shown in Figure 5.
Table 3.
Protein expression frequencies in 23 pairs of samples
| Protein expression | ||||
| Tissue | n | Positive (%) | Negative (%) | P value |
| Tumorous | 23 | 12 (52.2) | 11 (47.8) | 0.552 |
| Nontumorous | 23 | 14 (60.9) | 9 (39.1) | |
Table 4.
Association of secreted protein acidic and rich in cysteine methylation with protein expression
| Protein expression | ||||
| SPARC | n | Positive (%) | Negative (%) | P value |
| Methylated | 14 | 6 (42.9) | 8 (57.1) | 0.216 |
| Unmethylated | 32 | 20 (62.5) | 12 (37.5) | |
SPARC: Secreted protein acidic and rich in cysteine.
Figure 5.
Immunohistochemical analysis of secreted protein acidic and rich in cysteine expression. A: A tumor with positive staining; B: A tumor with negative result, but stromal tissues with positive signal (arrows); C: Nontumorous tissues with positive staining; D: Nontumorous tissues with negative staining; Scale bar: 50 μm.
Relationship between methylation and clinical data
We analyzed the association of SPARC methylation with clinicopathological parameters in patients with HCC. There was significant association between the methylation status and the pathological class. The SPARC methylation was more frequently observed in cases with a high pathologic grade (33 of 39, 84.6%) than in those with a low grade (12 of 21, 57.1%) (Table 5). However, there was no statistically significant correlation between the methylation status and other clinicopathologic factors.
Table 5.
Correlation between methylation status and clinicopathological data
| Parameters | n | Methylated | Unmethylated | P value |
| Age (yr) | 0.766 | |||
| > 53 | 30 | 23 | 7 | |
| ≤ 53 | 30 | 22 | 8 | |
| Gender | 0.835 | |||
| Male | 51 | 38 | 13 | |
| Female | 9 | 7 | 2 | |
| Tumor size (cm) | 1.000 | |||
| ≤ 5 | 20 | 15 | 5 | |
| > 5 | 40 | 30 | 10 | |
| Virus infection | 0.661 | |||
| HBV or HCV | 52 | 40 | 12 | |
| Negative | 8 | 5 | 3 | |
| Liver function | 1.000 | |||
| Child-Pugh A | 46 | 35 | 11 | |
| Child-Pugh B | 14 | 10 | 4 | |
| AFP (μg/L) | 0.125 | |||
| ≤ 400 | 35 | 29 | 6 | |
| > 400 | 23 | 15 | 8 | |
| Tumor number | 0.174 | |||
| Single | 35 | 24 | 11 | |
| Multiple | 25 | 21 | 4 | |
| Vascular invasion | 0.122 | |||
| Positive | 22 | 19 | 3 | |
| Negative | 38 | 26 | 12 | |
| Edmondson classification | 0.019 | |||
| I/II | 21 | 12 | 9 | |
| III/IV | 39 | 33 | 6 |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; AFP: Alpha-fetal protein.
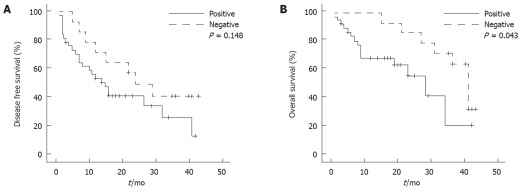
Prognostic value of SPARC methylation in HCC
We also divided all cases into two groups according to the methylation status of SPARC to determine whether this factor had prognostic value. The disease free survival between the two groups had no statistical difference. Patients whose primary tumors exhibited SPARC methylation had a lower overall survival rate after resection (28.0 mo vs 41.0 mo, P = 0.043, Table 6 and Figure 6). Five clinicopathological factors and methylation status of SPARC found to be prognostic on the univariate analysis were entered into a multivariate model to identify independent predictors of overall survival. The Cox’s multivariate proportional-hazard model indicated that the factors significantly affecting overall survival were tumor size, AFP level and SPARC methylation (Table 7).
Table 6.
Survival analysis of patients with different methylation status
| Gene | M/U | n |
Disease free survival |
Overall survival |
||||||
| Estimate (mo) | Scope (mo) | Log-Rank | P value | Estimate (mo) | Scope (mo) | Log-Rank | P value | |||
| SPARC | M | 37 | 15.0 | 9.6-20.4 | 2.094 | 0.148 | 28.0 | 17.8-38.2 | 4.096 | 0.043 |
| U | 14 | 24.0 | 12.6-35.5 | 41.0 | 36.5-45.5 | |||||
SPARC: Secreted protein acidic and rich in cysteine; M: Methylation; U: Unmethylation.
Figure 6.
Disease free (A) and overall (B) survival analysis of patients with different secreted protein acidic and rich in cysteine methylation status.
Table 7.
Cox regression model of overall survival
| Factors |
Univariate analysis |
Multivariate analysis |
||||
| RR | 95% CI | P value | RR | 95% CI | P value | |
| Methylation | ||||||
| Positive | 2.672 | 0.999-7.147 | 0.044 | 3.207 | 1.290-7.975 | 0.012 |
| Negative | 1 | 1 | ||||
| Tumor size (cm) | ||||||
| > 5 | 5.293 | 1.560-17.959 | 0.008 | 8.045 | 2.125-30.456 | 0.002 |
| ≤ 5 | 1 | 1 | ||||
| AFP (μg/L) | ||||||
| > 400 | 3.306 | 1.421-7.694 | 0.006 | 7.105 | 1.798-28.080 | 0.005 |
| ≤ 400 | 1 | 1 | ||||
| Age (yr) | ||||||
| > 53 | 0.663 | 0.279-1.576 | 0.353 | |||
| ≤ 53 | 1 | |||||
| Gender | ||||||
| Male | 1.104 | 0.373-3.266 | 0.859 | |||
| Female | 1 | |||||
| Tumor number | ||||||
| Multiple | 3.330 | 1.440-7.704 | 0.005 | |||
| Single | 1 | |||||
| Vascular invasion | ||||||
| Positive | 2.776 | 1.186-6.502 | 0.019 | |||
| Negative | 1 | |||||
| Edmondson classification | ||||||
| I/II | 0.379 | 0.147-0.982 | 0.046 | |||
| III/IV | 1 | |||||
AFP: Alpha-fetal protein; RR: Relative risk.
DISCUSSION
In this current study, we determined the methylation status of SPARC gene promoter in SMMC-7721 cell line and HCC tissues. The data suggested that in SMMC-7721 cell line, hypermethylation of the promoter was an important mechanism for SPARC downregulation, which was most likely involved in the development and progression of HCC. Moreover, the methylation frequency of SPARC was significantly higher in the HCC tissues than in the corresponding nontumorous tissues. The hypermethylation of SPARC was associated with pathological class and patients without SPARC methylation had higher rates of overall survival after resection. Our results showed that methylation of SPARC could be further evaluated as a tumor marker for the diagnosis and prognosis of HCC.
In some tumor cell lines, aberrant methylation of SPARC has been tested. Functional studies have shown that methylation of SPARC could induce gene silence and possess tumor suppressing effects[24-26]. Transcription factors were incapable of binding to the methylated DNA of their recognition sequences, therefore, the gene transcription was blocked[24,25]. However, the demethylating agent could convert the methylation status and restore the gene expression. SPARC involved in the occurrence and development of certain cancers[27-31]. In concordance with these studies, we observed that the loss of SPARC expression correlated with the aberrant methylation and this loss of expression could be rescued by the demethylating agent 5-Aza-CdR. These data suggested that hypermethylation of the promoter is also an important mechanism for SPARC inactivation in SMMC-7721 cell line. The results of our DNA bisulfite sequencing of the SPARC promoter also displayed that 5-Aza-CdR could convert the methylation status and affect the expression of SPARC.
We observed that SPARC methylation occurred more frequently in HCC tissues than in nontumorous tissues. We tested the same segments of putative CpG island near the transcription start site in HCC samples, and compared with the previous groups[15,32]. The results showed that SPARC methylation was also a relatively higher frequent incident in HCC and the sequencing results validated that there were high-density methylated CpG sites in the amplified region. The distinct methylation status of SPARC gene in the benign and malignant tissues was the prerequisite to determine it as an effective molecular biomarker. SPARC could discriminate HCC from the nontumorous tissues with a high sensitivity and a specificity, suggesting that SPARC methylation may be a promising epigenetic biomarker for the assistant diagnosis of HCC.
In this study, we observed that 65.0% of the HCC samples showed a relatively lower expression level of SPARC mRNA compared with the nontumorous tissues. On the contrary, previous groups have reported that SPARC was overexpressed in HCC tissues as compared with the nontumorous tissues, nevertheless, SPARC mRNA and protein were mainly detected in the tumor capsule, and fibrous bands within HCC[26,33]. SPARC was strongly expressed by the stromal myofibroblasts of HCC[26]. In our study, except for different patient population, we used exclusively tumors with more than 80% of epithelial tumor cells to test the SPARC mRNA expression, which could minimise the potential contamination of stromal cells in HCC. Some studies in other cancers have revealed aberrant hypermethylation of the SPARC promoter to be responsible for low levels of SPARC expression[15,16]. In concordance with these studies, we found that the SPARC expression of samples with methylation was significantly lower than that without methylation. Although there were other possible mechanisms for the downregulation of the SPARC expression, the concordance between the mRNA expression and the DNA methylation indicated that the gene was downregulated, at least partially, through the DNA methylation in HCC. We found no significant correlation between the SPARC protein expression and the DNA methylation. The regulation of the translation process or the degradation of protein might also influence the SPARC protein abundance in HCC tissues. On the other hand, the SPARC protein might be variably expressed by the heterogeneous hypermethylation in one allele of tumor cells. But, interestingly, we also found the SPARC expression in the stromal cells in HCC even though the tumor cells had a negative signal, which was accordant with the report[33].
We demonstrated that the pathological class was the only clinicopathological variable associated with the SPARC methylation and patients with the SPARC methylation tended to have a poorer overall survival after resection in this study. It may be explained by the function of this gene, which was involved in the tumor progression. SPARC could inhibit the progress of tumor by restraining the angiogenesis and affecting the extracellular matrix production[34-36]. Our results suggested a potential clinical use of SPARC methylation as a prognostic marker in patients with HCC. Because SPARC methylation was a kind of DNA marker, it will be possible to detect the status of SPARC methylation in peripheral blood in the future, which might be more convenient and less traumatic than using the pathological tissues. However, since the number of patients in this study is relatively small, these findings need to be verified in a study with more patients and a longer follow-up period.
In conclusion, the results in this study indicated that SPARC promoter hypermethylation in HCC was most likely related to a disease state, which may provide potential diagnostic or predictive markers of this disease.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world. The development of biomarkers for early diagnosis and accurate prognosis of HCC is important for improving patients’ survival. Aberrant DNA methylation of tumor suppressor genes could be used as a new marker for HCC in the future.
Research frontiers
It has been reported that secreted protein acidic and rich in cysteine (SPARC) has tumor suppressing properties to some cancers. Moreover, the SPARC promoter methylation is an important factor in the carcinogenesis of these cancers and may be a promising epigenetic marker for them. However, up to date, there have been few reports about the methylation status in HCC. In this study, the authors detected the status of SPARC methylation in HCC and estimated its clinical implication.
Innovations and breakthroughs
This is the first study to report that SPARC hypermethylation is a high frequent event in HCC. The downregulation of the SPARC mRNA expression in HCC is correlated with the SPARC methylation. The patients with methylated SPARC had a poorer overall survival than those without methylated SPARC.
Applications
The results in this study indicated that SPARC hypermethylation in HCC is most likely related to a disease state, which might be helpful for finding potential diagnostic or predictive markers of this disease.
Peer review
This is a good descriptive study in which authors investigate the methylation status of SPARC in HCC and evaluate its clinical implication.The results are interesting and suggest aberrant methylation is an important mechanism for SPARC inactivation in HCC and SPARC methylation may be a promising biomarker for the diagnosis and prognosis of HCC.
Footnotes
Supported by Tianjin Health Bureau for research projects, No. 09KY04, No. 2010KZ17 and No. 11KG112
Peer reviewer: Zenichi Morise, MD, PhD, Professor and Chairman, Department of Surgery Banbuntane Houtokukai Hospital, Fujita Health University School of Medicine, 3-6-10 Otobashi Nakagawa-ku, Nagoya, Aichi 454-8509, Japan
S- Editor Cheng JX L- Editor Ma JY E- Editor Li JY
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Iakova P, Timchenko L, Timchenko NA. Intracellular signaling and hepatocellular carcinoma. Semin Cancer Biol. 2011;21:28–34. doi: 10.1016/j.semcancer.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun S, Xu MZ, Poon RT, Day PJ, Luk JM. Circulating Lamin B1 (LMNB1) biomarker detects early stages of liver cancer in patients. J Proteome Res. 2010;9:70–78. doi: 10.1021/pr9002118. [DOI] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Suzuki T, Shimamura T, Furukawa H, Matsushita M, Todo S. Recurrence patterns after hepatectomy of hepatocellular carcinoma: implication of Milan criteria utilization. Ann Surg Oncol. 2009;16:1560–1571. doi: 10.1245/s10434-009-0407-7. [DOI] [PubMed] [Google Scholar]
- 7.Jin W, Lee JJ, Kim MS, Son BH, Cho YK, Kim HP. DNA methylation-dependent regulation of TrkA, TrkB, and TrkC genes in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2011;406:89–95. doi: 10.1016/j.bbrc.2011.01.116. [DOI] [PubMed] [Google Scholar]
- 8.Goeppert B, Schmezer P, Dutruel C, Oakes C, Renner M, Breinig M, Warth A, Vogel MN, Mittelbronn M, Mehrabi A, et al. Down-regulation of tumor suppressor A kinase anchor protein 12 in human hepatocarcinogenesis by epigenetic mechanisms. Hepatology. 2010;52:2023–2033. doi: 10.1002/hep.23939. [DOI] [PubMed] [Google Scholar]
- 9.Sun JZ, Yang XX, Li XH, Xu WW, Wang Y, Zhu W, Li M. Aberrant CpG island hypermethylation and down-regulation of Oct-6 mRNA expression in human hepatocellular carcinoma. Dig Dis Sci. 2011;56:3072–3077. doi: 10.1007/s10620-011-1686-y. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Dong H, Robertson K, Liu C. DNA methylation suppresses expression of the urea cycle enzyme carbamoyl phosphate synthetase 1 (CPS1) in human hepatocellular carcinoma. Am J Pathol. 2011;178:652–661. doi: 10.1016/j.ajpath.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamikihara T, Arima T, Kato K, Matsuda T, Kato H, Douchi T, Nagata Y, Nakao M, Wake N. Epigenetic silencing of the imprinted gene ZAC by DNA methylation is an early event in the progression of human ovarian cancer. Int J Cancer. 2005;115:690–700. doi: 10.1002/ijc.20971. [DOI] [PubMed] [Google Scholar]
- 12.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yiu GK, Chan WY, Ng SW, Chan PS, Cheung KK, Berkowitz RS, Mok SC. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159:609–622. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su GH, Hruban RH, Goggins M. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene. 2003;22:5021–5030. doi: 10.1038/sj.onc.1206807. [DOI] [PubMed] [Google Scholar]
- 16.Socha MJ, Said N, Dai Y, Kwong J, Ramalingam P, Trieu V, Desai N, Mok SC, Motamed K. Aberrant promoter methylation of SPARC in ovarian cancer. Neoplasia. 2009;11:126–135. doi: 10.1593/neo.81146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimura T, Nagahara M, Kuo C, Turner RR, Soon-Shiong P, Hoon DS. Lymphovascular invasion of colorectal cancer is correlated to SPARC expression in the tumor stromal microenvironment. Epigenetics. 2011;6:1001–1011. doi: 10.4161/epi.6.8.16063. [DOI] [PubMed] [Google Scholar]
- 18.Larson J, Yasmin T, Sens DA, Zhou XD, Sens MA, Garrett SH, Dunlevy JR, Cao L, Somji S. SPARC gene expression is repressed in human urothelial cells (UROtsa) exposed to or malignantly transformed by cadmium or arsenite. Toxicol Lett. 2010;199:166–172. doi: 10.1016/j.toxlet.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheetham S, Tang MJ, Mesak F, Kennecke H, Owen D, Tai IT. SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-Aza-2’deoxycytidine to increase SPARC expression and improve therapy response. Br J Cancer. 2008;98:1810–1819. doi: 10.1038/sj.bjc.6604377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez-Jiménez FJ, Caldés T, Iniesta P, Vidart JA, Garcia-Asenjo JL, Benito M. Overexpression of SPARC protein contrasts with its transcriptional silencing by aberrant hypermethylation of SPARC CpG-rich region in endometrial carcinoma. Oncol Rep. 2007;17:1301–1307. [PubMed] [Google Scholar]
- 21.Yang B, Du Z, Gao YT, Lou C, Zhang SG, Bai T, Wang YJ, Song WQ. Methylation of Dickkopf-3 as a prognostic factor in cirrhosis-related hepatocellular carcinoma. World J Gastroenterol. 2010;16:755–763. doi: 10.3748/wjg.v16.i6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Yang B, Du Z, Gao YT, Wang YJ, Jing X, Bai T. Identification and validation of specific methylation profile in bile for differential diagnosis of malignant biliary stricture. Clin Biochem. 2010;43:1340–1344. doi: 10.1016/j.clinbiochem.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Lou C, Du Z, Yang B, Gao Y, Wang Y, Fang S. Aberrant DNA methylation profile of hepatocellular carcinoma and surgically resected margin. Cancer Sci. 2009;100:996–1004. doi: 10.1111/j.1349-7006.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D, Da L, Tang H, Li T, Zhao M. CpG methylation plays a vital role in determining tissue- and cell-specific expression of the human cell-death-inducing DFF45-like effector A gene through the regulation of Sp1/Sp3 binding. Nucleic Acids Res. 2008;36:330–341. doi: 10.1093/nar/gkm1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Darwanto A, Linkhart TA, Sowers LC, Zhang L. Maternal cocaine administration causes an epigenetic modification of protein kinase Cepsilon gene expression in fetal rat heart. Mol Pharmacol. 2007;71:1319–1328. doi: 10.1124/mol.106.032011. [DOI] [PubMed] [Google Scholar]
- 26.Lau CP, Poon RT, Cheung ST, Yu WC, Fan ST. SPARC and Hevin expression correlate with tumour angiogenesis in hepatocellular carcinoma. J Pathol. 2006;210:459–468. doi: 10.1002/path.2068. [DOI] [PubMed] [Google Scholar]
- 27.Nagaraju GP, Sharma D. Anti-cancer role of SPARC, an inhibitor of adipogenesis. Cancer Treat Rev. 2011;37:559–566. doi: 10.1016/j.ctrv.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiMartino JF, Lacayo NJ, Varadi M, Li L, Saraiya C, Ravindranath Y, Yu R, Sikic BI, Raimondi SC, Dahl GV. Low or absent SPARC expression in acute myeloid leukemia with MLL rearrangements is associated with sensitivity to growth inhibition by exogenous SPARC protein. Leukemia. 2006;20:426–432. doi: 10.1038/sj.leu.2404102. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Hao C, Takahashi T, Shigematsu H, Shivapurkar N, Sathyanarayana UG, Iizasa T, Fujisawa T, Hiroshima K, Gazdar AF. Aberrant methylation of SPARC in human lung cancers. Br J Cancer. 2005;92:942–948. doi: 10.1038/sj.bjc.6602376. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Heller G, Schmidt WM, Ziegler B, Holzer S, Müllauer L, Bilban M, Zielinski CC, Drach J, Zöchbauer-Müller S. Genome-wide transcriptional response to 5-aza-2’-deoxycytidine and trichostatin a in multiple myeloma cells. Cancer Res. 2008;68:44–54. doi: 10.1158/0008-5472.CAN-07-2531. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Yu Q, Cho AH, Rondeau G, Welsh J, Adamson E, Mercola D, McClelland M. Survey of differentially methylated promoters in prostate cancer cell lines. Neoplasia. 2005;7:748–760. doi: 10.1593/neo.05289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Song J, Huang H, Li Z, Du Y, Cao J, Li M, Lv S, Lin H, Gong Y. Methylation of the SPARC gene promoter and its clinical implication in pancreatic cancer. J Exp Clin Cancer Res. 2010;29:28. doi: 10.1186/1756-9966-29-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Bail B, Faouzi S, Boussarie L, Guirouilh J, Blanc JF, Carles J, Bioulac-Sage P, Balabaud C, Rosenbaum J. Osteonectin/SPARC is overexpressed in human hepatocellular carcinoma. J Pathol. 1999;189:46–52. doi: 10.1002/(SICI)1096-9896(199909)189:1<46::AID-PATH392>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Puolakkainen PA, Brekken RA, Muneer S, Sage EH. Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res. 2004;2:215–224. [PubMed] [Google Scholar]
- 35.Yunker CK, Golembieski W, Lemke N, Schultz CR, Cazacu S, Brodie C, Rempel SA. SPARC-induced increase in glioma matrix and decrease in vascularity are associated with reduced VEGF expression and secretion. Int J Cancer. 2008;122:2735–2743. doi: 10.1002/ijc.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chlenski A, Cohn SL. Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol. 2010;21:55–65. doi: 10.1016/j.semcdb.2009.11.018. [DOI] [PubMed] [Google Scholar]