Abstract
AIM: To assess the effects and safety of Lactobacillus casei rhamnosus LCR35 complete freeze-dried culture (LCR35) in patients suffering from irritable bowel syndrome (IBS).
METHODS: A randomized, double-blind pilot study was performed in 50 patients complaining of IBS symptoms complying with Rome III criteria. Patients were allocated to receive either LCR35 (n = 25) at a minimum daily dose of 6 × 108 colony forming units or placebo (n = 25) for 4 wk. At inclusion, after treatment and 2 wk later, patients completed the IBS severity scale. Change from baseline in the IBS severity score at the end of treatment was the primary efficacy criterion. Changes were compared between groups in the whole population and in IBS subtypes (IBS with predominance of constipation, IBS with predominance of diarrhoea, mixed IBS, unsubtyped IBS). The presence of lactobacillus casei rhamnosus in stools was investigated at inclusion and at the end of treatment. The gastrointestinal quality of life questionnaire and the hospital anxiety and depression (HAD) scale were also completed.
RESULTS: Both groups were balanced for baseline characteristics. In 85% of patients, stool analyses showed that lactobacillus casei rhamnosus able to survive in the digestive tract. In the whole population, improvements in the IBS severity score did not differ significantly between treatments with a 25% decrease after 4-wk treatment, and a 15% decrease from baseline 2 wk later in both groups. In IBS subgroups, statistical analysis could not be performed due to small sample size, but a clinical response in favour of LCR35 was observed in IBS patients with predominance of diarrhoea: no change in the symptom severity score was seen with the placebo after 4 wk treatment, whereas a clinically relevant decrease occurred with LCR35 (-37% vs -3%). Furthermore, in spite of an increase in symptom intensity, the IBS severity score was maintained below the baseline value 2 wk later with LCR35 (-19% from baseline), whilst a slight 5% increase from baseline was observed with placebo. In the IBS subgroup with predominance of diarrhoea only, a clinically relevant decrease in abdominal pain severity score (-36%) was observed with LCR35, whereas no change occurred with placebo. In mixed IBS patients, the 20% and 30% decreases in the IBS severity score observed after treatment with LCR35 and placebo, respectively, were maintained 2 wk later in both groups. A clinical response slightly in favour of placebo was observed at the end of the treatment period in IBS patients with predominance of constipation (-41% vs -20%) and unsubtyped IBS patients (-47% vs -17%), with the same value maintained 2 wk later. In both groups, no clinically relevant changes were observed either for the gastrointestinal quality of life index or HAD score. Thus, these results suggest that sub-grouping of IBS patients may be important for optimizing treatment responses by the physician.
CONCLUSION: This pilot study suggests that LCR35 could have some efficacy in IBS patients complaining of diarrhoea. These preliminary results need to be confirmed in larger studies.
Keywords: Irritable bowel syndrome, Lactobacillus casei rhamnosus, Probiotics, Symptom severity score
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional bowel disorder, with an estimated worldwide prevalence of 10%-20% among adults and adolescents[1]. IBS is the most common diagnosis made by gastroenterologists. IBS contributes considerably to disability, absence from work or school and increased health-care costs[2]. As no curative treatment is available, therapy for IBS is palliative and supportive, targeting specific symptoms, but is notoriously unsatisfactory[3,4].
Studies have observed altered intestinal microflora in IBS patients and an increase in symptoms after enteric infections, suggesting that restoration of the intestinal microflora may be a useful therapeutic goal[5-8].
Lactobacilli are a component of the commensal microbiota of both the small and large intestinal tract of humans and animals. They are frequently used as probiotics and have a long history of safe consumption in food[9]. Probiotics, live microbiologic organisms found in foods and supplements, are supported by enough evidence to recommend their use in the treatment of IBS. This therapeutic class is gaining popularity for the treatment of multiple gastrointestinal disorders and a recent meta-analysis suggests that probiotics offer promise for the treatment of IBS[10]. Probiotics reportedly bind to small and large bowel epithelium and produce substances with antibiotic properties that may inhibit attachment and invasion by pathogenic organisms[11,12]. Probiotics may also modulate gastrointestinal luminal immunity by changing the cytokine and cellular milieu from a pro-inflammatory to anti-inflammatory state[13]. This immunomodulatory effect also attenuates the visceral hypersensitivity characteristic of IBS[7,14]. It has been speculated that each individual bacterial strain or a combination of strains may affect select subclasses of symptoms[15]. Whatever the underlying mechanism, in order to produce their health effects, the probiotic microorganisms must be able to survive within the gastrointestinal tract.
LCR35 complete freeze-dried culture has been successfully exploited commercially as a pharmaceutical product for its antidiarrhoeal properties for more than 50 years. In vitro investigations showed that this strain has probiotic activities such as the ability to adhere to intestinal cells and antibacterial activity against a large variety of pathogens[16]. Colonization by this probiotic in the gastrointestinal tracts of mice and humans has been studied and the findings suggest that LCR35 is able to survive in vivo[17]. In a study on mouse dendritic cells, Lactobacillus casei appears to be a probiotic which, in small concentrations, induces the production of large quantities of anti-inflammatory interleukins[18].
Thus, the therapeutic potential of probiotic bacteria - especially lactobacilli- reported in literature, as well as the research performed on LCR35, suggest a beneficial effect of this strain on the symptomatology of IBS patients.
The objective of this pilot study was to assess the efficacy and tolerability of the completely freeze-dried culture of Lactobacillus casei rhamnosus, LCR35, by measuring its effects on the symptomatology of IBS and evaluating its impact on the gastrointestinal quality of life and anxiety/depression level in patients suffering from IBS satisfying the Rome III diagnostic criteria.
MATERIALS AND METHODS
This was a prospective, multicentre, randomized, double-blind, placebo-controlled pilot trial on two parallel groups. Patients were recruited from the outpatient clinics of the Department of Gastroenterology of 3 university hospitals (in Clermont-Ferrand, Nice and Rouen) and one private medical centre in Clermont-Ferrand. The study was conducted in accordance with Good Clinical Practice (CPMP/ICH/135/95), the French regulations, and the Declaration of Helsinki and subsequent World Medical Assemblies. The trial was approved by the regional Ethics Committee (CPP Sud Est VI) on March 7th, 2008 and was registered by the French Health Authorities with the identifier number 2008-A00010-55.
Patient enrolment
Eligible patients were those fulfilling the Rome III criteria for IBS[19], whatever the subtype of IBS: IBS with predominance of constipation, IBS with predominance of diarrhoea, mixed IBS and unsubtyped IBS. At screening, the Hamilton scale[20] was used to exclude depressive patients. Inclusion criteria were: both genders, age between 18 and 70 years, availability of morphological, radiological and/or endoscopic data verifying the integrity of the digestive tract during the last 5 years, moderate symptom intensity (IBS severity score between 150 and 300 -see below-), efficient contraceptive method for women of child-bearing age. The non-inclusion criteria were: denied written informed consent, immunodeficiency or any serious illness or any progressive disease.
The following treatments were prohibited throughout the trial: other probiotics, antibiotics, anti-inflammatories, and any drugs aiming to treat IBS (antispasmodics, clays, etc.). Paracetamol was authorized to relieve pain at a daily dose ≤ 3 g/d; bisacodyl (no more than one tablet per day) and loperamide (≤ 6 capsules per day) could be used for no more than 2 consecutive days for constipation and diarrhoea, respectively. Psychotropic drugs (antidepressants or anxiolytics) were authorized if patients had been previously treated for several weeks without any modification of the dosage within the month preceding their enrolment into the study.
Procedures and treatment
After a screening visit (V1) performed 10 to 14 d before inclusion, patients had to attend 3 visits over a 6-wk period: V2 on day 0 involved randomization and treatment initiation; V3 was scheduled at the end of the 4-wk treatment period (between day 28-day 32) and V4 was planned 2 wk after the end of treatment (between day 42-day 46).
At screening, after obtaining informed consent, the Rome III criteria were checked. Patients were instructed not to change their eating habits as to dietary fibre intake except for fermented milk and any food supplement likely to contain probiotics which were forbidden throughout the entire study period.
At visit 2, each potentially eligible patient was evaluated by a full review of clinical history and physical examination and their transit was assessed using the Bristol stool form scale[21]. Each subject completed the IBS severity scale[22], the gastrointestinal quality of life index (GIQLI) questionnaire[23] and the hospital anxiety and depression (HAD) scale[24-26]. Subjects eligible for the treatment phase were identified by a serial number and were randomly assigned to receive either LCR35 complete freeze-dried culture or the placebo, in a 1:1 ratio. Each treatment was provided in gelatine capsules and 3 capsules had to be taken once daily in a fasting state for 4 wk. One capsule of LCR35 contained 250 mg of product (total freeze-dried culture of Lactobacillus casei variety rhamnosus with a concentration of at least 2 × 108 CFU). Placebo capsules were identical in all aspects to the verum, thus allowing effective blinding. All capsules had to be taken in the morning while fasting, with a glass of non-alcoholic drink at ambient temperature in order to avoid a decrease in the number of LCR35.
At visit 3, after 4 wk treatment, a clinical examination was performed and patients completed the IBS severity scale, the GIQLI questionnaire, the HAD scale and the Bristol stool form scale. They did the same at visit 4, 2 wk after the end of the treatment.
Adverse events and medication compliance were monitored throughout the study period.
Compliance was also evaluated by the presence or absence of Lactobacillus in the faeces which were collected at inclusion and at the end of the 4-wk treatment period. All samples were aliquoted into 2 faecal culture cups and frozen at -80 °C. After extraction of total bacterial DNA (kit QIAamp Mini Kit for stool QIAGEN), the presence of Lactobacillus casei variety rhamnosus was specifically determined by qualitative polymerase chain reaction (PCR - primer pairs hyb-21[27]) - cycles of amplification [(94 °C, 5 mn - 94 °C, 30 s; 56 °C, 30 s; 72 °C, 1 mn/kb) × 33, 72 °C, 7 mn].
Questionnaires
The IBS severity scoring system is a self-administered questionnaire initially developed and validated by Francis et al[22] of which the French version has been previously validated[28]. It is composed of: (1) two items concerning the presence of abdominal pain and bloating (response yes or no); (2) four visual analogue scales measuring intensity of pain, bloating, relief following defecation and impact of symptoms on general QoL; and (3) an item on the number of days of suffering during the preceding 10 d. It provides a quantitative score ranging from 0 to 500 enabling grouping patients by symptom severity from mild to severe forms [(0-150) = mild, (150-300) = moderate, > 300 = severe]. Furthermore, previous studies have shown a positive correlation between this severity score and QoL of IBS patients[28,29].
In this study, the IBS severity score was used as the primary efficacy variable. Patients with an IBS severity score reduced by 50% after 4 wk of treatment were considered “responders”.
The GIQLI[23] is a validated tool to measure quality of life related to gastrointestinal diseases. The GIQLI questionnaire includes 36 items asking about symptoms, physical status, emotions, social dysfunction, and effects of medical treatment. Higher scores, better GI-specific health-related quality of life.
The HAD scale[24-26] was designed to assess the contribution of mood disorder, especially anxiety and depression, in order to understand the experience of suffering in the setting of medical practice. The lower the HAD score, the lower the depression and anxiety level.
Ethical issues
All patients provided informed consent. Participation in the study was voluntary, and patients were allowed to withdraw at any point without giving an explanation.
Statistical analysis
For this pilot study, due to the lack of significant data in the literature, we arbitrarily considered that 60 subjects would be enrolled.
Statistical analysis was performed using version 9.1.3 Windows of SAS® software. Inclusion was considered as baseline.
The primary efficacy endpoint was the change in the IBS severity score at the end of the 4-wk treatment period. Other efficacy variables were considered as secondary: changes in the IBS severity composite score at the end of the study, changes in the IBS severity score referring to IBS subtypes, distribution of patients according to symptom severity classes, number of responders, changes in the abdominal pain severity score (sub-item of the IBS severity score; pain is one of the key features of many of the functional gastrointestinal disorders), changes in the GIQLI and HAD score.
Efficacy results were similar on the “full analysis set” (FAS) and the “per-protocol set”, therefore, only results based on the FAS are reported.
Absolute and relative changes from baseline in the IBS severity score, the GIQLI and the HAD score were compared between both treatment groups using the two-sided Student’s t-test with a 5% significance level. The same test was used to assess changes in the IBS severity score in the IBS sub-groups (IBS with predominance of constipation, IBS with predominance of diarrhoea, mixed IBS and unsubtyped IBS). The distribution of patients according to IBS severity score classes was described in both groups at each visit. In the whole population and in the four IBS sub-groups, the percentages of “responders” were compared between treatment groups using the χ2 test or the Fisher’s exact test. Data from the Bristol stool form scale could not be analysed because of an important number of missing data.
RESULTS
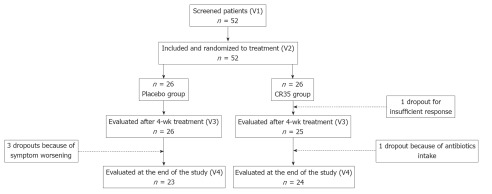
The flow of subjects through the protocol is described in Figure 1.
Figure 1.
Disposition of patients.
Fifty-two patients were screened for the study. All of them fulfilled the inclusion criteria and were randomized equally into two groups. Among the 52 included patients, 5 discontinued and 47 completed the study. Prior to unblinding of the data, 2 patients without primary criterion evaluation at V3 were excluded from the FAS, and 8 subjects were deemed non-evaluable because of major deviations (among them 3 were premature dropouts), thus providing a FAS of 50 patients (25 in each group) and a PP population of 44 (21 in the LCR35 group and 23 in the placebo group).
Baseline characteristics of IBS patients
Table 1 summarizes demographic data and disease-related baseline characteristics of the IBS patients. Except for a higher percentage of mixed IBS patients in the LCR35 group (44.0% vs 24.0%), no clinically relevant difference was observed between the groups. As required by the protocol, patients suffered from IBS symptoms of moderate intensity within the interval (150-300), with a mean value close to the upper values of the class in both groups.
Table 1.
Demographic and disease-related baseline characteristics (mean ± SD)
| Placebo (n = 25) | LCR35 (n = 25) | |
| Sex, n (%) | ||
| Male | 5 (20.0) | 10 (40.0) |
| Female | 20 (80.0) | 15 (60.0) |
| Age (yr) | 48.0 ± 10.8 | 46.1 ± 11.3 |
| Height (cm) | 163.2 ± 7.6 | 168.2 ± 7.6 |
| Weight (kg) | 65.5 ± 13.1 | 66.4 ± 14.9 |
| BMI (kg/m²) | 24.5 ± 4.0 | 23.4 ± 4.9 |
| IBS severity score | 247.1 ± 43.8 | 261.5 ± 39.4 |
| Abdominal pain score | 36.7 ± 20.6 | 44.6 ± 13.2 |
| GIQLI | 62.9 ± 8.6 | 63.9 ± 7.8 |
| HAD score | 16.5 ± 6.4 | 16.3 ± 6.5 |
| IBS subgroups | ||
| IBS with predominance of constipation, n (%) | 7 (28.0) | 4 (16.0) |
| IBS severity score | 270.4 ± 28.4 | 281.5 ± 9.9 |
| IBS with predominance of diarrhoea | 8 (32.0) | 7 (28.0) |
| IBS severity score | 259.6 ± 53.7 | 286.1 ± 11.2 |
| Abdominal pain score | 36.6 ± 27.2 | 51.4 ± 12.4 |
| Mixed IBS, n (%) | 6 (24.0) | 11 (44.0) |
| IBS severity score | 222.3 ± 36.7 | 245.0 ± 42.0 |
| Unsubtyped IBS, n (%) | 4 (16.0) | 3 (12.0) |
| IBS severity score | 218.5 ± 27.1 | 238.0 ± 63.6 |
NB: No statistical difference was found between the groups; BMI: Body mass index; IBS: Irritable bowel syndrome; GIQLI: Gastrointestinal quality of life index; HAD: Hospital anxiety and depression.
Compliance
The presence of Lactobacillus casei rhamnosus in stools was investigated in 27 patients (14 LCR35 and 13 Placebo). In 85% of patients treated with LCR35, Lactobacillus was found by qualitative PCR. In one patient in the placebo group, no data was available to explain the presence of Lactobacillus casei in the faeces collected before and after treatment. Such a result may reflect the presence of LCR35 at a commensal level in some people. Furthermore, in this study, patients suffering from IBS may have been previously treated with probiotics.
Response to treatment
In both groups, no clinically relevant changes vs baseline were observed during the study either for the GIQLI score or for the HAD score.
At the end of the treatment period, a similar improvement in the abdominal pain severity score was observed with the test drug (-13.1 ± 20.5) and the placebo (-11.9 ± 27.5). In patients with predominance of diarrhoea, no change in the abdominal pain severity score was observed with the placebo at the end of the 4-wk treatment period (-0.1 ± 26.5), whereas a clinically relevant decrease occurred with the test drug (-18.4 ± 26.3, i.e., 36%).
In the whole population, the improvements in the IBS severity score observed with LCR35 and placebo were not significantly different. Indeed, after a 25% decrease at the end of the treatment period (-63.2 ± 100.6 and -64.3 ± 95.9, respectively; P = 0.9692), a 15% decrease from baseline was observed 2 wk later in both treatment groups (-40.6 ± 110.1 and -36.0 ± 109.5, respectively; P = 0.8829).
Absolute and relative changes in the four IBS subgroups are presented in Table 2.
Table 2.
Absolute and relative changes from baseline in the irritable bowel syndrome severity score referring to irritable bowel syndrome type (mean ± SD)
|
Placebo (n = 25 ) |
LCR35 (n = 25) |
|||
| Absolute changes | Relative changes (%) | Absolute changes | Relative changes (%) | |
| IBS with predominance of constipation | n = 7 | n = 4 | ||
| Post-treatment (V3-V2) | -109.4 ± 93.1 | -41.0 ± 32.7 | -56.8 ± 43.9 | -20.5 ± 16.5 |
| End of study (V4-V2) | -61.0 ± 96.0 | -23.5 ± 35.0 | -27.5 ± 31.6 | -10.1 ± 11.3 |
| IBS with predominance of diarrhoea | n = 8 | n = 7 | ||
| Post-treatment (V3-V2) | -1.9 ± 82.8 | -3.1 ± 35.6 | -105.0 ± 128.4 | -36.6 ± 44.7 |
| End of study (V4-V2) | 23.9 ± 119.7 | 4.9 ± 46.8 | -54.9 ± 151.7 | -19.1 ± 53.5 |
| Mixed IBS | n = 6 | n = 11 | ||
| Post-treatment (V3-V2) | -70.0 ± 91.4 | -31.2 ± 38.8 | -50.3 ± 99.4 | -21.8 ± 39.7 |
| End of study (V4-V2) | -68.3 ± 110.6 | -30.7 ± 50.1 | -53.3 ± 97.4 | -20.5 ± 39.9 |
| Unsubtyped IBS | n = 4 | n = 3 | ||
| Post-treatment (V3-V2) | -101.8 ± 96.2 | -46.7 ± 46.7 | -22.0 ± 99.9 | -17.4 ± 45.9 |
| End of study (V4-V2) | -63.8 ± 97.7 | -31.3 ± 50.8 | 21.3 ± 140.8 | 4.3 ± 51.0 |
IBS: Irritable bowel syndrome.
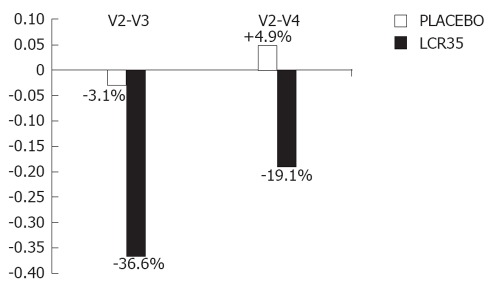
In IBS patients with predominance of diarrhoea, the clinical response was in favour of the active drug. Indeed, no change in the symptom severity score was observed with the placebo at the end of the 4-wk treatment period, whereas a more marked decrease occurred with the test drug (-36.6% vs -3.1%). Furthermore, in spite of an increase in the symptom intensity, the IBS severity score was maintained below the baseline value 2 wk later with the test drug (-19.1% from baseline), whilst a slight 4.9% increase from baseline was observed with the placebo.
Even if no statistical analysis could be performed due to the small sample size of this subgroup, the graphic representation of these results (Figure 2) clearly shows the differences in the clinical responses induced by the test drug and the placebo in IBS patients with predominance of diarrhoea.
Figure 2.
Relative changes in irritable bowel syndrome severity score between V2 and V3, V2 and V4 in irritable bowel syndrome patients with predominance of diarrhoea. V2: Baseline; V3: At the end of the 4-wk treatment period, a more marked decrease in irritable bowel syndrome (IBS) severity score occurred with the test drug (-36.6% vs -3.1% with placebo); V4: Two weeks later, the IBS severity score was maintained below baseline with the test drug (-19.1%), whilst it slightly increased over baseline (+4.9%) with placebo.
In mixed IBS patients, the response observed at the end of the treatment (a 20% and 30% decrease in the IBS severity score with LCR35 and placebo, respectively) was maintained at the end of the study for both treatment groups, with no relevant clinical difference between treatments.
A clinical response slightly in favour of placebo was observed at the end of the treatment period in IBS patients with predominance of constipation (-41% vs -20%) and unsubtyped IBS patients (-47% vs -17%). The same value was maintained 2 wk later.
After 4 wk of treatment, the patient distribution according to the IBS severity score classes was slightly in favour of the placebo (48% vs 32% of improved patients, Table 3), and this observation correlates with the results observed on the main criterion.
Table 3.
Distribution of patients according to the irritable bowel syndrome severity score classes n (%)
| Placebo (n = 25) | LCR35 (n = 25) | |
| Baseline IBS severity score (V2) | ||
| 150-300 (moderate symptoms) | 25 (100.0) | 25 (100.0) |
| Post-treatment IBS severity score (V3) | ||
| 0-150 (mild symptoms) | 12 (48.0) | 8 (32.0) |
| 150-300 (moderate symptoms) | 8 (32.0) | 13 (52.0) |
| > 300 (severe symptoms) | 5 (20.0) | 4 (16.0) |
| IBS severity score at the end of study (V4) | ||
| 0-150 (mild symptoms) | 9 (36.0) | 6 (24.0) |
| 150-300 (moderate symptoms) | 8 (32.0) | 13 (52.0) |
| > 300 (severe symptoms) | 8 (32.0) | 6 (24.0) |
IBS: Irritable bowel syndrome.
The results obtained on the responder rates were in accordance with the results reported above. Indeed, the percentage of patients with a 50% reduction in the IBS severity score was higher in the placebo group (40% vs 28%), except for IBS patients with predominance of diarrhoea who showed a responder rate higher with LCR35 compared to placebo (43% vs 12%).
Adverse events
There were no adverse effects attributable to treatment with either LCR35 or placebo.
DISCUSSION
The results of this placebo-controlled pilot study showed that IBS symptoms assessed by the IBS severity score did not improve with LCR35 complete freeze-dried culture when considering the whole population, and no clinically relevant changes vs baseline were observed either for the GIQLI score or the HAD score. Yet, when considering IBS subtyped patients, it can be seen on the graphic representation of the data that a deterioration in the baseline symptom score was never observed with the test drug, and the line graphs show that the evolution pattern of the IBS severity score differed between the IBS subtypes. Indeed, a clinical response in favour of LCR35 complete freeze-dried culture was observed in IBS patients with predominance of diarrhoea.
The efficacy of therapeutics for IBS is undoubtedly impacted by the heterogeneous pathogenesis of IBS, and up to now there is no recognised reference treatment for this pathology. Results observed in the present study are not surprising because the fact that subgroups of patients with IBS are likely to respond differently to a treatment is often discussed in the literature. Thus, sub-grouping of IBS patients may be important both for optimizing treatment responses by the practicing clinician as well as improving the outcome from clinical trials of novel therapeutic modalities. Thus, some authors also recommend that limiting trials to defined subgroups of patients should be considered to enhance homogeneity of the study population[30,31]. More recently, when validating the Rome III criteria, Longstreth et al[19] emphasize that “due to heterogeneity of IBS and to the fact that bowel pattern subtypes are highly instable, it may be desirable, in both research and practice, to base drug use on a stronger bowel pattern predominance”.
Many papers have discussed the difficulties of the methodology to be used in IBS clinical research, and recommendations have been drawn to minimize bias in trials of functional GI disorders. Nevertheless, there is no consensus on IBS clinical trial methodology; in particular, there is no standardized outcome assessments[10,32]. Major problems with clinical trial design are the multiple presentations of the disease and the placebo response which is extremely variable and high, up to 70%. Therefore, it is recommended that all IBS trials be placebo controlled and it is essential that clinical trials are conducted on consistently identified patients with clearly defined outcome measures. These outcome measures should not only deal with symptom relief but also improvement in quality of life[30]. As the symptomatology of IBS is highly unstable, the so-called placebo responses may equally well be the temporary spontaneous improvements that are part of the condition[33]. Furthermore, there is evidence that psychiatric disorders have an adverse influence on the outcome of irritable bowel syndrome. Thus, accurate measurement of psychological symptoms as predictors of outcome is an important aspect of trial design for IBS therapy, and selection criteria need to take both physical and psychological domains into account[34]. The results of the present study observed in the placebo group confirm the importance of the psychological impact in IBS patients.
The design of the present study complied with the recommendations in the literature. It was double blinded and placebo controlled and used internationally approved diagnostic criteria for a clinical trial in IBS (“Rome III criteria”[19,35]), in order to allow a homogeneous population to be selected. For the assessment of efficacy, a clear well defined outcome measure was chosen as the primary efficacy parameter. Indeed, the IBS severity scale is a tool which was described in the literature as the only IBS symptom severity scale “shown to be responsive to treatment effects”[36]. Thus, the study complied with the recommendations of the Rome Committee[37]. The duration of treatment was based on the evaluation of medicinal products recommendations with a main efficacy criterion assessed after a 4-wk treatment period[38]. As recommended in a recent meta-analysis highlighting important considerations for the design of probiotic controlled trials[32], every effort was made by the investigators to minimize loss-to-follow-up (none occurred in our study) and to adhere to “Intent-to-Treat” principles analyzing all subjects with the group to which they were originally assigned (our main analysis was done on the FAS set).
In our study population, the female predominance for IBS (70%), the mean age of 47.1 years, and the symptom severity as assessed by the IBS severity score were similar to data published in the literature and support the pertinence of our results. The IBS severity score at inclusion was close to the one reported in a French observational study on 1407 patients in gastroenterological practice (254.3 ± 41.9 with a range of 161-299 vs 268.5 ± 85.2 with a range of 10-487) but all of our patients had moderate symptom severity, whilst the observational study included 45% of patients with severe symptom intensity[39]. The distribution of patients according to IBS subtypes (IBS with predominance of constipation: 22%; IBS with predominance of diarrhoea: 30%; mixed IBS: 34%; unsubtyped IBS: 14%) was also similar to that of an observational study carried out in 1092 patients recruited by 159 GPs and 75 gastroenterologists (IBS with predominance of constipation: 22%; IBS with predominance of diarrhoea: 26%; mixed IBS: 29%; unsubtyped IBS: 22%)[40]. The mean value of the GIQLI score at inclusion showed clearly the negative impact of IBS on the QoL of our patients. The baseline value (63.4 ± 8.1) was lower than the value reported in patients in the study carried out to validate the French version of this questionnaire: the mean score was 126 for healthy individuals and 96 for patients[23].
Factors which might explain the absence of statistically significant results in the present trial are as follows: This study was a pilot study performed on a rather small sample size. The results in favour of the test drug might be confirmed with a statistically significant difference vs placebo in a future trial on a larger number of patients and, as discussed above, on a defined subgroup of patients (IBS patients with predominance of diarrhoea and mixed IBS subtypes).
Regarding the tool used to assess QoL, it must be pointed out that the GIQLI questionnaire is a generalist questionnaire for gastroenterological practice. As the QoL is known to be particularly altered in patients complaining of diarrhoea, it may be argued that this evaluation tool was not adapted to assess accurately the impact of diarrhoea on daily QoL.
In our study performed by gastroenterologists, patients suffered from marked IBS symptoms with a marked negative impact on QoL as shown by baseline values of IBS severity score (mean value close to the upper values of the moderate intensity class) and GIQLI (30% lower than in patients involved in the study which validated the French version of this questionnaire). The question of the likely impact of recruitment site has been often addressed in the literature[19].
In the French observational study carried out in 2000, the descriptive analysis of management practices demonstrated that patients who referred to gastroenterologists have a rather severe chronic form of IBS. Moreover, a search for a relationship between the qualitative score and the number of consultations nevertheless demonstrated that most patients first consult a general practitioner despite the fact that at that time there was access to specialists in the French healthcare system[39].
Two recent meta-analyses of randomized controlled trials on probiotics for the treatment of IBS showed heterogeneity across studies as to the outcome measures used to assess the severity of IBS symptoms, making it challenging to compare results across studies. Both meta-analyses selected the proportion of subjects with improvement in global IBS symptoms as the primary outcome to demonstrate that probiotics may improve IBS symptoms[10,32]. Thus, it is not possible to compare the results obtained with LCR35 complete freeze-dried culture in this study to results published for other probiotics.
The tolerability of LCR35 complete freeze-dried culture prescribed at the minimum daily dose of 6 × 108 CFU for 4 wk was excellent, and no adverse event was reported throughout the trial in the active group. This dose, used in several published studies, is the dose usually prescribed in daily practice for IBS patients[41-44]. The good tolerability displayed in this study is in accordance with the McFarland’s review of probiotics controlled trials which did not find any evidence of significant adverse effects due to these treatments[32]. Given their superior safety profile compared to drug therapies usually prescribed in IBS, and the efficacy results observed with some probiotics against all of the primary IBS symptoms[13], as well as the impact of many probiotics on “gas-related” symptoms[45], probiotics may ultimately prove more acceptable for long-term therapy than medications with adverse effects.
As functional bowel disorders are diseases without morbi-mortality, treatments prescribed should not be more deleterious than the disorder itself[46,47]. Therapies should focus on specific gastrointestinal dysfunctions (e.g., constipation, diarrhoea, pain), and medications only should be used when non-prescription remedies do not work or when symptoms are severe.
This study showed that in 85% of patients treated with LCR35, Lactobacillus was found in their stools with a concentration of at least 104 living bacteria per gram, indicating that survival in the digestive tract is possible.
As in any pilot study, this study did not aim to definitely demonstrate the efficacy of LCR35 complete freeze-dried culture in IBS patients. It was designed to test the trend in the magnitude of variation in clinical response measures, to evaluate the effect size in an attempt to predict an appropriate sample size and improve upon the study design prior to performance of a full-scale research project. Thus, it is not surprising that small sample size, a strong placebo effect and the lack of uniformity of patients led to results that did not reach statistical significance in the global population. Nevertheless, in IBS patients complaining of diarrhoea, the trend to lower global symptom score and abdominal pain sub-score (pain being the most bothersome symptom in IBS patients) after treatment observed with the test drug but not with the placebo, is an interesting observation suggesting that LCR35 complete freeze-dried culture might be useful in this subgroup of IBS patients. This observation made in sub-typed patients is in accordance with the fact that it is recognized that no drug is effective in treating all IBS symptoms because a variety of processes appear to be at work in this disorder and IBS sufferers are not a homogeneous population. As a precise characterization of patients is likely to lead to better therapeutic results, our results are encouraging and need to be confirmed in larger studies. Safety is a main concern in patients with gastrointestinal disorders, and deleterious adverse events are not acceptable in a relatively mild, non-fatal condition. The excellent safety profile of LCR35 complete freeze-dried culture shown in this study makes this probiotic strain, demonstrated to survive in the digestive tract, a reasonable choice for IBS.
ACKNOWLEDGMENTS
We are grateful to Lionel Bueno for his involvement in protocol writing. Dr. Raffaella Dainese is acknowledged for her involvement as co-investigator in the Department of Gastroenterology, Hôpital de l’Archet 2, Nice, France. Stéphane Lavigne and Brigitte Sarrazin-Rouland (ITEC Services) are acknowledged for data statistical analysis and for writing this manuscript, respectively.
COMMENTS
Background
Irritable bowel syndrome (IBS) is the most common diagnosis made by gastroenterologists. Despite its prevalence and its impact on quality of life and health expenditures, conventional medical treatment is notoriously unsatisfactory, and many patients try alternative or complementary therapies. Among them, probiotics are generating great interest.
Research frontiers
Studies have observed altered intestinal microflora in IBS patients and an increase in symptoms after enteric infections, suggesting that restoration of the intestinal microflora may be a useful therapeutic goal. One strategy to restore normal flora is the use of probiotics. Probiotics are beneficial bacteria or yeasts that are ingested to improve health. Probiotics are also known to modulate the immune response and reduce cytokine production. This pilot study investigated the efficacy and safety of Lactobacillus casei rhamnosus LCR35, a probiotic used for its antidiarrhoeal properties for more than 50 years and shown in vitro to adhere to intestinal cells and to display antibacterial activity against a large variety of pathogens. Major problems in clinical research on IBS are the multiple presentations of this disease, the high placebo response and the absence of any consensus on the main outcome measure.
Innovations and breakthroughs
This study showed a clinically significant improvement in global symptomatology, and especially in abdominal pain (the most bothersome symptom in this pathology), in one subgroup of patients called “IBS patients with predominance of diarrhoea”. Stool analysis demonstrated that Lactobacillus casei rhamnosus LCR35 was able to survive in the digestive tract. The improvement in symptom severity observed in sub-typed patients is in accordance with the fact that it is recognized that no drug is effective in treating all IBS symptoms because IBS sufferers are not a homogeneous population. The small sample size, a strong placebo effect and the lack of uniformity of patients may contribute to the absence of significant results in the global population. The clinical results and the excellent safety profile of LCR35 shown in this study make this probiotic strain a reasonable choice for IBS.
Applications
The findings in this pilot study indicate that subgrouping of patients with IBS may be important both for optimizing treatment responses by the practicing clinician as well as improving the outcome from future clinical trials on larger numbers of patients.
Peer review
In this pilot study, the authors evaluate the efficacy and safety profile of a newer probiotic in IBS patients. Treatment of IBS is still largely unsatisfactory, and thus newer treatments would add to the armamentarium of IBS therapy. The question posed by the authors is novel and well defined. However, the title should probably be changed to better reflect the nature of the study (e.g., “Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: A randomized, double-blind study”). The methods are appropriate and well described. The data are sound and well controlled. The discussion and conclusions are well balanced and adequately supported by the data. On the other hand, the sample size is small, though the authors have stated this clearly as a limitation of their study.
Footnotes
Supported by Laboratoires Lyocentre
Peer reviewers: Adolfo Benages, Professor, University of Valencia, Avda, Blasco Ibanez 15, 46010 Valencia, Spain; Mohammad Abdollahi, Professor, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran
S- Editor Cheng JX L- Editor Webster JR E- Editor Xiong L
References
- 1.Malinen E, Krogius-Kurikka L, Lyra A, Nikkilä J, Jääskeläinen A, Rinttilä T, Vilpponen-Salmela T, von Wright AJ, Palva A. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J Gastroenterol. 2010;16:4532–4540. doi: 10.3748/wjg.v16.i36.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paré P, Gray J, Lam S, Balshaw R, Khorasheh S, Barbeau M, Kelly S, McBurney CR. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther. 2006;28:1726–1735; discussion 1726-1735. doi: 10.1016/j.clinthera.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Cremonini F, Talley NJ. Treatments targeting putative mechanisms in irritable bowel syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005;2:82–88. doi: 10.1038/ncpgasthep0096. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A, Whorwell PJ. Irritable bowel syndrome: diagnosis and management. BMJ. 2006;332:280–283. doi: 10.1136/bmj.332.7536.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 6.Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 7.Quigley EM, Flourie B. Probiotics and irritable bowel syndrome: a rationale for their use and an assessment of the evidence to date. Neurogastroenterol Motil. 2007;19:166–172. doi: 10.1111/j.1365-2982.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- 8.Spiller RC. Role of infection in irritable bowel syndrome. J Gastroenterol. 2007;42 Suppl 17:41–47. doi: 10.1007/s00535-006-1925-8. [DOI] [PubMed] [Google Scholar]
- 9. Available from: http://www.accessdata.fda.gov/scripts/fcn/fcnNavigation.cfm?rpt=grasListing. [Google Scholar]
- 10.Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775–1780. doi: 10.1007/s10350-008-9335-z. [DOI] [PubMed] [Google Scholar]
- 11.Johansson ML, Molin G, Jeppsson B, Nobaek S, Ahrné S, Bengmark S. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effect on the indigenous flora. Appl Environ Microbiol. 1993;59:15–20. doi: 10.1128/aem.59.1.15-20.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alander M, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Recovery of Lactobacillus rhamnosus GG from human colonic biopsies. Lett Appl Microbiol. 1997;24:361–364. doi: 10.1046/j.1472-765x.1997.00140.x. [DOI] [PubMed] [Google Scholar]
- 13.O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M. Probiotics and irritable bowel syndrome: rationale, putative mechanisms, and evidence of clinical efficacy. J Clin Gastroenterol. 2006;40:264–269. doi: 10.1097/00004836-200603000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Floch MH. Use of diet and probiotic therapy in the irritable bowel syndrome: analysis of the literature. J Clin Gastroenterol. 2005;39:S243–S246. doi: 10.1097/01.mcg.0000156104.67505.5b. [DOI] [PubMed] [Google Scholar]
- 16.Forestier C, De Champs C, Vatoux C, Joly B. Probiotic activities of Lactobacillus casei rhamnosus: in vitro adherence to intestinal cells and antimicrobial properties. Res Microbiol. 2001;152:167–173. doi: 10.1016/s0923-2508(01)01188-3. [DOI] [PubMed] [Google Scholar]
- 17.de Champs C, Maroncle N, Balestrino D, Rich C, Forestier C. Persistence of colonization of intestinal mucosa by a probiotic strain, Lactobacillus casei subsp. rhamnosus Lcr35, after oral consumption. J Clin Microbiol. 2003;41:1270–1273. doi: 10.1128/JCM.41.3.1270-1273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 19.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 21.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 22.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 23.Slim K, Bousquet J, Kwiatkowski F, Lescure G, Pezet D, Chipponi J. [First validation of the French version of the Gastrointestinal Quality of Life Index (GIQLI)] Gastroenterol Clin Biol. 1999;23:25–31. [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lépine JP, Godchau M, Brun P, Lempérière T. [Evaluation of anxiety and depression among patients hospitalized on an internal medicine service] Ann Med Psychol (Paris) 1985;143:175–189. [PubMed] [Google Scholar]
- 27.Coudeyras S, Marchandin H, Fajon C, Forestier C. Taxonomic and strain-specific identification of the probiotic strain Lactobacillus rhamnosus 35 within the Lactobacillus casei group. Appl Environ Microbiol. 2008;74:2679–2689. doi: 10.1128/AEM.02286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coffin B, Dapoigny M, Cloarec D, Comet D, Dyard F. Relationship between severity of symptoms and quality of life in 858 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2004;28:11–15. doi: 10.1016/s0399-8320(04)94834-8. [DOI] [PubMed] [Google Scholar]
- 29.Sabate JM, Veyrac M, Mion F, Siproudhis L, Ducrotte P, Zerbib F, Grimaud JC, Dapoigny M, Dyard F, Coffin B. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:484–490. doi: 10.1111/j.1365-2036.2008.03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomised controlled trials. Gut. 2001;48:272–282. doi: 10.1136/gut.48.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehead WE. Patient subgroups in irritable bowel syndrome that can be defined by symptom evaluation and physical examination. Am J Med. 1999;107:33S–40S. doi: 10.1016/s0002-9343(99)00078-9. [DOI] [PubMed] [Google Scholar]
- 32.McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650–2661. doi: 10.3748/wjg.14.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkey CJ. Irritable bowel syndrome clinical trial design: future needs. Am J Med. 1999;107:98S–102S. doi: 10.1016/s0002-9343(99)00087-x. [DOI] [PubMed] [Google Scholar]
- 34.Creed F. The relationship between psychosocial parameters and outcome in irritable bowel syndrome. Am J Med. 1999;107:74S–80S. doi: 10.1016/s0002-9343(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 35.Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–1551. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 36.Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable bowel syndrome: end points and regulatory hurdles. Gastroenterology. 2008;135:1877–1891. doi: 10.1053/j.gastro.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, Peterson W, Quigley E, Schoenfeld P, Schuster M, Talley N. Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S7–26. doi: 10.1016/s0002-9270(02)05657-5. [DOI] [PubMed] [Google Scholar]
- 38.Committee for Proprietary Medical Products (CPMP) Points to consider on the evaluation of medicinal products for the treatment of irritable bowel syndrome. London, England: European Agency for the Evaluation of Medicinal Products;; 2003. [Google Scholar]
- 39.Dapoigny M, Dyard F, Grimaud JC, Guyot P, van Ganse E. [Irritable bowel syndrome and healthcare consumption. An observational study in private gastroenterology] Gastroenterol Clin Biol. 2003;27:265–271. [PubMed] [Google Scholar]
- 40.Dapoigny M, Vray M, Albert-Marty A. P.31 Etude observationnelle des troubles fonctionnels intestinaux (TFI) définis selonles critères deRomeIII (CRIII) Gastroenterol Clin Biol. 2009;33 Suppl 1:A34. [Google Scholar]
- 41.Halpern GM, Prindiville T, Blankenburg M, Hsia T, Gershwin ME. Treatment of irritable bowel syndrome with Lacteol Fort: a randomized, double-blind, cross-over trial. Am J Gastroenterol. 1996;91:1579–1585. [PubMed] [Google Scholar]
- 42.Niv E, Naftali T, Hallak R, Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome--a double blind, placebo-controlled, randomized study. Clin Nutr. 2005;24:925–931. doi: 10.1016/j.clnu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 44.Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, Kim YH, Kim JJ, Rhee JC, Rhee PL. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53:2714–2718. doi: 10.1007/s10620-007-0196-4. [DOI] [PubMed] [Google Scholar]
- 45.Quigley EM. Germs, gas and the gut; the evolving role of the enteric flora in IBS. Am J Gastroenterol. 2006;101:334–335. doi: 10.1111/j.1572-0241.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 46.Farthing MJ. Treatment options in irritable bowel syndrome. Best Pract Res Clin Gastroenterol. 2004;18:773–786. doi: 10.1016/j.bpg.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Bergmann JF. [Functional bowel disorders: caring without understanding and treating without curing?] Gastroenterol Clin Biol. 2003;27:263–264. [PubMed] [Google Scholar]